Abstract
Background:
Despite the efficacy of diabetes prevention programs, only an estimated 5% of people with pre-diabetes actually participate. Mobile health (mHealth) holds promise to engage patients with pre-diabetes into lifestyle modification programs by decreasing the referral burden, centralizing remote enrollment, removing the physical requirement of a brick-and-mortar location, lowering operating costs through automation, and reducing time and transportation barriers.
Methods:
Non-randomized implementation study enrolling patients with pre-diabetes from a large health care organization. Patients were exposed to a text message–based program combining live human coaching guidance and support with automated scheduled, interactive, data-driven, and on-demand messages. The primary analysis examined predicted weight outcomes at 6 and 12 months. Secondary outcomes included predicted changes in HbA1c and minutes of exercise at 6 and 12 months.
Results:
Of the 163 participants included in the primary analysis, participants had a mean predicted weight loss of 5.5% at six months (P < .001) and of 4.3% at 12 months (P < .001). We observed a decrease in predicted HbA1c from 6.1 at baseline to 5.8 at 6 and 12 months (P < .001). Activity minutes were statistically similar from a baseline of 155.5 minutes to 146.0 minutes (P = .567) and 142.1 minutes (P = .522) at 6 and 12 months, respectively, for the overall cohort.
Conclusions:
In this real-world implementation of the myAgileLife Diabetes Prevention Program among patients with pre-diabetes, we observed significant decreases in weight and HbA1c at 6 and 12 months. mHealth may represent an effective and easily scalable potential solution to deliver impactful diabetes prevention curricula to large numbers of patients.
Keywords: pre-diabetic state, mobile applications, text messaging, weight loss, exercise
Introduction
In the United States, pre-diabetes affects more than 84 million adults. 1 Without intervention, an estimated 6% to 20% of these people will develop diabetes annually, with a cumulative incidence of 62% over 15 years.2 -4 Interrupting the progression from pre-diabetes to diabetes has profound human and economic consequences, as diabetes currently accounts for a loss of 4.4 years of life per individual with diabetes and the cost of care in the United States has skyrocketed to more than 327 billion dollars annually.5,6 In the landmark Diabetes Prevention Program (DPP) trial, the progression to diabetes was attenuated by 58% via intensive lifestyle support. 7 This study provided the foundation for the creation of the National Diabetes Prevention Program (NDPP) in 2010, representing a partnership between the Centers for Disease Control and Prevention (CDC) and both public and private organizations offering an evidence-based framework to help prevent or delay the development of type 2 diabetes. 8 In 2016, the CDC provided the Prevent T2 curriculum based on data from the original DPP trial and its follow-up studies combined with newer literature on self-efficacy, physical activity, and diet. 9 The curriculum promotes modest weight loss and increased physical activity over a 12-month lifestyle change focused program. 8 Despite the efficacy of DPP programs across various implementations, it is estimated that only 5% of people with pre-diabetes are actually referred to a certified DPP. 10 Numerous barriers to joining and completing a DPP exist, starting with the limited time patients may have with their clinician during the visit at which a diagnosis of pre-diabetes is made to discuss options. Furthermore, limited funding, staff, and physical space prevent widespread implementation of DPPs in environments in which diabetes prevention is desperately needed.11,12 Third, even when patients are enrolled into in-person programs, participation and adherence are poor due to time and travel requirements.13,14
Mobile health holds promise to engage patients with pre-diabetes into lifestyle modification programs by decreasing the referral burden on the clinician, centralizing remote enrollment, removing the physical requirement of a brick-and-mortar location, lowering operating costs through automation, and reducing time and transportation barriers. mHealth is the use of mobile phones to provide public health and medical solutions in a scalable and cost-effective manner. Mobile apps, text messaging–based interventions, integrated web programs, web-connected glucometers, and wearable activity trackers have all been developed to assist patients with the complex task of managing diabetes and maintaining pre-diabetes lifestyle changes.5,10,15 -19 mHealth can also be integrated into electronic medical records (EMRs) and health care data systems to refer patients to DPPs without adding to the stretched capacities of health care providers. The myAgileLife DPP is a mobile short message service (SMS) text messaging–based coaching service and support system designed to help people with pre-diabetes prevent or delay the onset of type II diabetes. It can be integrated into health care systems so that patients can be referred without face-to-face consultation with a health care provider. The primary aim of this study is to evaluate the impact of the myAgileLife DPP on weight loss in a real-world health care setting. Secondary aims include assessing the impact of this program on HbA1C and exercise patterns.
Methods
This is a non-randomized mobile SMS text messaging–based DPP intervention conducted between January 2017 and November 2019. Eligible patients from a single, large health care organization in Southern California with pre-diabetes were solicited and then self-selected into the 12-month myAgileLife DPP. The primary analysis examined predicted weight outcomes at 6 and 12 months. Secondary outcomes included predicted changes in HbA1c and minutes of exercise at 6 and 12 months. In an exploratory outcome, we describe the association between frequency of program engagement with changes in weight, HbA1c, and minutes of exercise. All study activities were approved by the institutional review board (IRB) at the Keck School of Medicine of University of Southern California (USC).
Study Population
The study cohort represents a convenience sample of patients from one of the largest and most comprehensive medical groups in Southern California. The medical group is spread more than 22 locations offering services such as primary and specialty care, laboratory, physical therapy, radiology, pharmacy, and urgent care. Eligibility criteria were (1) age ≥18 years, (2) body mass index (BMI) of ≥25 kg/m2 (≥23 kg/m2 for Asian American), and (3) a positive screening for pre-diabetes in the form of a fasting glucose of 100 to 125 mg/dL or a hemoglobin A1c of 5.7 to 6.4. A list of eligible patients was generated based on (1) laboratory data showing evidence of pre-diabetes, (2) the addition of “pre-diabetes” to a patient problem list in the EMR, or (3) direct provider referral. Health coaches contacted potential subjects to confirm eligibility and to describe the myAgileLife DPP. Patients were not eligible if they were pregnant at time of enrollment or had a previous diagnosis of either type I or type II diabetes prior to enrollment. The recruitment process was stepwise and multi-modal in which outreach was attempted first via phone, and if unsuccessful was followed by a letter and up to three phone calls. No in-person visits were required. Patients were advised to self-enroll into the program via the Internet or mobile web portal (myagilelife.com). Upon enrollment, participants received a text message asking them to reply YES to opt into the year-long program. This opt-in step confirmed they knew how to use the SMS-based platform on which the program was delivered. To be included in the primary analysis, participants needed to have two or more weight records and/or HbA1c records to calculate changes over time.
The Intervention
The myAgileLife DPP is a mobile SMS text messaging–based coaching service for patients with pre-diabetes. The Program combines live human coaching guidance and support with automated scheduled, interactive, data-driven, and on-demand messages, all delivered via the same, text messaging–based conversation and augmented by web-based resources and content. The data-driven messages were developed through an iterative process combining currently available materials from the National Diabetes Education Program (NDEP) with multi-disciplinary expert opinion from an endocrinologist, a qualitative researcher, and a certified diabetes educator. 20 myAgileLife DPP delivers the CDC’s NDPP Prevent T2 curriculum accompanied by comprehensive and timely support designed to keep participants engaged and focused on a daily basis. The full program duration is one year, divided up into 26 sessions that span one, two, or three weeks, depending on the depth and intensity of the Prevent T2 Session content for that session. Each participant is assigned a certified DPP lifestyle coach who reaches out at least once per session, and more often if necessary, to discuss associated topics, content, and lifestyle change recommendations. Participants receive up to three automated, personalized text messages per day that supplement their interaction with the lifestyle coach and ensure comprehensive and consistent coverage of the Prevent T2 curriculum.
Participants receive a weekly automated coach check-in with live coach follow-up as required to encourage, remind, and reinforce the logging and tracking of program data. During the “Introduction Session,” participants are directed to the secure member portal to set a six-month weight loss goal and a weekly activity minutes goal (both of which are required to participate in the program) and can optionally set goals for steps-per-day and distance-per-week. Participants have access to a member portal that includes a wide range of tools and content, including but not limited to DPP Session Summaries and materials, an online journal, online Action Plans, a configurable messaging calendar, and a Healthy Living library with educational content on food, exercise, diabetes/pre-diabetes, and lifestyle change.
The text-based coaching dialogue encourages participant interaction through quizzes, polls, and questions designed to reinforce the Prevent T2 curriculum content—each of which triggers immediate and relevant messages when the participant responds. The program continuously monitors progress data (weight, activity minutes, etc) by participants and sends dynamic messages on an automated basis to remind and/or reinforce these key behaviors. Participants are provided keywords they can text in at any time to trigger immediate in-the-moment responses that are tailored to the participant’s individual need. The full list of keywords for this program include MOOD, MOTIVATE, SHOPPING, EAT OUT, CHALLENGE, RISKS, SLIP UP, CUES, and GREAT.
Measures
The primary outcome was predicted weight change (lbs) at 6 and 12 months. Participants were asked to provide weight and activity minutes on a weekly basis, which they could do at any time on a self-reported basis via text, via their health coach, and/or directly in the member portal. Within the member portal, participants could also pair one or more personal monitoring devices such as fitness watches, fitness bands, and smart scales, so that their weight and activity data would be uploaded automatically by their device. For the program analysis, weight measured during medical visits at facilities within participant’s program duration was also incorporated into the logged weight data. For HbA1c, records measured during medical visits at facilities within 180 days before and after the program duration were analyzed. If multiple records exist around the program start and end date, records that were closest to these dates, either before or after, were treated as the base and final HbA1c measurements. Session attendance was defined as having any interaction from logging weights, logging activity minutes, or sending in-bound text messages (ie, ad hoc, keywords, and quizzes) at least once during each session. Demographic data were obtained using a self-reported baseline questionnaire at enrollment, which included reporting of participant’s start weight for this trial.
Program engagement was defined as any participant-initiated interaction including in-bound text messages, logging activity minutes, and/or logging weight. Level of engagement was calculated based on the number of sessions with one or more activity logs over the total number of sessions during program duration per each individual. To assess the relationships between the level of engagement and measurement change, patients were divided into two groups (high or low) based on the 50th percentile of their level of engagement.
Statistical Analysis
Participants with two or more weight assessments (including the self-reported baseline weight) within their program duration were included in the primary analysis. Importantly, not all participants logged the same number of weight entries, nor did they log them at the same time and frequency. To minimize the impact of this on our findings, we looked at the EMR for any additional weight entries and ran a multilevel regression model to generate predicted weight loss for the entire cohort. The study used the multilevel mixed-effects regression model by including all available logged weights for up to 12 months of follow-up as the study outcome. The model applied random intercepts and random slopes to adjust for within-participant correlations of the outcome. The model was adjusted for baseline weight, sex, race, and ethnicity (Hispanic, Non-Hispanic white, and non-Hispanic other) and total weeks in the program. To account for the anticipated non-linear trajectories of weight change over time, we included a weeks-squared term to calculate a polynomial model instead of a simple linear model. The following is the equation of the model that we used.
Y is the outcome measure.
i indexes the individual.
w indexes the week.
X is time-invariant covariates at the individual level (ie, race/ethnicity, sex, weeks enrolled, and baseline measurement).
W is indicator for the week where the observed value is measured.
W2 is the squared of the indicator of the week.
In a secondary analysis focused on participant engagement as a moderator of program effect, the models included interaction terms of weeks and weeks-squared by the indicator for the levels of engagement of each type of logged activities. From the model, predicted changes at 6 and 12 months from baseline weight were obtained for the overall program and by the level of engagement, and then tested for statistical differences in time and across engagement groups. All analyses were performed using Stata 15 with alpha set at 0.05.
Results
Between January 2017 and November 2019, a total of 366 patients were assessed for eligibility and registered to participate (Figure 1). All received a text-message to opt-in the program, and 309 (84%) completed the opt-in process. Opt-in participants had a mean age of 53 (SD = 12) years, BMI of 32.2 (SD = 6.5), and HbA1c of 5.9% (SD = 0.3); 63% were female, 25% were Hispanic, and 35% were white (Table 1). The mean self-reported start weight was 198lb (SD = 48). Of these participants, 146 were excluded from the primary analysis as they did not log at least two weights or two HbA1c. Subjects included in the primary analysis were similar in terms of demographics and initial weight to those who were excluded.
Figure 1.
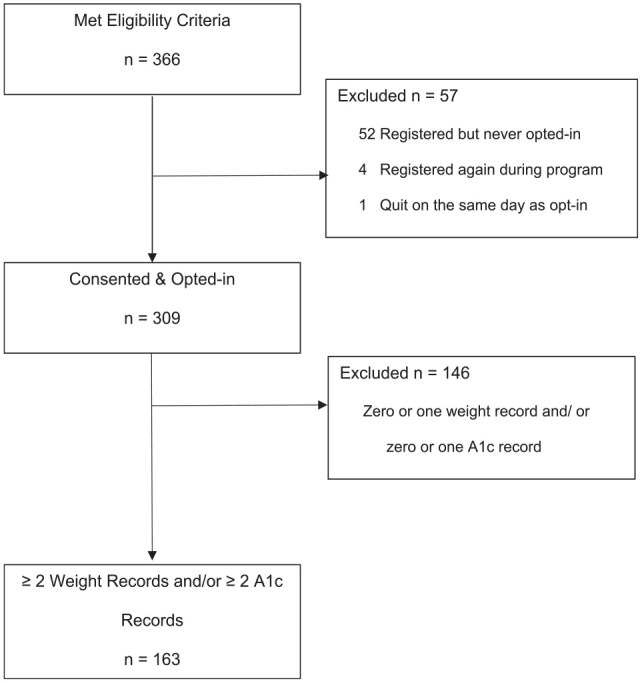
Flow diagram of participant flow through text message–based diabetes prevention program.
Table 1.
Baseline Characteristics of Text Messaging–Based Diabetes Prevention Program Participants.
| Characteristics | Consented for text-messaged DPP (n = 309) | Included in primary analysis (n = 163) | Excluded from primary analysis (n = 146) | Included vs excluded, P value |
|---|---|---|---|---|
| Female, n (%) | 194 (62.8) | 105 (64.4) | 89 (61.0) | .53 |
| Age, years, mean ± SD | 52.8 ± 12.0 | 52.8 ± 12.1 | 52.8 ± 12.0 | .99 |
| Ethnicity, n (%) | ||||
| Hispanic | 76 (24.6) | 32 (19.6) | 44 (30.1) | .07 |
| Non-Hispanic | 171 (55.3) | 100 (61.4) | 71 (48.6) | |
| Declined/Unknown | 30 (9.7) | 17 (10.4) | 13 (8.9 | |
| Missing | 23 (10.4) | 14 (8.6) | 18 (12.3) | |
| Race, n (%) | ||||
| Black | 19 (6.2) | 11 (6.8) | 8 (5.5) | .27 |
| White | 107 (34.6) | 59 (36.2) | 48 (32.9) | |
| Asian | 44 (14.2) | 26 (16.0) | 18 (12.3) | |
| Other | 74 (24.0) | 32 (19.6) | 42 (28.8) | |
| Declined/Unknown | 33 (10.7) | 21 (12.9) | 12 (8.2) | |
| Missing | 32 (10.4) | 14 (8.6) | 18 (12.3) | |
| Base weight, lb, mean ± SD | 197.7 ± 47.6 | 201.8 ± 50.5 | 193.1 ± 43.8 | .11 |
| Base BMI, mean ± SD (n = 276) | 32.2 ± 6.5 | 32.8 ± 6.9 | 31.5 ± 5.9 | .09 |
| Base HbA1c %, mean ± SD (n = 268) | 5.9 ± 0.3 | 6.0 ± 0.3 | 5.9 ± 0.3 | .47 |
Abbreviations: DPP, diabetes prevention program; SD, standard deviation; BMI, body mass index.
Of the 163 participants included in the primary analysis, 54% completed the 12-month program (Table 2). The mean program duration was 265 days (SD = 125) with two participants exceeding the 365-day mark. The mean number of attended sessions was 12 (SD = 8). For program engagement, participants sent text messages into the automated system during 82% of sessions attended (SD = 24), logged activity minutes at 61% of sessions attended (SD = 35), and logged weight at 72% of sessions attended (SD = 25). Participants had a mean predicted weight loss of 5.5% at six months (P < .001) and of 4.3% at 12 months (P < .001) (Table 3). We observed a decrease in predicted HbA1c from 6.1 at baseline to 5.8 at six months and at 12 months (P < .001). Activity minutes were statistically similar from a baseline of 155.5 minutes to 146.0 minutes (P = .567) and 142.1 minutes (P = .522) at six and 12 months, respectively, for the overall cohort. The diagram of these predictive models overlaying their scatter plot is presented in Supplemental Figures 1–3. The observed values for the full sample and the completer sample from available data are also presented in Supplemental Table 1.
Table 2.
Program Outcomes Description (N = 163).
| Primary analysis sample | |
|---|---|
| Completed the 12-month program, n (%) | 87 (54.4) |
| Program duration, days, mean ± SD | 264.5 ± 125.1 |
| Sessions attended, mean ± SD | 12.4 ± 8.3 |
| % of attended session with in-bound messages | 81.8% ± 23.6 |
| % of attended session with activity minutes logged | 61.4% ± 35.1 |
| % of attended session with weight logged | 71.7% ± 24.9 |
Abbreviation: SD, standard deviation.
Table 3.
Predicted Changes of Study Outcomes at six and 12 Months (N = 163).
| Program time | P value | ||||
|---|---|---|---|---|---|
| 0 months | 6 months | 12 months | 0 vs 6 | 0 vs 12 | |
| % weight loss (n = 158) | −0.3 ± 0.2 | −5.5 ± 0.4 | −4.3 ± 0.7 | <.001 | <.001 |
| HbA1c (n = 77) |
6.1 ± 0.0 | 5.8 ± 0.1 | 5.8 ± 0.1 | <.001 | <.001 |
| Minute exercise (n = 139) | 155.5 ± 8.4 | 146.0 ± 14.6 | 142.1 ± 21.8 | .567 | .522 |
In a secondary analysis focused on participant engagement as a moderator of program effect, the outcomes based on level of engagement results from the multilevel regression models did not show statistically significant differences for weight loss between high and low engagers but did show a statistically significant difference in minutes of exercise at six months (186.6 minutes for high engagers vs 111.7 minutes for low engagers; P = .008) and HbA1c (5.7 for high engagers vs 6.0 for low engagers; P = .020). Full results from the models are described in Table 4.
Table 4.
Predicted Changes of Study Outcomes at 6 and 12 Months, High versus Low Engagement.
| High engagement | Low engagement | P value a | ||||||
|---|---|---|---|---|---|---|---|---|
| 0 months | 6 months | 12 months | 0 months | 6 months | 12 months | 6 months | 12 months | |
| % weight loss (n = 158) | −0.2 | −5.5 | −5.1 | −0.3 | −5.5 | −3.4 | .984 | .228 |
| HbA1c (n = 77) |
6.1 | 5.7 | 5.8 | 6.1 | 6.0 | 5.8 | .020 | .949 |
| Minute exercise (n = 139) | 167.3 | 186.6 | 164.9 | 147.2 | 111.7 | 121.9 | .008 | .327 |
High versus low engagement at the specific month.
Discussion
We observed significant decreases in weight and HbA1c among patients with pre-diabetes who participated in the 12-month myAgileLife DPP in this pragmatic application of a mobile SMS text messaging–based DPP in a single, large health care organization in Southern California. Our findings are in line with other published reports of in-person, web, and mobile-based interventions reporting an average 3% to 5% weight loss.21 -26 Furthermore, we observed relatively high retention as over half of the enrolled patients completed the program. Together, this suggests that the desire for mobile DPP programs is strong, and if deployed widely, acceptance may be high among a patient population that has traditionally been difficult to reach and engage.
In addition to describing a successful program, this study adds to our understanding of the role of engagement on the efficacy of mHealth-based lifestyle interventions by providing novel, nuanced information regarding the association between level of engagement and predicted weight loss. Not only was weight loss greater at six months among patients who logged more activity minutes and among patients who interacted more frequently via text message (9.3 and 5.6 pounds more weight lost, respectively), but higher engagement predicted that the weight loss would maintain at 12 months. For example, patients in the lower engagement group not only did not lose as much weight during the program, but also regressed to near baseline weight by program end. This is in contrast to the patients in the highest engagement group who not only lost more weight during the program but kept a good portion of it off by program end (−5.3% weight change at 12 mon). The long-lasting impact of maintaining engagement with participants has not been previously reported in a mobile DPP and is likely of great importance to developers of similar programs targeting weight loss, to researchers studying program effectiveness, and to patients who want and need help. The most prominent impact of engagement was seen in exercise as high engagers increased their exercise minutes to more than 200 minutes per DPP session while low engagers decreased their activity. Across the board, outcomes were best at the six-month point, and then, there was a regression to the baseline. This suggests that strategies to increase engagement and retention in later months of this and similar programs must be developed and investigated to capitalize fully on mHealth’s potential impact for long-term change.
One important feature distinguishing this program from similar interventions was that there was no requirement for in-person interaction at any point. The outreach and enrollment were conducted remotely, weight loss was recorded via the web portal and smart devices, and all aspects of the DPP content were delivered via text message. Even the interactions with each individual’s assigned certified DPP Lifestyle Coach who reached out at least once per session occurred via live ad hoc text. Removing the need for any face-to-face interaction expands the potential reach of the program. This is especially important in our current medical environment in which office appointments and face-to-face clinical interactions have been dramatically reduced due to COVID-19. Moin et al 27 were also able to remove the in-person requirement for their program; however, they still required scheduled meetings via virtual visit, which is likely a barrier for some portion of the target population, and their results and findings are limited to a Veterans’ Affairs population. The myAgileLife DPP allows people to engage in the moment, over time, on their own schedule without having to carve out specific days/times for a virtual visit. In addition, the study population in this effort provides more evidence that an entirely mHealth-based DPP can effectively reach DPP goals.
While results in this pragmatic trial are promising, there are some notable limitations. There was no way to assess whether the delivered content was actually being read or understood by the participants. However, we observed a high active engagement rate during attended sessions implying that patients were active participants. Another related limitation is that there is no existing definition to define session attendance for a fully mHealth-based program, as one cannot simply take role as in in-person or virtual programs. An option would be to simply count any receipt of messages in the session as attendance but there is no objective way to track whether a participant actually viewed the content of the DPP texts. Instead, we chose to define engagement more actively—any participant-initiated interaction from logging weights, logging activity minutes, or sending in-bound text messages (ie, ad hoc, keywords, and quizzes) at least once during each session. Furthermore, we conducted various sensitivity checks using different definitions of attendance, and all resulted in the same magnitude and direction of findings. An additional limitation is that in this real-world implementation, there was no comparison group to compare the observed weight loss. Finally, as the study population reflects a self-selected group of participants from a larger cohort that was invited to participate, it is possible they were more motivated to improve their health than the average patient. However, this would still be representative of the type of patient that might enroll in any large, automated roll-out of a similar program.
Conclusions
In this real-world implementation of the myAgileLife DPP among patients with pre-diabetes, we observed significant decreases in weight and HbA1c at six and 12 months. In an exploratory analysis, the success of the program was more pronounced among patients with high levels of engagement. Strategies to generate and maintain engagement should be a focus for groups developing similar mHealth solutions. Although more work was needed to confirm our findings, mHealth appears to be an effective and easily scalable potential solution to deliver impactful diabetes prevention curricula to large numbers of deserving patients.
Supplemental Material
Supplemental material, sj-docx-1-dst-10.1177_19322968231162601 for Implementation and Evaluation of an Automated Text Message–Based Diabetes Prevention Program for Adults With Pre-diabetes by Sanjay Arora, Chun Nok Lam, Elizabeth Burner and Michael Menchine in Journal of Diabetes Science and Technology
Footnotes
Abbreviations: CDC, Centers for Disease Control and Prevention; DPP, diabetes prevention program; mHealth, mobile health; NDDP, National Diabetes Prevention Program.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Drs. Arora and Menchine serve as consultants to Agile Health. All other authors have no relevant conflicts of interest to disclose.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Sanjay Arora  https://orcid.org/0000-0002-7234-2398
https://orcid.org/0000-0002-7234-2398
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2017. Atlanta, GA: Centers for Disease Control and Prevention; US Department of Health and Human Services; 2017. [Google Scholar]
- 2. Rasmussen SS, Johansen NB, Witte DR, et al. Incidence of register-based diabetes 10 years after a stepwise diabetes screening programme: the ADDITION-Denmark study. Diabetologia. 2016;59(5):989-997. [DOI] [PubMed] [Google Scholar]
- 3. Forouhi NG, Luan J, Hennings S, Wareham NJ. Incidence of type 2 diabetes in England and its association with baseline impaired fasting glucose: the Ely study 1990–2000. Diabet Med. 2007;24(2):200-207. [DOI] [PubMed] [Google Scholar]
- 4. Diabetes Prevention Program Research Group. Long-term effects of lifestyle intervention or metformin on diabetes development and microvascular complications over 15-year follow-up: the Diabetes Prevention Program Outcomes Study. Lancet Diabetes Endocrinol. 2015;3(11):866-875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Alva ML, Hoerger TJ, Zhang P, Cheng YJ. State-level diabetes-attributable mortality and years of life lost in the United States. Ann Epidemiol. 2018;28(11):790-795. [DOI] [PubMed] [Google Scholar]
- 6. American Diabetes Association. Economic costs of diabetes in the US in 2017. Diabetes Care. 2018;41(5):917-928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or Metformin. N Engl J Med. 2002;346(6):393-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ely EK, Gruss SM, Luman ET, et al. A national effort to prevent type 2 diabetes: participant-level evaluation of CDC’s National Diabetes Prevention Program. Diabetes Care. 2017;40(10):1331-1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Centers for Disease Control and Prevention. Prevent T2 frequently asked questions. https://www.cdc.gov/diabetes/prevention/resources/t2faq.html. Published 2021. Accessed December 5, 2022.
- 10. Venkataramani M, Pollack CE, Yeh HC, Maruthur NM. Prevalence and correlates of diabetes prevention program referral and participation. Am J Prev Med. 2019;56(3):452-457. [DOI] [PubMed] [Google Scholar]
- 11. Thomas T, Samuel-Hodge CD, Porterfield DS, Alva ML, Leeman J. Scaling up diabetes prevention programs in North Carolina: perceptions of demand from potential program recipients and providers. Diabetes Educ. 2019;45(1):116-124. [DOI] [PubMed] [Google Scholar]
- 12. Bowen ME, Schmittdiel JA, Kullgren JT, Ackermann RT, O’Brien MJ. Building toward a population-based approach to diabetes screening and prevention for US adults. Curr Diab Rep. 2018;18(11):104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jackson SL, Long Q, Rhee MK, et al. Weight loss and incidence of diabetes with the Veterans Health Administration MOVE! lifestyle change programme: an observational study. Lancet Diabetes Endocrinol. 2015;3(3):173-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Griauzde D, Kullgren JT, Liestenfeltz B, et al. A mobile phone-based program to promote healthy behaviors among adults with prediabetes who declined participation in free diabetes prevention programs: mixed-methods pilot randomized controlled trial. JMIR Mhealth Uhealth. 2019;7(1):e11267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Norris SL, Zhang X, Avenell A, Gregg E, Schmid CH, Lau J. Long-term non-pharmacological weight loss interventions for adults with prediabetes. Cochrane Database Syst Rev. 2005;2:CD005270. [DOI] [PubMed] [Google Scholar]
- 16. Diabetes Prevention Program (DPP) Research Group. The Diabetes Prevention Program (DPP): description of lifestyle intervention. Diabetes Care. 2002;25(12):2165-2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Messina J, Campbell S, Morris R, Eyles E, Sanders C. A narrative systematic review of factors affecting diabetes prevention in primary care settings. PLoS ONE. 2017;12(5):e0177699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rehm CD, Marquez ME, Spurrell-Huss E, Hollingsworth N, Parsons AS. Lessons from launching the diabetes prevention program in a large integrated health care delivery system: a case study. Popul Health Manag. 2017;20(4):262-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Arora S, Peters AL, Burner E, Lam CN, Menchine M. Trial to examine text message-based mHealth in emergency department patients with diabetes (TExT-MED): a randomized controlled trial. Ann Emerg Med. 2014;63(6):745-754.e6. doi: 10.1016/j.annemergmed.2013.10.012. Erratum in: Ann Emerg Med. 2017;69(6):802. [DOI] [PubMed] [Google Scholar]
- 21. Block G, Azar KM, Romanelli RJ, et al. Diabetes prevention and weight loss with a fully automated behavioral intervention by email, web, and mobile phone: a randomized controlled trial among persons with prediabetes. J Med Internet Res. 2015;17(10):e240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Castro Sweet CM, Chiguluri V, Gumpina R, et al. Outcomes of a digital health program with human coaching for diabetes risk reduction in a Medicare population. J Aging Health. 2018;30:692-710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shirinzadeh M, Afshin-Pour B, Angeles R, Gaber J, Agarwal G. The effect of community-based programs on diabetes prevention in low- and middle-income countries: a systematic review and meta-analysis. Global Health. 2019;15(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Aziz Z, Absetz P, Oldroyd J, Pronk NP, Oldenburg B. A systematic review of real-world diabetes prevention programs: learnings from the last 15 years. Implement Science. 2015;10:172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Joiner KL, Nam S, Whittemore R. Lifestyle interventions based on the diabetes prevention program delivered via eHealth: a systematic review and meta-analysis. Prev Med. 2017;100:194-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McTigue KM, Conroy MB, Hess R, et al. Using the internet to translate an evidence-based lifestyle intervention into practice. Telemed J E Health. 2009;15(9):851-858. [DOI] [PubMed] [Google Scholar]
- 27. Moin T, Damschroder LJ, AuYoung M, et al. Results from a trial of an online diabetes prevention program intervention. Am J Prev Med. 2018;55(5):583-591. doi: 10.1016/j.amepre.2018.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-dst-10.1177_19322968231162601 for Implementation and Evaluation of an Automated Text Message–Based Diabetes Prevention Program for Adults With Pre-diabetes by Sanjay Arora, Chun Nok Lam, Elizabeth Burner and Michael Menchine in Journal of Diabetes Science and Technology


