Abstract
Objective
Patients with systemic lupus erythematosus (SLE) have an increased risk of cardiovascular and cerebrovascular events (CCEs). Furthermore, CCE was a significant factor contributing to mortality in patients with SLE. However, no clinical model exists that can predict which patients are at high risk. The purpose of this study was to develop a practical model for predicting the risk of CCE in people with SLE.
Methods
This study was based on the Chinese SLE Treatment and Research Group cohort. A total of 2399 patients, who had a follow-up period of over 3 years and were diagnosed with SLE for less than 1 year at the start of the study, were included. Cox proportional hazards regression and least absolute shrinkage and selection operator regression were used to establish the model. Internal validation was performed, and the predictive power of the model was evaluated.
Results
During the follow-up period, 93 patients had CCEs. The prediction model included nine variables: male gender, smoking, hypertension, age of SLE onset >40, cutaneous involvement, arthritis, anti-β2GP1 antibody positivity, high-dose glucocorticoids and hydroxychloroquine usage. The model’s C index was 0.801. Patients with a prognostic index over 0.544 were classified into the high-risk group.
Conclusion
We have developed a predictive model that uses clinical indicators to assess the probability of CCE in patients diagnosed with SLE. This model has the ability to precisely predict the risk of CCE in patients with SLE. We recommended using this model in the routine assessment of patients with SLE.
Keywords: Systemic Lupus Erythematosus, Cardiovascular Diseases, Autoimmune Diseases
WHAT IS ALREADY KNOWN ON THIS TOPIC
Individuals diagnosed with systemic lupus erythematosus (SLE) experience a greater frequency of cardiovascular and cerebrovascular event (CCE), which significantly adds to mortality among SLE.
There is currently no clinical prediction model available that can identify those high-risk patients.
WHAT THIS STUDY ADDS
A new prediction model with clinical indicators was developed and validated in this study.
For the convenience of clinical practice, we also proposed the risk stratification based on the model.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
The risk of CCE can be evaluated for patients when they are diagnosed with SLE.
Closer monitoring and tighter control of the risk factors were recommended for the high-risk patients.
Introduction
Systemic lupus erythematosus (SLE) is an autoimmune disease that involves multiple organs.1 2 Organ damages result from autoimmune reactions with one’s own tissue and cause the majority of harm to health and life quality. About 7.2% of Chinese patients with SLE have cardiovascular involvement.3 The risk of cardiovascular disease (CVD) in patients with SLE has been reported to increase by two times.4 Previous research also demonstrated that cardiovascular and cerebrovascular events (CCEs) were the fourth leading cause of death of Chinese patients with SLE, after malignancy, infections and active lupus itself.3 Meanwhile, the CVD-specific standardised mortality ratio of patients with SLE was reported to be 2.25.5 The mechanisms that drive CCE development in SLE are complex and not entirely understood. Antiphospholipid antibodies (aPLs) have been found to play a role in causing CCE in SLE.6 A prior study has revealed that endothelial dysfunction contributes to the pathogenesis of CCE in SLE.7 Traditional risk factors for CCE, including age, hypertension, diabetes mellitus and hyperlipidaemia, have been proven to cause a higher incidence of CCE in patients with SLE.8 9 Non-traditional risk factors like renal involvement, aPLs positivity and overproduction of C reactive protein have also been reported.10,13 Due to the high incidence of cardiovascular events in patients with SLE, which lead to adverse outcomes, there is a pressing requirement for a practical clinical prediction model. However, traditional models, like Framingham and SCORE (Systematic COronary Risk Evaluation), do not include SLE-specific risk factors, which limits their applicability in these patients.14 15 The conventional risk score systems were also proven to underperform in patients with SLE.16
To our knowledge, there is currently no clinical prediction model for CCE in patients with SLE. The aim of this study is to establish a practical prediction model based on the Chinese SLE Treatment and Research Group (CSTAR) cohort to instruct early detection and intervention for high-risk patients.
Methods
Patients
This study is based on CSTAR, which is the largest multicentre cohort of Chinese patients with SLE with 331 rheumatology centres nationwide participating. The enrolled patients are mainly Chinese patients with SLE from provinces across the country, and all of them have visited the rheumatology centres of CSTAR. The inclusion criteria were fulfilment of the 2012 Systemic Lupus International Collaborating Clinics (SLICC) classification criteria for SLE.17 18 In addition, only those who had a complete follow-up period of more than 3 years and were diagnosed with SLE for less than 1 year at the beginning of the study were included. Patients with prior CCEs before cohort entry were excluded. A total of 2399 patients were ultimately enrolled in this study. Prior to their registration, all patients have provided signed written informed permission.
Data collection
The previously designed protocol was uniformly performed in all centres of CSTAR for data acquisition and evaluation.2 The baseline was defined as the first time the patient visited CSTAR rheumatology centres. The baseline and follow-up evaluations were prospectively collected, including demographic characteristics, SLE manifestations, laboratory exams, autoimmune antibodies, medical history and treatment strategies. Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI-2K) was used to define SLE disease activity state.18 The demographic data were recorded based on self-reports and the data from China Medical Insurance Bureau. The manifestations of SLE were recorded according to 2012 SLICC classification criteria for SLE.18 The autoantibodies were detected according to the consensus on quality control of China.19 CSTAR investigators were blinded with regard to the outcomes reviewed when recording the clinical evaluation data, and all data were finally classified in a structured and standardised format.
Clinical outcome
The study’s endpoint was the first occurrence of CCE after baseline. CCE included stroke, heart failure (HF) events caused by ischaemic disease, cardiac mortality and acute coronary syndromes (ACS).20 The CCEs were diagnosed by qualified medical institutions or reported as the cause of death of the patients. The CCEs were diagnosed and reported in the centres of CSTAR. The death causes were collected by Chinese Center for Disease Control and Prevention and reported to CSTAR.
Statistical analysis
When demonstrating the baseline data, categorical data were presented as percentages, and continuous data in normal distribution were shown as mean and SE. Student’s t-test was performed to compare continuous variables in the normal distribution, and Pearson χ2 test or Fisher’s exact test was used for categorical variables. Univariate Cox regression was used for estimating the HR of each candidate variable, and multivariate Cox regression was performed to establish the model. All of the statistical analysis was performed with R V.4.3.1.
Development and validation of the prediction model
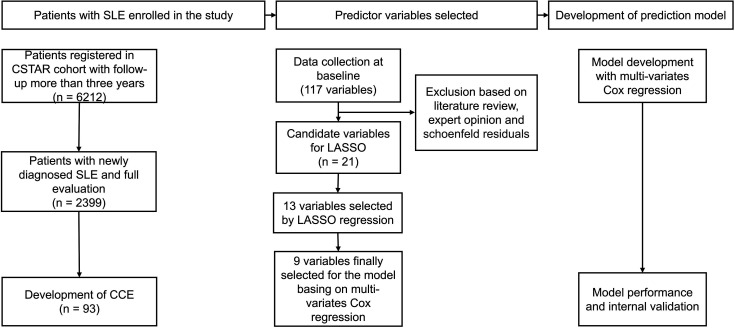
The design of this study was shown in figure 1. The least absolute shrinkage and selection operator (LASSO) Cox model was used to select the most predictive variables from the 21 potential candidate variables selected according to the expert opinions. The lambda was determined through 10-fold cross validation. The 13 variables that were derived from the LASSO regression were subjected to multivariate Cox regression. Ultimately, nine significant variables were incorporated into the final prediction model. The Cox proportional hazards assumption for each covariate was tested using Schoenfeld residuals. The cumulative risk of CCE occurrence in patients with SLE was calculated according to the following formula, in which S0(t) referred to the average survival probability at time t and the prognostic index was the sum of the variables multiplied by their coefficients.
Figure 1. Flow chart of the study design. The study included 2399 newly diagnosed patients with systemic lupus erythematosus (SLE) with a full evaluation, of whom 93 experienced cardiovascular and cerebrovascular events (CCEs) during the follow-up. We input 21 candidate variables into the least absolute shrinkage and selection operator (LASSO) regression model and multivariate Cox regression model, ultimately selecting 9 variables for model construction. Internal validation was performed, and model performance was then evaluated. CSTAR, Chinese SLE Treatment and Research Group.
Internal validation was performed with the bootstrap method, and the performance of the model was evaluated with Harrell’s concordance index and calibration curve.21 The decision curve analysis performed to assess the net benefit of our model.
Risk stratification
In order to stratify the risk of CCE development in patients with SLE, the ROC (receiver operating characteristic) curve of the model was plotted, and the point (0.544) with the maximum Youden’s index was selected as the cut-off point for high risk.
Results
Characteristics of patients with SLE on registration
Among the 2399 patients with SLE included in this study, 93 experienced CCEs (stroke=28, HF caused by ischaemic disease=8, cardiac mortality=9 and ACS=48) during the follow-up period. All the baseline characteristics of the patients are shown in table 1. At baseline, the mean age of the patients was 32.2 years, and only 7.6% of them were men. As displayed in table 1, the average time interval from SLE onset to the baseline was only 0.18 years, and the mean SLEDAI was 8.00, indicating those newly diagnosed patients with SLE were in a relatively active state on registration. In this study, the cohort’s median follow-up was 4.81 years.
Table 1. Demographic and clinical data at baseline patients.
| Overall | Without CCE | With CCE | P value | |
| n | 2399 | 2306 | 93 | |
| Demographic characteristics | ||||
| Male sex, N (%) | 183 (7.6) | 164 (7.1) | 19 (20.4) | <0.001 |
| Age, mean (SD) | 33.21 (12.75) | 32.68 (12.18) | 46.36 (18.27) | <0.001 |
| Obesity, N (%) | 247 (10.3) | 229 (9.9) | 18 (19.4) | 0.006 |
| Traditional CCE risk factors | ||||
| Hypertension, N (%) | 240 (10.0) | 216 (9.4) | 24 (25.8) | <0.001 |
| Diabetes mellitus, N (%) | 41 (1.7) | 36 (1.6) | 5 (5.4) | 0.018 |
| Smoking, N (%) | 53 (2.2) | 42 (1.8) | 11 (11.8) | <0.001 |
| Hyperlipidaemia, N (%) | 246 (10.3) | 232 (10.1) | 14 (15.1) | 0.167 |
| SLE-related characteristics | ||||
| Age at SLE onset >40, N (%) | 571 (23.8) | 517 (22.4) | 54 (58.1) | <0.001 |
| Duration of SLE (mean (SD)) | 0.18 (0.37) | 0.18 (0.38) | 0.18 (0.24) | 0.986 |
| Cutaneous involvement, N (%) | 566 (23.6) | 551 (23.9) | 15 (16.1) | 0.109 |
| Nonscarring alopecia, N (%) | 950 (39.6) | 919 (39.9) | 31 (33.3) | 0.249 |
| Oral or nasal ulcers, N (%) | 204 (8.5) | 196 (8.5) | 8 (8.6) | 1.000 |
| Arthritis, N (%) | 682 (28.4) | 666 (28.9) | 16 (17.2) | 0.020 |
| Serositis, N (%) | 288 (12.0) | 273 (11.8) | 15 (16.1) | 0.278 |
| Nephritis, N (%) | 834 (34.8) | 795 (34.5) | 39 (41.9) | 0.171 |
| Neuropsychiatric SLE, N (%) | 67 (2.8) | 63 (2.7) | 4 (4.3) | 0.562 |
| Anaemia, N (%) | 655 (27.3) | 612 (26.5) | 43 (46.2) | <0.001 |
| Leucopenia, N (%) | 380 (15.8) | 364 (15.8) | 16 (17.2) | 0.824 |
| Thrombocytopenia, N (%) | 367 (15.3) | 345 (15.0) | 22 (23.7) | 0.033 |
| Hypocomplementaemia, N (%) | 1394 (58.1) | 1339 (58.1) | 55 (59.1) | 0.921 |
| SLE disease activity index, mean (SD) | 8.00 (7.05) | 7.98 (7.04) | 8.54 (7.20) | 0.454 |
| Antibody positivity | ||||
| ANA, N (%) | 2334 (97.3) | 2241 (97.2) | 93 (100.0) | 0.188 |
| Anti-dsDNA, N (%) | 1697 (70.7) | 1635 (70.9) | 62 (66.7) | 0.445 |
| Anti-Sm, N (%) | 915 (38.1) | 880 (38.2) | 35 (37.6) | 1.000 |
| Anti-SSA, N (%) | 762 (31.8) | 721 (31.3) | 41 (44.1) | 0.013 |
| Anti-SSB, N (%) | 266 (11.1) | 251 (10.9) | 15 (16.1) | 0.158 |
| Anti-rRNP, N (%) | 289 (12.0) | 280 (12.1) | 9 (9.7) | 0.580 |
| Anti-RNP, N (%) | 494 (20.6) | 469 (20.3) | 25 (26.9) | 0.162 |
| LA, N (%) | 105 (4.4) | 98 (4.2) | 7 (7.5) | 0.209 |
| ACL, N (%) | 185 (7.7) | 170 (7.4) | 15 (16.1) | 0.004 |
| Anti-β2GP1, N (%) | 195 (8.1) | 179 (7.8) | 16 (17.2) | 0.002 |
| Treatment | ||||
| Glucocorticoids, N (%) | 1599 (66.7) | 1531 (66.4) | 68 (73.1) | 0.216 |
| Maximal glucocorticoids dose, mg/d, mean (SD) | 45.89 (150.1) | 43.75 (143.8) | 98.95 (257.8) | 0.001 |
| High-dose glucocorticoids, N (%) | 179 (7.5) | 162 (7.0) | 17 (18.3) | <0.001 |
| HCQ, N (%) | 1398 (58.3) | 1356 (58.8) | 42 (45.2) | 0.012 |
| MTX, N (%) | 150 (6.3) | 147 (6.4) | 3 (3.2) | 0.312 |
| CTX, N (%) | 344 (14.3) | 316 (13.7) | 28 (30.1) | <0.001 |
| MMF, N (%) | 283 (11.8) | 276 (12.0) | 7 (7.5) | 0.255 |
| CsA, N (%) | 83 (3.5) | 80 (3.5) | 3 (3.2) | 1.000 |
| TAC, N (%) | 78 (3.3) | 77 (3.3) | 1 (1.1) | 0.364 |
ACLanticardiolipin antibodyANAantinuclear antibodyCCEscardiovascular and cerebrovascular eventsCsAcyclosporin ACTXcyclophosphamideHCQhydroxychloroquineLAlupus anticoagulantMMFmycophenolate mofetilMTXmethotrexateSLEsystemic lupus erythematosusTACtacrolimus
Selection of candidate variables
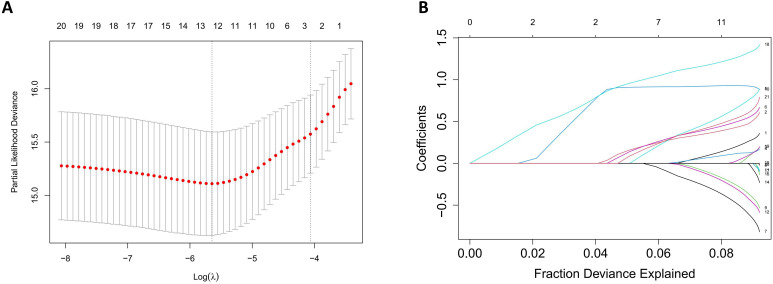
First of all, 21 variables were selected based on experts’ opinions (online supplemental table 1). Univariate Cox proportional hazard regression was performed to analyse the HR of each single variable, and the results are shown in table 2. To further filter variables and avoid overfitting issues, we entered 21 variables into the LASSO regression model, which can filter out the variables most relevant to the endpoint, reducing the complexity of the model (figure 2). Thirteen variables were then selected and included in the multivariate Cox regression from which nine statistically significant variables were included in the final predict model. Detailed statistical results are available in online supplemental table 2.
Table 2. Analysis for risk of cardiovascular and cerebrovascular event (CCE) development with univariate Cox proportional hazards regression models.
| Characteristics | HR (95% CI) | P value |
| Male gender | 3.25 (1.96 to 5.38) | <0.001 |
| Age of SLE onset >40 | 4.98 (3.3 to 7.53) | <0.001 |
| Obesity | 1.98 (1.18 to 3.31) | 0.0096 |
| Hypertension | 2.96 (1.86 to 4.72) | <0.001 |
| Diabetes mellitus | 3.42 (1.39 to 8.42) | 0.0076 |
| Hyperlipidaemia | 1.07 (0.6 to 1.91) | 0.82 |
| Smoking | 6.58 (3.51 to 12.36) | <0.001 |
| Arthritis | 0.49 (0.29 to 0.84) | 0.0094 |
| Serositis | 1.32 (0.76 to 2.29) | 0.33 |
| Oral or nasal ulcers | 0.89 (0.43 to 1.85) | 0.76 |
| Cutaneous involvement | 0.46 (0.26 to 0.8) | 0.0063 |
| Nonscarring alopecia | 0.78 (0.51 to 1.2) | 0.25 |
| Nephritis | 1.25 (0.83 to 1.88) | 0.29 |
| Neuropsychiatric SLE | 1.38 (0.51 to 3.76) | 0.53 |
| Thrombocytopenia | 1.66 (1.03 to 2.68) | 0.038 |
| Leucopenia | 1.06 (0.62 to 1.81) | 0.84 |
| LA | 1.85 (0.86 to 4) | 0.12 |
| ACL | 2.17 (1.25 to 3.77) | 0.0062 |
| Anti-β2GP1 | 2.32 (1.35 to 3.98) | 0.0022 |
| High-dose glucocorticoids | 2.58 | <0.001 |
| HCQ usage | 0.66 (0.44 to 1) | 0.052 |
ACLanticardiolipin antibodyHCQhydroxychloroquineLAlupus anticoagulantSLEsystemic lupus erythematosus
Figure 2. Variable selection with the least absolute shrinkage and selection operator (LASSO) regression model. (A) 10-fold cross validation in the LASSO regression model. The solid vertical line with a red dot represents the cross-validation curve and the SE of partial likelihood deviance. The vertical dot line represents the optimal lambda value. The lambda of 0.00293 was selected for the LASSO regression. (B) The coefficient profiles of the 21 candidate variables. The L1 norm is a regularisation term to prevent overfitting problems. Each coloured line represents a candidate variable.
Development of the predict model
The whole set of data (2399 patients with 93 events) was used to establish the model, since there were no missing data. The nine risk factors included were male gender, smoking, hypertension, age of SLE onset >40, cutaneous involvement, arthritis, anti-β2GP1 antibody positivity, high-dose glucocorticoids and hydroxychloroquine (HCQ) usage, among which cutaneous involvement, arthritis and HCQ usage were protective. The HRs were calculated by fitting the multivariate Cox model and shown in table 3. The cumulative risk for CCE occurrence was calculated according to the formula in the method. The formula of prognostic index was demonstrated in online supplemental figure 1. All the variables were coded in binary.
Table 3. Risk prediction model for cardiovascular and cerebrovascular event development in systemic lupus erythematosus (SLE).
| Predictors | HR (95% CI) | Beta coefficient | P value |
| Age of SLE onset >40 | 4.148 (2.712 to 6.345) | 1.423 | <0.001 |
| Anti-β2GP1 | 2.695 (1.565 to 4.640) | 0.991 | <0.001 |
| Smoking | 2.453 (1.152 to 5.224) | 0.897 | 0.02 |
| High-dose glucocorticoids | 2.101 (1.22 to 3.618) | 0.743 | 0.0074 |
| Male gender | 1.888 (1.041 to 3.423) | 0.635 | 0.036 |
| Hypertension | 1.852 (1.133 to 3.027) | 0.616 | 0.014 |
| HCQ usage | 0.578 (0.381 to 0.878) | −0.548 | 0.01 |
| Cutaneous involvement | 0.504 (0.286 to 0.887) | −0.685 | 0.017 |
| Arthritis | 0.435 (0.253 to 0.749) | −0.832 | 0.0027 |
HCQhydroxychloroquine
Evaluation the performance of the model
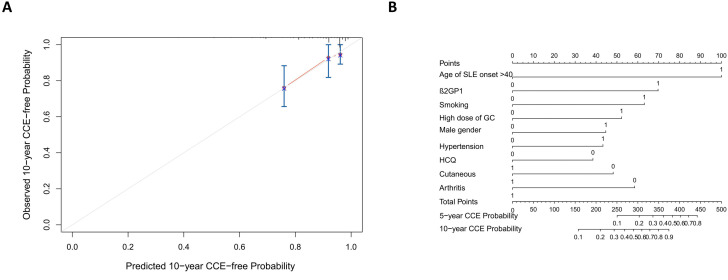
The final model’s C-index was 0.801, indicating that it had strong prediction power as a whole. The R2 of the model was 0.05 (max possible=0.432). The calibration plot of 10-year risk of CCEs, which compared the actual events with the predicted risk, was used for internal validation. All the evaluations proved that the model was accurate and stable (figure 3A). For clinical practice convenience, we plotted the nomogram (figure 3B and figure 4A). The results indicated that the model performed well, and it was beneficial to identify patients with SLE that were susceptible to CCE with the model.
Figure 3. Validation of the risk prediction model. (A) Calibration curve of the model. The patients were randomly divided into three groups, and the predicted probability was compared with the actual probability to validate the model. The red solid line represents the performance of the model, and the grey line represents a perfect model. (B) Nomogram of the model. Point for each variable can be calculated, and the total points can match the cumulative incidence of cardiovascular and cerebrovascular event (CCE) in 5 and 10 years. HCQ, hydroxychloroquine; SLE, systemic lupus erythematosus. GC, glucocorticoids.
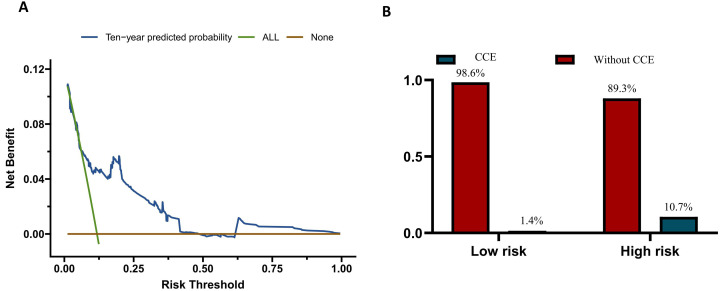
Figure 4. Clinical utilisation of the cardiovascular and cerebrovascular event (CCE) prediction model. (A) Decision curve analysis of the prediction model. The brown line illustrates the net benefit when none of the patients with systemic lupus erythematosus (SLE) were intervened to prevent CCEs, while the green line assumes that all the patients were intervened. The risk threshold of X-axis correlates with the cost to benefit ratio of intervening the patients that are predicted to be susceptible to CCE. The blue curve above the other two curves indicates the positive net benefit when patients with SLE are screened for potential CCE risk according to the model. (B) The risk stratification of the model. The prognostic index was calculated for each patient. The cut-off values are set as −0.376 and 0.663 to define the three risk-strata. The proportion of CCEs occurence in different groups was labelled in the figure.
Risk stratification
To decide the threshold that defined different risk groups of patients with SLE, the prognostic index was used to predict CCEs, and the ROC curve was plotted (online supplemental figure 2). The patients were divided into low-risk (n=636) and high-risk group (n=1763), with cut-off values of prognostic index at 0.544. The high-risk group had 636 patients, 68 (10.7%) of whom experienced CCEs during follow-up, whereas there were 1763 patients in the low-risk group, with only 25 (1.4%) of them developing CCEs (figure 4B). The model recommends screening patients of high-risk group and implementing lifestyle and pharmacological interventions proactively to minimise the occurrence of CCEs. For the ease of clinical practice, we recommended intervening patients with total points over 150 according to the nomogram (figure 3B). In this condition, we could identify 73.1% of the patients with SLE who developed CCEs. The model recommends screening patients in the high-risk group and implementing lifestyle and pharmacological interventions proactively to minimise the occurrence of CCEs. Therefore, it was acceptable to closely monitor and manage 9–10 high-risk patients with SLE to prevent 1 CCE case.
Discussion
This is a study based on the largest prospective Chinese SLE cohort, CSTAR. In this study, we created a useful and effective prediction model for proactively screening potential CCE patients when they are initially diagnosed with SLE. To our knowledge, this was the very first study aiming to establish a clinical prediction model for CCEs in patients with SLE. Besides, this study demonstrated the demographic and clinical features of patients with SLE-CCE and discovered significant risk factors for CCE occurrence. The multivariate Cox model revealed six independent risk factors: male gender, smoking, hypertension, age of SLE onset over 40, anti-β2GP1 antibody positivity and high-dose glucocorticoids. In addition, the model identified three protective factors: cutaneous involvement, arthritis and HCQ usage. Three of the identified factors were widely accepted demographic risk factors for CCE, while the others were associated with SLE, suggesting that the model could comprehensively assess the CCE risk of patients with SLE.
The conventional prediction model of CCEs focused mainly on demographic characteristics and metabolic disorders. The Framingham model was the most widely accepted and applied model for coronary heart disease, including age, sex, high-density lipoprotein, total cholesterol, blood pressure, smoking and diabetes.14 22 All of these factors have been demonstrated to be significantly correlated with CCEs.23,26 We performed univariate and multivariate Cox regressions and identified all these variables as risk factors (tables2 3). Among the traditional risk factors, smoking (HR=2.453), hypertension (HR=1.852) and male gender (HR=1.89) were selected for the model. These traditional risk factors displayed a relatively strong impact. It is worth noting that other traditional risk factors, such as diabetes, though having a high HR (3.42), were excluded by LASSO regression due to their low prevalence in the cohort, which limited their predictive ability. Four variables associated with SLE disease, including anti-β2GP1, arthritis, cutaneous involvement and age of SLE onset >40, were included in the final model. Cutaneous involvement (HR=0.504) and arthritis (HR=0.435) were recognised as protective factors in this study. We believed that the protective effect of these two variables might be attributed to the relatively milder condition of patients with arthritis or skin lesions as the primary manifestations.2 27 28 The overall HR for the composite CVD endpoint has also been proven to be significantly lower for cutaneous lupus than for SLE.29 The lower intensity of treatment in these patients might also play a role.30 Anti-β2GP1 antibody was proven to be a strong risk factor for CCE (HR=2.695). β2GP1 is an apolipoprotein that binds to oxidised LDL deposited in the arterial wall.31 Anti-β2GP1 antibody positivity has been proven to accelerate atheroma.32 Furthermore, anti-β2GP1 was one of the most important antibodies in the aPL spectrum for APS diagnosis. It was also confirmed that aPLs promoted thrombus formation.33 Furthermore, studies have reported that anti-β2GP1 antibody increases the risk of stroke and intractable headaches in patients with SLE, with its potency surpassing that of lupus anticoagulant and anti-cardiolipin antibody.34 Though all three aPLs are risk factors for CCEs, the LASSO regression included only anti-β2GP1 antibody in the model because of its stronger impact. However, aPLs were less significant when predicting mortality related to CCE and got excluded. The results indicate that the CCEs caused from aPLs might be less fetal.
Two treatment-related variables, high-dose glucocorticoids and HCQ usage, were included. High-dose glucocorticoids was identified as a risk factor for CCEs (HR=2.101). Though glucocorticoids minimised the inflammatory response, which might suppress atherogenesis, it was related to conventional risk factors like hyperlipidaemia, obesity and insulin resistance.35 Besides, it was also reported that glucocorticoid-induced tumour necrosis factor receptor family-related protein could directly drive atherogenesis.36 37 HCQ usage was defined as a protective factor against CCE in this model (HR=0.578). Several previous studies have reported that HCQ has metabolic and cardiovascular benefits.38,40 HCQ has also been reported as a protective factor against CVD in patients with rheumatoid arthritis.41
The largest prospective SLE cohort in China served as the basis for this study, and the inclusion of only newly diagnosed patients with SLE maximised the assurance of complete patient evaluations, consistent medical backgrounds and minimised confounding factors. However, this study had several limitations. First, only internal validation of this model was performed, and further external validation was needed. Second, the cohort had a median follow-up of 4.81 years, which may be insufficient for monitoring CCEs. Third, only baseline data were used for model development, so changes in the disease condition and treatment plans of the patients during the disease course were not evaluated in this study. Subsequent research based on time series models might address this issue. In addition, the isotypes of antiphospholipids antibodies were not recorded in our cohort, which limited the predictive ability of our model. Moreover, most patients enrolled in our cohort are Chinese. The racial homogeneity of our cohort might limit the generalisability of the results in patients from other racial backgrounds. Finally, this was a retrospective study, the records of treatment were not precise enough, and using baseline treatment as predictors might underestimate the impact of treatment on the risk of CCE.
Conclusion
In conclusion, we developed the first clinical prediction model for CCEs in patients with SLE and performed internal validation of the model. The model is based on the multicentre prospective cohort and could help identify high-risk patients in clinical practice. We recommended the application of this model in the routine assessment of patients with SLE, and we also recommended that those high-risk patients need closer monitoring and tighter control of the risk factors.
supplementary material
Acknowledgements
We appreciate every physician, research nurse and coordinator involved in the CSTAR-PAH cohort study. We also sincerely thank all patients who were enrolled in this study.
Footnotes
Funding: This study was supported by the Chinese National Key Technology R&D Program, Ministry of Science and Technology (2021YFC2501300), Beijing Municipal Science & Technology Commission (Z201100005520022, Z201100005520023, Z201100005520025, Z201100005520026, Z201100005520027), CAMS Innovation Fund for Medical Sciences (CIFMS) (2021-I2M-1-005, 2022-I2M-1-004), National High Level Hospital Clinical Research Funding (2022-PUMCH-A-038, 2022-PUMCH-B-013, 2022-PUMCH-C-002, 2022-PUMCH-D-009).
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Not applicable.
Ethics approval: This study was approved by the Peking Union Medical College Hospital Institutional Review Board and Ethical Board (ethical number: JS-2038). Participants gave informed consent to participate in the study before taking part.
Contributor Information
Can Huang, Email: huang_can@yeah.net.
Yutong Li, Email: 812309036@qq.com.
Ziqian Wang, Email: evalaver@163.com.
Shudian Lin, Email: linsd0110@163.com.
Jiu-Liang Zhao, Email: zjlpumc@163.com.
Qian Wang, Email: zhengaqian@sina.com.
Xinping Tian, Email: tianxp6@126.com.
Yanhong Wang, Email: wyhong826@163.com.
Xinwang Duan, Email: 13970085678@163.com.
Yongfu Wang, Email: wyf5168@hotmail.com.
Cheng Zhao, Email: zhaochenggx@163.com.
Zhenbiao Wu, Email: wuzhenbiao@fmmu.edu.cn.
Jian Xu, Email: Jianxu777@126.com.
Chen Han, Email: 764676800@qq.com.
Min Yang, Email: minyanggz@yahoo.com.
Rui Wu, Email: tcmclinic@163.com.
Xiaofeng Zeng, Email: zengxfpumc@163.com.
Mengtao Li, Email: mengtao.li@cstar.org.cn.
Data availability statement
Data are available upon reasonable request.
References
- 1.Kiriakidou M, Ching CL. Systemic Lupus Erythematosus. Ann Intern Med. 2020;172:ITC81–96. doi: 10.7326/AITC202006020. [DOI] [PubMed] [Google Scholar]
- 2.Li M, Wang Y, Zhao J, et al. Chinese SLE Treatment and Research Group (CSTAR) Registry 2009-2019: Major Clinical Characteristics of Chinese Patients with Systemic Lupus Erythematosus. Rheumatol Immunol Res . 2021;2:43–7. doi: 10.2478/rir-2021-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang Z, Li M, Ye Z, et al. Long-term Outcomes of Patients with Systemic Lupus Erythematosus: A Multicenter Cohort Study from CSTAR Registry. Rheumatol Immunol Res. 2021;2:195–202. doi: 10.2478/rir-2021-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lu X, Wang Y, Zhang J, et al. Patients with systemic lupus erythematosus face a high risk of cardiovascular disease: A systematic review and Meta-analysis. Int Immunopharmacol. 2021;94:107466. doi: 10.1016/j.intimp.2021.107466. [DOI] [PubMed] [Google Scholar]
- 5.Lee YH, Choi SJ, Ji JD, et al. Overall and cause-specific mortality in systemic lupus erythematosus: an updated meta-analysis. Lupus (Los Angel) 2016;25:727–34. doi: 10.1177/0961203315627202. [DOI] [PubMed] [Google Scholar]
- 6.Frostegård J. Systemic lupus erythematosus and cardiovascular disease. J Intern Med. 2023;293:48–62. doi: 10.1111/joim.13557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mak A, Chan JKY. Endothelial function and endothelial progenitor cells in systemic lupus erythematosus. Nat Rev Rheumatol. 2022;18:286–300. doi: 10.1038/s41584-022-00770-y. [DOI] [PubMed] [Google Scholar]
- 8.Sazliyana S, Mohd Shahrir MS, Kong CTN, et al. Implications of immunosuppressive agents in cardiovascular risks and carotid intima media thickness among lupus nephritis patients. Lupus (Los Angel) 2011;20:1260–6. doi: 10.1177/0961203311411347. [DOI] [PubMed] [Google Scholar]
- 9.Chung CP, Avalos I, Oeser A, et al. High prevalence of the metabolic syndrome in patients with systemic lupus erythematosus: association with disease characteristics and cardiovascular risk factors. Ann Rheum Dis. 2007;66:208–14. doi: 10.1136/ard.2006.054973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Skamra C, Ramsey-Goldman R. Management of cardiovascular complications in systemic lupus erythematosus. Int J Clin Rheumtol. 2010;5:75–100. doi: 10.2217/ijr.09.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Symmons DPM, Gabriel SE. Epidemiology of CVD in rheumatic disease, with a focus on RA and SLE. Nat Rev Rheumatol. 2011;7:399–408. doi: 10.1038/nrrheum.2011.75. [DOI] [PubMed] [Google Scholar]
- 12.Sinicato NA, da Silva Cardoso PA, Appenzeller S. Risk factors in cardiovascular disease in systemic lupus erythematosus. Curr Cardiol Rev. 2013;9:15–9. doi: 10.2174/157340313805076304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tektonidou MG. Cardiovascular disease risk in antiphospholipid syndrome: Thrombo-inflammation and atherothrombosis. J Autoimmun. 2022;128:102813. doi: 10.1016/j.jaut.2022.102813. [DOI] [PubMed] [Google Scholar]
- 14.KANNEL WB, DAWBER TR, KAGAN A, et al. Factors of risk in the development of coronary heart disease--six year follow-up experience. The Framingham Study. Ann Intern Med . 1961;55:33–50. doi: 10.7326/0003-4819-55-1-33. [DOI] [PubMed] [Google Scholar]
- 15.Conroy RM, Pyörälä K, Fitzgerald AP, et al. Estimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur Heart J. 2003;24:987–1003. doi: 10.1016/s0195-668x(03)00114-3. [DOI] [PubMed] [Google Scholar]
- 16.Drosos GC, Konstantonis G, Sfikakis PP, et al. Underperformance of clinical risk scores in identifying vascular ultrasound-based high cardiovascular risk in systemic lupus erythematosus. Eur J Prev Cardiol. 2021;28:346–52. doi: 10.1093/eurjpc/zwaa256. [DOI] [PubMed] [Google Scholar]
- 17.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 18.Petri M, Orbai A-M, Alarcón GS, et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012;64:2677–86. doi: 10.1002/art.34473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Clinical Research Center for Dermatologic and Immunologic Diseases (Peking Union Medical College Hospital); Experimental Diagnosis Research Committee, Rheumatology and Immunology Physicians Committee of Chinese Medical Doctor Association, Autoantibodies Detection Committee, & Chinese Rheumatism Data Center Expert consensus on quality control for detecting autoantibodies. Zhonghua Nei Ke Za Zhi. 2023;62:1418–22. doi: 10.3760/cma.j.cn112138-20230619-00320. [DOI] [PubMed] [Google Scholar]
- 20.Sánchez-de-la-Torre M, Gracia-Lavedan E, Benitez ID, et al. Adherence to CPAP Treatment and the Risk of Recurrent Cardiovascular Events: A Meta-Analysis. JAMA. 2023;330:1255–65. doi: 10.1001/jama.2023.17465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harrell FE, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–87. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 22.Tsao CW, Vasan RS. Cohort Profile: The Framingham Heart Study (FHS): overview of milestones in cardiovascular epidemiology. Int J Epidemiol. 2015;44:1800–13. doi: 10.1093/ije/dyv337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Strain WD, Paldánius PM. Diabetes, cardiovascular disease and the microcirculation. Cardiovasc Diabetol. 2018;17:57. doi: 10.1186/s12933-018-0703-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grundy SM. Correction to: 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139:e1082–143. doi: 10.1161/CIR.0000000000000698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao D, Liu J, Wang M, et al. Epidemiology of cardiovascular disease in China: current features and implications. Nat Rev Cardiol. 2019;16:203–12. doi: 10.1038/s41569-018-0119-4. [DOI] [PubMed] [Google Scholar]
- 26.Kodama S, Saito K, Tanaka S, et al. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: a meta-analysis. JAMA. 2009;301:2024–35. doi: 10.1001/jama.2009.681. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Y-P, Wu J, Han Y-F, et al. Pathogenesis of cutaneous lupus erythema associated with and without systemic lupus erythema. Autoimmun Rev. 2017;16:735–42. doi: 10.1016/j.autrev.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 28.Drucker AM, Su J, Mussani F, et al. Prognostic implications of active discoid lupus erythematosus and malar rash at the time of diagnosis of systemic lupus erythematosus: Results from a prospective cohort study. Lupus (Los Angel) 2016;25:376–81. doi: 10.1177/0961203315610645. [DOI] [PubMed] [Google Scholar]
- 29.Hesselvig JH, Ahlehoff O, Dreyer L, et al. Cutaneous lupus erythematosus and systemic lupus erythematosus are associated with clinically significant cardiovascular risk: a Danish nationwide cohort study. Lupus (Los Angel) 2017;26:48–53. doi: 10.1177/0961203316651739. [DOI] [PubMed] [Google Scholar]
- 30.Li M, Zhao Y, Zhang Z, et al. 2020 Chinese Guidelines for the Diagnosis and Treatment of Systemic Lupus Erythematosus. Rheumatol Immunol Res . 2020;1:5–23. doi: 10.2478/rir-2020-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matsuura E, Koike T. Accelerated atheroma and anti-beta2-glycoprotein I antibodies. Lupus (Los Angel) 2000;9:210–6. doi: 10.1191/096120300678828262. [DOI] [PubMed] [Google Scholar]
- 32.Karpouzas GA, Ormseth SR, Hernandez E, et al. Beta-2-glycoprotein-I IgA antibodies predict coronary plaque progression in rheumatoid arthritis. Semin Arthritis Rheum. 2021;51:20–7. doi: 10.1016/j.semarthrit.2020.10.003. [DOI] [PubMed] [Google Scholar]
- 33.Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS) J Thromb Haemost. 2006;4:295–306. doi: 10.1111/j.1538-7836.2006.01753.x. [DOI] [PubMed] [Google Scholar]
- 34.Hawro T, Bogucki A, Krupińska-Kun M, et al. Intractable headaches, ischemic stroke, and seizures are linked to the presence of anti-β2GPI antibodies in patients with systemic lupus erythematosus. PLoS One. 2015;10:e0119911. doi: 10.1371/journal.pone.0119911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Karp I, Abrahamowicz M, Fortin PR, et al. Recent corticosteroid use and recent disease activity: independent determinants of coronary heart disease risk factors in systemic lupus erythematosus? Arthritis Rheum. 2008;59:169–75. doi: 10.1002/art.23352. [DOI] [PubMed] [Google Scholar]
- 36.Bosmans LA, Shami A, Atzler D, et al. Glucocorticoid induced TNF receptor family-related protein (GITR) - A novel driver of atherosclerosis. Vasc Pharmacol. 2021;139:106884. doi: 10.1016/j.vph.2021.106884. [DOI] [PubMed] [Google Scholar]
- 37.Shami A, Atzler D, Bosmans LA, et al. Glucocorticoid-induced tumour necrosis factor receptor family-related protein (GITR) drives atherosclerosis in mice and is associated with an unstable plaque phenotype and cerebrovascular events in humans. Eur Heart J. 2020;41:2938–48. doi: 10.1093/eurheartj/ehaa484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rempenault C, Combe B, Barnetche T, et al. Metabolic and cardiovascular benefits of hydroxychloroquine in patients with rheumatoid arthritis: a systematic review and meta-analysis. Ann Rheum Dis. 2018;77:98–103. doi: 10.1136/annrheumdis-2017-211836. [DOI] [PubMed] [Google Scholar]
- 39.Cordova Sanchez A, Khokhar F, Olonoff DA, et al. Hydroxychloroquine and Cardiovascular Events in Patients with Rheumatoid Arthritis. Cardiovasc Drugs Ther. 2024;38:297–304. doi: 10.1007/s10557-022-07387-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu D, Li X, Zhang Y, et al. Chloroquine and hydroxychloroquine are associated with reduced cardiovascular risk: a systematic review and meta-analysis. Drug Des Devel Ther. 2018;12:1685–95. doi: 10.2147/DDDT.S166893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li C, Wang XR, Ji HJ, et al. Cardiovascular disease in rheumatoid arthritis: medications and risk factors in China. Clin Rheumatol. 2017;36:1023–9. doi: 10.1007/s10067-017-3596-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request.