Abstract
Background:
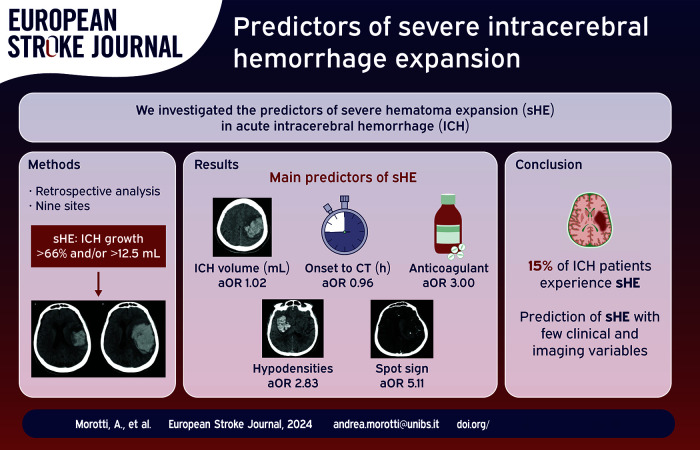
Severe hematoma expansion (sHE) has the strongest impact on intracerebral hemorrhage (ICH) outcome. We investigated the predictors of sHE.
Methods:
Retrospective analysis of ICH patients admitted at nine sites in Italy, Germany, China, and Canada. The following imaging features were analyzed: non-contrast CT (NCCT) hypodensities, heterogeneous density, blend sign, irregular shape, and CT angiography (CTA) spot sign. The outcome of interest was sHE, defined as volume increase >66% and/or >12.5 from baseline to follow-up NCCT. Predictors of sHE were explored with logistic regression.
Results:
A total of 1472 patients were included (median age 73, 56.6% males) of whom 223 (15.2%) had sHE. Age (odds ratio (OR) per year, 95% confidence interval (CI), 1.02 (1.01–1.04)), Anticoagulant treatment (OR 3.00, 95% CI 2.09–4.31), Glasgow Coma Scale (OR 0.93, 95% CI 0.89–0.98), time from onset/last known well to imaging, (OR per h 0.96, 95% CI 0.93–0.99), and baseline ICH volume, (OR per mL 1.02, 95% CI 1.02–1.03) were independently associated with sHE. Ultra-early hematoma growth (baseline volume/baseline imaging time) was also a predictor of sHE (OR per mL/h 1.01, 95% CI 1.00–1.02). All NCCT and CTA imaging markers were also predictors of sHE. Amongst imaging features NCCT hypodensities had the highest sensitivity (0.79) whereas the CTA spot sign had the highest positive predictive value (0.51).
Conclusions:
sHE is common in the natural history of ICH and can be predicted with few clinical and imaging variables. These findings might inform clinical practice and future trials targeting active bleeding in ICH.
Keywords: Intracerebral hemorrhage, stroke, hematoma expansion, spot sign, hypodensities
Graphical abstract.
Introduction
Intracerebral hemorrhage (ICH) is one of the deadliest types of stroke, with high short term mortality and severe neurological sequelae in the majority of survivors. 1 Hematoma expansion (HE) is a potentially preventable determinant of poor outcome and represents therefore a compelling therapeutic target. 2 However, the HE-outcome relationship is not linear, and only severe HE (sHE), defined as hematoma volume increase >66% and/or >12.5 mL, has a significant prognostic impact. 3 Previous studies and prediction models focused on the more commonly used definition of HE (volume increase >33% and or >6 mL), whereas predictors of sHE remain poorly characterized. 4 We aimed to describe the clinical and imaging variables associated with sHE.
Methods
Study population
Patients admitted for spontaneous non-traumatic ICH at nine sites in Italy, China, Germany, and Canada were retrospectively selected from ongoing ICH registries. Written informed consent was obtained by patients, relatives, or waived by the Institutional Review Board. All the study procedures were approved by the local authorities at each participating institution: Arcispedale S. Anna, Ferrara, Italy (PN 26032009-15122011, 2010–2019); IRCCS Mondino Foundation, Pavia, Italy (PN 0035588/22, 2017–2019); ASST Spedali Civili, Brescia and Fondazione Poliambulanza, Brescia, Italy (PN 4067-08052020, 2020–2021); IRCCS Istituto delle Scienze Neurologiche, Bologna, Italy (DL 196/2003, 2015–2019); Charitè Hospital, Berlin, Germany (PN EA1/035/20, 2014–2019), University of Perugia/Azienda Ospedaliera Santa Maria Della Misericordia (DL 196/2003, 2019–2022); The First Affiliated Hospital of Chongqing Medical University, Chongqing, China (PN 2017-075, 2011-2015); McMaster University/Population Health Research Institute, Hamilton, Ontario, Canada (PN 3253, 2010–2016). For the present analysis we included patients with primary spontaneous intracerebral hemorrhage and (1) baseline non-contrast CT (NCCT) acquired within 24 h from symptoms onset or time last seen well (LSW), (2) availability of follow-up NCCT within 24–72 h from symptoms onset or time LSW, and (3) age > 18. Patients with any of the following were excluded: (1) traumatic brain injury, macrovascular lesions or other brain disease underlying the acute ICH, (2) missing or poor quality NCCT imaging, (3) missing clinical variables and 4) surgical treatment before follow-up imaging.
Requests to access the dataset may be sent to the corresponding author.
Clinical variables
Clinical data were collected by trained investigators through medical charts, patients, and family members interviews. Mortality at 90 days was assessed through phone calls, outpatient service evaluations, or querying the national social security database when available.
Imaging analysis
All patients underwent baseline and follow-up NCCT according to local stroke imaging protocols. The imaging analysis included ICH volume calculation (semi-automated, planimetric softwares), presence of intraventricular hemorrhage (IVH), and ICH location. 5 NCCT features (hypodensities, heterogeneous density, irregular shape, and blend sign) and computed tomography angiography (CTA) markers (spot sign) of sHE were also evaluated by trained raters, using validated standardized diagnostic criteria.6–8 The diagnostic criteria for NCCT and CTA markers and an illustrative example of all these radiological features are reported in Supplemental eTable 1 and eFigure 1 respectively.
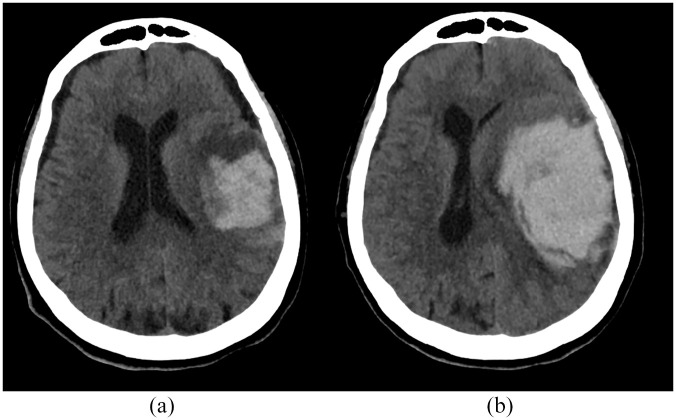
The imaging rating was performed by different trained investigators at each participating institution. Good inter-rater reliability for NCCT markers and spot sign evaluation has been previously demonstrated.9,10 However, inter-rater reliability for ICH volume quantification and sHE imaging markers was tested, comparing the results of two raters from different institutions (AM and FM) in a subgroup of randomly selected cases (n = 50). The occurrence of sHE was the main outcome of interest, defined as volume increase >66% and/or >12.5 mL from baseline to follow-up NCCT. 3 Figure 1 shows an illustrative example of sHE. All the investigators and imaging raters were blinded to the results of follow-up imaging.
Figure 1.
Severe hematoma expansion. Baseline volume 38 mL (a), follow-up volume 119 mL (b).
Statistical analysis
Categorical variables were expressed as n (%) and compared with Chi Square test. Continuous variables were summarized as medians (interquartile range, IQR) and compared with Mann-Whitney test. Inter-rater reliability was measured with intraclass correlation coefficient (ICC) for ICH volume quantification and Cohen K for NCCT markers and CTA spot sign detection. 11
Predictors of sHE were explored with logistic regression (backward elimination at p < 0.1), adjusting for baseline ICH volume, antithrombotic treatment, baseline imaging time, and variables with p < 0.1 in univariate analysis. In another analysis, we also included NCCT and CTA imaging features in logistic regression and calculated their diagnostic performance for sHE. To avoid multicollinearity, because of the wide overlap between different imaging markers, every imaging feature was included separately in logistic regression models. Every participant’s predicted probability of sHE was calculated using individual data and logistic regression estimates derived from a model incorporating clinical (age, GCS, imaging time from onset/last known well) and imaging variables (ICH volume, hypodensities, and spot sign). The predicted probability of sHE was expressed as a continuous variable ranging from 0 to 1. The discriminative ability of our model was tested with receiver operating characteristic curve (ROC) and area under the curve (AUC).
Several secondary analyses were performed, as follows. (1) The logistic regression model and ROC curve were repeated restricting the analysis to subjects with baseline ICH volume ⩽30 mL 3 . (2) The same prediction model was also tested using the traditional definition of HE (volume increase >33% and or >6 mL from baseline to follow-up imaging). (3) We tested the predictive ability of ultra-early hematoma growth (uHG), a surrogate measure of the speed of active bleeding, quantified dividing baseline ICH volume by onset to baseline imaging time, and measured in mL/h. 12 (4) The main logistic regression models were repeated after follow-up volume imputation, 13 to include subjects with missing or poor quality follow-up imaging. (5) The analysis was restricted to supratentorial ICH and ICH location (lobar vs nonlobar) was included as a covariate in logistic regression. 14
SPSS V 25.0 was used for all the analyses. Statistical significance was set at p < 0.05.
Results
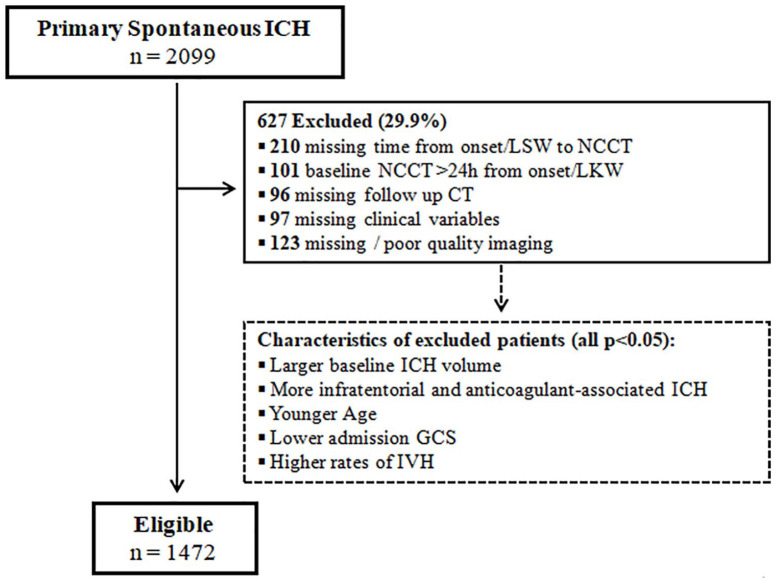
A total of 1472 patients were included, of whom 223 (15.2%) had sHE. Figure 2 shows the selection flowchart and comparison between included and excluded subjects whereas Table 1 summarizes the study cohort characteristics. The inter-rater reliability was good for ICH volume quantification (ICC 0.93, 95% confidence interval (CI) 0.88–0.96) and for detection of NCCT and CTA markers (Cohen K >0.80 for all imaging features). Patients with sHE had larger ICH volume at baseline, lower GCS, were older, more frequently on anticoagulant therapy and had higher mortality. Among the 222 patients with anticoagulant treatment, the majority were on vitamin K antagonists (155, 69.8%) and the remaining were on direct oral anticoagulants and the frequency of sHE in these two groups was similar (30.3% vs 31.0% respectively, p = 0.956).
Figure 2.
Selection flowchart.
GCS: Glasgow Coma Scale; NCCT: non-contrast computed tomography; IVH: intraventricular hemorrhage; IQR: interquartile range; LKW: last known well; ICH: intracerebral hemorrhage.
Table 1.
Population characteristics.
| ALL (n = 1472) | Severe HE | |||
|---|---|---|---|---|
| No (n = 1249) | Yes (n = 223) | p | ||
| Age, median (IQR), year | 73 (63–81) | 72 (61–80) | 77 (69–82) | <0.001 |
| Sex, male, n (%) | 833 (56.6) | 706 (56.5) | 127 (57.0) | 0.906 |
| History of hypertension, n (%) | 1135 (77.1) | 953 (76.3) | 182 (81.6) | 0.082 |
| Antiplatelet treatment, n (%) | 401 (27.2) | 331 (26.5) | 70 (31.4) | 0.131 |
| Anticoagulant treatment, n (%) | 222 (15.1) | 153 (12.2) | 69 (30.9) | <0.001 |
| SBP, median (IQR), mmHg | 165 (143–190) | 163 (142–190) | 170 (150–190) | 0.508 |
| GCS, median (IQR) | 14 (11–15) | 14 (11–15) | 12 (9–15) | <0.001 |
| Time from onset/LSW to NCCT, median (IQR), h | 3.8 (2.1–8.9) | 3.9 (2.2–9.0) | 3.4 (2.0–7.2) | 0.070 |
| Baseline ICH volume, median (IQR), mL | 13.4 (5.6–32.2) | 12.0 (5.4–26.3) | 36.6 (11.6–61.3) | <0.001 |
| ICH location | 0.291 | |||
| Lobar, n (%) | 632 (42.9) | 526 (42.1) | 106 (47.5) | |
| Deep, n (%) | 735 (49.9) | 632 (50.6) | 103 (46.2) | |
| Cerebellar, n (%) | 54 (3.7) | 49 (3.9) | 5 (2.2) | |
| Brainstem, n (%) | 51 (3.5) | 42 (3.4) | 9 (4.0) | |
| IVH, n (%) | 475 (32.3) | 380 (30.4) | 95 (42.6) | <0.001 |
| NCCT hypodensities, n (%) | 749 (50.9) | 574 (46.0) | 175 (78.5) | <0.001 |
| NCCT blend sign, n (%) | 206 (14.0) | 145 (11.6) | 61 (27.4) | <0.001 |
| NCCT heterogeneous density, n (%) | 469 (31.9) | 340 (27.2) | 129 (57.8) | <0.001 |
| NCCT irregular shape, n (%) | 517 (35.1) | 394 (31.5) | 12 (55.2) | <0.001 |
| CTA spot sign, n (%) | 109/551 (7.4) | 54 /436 (12.4) | 55/115 (45.8) | <0.001 |
| Mortality at 90 days, n (%) | 328 (22.3) | 204 (16.3) | 124 (55.6) | <0.001 |
ICH: intracerebral hemorrhage; SBP: systolic blood pressure; GCS: Glasgow Coma Scale; NCCT: non-contrast computed tomography; CTA: computed tomography angiography; IVH: intraventricular hemorrhage; IQR: interquartile range; LKW: last known well.
CTA was available in 551 (37.4%) patients. All imaging markers were more common in patients with sHE. These associations remained significant after adjustment for potential confounders in logistic regression, as summarized in Tables 2 and 3. Shorter time from onset/LSW to NCCT was also independently associated with higher odds of sHE. Amongst imaging features, hypodensities had the highest sensitivity (0.79) whereas the spot sign had the highest positive predictive value (0.51) for sHE. The diagnostic performance of imaging markers for sHE is provided in Table 4.
Table 2.
Predictors of severe ICH expansion.
| OR (95% CI) | p | |
|---|---|---|
| Age, years | 1.02 (1.01–1.04) | 0.001 |
| Anticoagulant treatment | 3.00 (2.09–4.31) | <0.001 |
| GCS | 0.93 (0.89–0.98) | 0.002 |
| Time from onset/LKW to NCCT, h | 0.96 (0.93–0.99) | 0.009 |
| Baseline ICH volume, mL | 1.02 (1.02–1.03) | <0.001 |
ICH: intracerebral hemorrhage; GCS: Glasgow Coma Scale; NCCT: non-contrast computed tomography; LKW: last known well; OR: odds ratio; CI: confidence interval.
Variables included: age, hypertension, anticoagulant, GCS, time from onset/LKW to NCCT, baseline ICH volume, IVH.
Table 3.
Prediction of severe ICH expansion with imaging markers.
| OR (95% CI) | p | |
|---|---|---|
| NCCT markers (n = 1472) | ||
| Hypodensities | 2.83 (1.98–4.06) | <0.001 |
| Blend sign | 2.19 (1.48–3.23) | <0.001 |
| Heterogeneous density | 2.34 (1.70–3.25) | <0.001 |
| Irregular shape | 1.79 (1.29–2.48) | 0.001 |
| CTA markers (n = 551) | ||
| Spot Sign | 5.11 (3.12–8.36) | <0.001 |
ICH: intracerebral hemorrhage; NCCT: non-contrast computed tomography; CTA: computed tomography angiography; OR: odds ratio; CI: confidence interval.
Logistic regression adjusted for age, hypertension, anticoagulant, GCS, time from onset/LKW to NCCT, baseline ICH volume, presence of intraventricular hemorrhage.
Table 4.
Diagnostic accuracy of imaging markers for sHE..
| Sensitivity | Specificity | PPV | NPV | |
|---|---|---|---|---|
| NCCT markers (n = 1472) | ||||
| Hypodensities | 0.79 | 0.54 | 0.23 | 0.93 |
| Blend sign | 0.27 | 0.88 | 0.30 | 0.87 |
| Heterogeneous density | 0.58 | 0.73 | 0.28 | 0.91 |
| Irregular shape | 0.55 | 0.69 | 0.24 | 0.90 |
| CTA markers (n = 551) | ||||
| Spot Sign | 0.48 | 0.88 | 0.51 | 0.86 |
ICH: intracerebral hemorrhage; NCCT: non-contrast computed tomography; CTA: computed tomography angiography; PPV: positive predictive value; NPV: negative predictive value.
A prediction model incorporating clinical (age, anticoagulation, GCS, and time from onset/LSW to NCCT) and imaging predictors (ICH volume, hypodensities and spot sign) had good discrimination for sHE (AUC 0.81, 95% CI 0.76–0.85). This prediction model showed high discriminative ability also for HE defined as volume increase >33% and or >6 mL (AUC 0.85, 95% CI 0.82–0.88).
In the subgroup of patients with baseline ICH volume ⩽30 mL the main predictors of sHE were age, anticoagulant treatment, NCCT hypodensities, and CTA spot sign. The prediction model based on these clinical and imaging variables had good discrimination for sHE (AUC 0.83, 95% CI 0.79–0.89). Secondary analyses showed also that uHG was an independent predictor of sHE (odds ratio per mL/h, OR 1.01, 95% CI 1.00–1.02, p = 0.007). The presence of uHG > 5 mL/h was associated with a greater than twofold increase in the odds of experiencing sHE (OR 2.42, 95% CI 1.74–3.35, p < 0.001).
Finally, all the main findings of our analysis were confirmed after follow-up volume imputation (data not shown) and inclusion of ICH location in logistic regression. In particular, the overall risk of sHE was similar in lobar and nonlobar ICH (16.8% vs 14.0%, p = 0.158) and lobar ICH location was not associated with higher odds of sHE (OR 1.08, 95% CI 0.79–1.50, p = 0.623).
Discussion
Age, GCS and known predictors of HE such as baseline volume, anticoagulation, imaging markers and time were associated with sHE.2,15 uHG, an indirect measure of the speed of active bleeding and validated predictor of HE, 12 was also independently associated with sHE. An inverse association between GCS and the odds of HE has also been previously reported, although the underlying biological mechanisms remains unclear. 16 Admission GCS might simply be the epiphenomenon of other factors associated with HE such as ICH volume and location. Other mechanisms might explain the age-sHE relationship. Age correlates with brain atrophy, which in turn might predispose to sHE because of a diminished resistance to the force of active bleeding. 17 Our findings might have important implications, as accurate prediction of HE is a clinical research priority. Patients at high risk of sHE are more likely to experience clinical deterioration, and therefore might be selected for more intensive monitoring in settings with limited resources. Our results might also inform future trials, as patients with high risk of sHE are probably more likely to benefit from medical therapies targeting hematoma growth. Our analyses demonstrated that it is feasible to predict sHE with few clinical and imaging variables, also in the subgroup of patients more likely to benefit from HE prevention because presenting with a small baseline volume. There might also be value in a retrospective analysis of previous neutral randomized controlled trials, as some therapeutic strategies might have an impact on sHE only and not on any degree of HE.18,19 Finally, multiple HE prediction tools and nomograms have been developed and it might be of interest to test their diagnostic performance for sHE. 4
Some limitations of our analyses should be acknowledged. First, selection bias in favor of less severely affected patients might have occurred, as the availability of follow-up NCCT is mandatory for HE assessment. Second, we were unable to precisely account for treatment variables that have a significant impact on the risk of HE such as blood pressure levels and fluctuations and coagulopathy reversal.2,20–22 Third, the CTA acquisition protocol was not standardized and might have influenced the diagnostic accuracy of the spot sign. 7 Fourth, data collection occurred over a long time period, with several relevant changes in guidelines recommendations.23–26
Conclusion
sHE is common after ICH and can be predicted with few clinical and imaging variables. These findings might inform clinical practice and improve the identification of patients more likely to benefit from treatments targeting HE in clinical trials.
Supplemental Material
Supplemental material, sj-doc-1-eso-10.1177_23969873241247436 for Predictors of severe intracerebral hemorrhage expansion by Andrea Morotti, Qi Li, Jawed Nawabi, Giorgio Busto, Federico Mazzacane, Anna Cavallini, Ashkan Shoamanesh, Mauro Morassi, Frieder Schlunk, Laura Piccolo, Giacomo Urbinati, Debora Pezzini, Maurizio Paciaroni, Enrico Fainardi, Ilaria Casetta, Alessandro Padovani and Andrea Zini in European Stroke Journal
Footnotes
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr. Morotti has received expert meeting and advisory board honoraria from EMG-REG International and AstraZeneca. Dr. Shoamanesh has received speaker and consultation fees from AstraZeneca, Takeda Pharmaceuticals; and research funding from Alexion and Octapharma Canada. Dr. Paciaroni has received honoraria as a member of the speaker bureau of Sanofi-Aventis, BMS, Daiichi Sankyo, Pfizer, and iRhythm. Dr. Zini has received speaker and consultation fees from Alexion, CLS-Behring, Boehringer-Ingelheim. All the other authors report no disclosures.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed consent: Written informed consent was obtained by patients or caregivers, or waived by the Institutional Review Boards.
Ethical approval: Arcispedale S. Anna, Ferrara, Italy (PN 26032009-15122011); IRCCS Mondino Foundation, Pavia, Italy (PN 0035588/22); ASST Spedali Civili, Brescia and Fondazione Poliambulanza, Brescia, Italy (PN 4067-08052020); IRCCS Istituto delle Scienze Neurologiche, Bologna, Italy (DL 196/2003); Charitè Hospital, Berlin, Germany (PN EA1/035/20), University of Perugia/Azienda Ospedaliera Santa Maria Della Misericordia (DL 196/2003); The First Affiliated Hospital of Chongqing Medical University, Chongqing, China (PN 2017-075); McMaster University/Population Health Research Institute, Hamilton Ontario, Canada (PN 3253).
Guarantors: Andrea Morotti—Andrea Zini
Contributorship: AM designed the study, performed statistical analyses, and drafted the manuscript. AZ supervised the study and all authors collected data, analyzed images, reviewed and edited the manuscript, and approved the final version of the manuscript.
ORCID iDs: Andrea Morotti  https://orcid.org/0000-0002-6558-1155
https://orcid.org/0000-0002-6558-1155
Qi Li  https://orcid.org/0000-0002-9144-148X
https://orcid.org/0000-0002-9144-148X
Anna Cavallini  https://orcid.org/0000-0002-5227-1502
https://orcid.org/0000-0002-5227-1502
Ashkan Shoamanesh  https://orcid.org/0000-0002-2802-1626
https://orcid.org/0000-0002-2802-1626
Maurizio Paciaroni  https://orcid.org/0000-0002-5483-8795
https://orcid.org/0000-0002-5483-8795
Enrico Fainardi  https://orcid.org/0000-0003-0477-724X
https://orcid.org/0000-0003-0477-724X
Ilaria Casetta  https://orcid.org/0000-0003-4099-8875
https://orcid.org/0000-0003-4099-8875
Supplemental material: Supplemental material for this article is available online.
References
- 1. Puy L, Parry-Jones AR, Sandset EC, et al. Intracerebral haemorrhage. Nat Rev Dis Prim 2023; 9: 14. [DOI] [PubMed] [Google Scholar]
- 2. Morotti A, Boulouis G, Dowlatshahi D, et al. Intracerebral haemorrhage expansion: definitions, predictors, and prevention. Lancet Neurol 2023; 22: 159–171. [DOI] [PubMed] [Google Scholar]
- 3. Morotti A, Boulouis G, Nawabi J, et al. Association between hematoma expansion severity and outcome and its interaction with baseline intracerebral hemorrhage volume. Neurology 2023; 101: E1606–E1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yogendrakumar V, Moores M, Sikora L, et al. Evaluating hematoma expansion scores in acute spontaneous intracerebral hemorrhage a systematic scoping review. Stroke 2020; 51: 1305–1308. [DOI] [PubMed] [Google Scholar]
- 5. Falcone GJ, Biffi A, Brouwers HB, et al. Predictors of hematoma volume in deep and lobar supratentorial intracerebral hemorrhage. JAMA Neurol 2013; 70: 988–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Demchuk AM, Dowlatshahi D, Rodriguez-Luna D, et al. Prediction of haematoma growth and outcome in patients with intracerebral haemorrhage using the CT-angiography spot sign (PREDICT): a prospective observational study. Lancet Neurol 2012; 11: 307–314. [DOI] [PubMed] [Google Scholar]
- 7. Rodriguez-Luna D, Coscojuela P, Rodriguez-Villatoro N, et al. Multiphase CT angiography improves prediction of intracerebral hemorrhage expansion. Radiology 2017; 285: 932–940. [DOI] [PubMed] [Google Scholar]
- 8. Morotti A, Boulouis G, Dowlatshahi D, et al. Standards for detecting, interpreting, and reporting noncontrast computed tomographic markers of intracerebral hemorrhage expansion. Ann Neurol 2019; 86: 480–492. [DOI] [PubMed] [Google Scholar]
- 9. Dowlatshahi D, Morotti A, Al-Ajlan FS, et al. Interrater and intrarater measurement reliability of noncontrast computed tomography predictors of intracerebral hemorrhage expansion. Stroke 2019; 50: 1260–1262. [DOI] [PubMed] [Google Scholar]
- 10. Morotti A, Brouwers HB, Romero JM, et al. Intensive blood pressure reduction and spot sign in intracerebral hemorrhage: a secondary analysis of a randomized clinical trial. JAMA Neurol 2017; 74: 950–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Benomar A, Zarour E, Létourneau-Guillon L, et al. Measuring interrater reliability. Radiology 2023; 309: e230492. [DOI] [PubMed] [Google Scholar]
- 12. Rodriguez-luna D, Hill MD, Dowlatshahi D, et al. Ultraearly hematoma growth in active intracerebral hemorrhage. Neurology 2016; 87: 357–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Newgard CD, Lewis RJ. Missing data: how to best account for what is not known. JAMA 2015; 314: 940–941. [DOI] [PubMed] [Google Scholar]
- 14. Seiffge DJ, Polymeris AA, Law ZK, et al. Cerebral amyloid angiopathy and the risk of hematoma expansion. Ann Neurol 2022; 92: 921–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Al-Shahi Salman R, Frantzias J, Lee RJ, et al. Absolute risk and predictors of the growth of acute spontaneous intracerebral haemorrhage: a systematic review and meta-analysis of individual patient data. Lancet Neurol 2018; 17: 885–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yao X, Xu Y, Siwila-Sackman E, et al. The HEP score: a nomogram-derived hematoma expansion prediction scale. Neurocrit Care 2015; 23: 179–187. [DOI] [PubMed] [Google Scholar]
- 17. Schlunk F, Greenberg SM. The pathophysiology of intracerebral hemorrhage formation and expansion. Transl Stroke Res 2015; 6: 257–263. [DOI] [PubMed] [Google Scholar]
- 18. Mayer SA, Brun NC, Begtrup K, et al. Efficacy and safety of recombinant activated factor vii for acute intracerebral hemorrhage. N Engl J Med 2008; 358: 2127–2137. [DOI] [PubMed] [Google Scholar]
- 19. Qureshi AI, Palesch YY, Barsan WG, et al. Intensive blood-pressure lowering in patients with acute cerebral hemorrhage. N Engl J Med 2016; 375: 1033–1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kuramatsu JB, Gerner ST, Schellinger PD, et al. Anticoagulant reversal, blood pressure levels, and anticoagulant resumption in patients with anticoagulation-related intracerebral hemorrhage. JAMA 2015; 313: 824. [DOI] [PubMed] [Google Scholar]
- 21. Ma L, Hu X, Song L, et al. The third intensive care bundle with blood pressure reduction in acute cerebral haemorrhage trial (INTERACT3): an international, stepped wedge cluster randomised controlled trial. Lancet 2023; 402: 27–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sheth KN, Solomon N, Alhanti B, et al. Time to anticoagulation reversal and outcomes after intracerebral hemorrhage. JAMA Neurol. Epub ahead of print 9 February 2024. DOI: 10.1001/jamaneurol.2024.0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Greenberg SM, Ziai WC, Cordonnier C, et al. 2022 Guideline for the management of patients with spontaneous intracerebral hemorrhage: a guideline from the american heart association/american stroke association. Stroke 2022; 53: e282–e361. [DOI] [PubMed] [Google Scholar]
- 24. Hemphill JC, Greenberg SM, Anderson CS, et al. Guidelines for the management of spontaneous intracerebral hemorrhage. Stroke 2015; 46: 2032–2060. [DOI] [PubMed] [Google Scholar]
- 25. Sandset EC, Anderson CS, Bath PM, et al. European Stroke Organisation (ESO) guidelines on blood pressure management in acute ischaemic stroke and intracerebral haemorrhage. Eur Stroke J 2021; 6: II. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26. Shoamanesh A, Patrice Lindsay M, Castellucci LA, et al. Canadian stroke best practice recommendations: management of spontaneous intracerebral hemorrhage, 7th Edition update 2020. Int J Stroke 2021; 16: 321–341. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-doc-1-eso-10.1177_23969873241247436 for Predictors of severe intracerebral hemorrhage expansion by Andrea Morotti, Qi Li, Jawed Nawabi, Giorgio Busto, Federico Mazzacane, Anna Cavallini, Ashkan Shoamanesh, Mauro Morassi, Frieder Schlunk, Laura Piccolo, Giacomo Urbinati, Debora Pezzini, Maurizio Paciaroni, Enrico Fainardi, Ilaria Casetta, Alessandro Padovani and Andrea Zini in European Stroke Journal