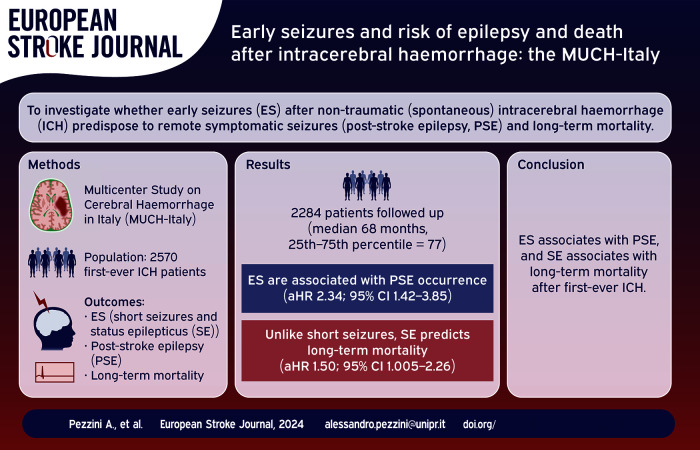
Abstract
Introduction:
It is unclear which patients with non-traumatic (spontaneous) intracerebral haemorrhage (ICH) are at risk of developing acute symptomatic seizures (provoked seizures occurring within the first week after stroke onset; early seizures, ES) and whether ES predispose to the occurrence of remote symptomatic seizures (unprovoked seizures occurring more than 1 week after stroke; post-stroke epilepsy, PSE) and long-term mortality.
Patients and Methods:
In the setting of the Multicenter Study on Cerebral Haemorrhage in Italy (MUCH-Italy) we examined the risk of ES and whether they predict the occurrence of PSE and all-cause mortality in a cohort of patients with first-ever spontaneous ICH and no previous history of epilepsy, consecutively hospitalized in 12 Italian neurological centers from 2002 to 2014.
Results:
Among 2570 patients (mean age, 73.4 ± 12.5 years; males, 55.4%) 228 (8.9%) had acute ES (183 (7.1%) short seizures and 45 (1.8%) status epilepticus (SE)). Lobar location of the hematoma (OR, 1.49; 95% CI, 1.06–2.08) was independently associated with the occurrence of ES. Of the 2,037 patients who were followed-up (median follow-up time, 68.0 months (25th–75th percentile, 77.0)), 155 (7.6%) developed PSE. ES (aHR, 2.34; 95% CI, 1.42–3.85), especially when presenting as short seizures (aHR, 2.35; 95% CI, 1.38–4.00) were associated to PSE occurrence. Unlike short seizures, SE was an independent predictor of all-cause mortality (aHR, 1.50; 95% CI, 1.005–2.26).
Discussion and Conclusion:
The long-term risk of PSE and death after an ICH vary according to ES subtype. This might have implications for the design of future clinical trials targeting post-ICH epileptic seizures.
Keywords: Cerebral haemorrhage, risk factors in epidemiology, epilepsy
Graphical abstract.
Introduction
One in every six people experience a stroke during their lifetime, and many stroke survivors suffer from major disability afterwards. 1 Intracerebral haemorrhage (ICH), the most severe form of stroke, accounts for 10%–15% of all cases in high income countries and its rate is expected to double in the next 50 years because of the increasing aging population. 2 The disease is associated with a high short-term case fatality rate, 1-year and 5-year survival rate of 46% and 29%, respectively, 1 and high long-term morbidity. Studies of patients with an ICH have consistently documented future risk of seizures and epilepsy among these subjects, 3 which affect patients’ quality of life, 4 increase the economic burden and result in a worse prognosis.5–8 Fewer studies have been conducted to identify which patients are more likely to develop acute symptomatic seizures (provoked seizures occurring within the first week after stroke onset; early seizures, ES) and to assess whether ES contribute to remote symptomatic seizures (unprovoked seizures occurring more than 1 week after stroke; post-stroke epilepsy, PSE) and mortality after an ICH. Furthermore, it is currently unknown whether the risk of death or epilepsy after an ICH varies by acute symptomatic seizure subtypes. This is a relevant issue when considering the risks and benefits associated with possible long-term treatment with anti-seizure medications (ASMs) in these patients. One such analysis, recently conducted on a large cohort of patients with acute ischemic stroke, showed that acute symptomatic status epilepticus (SE), as opposed to short acute symptomatic seizures, is associated with a high risk of post-stroke epilepsy and mortality. 9 Here, we aimed at testing the same hypothesis in an unselected, large cohort of patients with spontaneous ICH enrolled in the setting of the Multicenter Study on Cerebral Haemorrhage in Italy (MUCH-Italy). We also assessed predictive factors of ES and PSE.
Methods
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Study design and participants selection
The MUCH-Italy is a countrywide network of neurological centres designed to investigate epidemiology, risk factors and consequences of ICH in the setting of a hospital-based, multicentre, prospectively-recruiting, observational study. 10 Written informed consent was obtained for all participants (or next of kin). For the purpose of the present analysis, we screened data sets from patients with acute ICH consecutively admitted from 1 January 2002 to 31 July 2014. Eligibility for study participation required neuroimaging (CT or MRI) confirmation of haemorrhagic stroke. Exclusion criteria included the presence of trauma, brain tumour, haemorrhagic transformation of a cerebral infarction, vascular malformation or any other perceived cause of secondary ICH. As the primary goal of this study was to investigate post-ICH seizures, we excluded all patients with history of seizures or epilepsy prior to index ICH (diagnostic information derived from medical records or clinical interview with the patients or next-of-kin).
All patients underwent the first CT scan at admission and a follow-up CT scan at 24 h of symptom onset. Lesion volumes were estimated with the ABC/2 method. 11 Hematoma expansion (HE) was defined as absolute growth of > 6 mL or relative growth of > 33% from the first CT to the follow-up CT. 12 Twelve pre-specified medical complications were recorded for all patients after daily examinations, performed by specially trained physicians, nurses, and physiotherapists 13 (Supplemental Table 1). Medical complications were considered to have a potential pathogenic role and were included in the analysis when they occurred within the first 7 days after the index ICH and, in the subgroup of patients who developed ES, before the occurrence of the latter.
Risk factor definition
A history of vascular risk factors was defined as the presence of these predisposing conditions, either in the personal medical history or when identified during hospital stay 3 (Supplemental Methods).
Outcomes
Follow-up evaluations were conducted at 3 months and then annually, and outcome events were classified by using information from interviews (directly during follow-up visits or by telephone) with patients, next of kin, witnesses, and attending physicians or from hospital/general practitioner records. The primary end points were (1) any acute symptomatic seizures (ES) as a composite of short seizures and SE occurring within 7 days since index ICH and (2) remote symptomatic seizures (late seizures or PSE) as spontaneous unprovoked seizures occurring >7 days after stroke. 14 A single remote symptomatic seizure was classified as poststroke epilepsy because of the high (>60%) recurrence risk.15,16 For the categorization into SE or short seizures (i.e. not fulfilling criteria for SE), we used the revised definition of the International League Against Epilepsy (ILAE) 17 ; non-convulsive SE was defined electroencephalographically according to the Salzburg criteria. 18 SE was only diagnosed in cases with clinical signs or symptoms suggestive of convulsive or non-convulsive SE.
Statistical analysis
We used the Pearson χ 2 test, the t test for independent samples or the ANOVA test, as appropriate, to describe baseline demographic and clinical data. To evaluate variables that are associated with the risk of acute symptomatic seizures as well as with seizure subtypes (short seizures or SE), we first used univariable logistic regression and subsequently analysed all significant (p < 0.1) variables in a multivariable logistic regression model. The occurrence of acute symptomatic seizures was compared using logistic regression, because time-to-event was <1 week and thus pragmatically classified as binomial.
We applied a similar two-step approach using Cox regression to assess factors associated with time to PSE and time to death (separate models for the two outcome measures), as well as time to post-ICH IS and time to recurrent ICH. Candidate independent variables for multivariable modelling included all those with p < 0.1 for association with the outcome of interest. Duration of follow-up was calculated in person-months by using the follow-up of each participant from baseline examination until death, first remote symptomatic seizure, or most recent censored follow-up assessment. Two-sided values of p < 0.05 were considered significant. Statistical analyses were conducted with the software SPSS (version 27.0).
Results
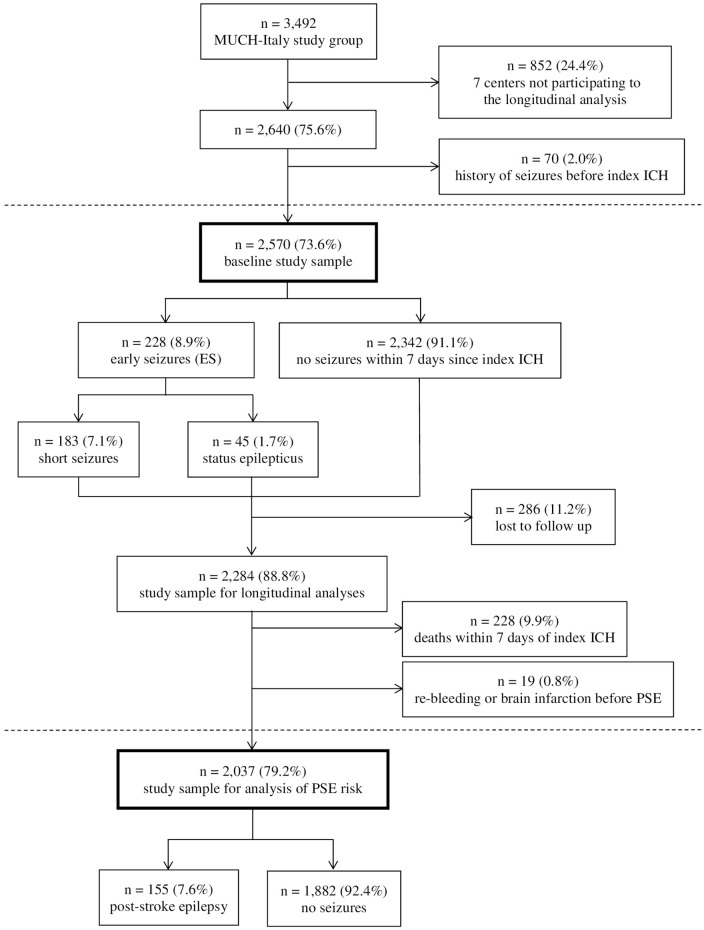
Twelve of the nineteen centres comprising the MUCH-Italy network participated to the present study. The remaining seven centres were unable to provide a systematic follow-up evaluation of the patients enrolled because of bureaucratic and administrative issues and, therefore, did not contribute to the present analysis. In all, of the 3492 patients with first-ever ICH included in the MUCH-Italy database, 2640 (75.6%) were considered for participation. Among them, 70 (2.0%) were excluded because of their history of seizures or epilepsy prior to index ICH, leaving 2570 (73.6%) patients (mean age, 73.4 ± 12.5 years; males, 55.4%) eligible for inclusion. Of these, 228 (8.9%) were diagnosed with acute ES. They were categorized as short seizures in 183 (7.1%) individuals and SE in 45 (1.7%; convulsive SE, n = 37 (82.2%); non-convulsive SE, n = 8 (17.8%)). After the exclusion of 286 (11.2%) patients who were lost to follow-up, 2284 (mean age, 73.7 ± 12.5 years; males, 55.3%) ICH survivors qualified for the longitudinal analysis and were followed for 6,685 patient-years (median follow-up time, 68.0 months (25th–75th percentile, 77.0) in patients who did not experience outcome events, with a maximum follow-up of 17 years). For the analysis of long-term risk of PSE we excluded from this cohort 228 (9.9%) patients who died within 7 days of the index haemorrhage and 19 (0.8%) patients who developed cerebral re-bleeding or brain infarction during follow-up before or at the same time as late seizures. Among the remaining 2037 (79.2%) patients we diagnosed late seizures in 155 (7.6%) cases, with median time to event of 10 months (25th to 75th percentile, 34 months; Figure 1).
Figure 1.
Participants’ enrolment and eligibility criteria flow chart. Flow-chart summarizing sequential application of eligibility/exclusion criteria leading to definition of final study population. Solid bordered boxes report number of patients fulfilling eligibility criteria at each stage.
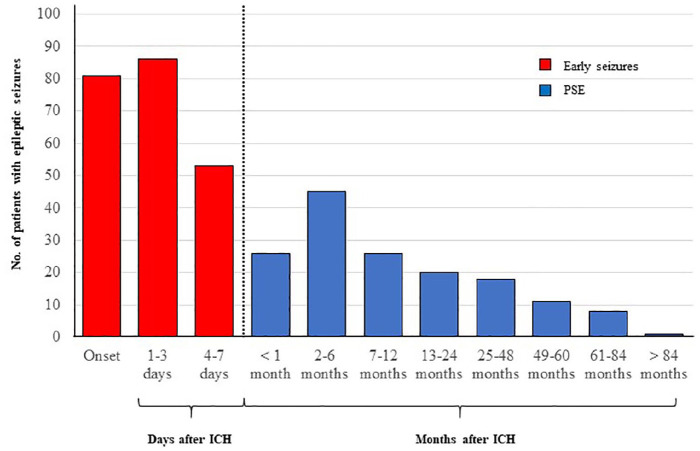
All ES patients received treatment with antiepileptic drugs at time of seizure diagnosis. Among PSE patients, 85 (54.8%) were still receiving antiepileptic treatment at time of recurrent seizures (Supplemental Table 2). Detailed information about temporal distribution of ES and PSE are shown in Figure 2.
Figure 2.
Post-ICH seizure diagnosis incidence over time. The incidence of newly-diagnosed seizure disorders at different time points following intracerebral haemorrhage (ICH). The dashed vertical line identifies the cut-off for definition of early versus late seizure (i.e. within or beyond 7 days from onset of ICH symptoms).
PSE: post-stroke epilepsy.
Factors associated with ES and their subtypes
Demographic and baseline clinical characteristics of the study group, stratified by occurrence of ES and its subtypes (short seizures and SE), are summarised in Table 1. Patients who developed ES had more frequently prior history of cancer, larger hematomas, higher NIHSS scores on admission, lobar location of the hematoma, and more often developed medical complications in the acute phase of cerebral bleeding. These findings were more robust among patients who developed SE, who also more frequently underwent surgical evacuation of the hematoma than patients without ES (Table 1).
Table 1.
Demographic and baseline clinical characteristics of the study group, stratified by occurrence of early seizures and their subtypes.
| Variable | No-seizures (n = 2342) | Early seizures (n = 228) | p-Value | Short seizures (n = 187) | Status epilepticus (n = 45) |
|---|---|---|---|---|---|
| Age, years ± SD | 73.4 ± 12.6 | 73.3 ± 12.1 | 0.844 | 73.3 ± 12.3 | 73.0 ± 11.5 |
| Sex, Male | 1296 (55.3) | 128 (56.1) | 0.816 | 103 (56.3) | 25 (55.6) |
| Coronary artery disease | 400 (17.1) | 43 (18.9) | 0.497 | 34 (18.6) | 9 (20.0) |
| Hystory of cancer | 159 (6.8) | 24 (10.5) | 0.036 | 18 (9.8) | 6 (13.3) |
| Hypertension | 0.409 | ||||
| Non-hypertensive | 629 (26.9) | 52 (22.8) | 42 (23.0) | 10 (22.2) | |
| Hypertensive under treatment | 1401 (59.8) | 145 (63.6) | 113 (61.7) | 32 (71.1) | |
| Hypertensive not under treatment | 312 (13.3) | 31 (13.6) | 28 (15.3) | 3 (6.7) | |
| Diabetes | 0.334 | ||||
| Non-diabetic | 1894 (80.9) | 179 (78.5) | 147 (80.3) | 32 (71.1) | |
| Diabetic under treatment | 374 (16.0) | 44 (19.3) | 32 (17.5) | 12 (26.7) | |
| Diabetic not under treatment | 74 (3.2) | 5 (2.2) | 4 (2.2) | 1 (2.2) | |
| Hypercholesterolemia | 0.353 | ||||
| Non-hypercholesterolemic | 1759 (75.1) | 162 (71.1) | 129 (70.5) | 33 (73.3) | |
| Hypercholesterolemic under treatment with statins | 423 (18.1) | 46 (20.2) | 35 (19.1) | 11 (24.4) | |
| Hypercholesterolemic not under treatment | 160 (6.8) | 20 (8.8) | 19 (10.4) | 1 (2.2) | |
| Smoking | 0.736 | ||||
| Never smoker | 1735 (74.1) | 174 (76.3) | 138 (75.4) | 36 (80.0) | |
| Former smoker | 349 (14.9) | 30 (13.2) | 25 (13.7) | 5 (11.1) | |
| Current smoker | 258 (11.0) | 24 (10.5) | 20 (10.9) | 4 (8.9) | |
| Alcohol, heavy intake | 298 (12.7) | 32 (14.0) | 0.572 | 28 (15.3) | 4 (8.9) |
| Antithrombotic therapy | |||||
| Antiplatelet agents | 770 (32.9) | 81 (35.5) | 0.417 | 66 (36.1) | 15 (33.3) |
| Oral anticoagulants | 301 (12.9) | 28 (12.3) | 0.805 | 19 (10.4) | 9 (20.0) |
| Hematoma location a | 0.001 | ||||
| Deep | 1280 (54.8) | 98 (43.0) | 84 (45.9) | 14 (31.1) | |
| Lobar | 1056 (45.2) | 130 (57.0) | 99 (54.1) | 31 (68.9) | |
| Hematoma expansion | 567 (25.1) | 59 (26.5) | 0.667 | 46 (25.6) | 13 (30.2) |
| Hematoma volume, mL | 45.0 ± 46.1 | 56.2 ± 37.8 | 0.003 | 57.0 ± 36.0 | 52.0 ± 43.0 |
| Hematoma evacuation | 54 (2.3) | 10 (4.4) | 0.055 | 7 (3.9) | 3 (6.8) |
| NIHSS score, median (IQR) | 13 (12) | 15 (9) | ⩽0.001 | 15 (9) | 18 (7) |
| Medical complications b | 1076 (45.9) | 125 (54.8) | 0.010 | 99 (54.1) | 26 (57.8) |
NIHSS: National Institute of Health Stroke Scale.
6 [0.2%] patients in the overall sample were categorized as mixed ICH.
Within the first 7 days since stroke occurrence.
In multivariable regression model only lobar location of the hematoma (OR, 1.49; 95% CI, 1.06–2.08) was independently associated with ES. In the analysis stratified by ES subtype, we identified hematoma volume (OR, 1.004; 95% CI, 1.0001–1.007 for each 1-ml increase) as independently associated with risk of short seizures and lobar location of the hematoma (OR, 3.48; 95% CI, 1.47–8.26), NIHSS scores on admission (OR, 1.11; 95% CI, 1.04–1.17 for each 1-point score increase) and surgical evacuation of the hematoma (OR, 4.20; 95% CI, 1.15–15.37) with risk of SE.
Long-term Risk of Post-stroke Epilepsy
The risk of ICH survivors having PSE following ES was 9.2% after 1 year and 11.8% after 5 years of the index stroke. This risk was lower in individuals without acute symptomatic seizures (4.3% after 1 year; 5.7% after 5 years; Supplemental Figure 1A). Demographic and clinical characteristics of the study group stratified by the occurrence of PSE are summarized in Supplemental Table 3 while the cumulative risk of PSE is summarized in Supplemental Figure 2. Among those who were alive at 7 days, we identified AF, characteristics of the index haemorrhage (lobar location of the hematoma, hematoma volume and HE), and ES and its subtypes (short seizures or SE) as related to PSE (all p < 0.01) in unadjusted Cox analysis. In multivariable Cox regression model, along with lobar location of the hematoma (HR, 1.90; 95% CI, 1.27–2.84), ES were independently associated with PSE compared to the subgroup of patients without ES (HR, 2.34; 95% CI, 1.42–3.85) an association that was mainly driven by short seizures (HR, 2.35; 95% CI, 1.38–4.00; Table 2).
Table 2.
Multivariable Cox regression model of time to post-stroke epilepsy.
| Variable | aHR (95% CI) | p Value |
|---|---|---|
| History of cancer | 1.50 (0.83–2.70) | 1.174 |
| Atrial fibrillation | 1.46 (0.88–2.42) | 0.143 |
| Hematoma location | 0.002 | |
| Deep | 1 | |
| Lobar | 1.90 (1.27–2.84) | |
| Hematoma expansion | 1.35 (0.81–2.24) | 0.237 |
| Hematoma volume | 1.003 (0.99–1.007) | 0.166 |
| Early seizures | 2.34 (1.42–3.85) | 0.001 |
| Early seizures, subtypes | ||
| Short seizures | 2.35 (1.38–4.00) | 0.002 |
| Status epilepticus | 2.27 (0.71–7.28) | 0.166 |
All-cause Mortality
All-cause mortality after ES was 47.2% after 1 year and 60.0% after 5 years compared to 41.7% after 1 year and 53.5% after 5 years in subjects without ES (Supplemental Figure 1B). After the exclusion from the overall cohort of 20 patients who developed cerebral re-bleeding or brain infarction during follow-up, we compared the characteristics of patients who were alive at the end of follow-up with those of patients who died. The results are summarized in Supplemental Table 4. Age (HR, 1.018; 95% CI, 1.013–1.024), history of ischemic heart disease (HR, 1.17; 95% CI, 1.001–1.37), NIHSS score on admission (HR, 1.03; 95% CI, 1.02–1.04 for each 1-point increase) and HE (HR, 2.09; 95% CI, 1.81–2.40) were independently and directly associated with all-cause mortality in multivariable Cox regression analysis, whereas the use of anti-thrombotic agents and statins turned out to have a protective effect (HR, 0.66; 95% CI, 0.52–0.84; HR, 0.51; 95% CI, 0.39–0.66, respectively). There was no association between overall ES and all-cause mortality compared to patients without ES (HR, 0.88; 95% CI, 0.71–1.08). However, we observed a differential effect on the long-term risk of death by seizure subtype. Unlike short seizures, which did not appear to be independently associated (HR, 1.04; 95% CI, 0.82–1.32), SE was found to predict all-cause mortality after adjustment for other covariates (HR, 1.50; 95% CI, 1.005–2.26; Table 3).
Table 3.
Multivariable Cox regression model of time to death.
| Variable | aHR (95% CI) | p Value |
|---|---|---|
| Age | 1.018 (1.013–1.024) | ⩽0.001 |
| Coronary artery disease | 1.17 (1.001–1.37) | 0.049 |
| Antithrombotic therapy | 0.66 (0.52–0.84) | 0.001 |
| Statins | 0.51 (0.39–0.66) | ⩽0.001 |
| NIHSS score | 1.03 (1.02–1.04) | ⩽0.001 |
| Hematoma expansion | 2.09 (1.81–2.40) | ⩽0.001 |
| Early seizures | 0.88 (0.71–1.08) | 0.246 |
| Early seizures, subtypes | ||
| Short seizures | 1.04 (0.82–1.32) | 0.697 |
| Status epilepticus | 1.50 (1.005–2.26) | 0.049 |
NIHSS: National Institute of Health Stroke Scale.
Impact of PSE on the risk of recurrent ICH and ischemic stroke
Overall, in the group of patients who developed PSE, we detected recurrent ICH in 27 (17.4%) cases compared to 170 (8.1%) cases in the group of patients who did not develop PSE (p ⩽ 0.001) and IS in 15 (9.7%) cases vs 82 (3.9%; p = 0.001) cases. By the Cox’s model, we estimated that the relative hazard of IS for patients in the PSE group was 2.32 (95% CI, 1.18–4.44) compared with patients in the non-PSE group. Conversely, we did not detect an association of PSE with recurrent ICH (HR, 1.24; 95% CI, 0.73–2.11).
Discussion
In the present analysis we used data from a large multicenter Italian cohort of patients with first-ever spontaneous ICH to assess the effect of selected variables in predicting the occurrence of early symptomatic seizures and their specific subtypes and to evaluate the impact of the latter on PSE and long-term mortality. Our main findings can be summarized as follows.
First, the occurrence of overall ES in the acute phase of ICH was associated with cortical-subcortical (lobar) location of bleeding. In the analysis stratified by ES subtypes, while hematoma volume was directly related to short seizures, lobar location of the haemorrhage, increasing admission NIHSS score and surgical evacuation of the hematoma were independently associated to SE. Overall, these findings are consistent with those of previous reports19–23 and support the prevailing idea that the cortical irritation from the blood products or from the mechanical effects of surgery result in decrease of the seizure threshold.
Second, ES are a strong predictor of PSE. Gliosis, deafferentation, selective neuronal loss, chronic inflammation, angiogenesis, neurodegeneration, collateral synaptic sprouting and altered synaptic plasticity have been advocated to explain the occurrence of epileptogenesis after stroke.24,25 Seizures also exacerbate the disruption of the blood–brain barrier and promote brain inflammation, which has been implicated as a nidus for the development of PSE. 26 Lastly, hemosiderin deposits after ICH may result in increasing neuronal excitability. 27 Our findings are in line with those of most prior studies24,28,29 which, with few exceptions, 30 have reported an association between ES and post-ICH epilepsy. We further observed that the impact of ES on the risk of PSE overcomes that of lobar location of the hematoma, the only other variable independently associated with epilepsy after ICH. These results are substantially in line with those on the basis of which the prognostic CAVE score was created, which identified ES as the strongest, independent predictor of the risk of post-ICH epilepsy. 31
Third, SE occurring within the first week after cerebral bleeding, as opposed to short seizures, is an independent predictor of all-cause mortality after correction for demographic and clinical characteristics, risk factors and comorbidities. The lack of association between ES and all-cause mortality is, therefore, mainly due to the prominent effect of acute symptomatic short seizures. SE is an uncommon, life-threatening condition, caused by acute stroke in approximately 22% of the cases in adults, 32 which has been reported to occur in 1.5% of the overall stroke population and to have a negative impact on long-term patient’s prognosis.33–35 Early-onset SE has been identified as an independent predictor of in-hospital mortality in many patient populations, including traumatic brain injury, epilepsy, acute IS, and subarachnoid haemorrhage.36–40 Conversely, its effect in patients with ICH is much less clear. Data of the literature vary according to study design and availability of EEG in the clinical setting, a few reports have focused on the impact of early-onset SE on short-term clinical outcome 40 and none of them have explored the long-term effect compared to that of other seizure subtypes. Hence, the present analysis yields essential new information on the influence of ES on the long-term outcome after ICH. Our findings reinforce and extend those of the recent meta-analysis of Misra and co-workers, which combines data on the impact of SE on all-cause mortality from cohorts of patients with ischemic stroke, subarachnoid haemorrhage, small series of patients with ICH, and which targets in-hospital and 30-day mortality. 40 Given the observational nature of our study, we can only advocate theoretical arguments to explain the increased long-term risk of death following acute symptomatic SE in patients with ICH. First, the persistence of epileptic activity is responsible for well-known neurotoxic effects,20,41 which, in turn, may adversely affect patients’ outcome. Also, cerebral oedema, collapse of cellular ion gradients and mitochondrial dysfunction from repeated seizure activity may contribute to secondary irreversible brain damage (gliotic scarring). Second, medical complications during hospital stay occur more frequently and can be misdiagnosed (and, thus, not properly managed) when SE happens in the acute phase of stroke. Accordingly, in our cohort we observed a higher prevalence of such complications in the SE subgroup (58.5%) than in the other patients’ subgroups (36.9%; p = 0.005). Third, SE and its pharmacological treatment may limit patient cooperation during rehabilitation with further risk of long-term complications and mortality. On the other hand, in contrast, we cannot theoretically rule out the possibility that treatment of SE has at least partially masked and reduced the impact of the latter on long-term mortality.
Finally, our findings suggest that patients with spontaneous ICH who develop PSE are at increased risk of IS. Whether this depends on hemodynamic changes, brain–blood barrier dysfunction, the effect of covert cerebrovascular lesions or of other coexistent pro-thrombotic conditions 42 in patients with PSE is difficult to determine based on our data. Notwithstanding, the clinical implication of the above is that PSE should be conceptualized as a biomarker for subsequent brain ischemia.
Strength and limitations
The MUCH-Italy combines the advantages of a prospective, multicentric, hospital-based recruitment, enabling a large sample size with detailed and standardized data collection with few missing data and a long-term systematic assessment of outcome events. But our study also has some limitations. First, it covers a long period of time which makes it subject to variability in diagnostic technologies and estimation of the prevalence of historical risk factors and therapeutic options. Furthermore, data on some variables (i.e. smoking habit and alcohol intake) were self-reported which cannot exclude the unavoidable risk of misclassification. Second, we only considered clinically apparent seizures and may have underestimated the incidence of non-convulsive seizures. Using continuous EEG after stroke might have increased the detection of seizures with subtle or no clinical signs but it appears non-feasible in the setting of a long-lasting multicentre study not designed to systematically detect asymptomatic seizures. Third, the study protocol did not include any formal assessment, other than the clinical interview with the patients or their relatives, of individual non-compliance with AEMs, which may have artificially influenced the long-term risk of PSE and death. Fourth, since we did not include MRI data in our analysis, we cannot estimate how relevant imaging biomarkers of cerebral vascular disease at baseline might be in predicting the risk of outcome events over time. Finally, our results are based on an Italian population and might not be generalizable to other countries or ethnicities.
Conclusion
In a large cohort of Italian patients with ICH we found that ES are strong predictors of PSE, SE occurring within the first week after cerebral bleeding, as opposed to short seizures, is an independent predictor of all-cause mortality and the occurrence of PSE predicts subsequent brain ischemia. Therefore, although it remains unclear whether long-term treatment of acute seizures might lead to improved outcomes after ICH,43,44 it seems likely that this varies according to the type of ES themselves. Future clinical trials targeting ES after acute ICH should take into account the differential effect that seizure subtypes may have on the long-term outcome of these patients.
Supplemental Material
Supplemental material, sj-docx-1-eso-10.1177_23969873241247745 for Early seizures and risk of epilepsy and death after intracerebral haemorrhage: The MUCH Italy by Alessandro Pezzini, Barbara Tarantino, Marialuisa Zedde, Simona Marcheselli, Giorgio Silvestrelli, Alfonso Ciccone, Maria Luisa DeLodovici, Lucia Princiotta Cariddi, Simone Vidale, Maurizio Paciaroni, Cristiano Azzini, Marina Padroni, Massimo Gamba, Mauro Magoni, Massimo Del Sette, Rossana Tassi, Ivo Giuseppe De Franco, Anna Cavallini, Rocco Salvatore Calabrò, Manuel Cappellari, Elisa Giorli, Giacomo Giacalone, Corrado Lodigiani, Mara Zenorini, Francesco Valletta, Gianni Cutillo, Guido Bonelli, Giorgia Abrignani, Paola Castellini, Antonio Genovese, Lilia Latte, Maria Claudia Trapasso, Chiara Ferraro, Francesco Piancatelli, Rosario Pascarella, Ilaria Grisendi, Federica Assenza, Manuela Napoli, Claudio Moratti, Maurizio Acampa and Mario Grassi in European Stroke Journal
Supplemental material, sj-docx-2-eso-10.1177_23969873241247745 for Early seizures and risk of epilepsy and death after intracerebral haemorrhage: The MUCH Italy by Alessandro Pezzini, Barbara Tarantino, Marialuisa Zedde, Simona Marcheselli, Giorgio Silvestrelli, Alfonso Ciccone, Maria Luisa DeLodovici, Lucia Princiotta Cariddi, Simone Vidale, Maurizio Paciaroni, Cristiano Azzini, Marina Padroni, Massimo Gamba, Mauro Magoni, Massimo Del Sette, Rossana Tassi, Ivo Giuseppe De Franco, Anna Cavallini, Rocco Salvatore Calabrò, Manuel Cappellari, Elisa Giorli, Giacomo Giacalone, Corrado Lodigiani, Mara Zenorini, Francesco Valletta, Gianni Cutillo, Guido Bonelli, Giorgia Abrignani, Paola Castellini, Antonio Genovese, Lilia Latte, Maria Claudia Trapasso, Chiara Ferraro, Francesco Piancatelli, Rosario Pascarella, Ilaria Grisendi, Federica Assenza, Manuela Napoli, Claudio Moratti, Maurizio Acampa and Mario Grassi in European Stroke Journal
Acknowledgments
None.
Footnotes
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr Ciccone reports grants from Daiichi-Sankyo; grants from Italfarmaco; and grants from Alexion Pharmaceuticals.
Dr Paciaroni reports compensation from SANOFI-AVENTIS U.S. LLC for other services; compensation from PFIZER CANADA INC for other services; compensation from iRhythm Technologies for other services; compensation from Daiichi Sankyo Europe GmbH for other services; and compensation from Bristol-Myers Squibb for other services.
The other Authors have nothing to disclose.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical approval: Ethical approval for this study was obtained from the Institutional Ethical Standards Committee on human experimentation at Brescia University Hospital.
Informed consent: Written informed consent was obtained for all participants (or next of kin).
Guarantor: A.P
Contributorship: A.P. had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: A.P., M.G. Acquisition of data: All authors. Interpretation of data: A.P., M.G. Drafting of the manuscript: A.P. Critical revision of the manuscript for important intellectual content: All authors. Data analysis: A.P., M.G. Statistical analysis: M.G, A.P. Administrative, technical, or material support: A.P. Study supervision: A.P.
ORCID iDs: Alessandro Pezzini  https://orcid.org/0000-0001-8629-3315
https://orcid.org/0000-0001-8629-3315
Maurizio Paciaroni  https://orcid.org/0000-0002-5483-8795
https://orcid.org/0000-0002-5483-8795
Rossana Tassi  https://orcid.org/0000-0002-5906-8718
https://orcid.org/0000-0002-5906-8718
Anna Cavallini  https://orcid.org/0000-0002-5227-1502
https://orcid.org/0000-0002-5227-1502
Manuel Cappellari  https://orcid.org/0000-0002-3534-3201
https://orcid.org/0000-0002-3534-3201
Antonio Genovese  https://orcid.org/0000-0003-0490-187X
https://orcid.org/0000-0003-0490-187X
Francesco Piancatelli  https://orcid.org/0009-0001-0976-4061
https://orcid.org/0009-0001-0976-4061
Rosario Pascarella  https://orcid.org/0000-0002-0512-9298
https://orcid.org/0000-0002-0512-9298
Maurizio Acampa  https://orcid.org/0000-0003-4149-1785
https://orcid.org/0000-0003-4149-1785
Supplemental material: Supplemental material for this article is available online.
References
- 1. GBD 2019 Stroke Collaborators. Global, regional, and national burden of stroke and its risk factors, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol 2021; 20: 795–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Qureshi AI, Tuhrim S, Broderick JP, et al. Spontaneous intracerebral haemorrhage. N Engl J Med 2001; 344: 1450–1460. [DOI] [PubMed] [Google Scholar]
- 3. Guo X, Zhong R, Han Y, et al. Incidence and relevant factors for seizures after spontaneous intracerebral haemorrhage: a systematic review and meta-analysis. Seizure 2022; 101: 30–38. [DOI] [PubMed] [Google Scholar]
- 4. Hamer HM. Seizures and epilepsies after stroke. Nervenarzt 2009; 80: 405–414. [DOI] [PubMed] [Google Scholar]
- 5. Gilmore E, Choi HA, Hirsch LJ, et al. Seizures and CNS haemorrhage: spontaneous intracerebral and aneurysmal subarachnoid haemorrhage. Neurologist 2010; 16: 165–175. [DOI] [PubMed] [Google Scholar]
- 6. Li Z, Zhao X, Wang Y, et al. Association between seizures and outcomes among intracerebral haemorrhage patients: the China National Stroke Registry. J Stroke Cerebrovasc Dis 2015; 24: 455–464. [DOI] [PubMed] [Google Scholar]
- 7. Madzar D, Kuramatsu JB, Gollwitzer S, et al. Seizures among long-term survivors of conservatively treated ICH patients: incidence, risk factors, and impact on functional outcome. Neurocrit Care 2014; 21: 211–219. [DOI] [PubMed] [Google Scholar]
- 8. Woo KM, Yang SY, Cho KT. Seizures after spontaneous intracerebral haemorrhage. J Korean Neurosurg Soc 2012; 52: 312–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sinka L, Abraira L, Imbach LL, et al. Association of mortality and risk of epilepsy with type of acute symptomatic seizure after ischemic stroke and an updated prognostic model. JAMA Neurol 2023; 80: 605–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pezzini A, Grassi M, Paciaroni M, et al. ; Multicentre Study on Cerebral Haemorrhage in Italy (MUCH-Italy) Investigators. Obesity and the risk of intracerebral haemorrhage: the multicenter study on cerebral haemorrhage in Italy. Stroke 2013; 44: 1584–1589. [DOI] [PubMed] [Google Scholar]
- 11. Kothari RU, Brott T, Broderick JP, et al. The ABCs of measuring intracerebral haemorrhage volumes. Stroke 1996; 27: 1304–1305. [DOI] [PubMed] [Google Scholar]
- 12. Wada R, Aviv RI, Fox AJ, et al. CT angiography “spot sign” predicts hematoma expansion in acute intracerebral haemorrhage. Stroke 2007; 38: 1257–1262. [DOI] [PubMed] [Google Scholar]
- 13. Pezzini A, Grassi M, Del Zotto E, et al. Complications of acute stroke and the occurrence of early seizures. Cerebrovasc Dis 2013; 35: 444—450. [DOI] [PubMed] [Google Scholar]
- 14. Beghi E, Carpio A, Forsgren L, et al. Recommendation for a definition of acute symptomatic seizure. Epilepsia 2010; 51(4): 671–675. [DOI] [PubMed] [Google Scholar]
- 15. Hesdorffer DC, Benn EKT, Cascino GD, et al. Is a first acute symptomatic seizure epilepsy? Mortality and risk for recurrent seizure. Epilepsia 2009; 50: 1102–1108. [DOI] [PubMed] [Google Scholar]
- 16. Fisher RS, Acevedo C, Arzimanoglou A, et al. ILAE official report: a practical clinical definition of epilepsy. Epilepsia 2014; 55: 475–482. [DOI] [PubMed] [Google Scholar]
- 17. Trinka E, Cock H, Hesdorffer D, et al. A definition and classification of status epilepticus: report of the ILAE Task Force on Classification of Status Epilepticus. Epilepsia 2015; 56: 1515–1523. [DOI] [PubMed] [Google Scholar]
- 18. Leitinger M, Trinka E, Gardella E, et al. Diagnostic accuracy of the Salzburg EEG criteria for non-convulsive status epilepticus: a retrospective study. Lancet Neurol 2016; 15: 1054–1062. [DOI] [PubMed] [Google Scholar]
- 19. Derex L, Rheims S, Peter-Derex L. Seizures and epilepsy after intracerebral haemorrhage: an update. J Neurol 2021; 268: 2605–2615. [DOI] [PubMed] [Google Scholar]
- 20. Vespa PM, O’Phelan K, Shah M, et al. Acute seizures after intracerebral haemorrhage: a factor in progressive midline shift and outcome. Neurology 2003; 60: 441–446. [DOI] [PubMed] [Google Scholar]
- 21. De Herdt V, Dumont F, Henon H, et al. Early seizures in intracerebral haemorrhage: incidence, associated factors, and outcome. Neurology 2011; 77: 1794–1780. [DOI] [PubMed] [Google Scholar]
- 22. Nilo A, Pauletto G, Lorenzut S, et al. Post-stroke status epilepticus: time of occurrence may be the difference? J Clin Med 2023; 12: 769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang H, Chen D, Tan G, et al. Incidence rate and risk factors of status epilepticus after stroke. Seizure 2021; 91: 491–498. [DOI] [PubMed] [Google Scholar]
- 24. Ferlazzo E, Gasparini S, Beghi E, et al. Epilepsy in cerebrovascular diseases: review of experimental and clinical data with meta-analysis of risk factors. Epilepsia 2016; 57: 1205–1214. [DOI] [PubMed] [Google Scholar]
- 25. Li S, Overman JJ, Katsman D, et al. An age-related sprouting transcriptome provides molecular control of axonal sprouting after stroke. Nat Neurosci 2010; 13: 1496–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Marchi N, Granata T, Ghosh C, et al. Blood-brain barrier dysfunction and epilepsy: pathophysiologic role and therapeutic approaches. Epilepsia 2012; 53: 1877–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kamp MA, Dibue M, Schneider T, et al. Calcium and potassium channels in experimental subarachnoid haemorrhage and transient global ischemia. Stroke Res Treat 2012; 2012: 382146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lahti AM, Saloheimo P, Huhtakangas J, et al. Poststroke epilepsy in long-term survivors of primary intracerebral haemorrhage. Neurology 2017; 88: 2169–2175. [DOI] [PubMed] [Google Scholar]
- 29. Doria JW, Forgacs PB. Incidence, implications, and management of seizures following ischemic and hemorrhagic stroke. Curr Neurol Neurosci Rep 2019; 19(7): 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rossi C, De Herdt V, Dequatre-Ponchelle N, et al. Incidence and predictors of late seizures in intracerebral haemorrhages. Stroke 2013; 44: 1723–1725. [DOI] [PubMed] [Google Scholar]
- 31. Haapaniemi E, Strbian D, Rossi C, et al. The CAVE score for predicting late seizures after intracerebral hemorrhage. Stroke 2014; 45(7): 1971–1976. [DOI] [PubMed] [Google Scholar]
- 32. Labovitz DL, Hauser WA, Sacco RL. Prevalence and predictors of early seizure and status epilepticus after first stroke. Neurology 2001; 57: 200–206. [DOI] [PubMed] [Google Scholar]
- 33. Knake S, Rochon J, Fleischer S, et al. Status epilepticus after stroke is associated with increased long-term case fatality. Epilepsia 2006; 47: 2020–2026. [DOI] [PubMed] [Google Scholar]
- 34. Waterhouse E, Vaughan J, Barnes T, et al. Synergistic effect of status epilepticus and ischemic brain injury on mortality. Epilepsy Res 1998; 29: 175–183. [DOI] [PubMed] [Google Scholar]
- 35. Santamarina E, Abraira L, Toledo M, et al. Prognosis of post-stroke status epilepticus: effects of time difference between the two events. Seizure 2018; 60: 172–177. [DOI] [PubMed] [Google Scholar]
- 36. Dhakar MB, Sivakumar S, Bhattacharya P, et al. A retrospective cross-sectional study of the prevalence of generalized convulsive status epilepticus in traumatic brain injury: United States 2002–2010. Seizure 2015; 32: 16–22. [DOI] [PubMed] [Google Scholar]
- 37. Kennedy JD, Gerard EE. Continuous EEG monitoring in the intensive care unit. Curr Neurol Neurosci Rep 2012; 12: 419–428. [DOI] [PubMed] [Google Scholar]
- 38. Sethi NK, Rapaport BS, Solomon GE. An audit of continuous EEG monitoring in the neurological-neurosurgical intensive care unit. J Clin Neurophysiol 2014; 31: 416–417. [DOI] [PubMed] [Google Scholar]
- 39. van Walraven C, Dhalla IA, Bell C, et al. Derivation and validation of an index to predict early death or unplanned readmission after discharge from hospital to the community. CMAJ 2010; 182: 551–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Misra S, Kasner SE, Dawson J, et al. Outcomes in patients with poststroke seizures: a systematic review and meta-analysis. JAMA Neurol 2023; 80: e233240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Meldrum BS, Vigouroux RA, Brierley JB. Systemic factors and epileptic brain damage. Arch Neurol 1973; 29: 82–87. [DOI] [PubMed] [Google Scholar]
- 42. Brigo F, Lattanzi S. Poststroke seizures as stroke mimics: clinical assessment and management. Epilepsy Behav 2020; 104: 106297. [DOI] [PubMed] [Google Scholar]
- 43. Jones FJS, Sanches PR, Smith JR, et al. Seizure prophylaxis after spontaneous intracerebral haemorrhage. JAMA Neurol 2021; 78: 1128–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Peter-Derex L, Philippeau F, Garnier P, et al. Safety and efficacy of prophylactic levetiracetam for prevention of epileptic seizures in the acute phase of intracerebral haemorrhage (PEACH): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Neurol 2022; 21: 781–791. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-eso-10.1177_23969873241247745 for Early seizures and risk of epilepsy and death after intracerebral haemorrhage: The MUCH Italy by Alessandro Pezzini, Barbara Tarantino, Marialuisa Zedde, Simona Marcheselli, Giorgio Silvestrelli, Alfonso Ciccone, Maria Luisa DeLodovici, Lucia Princiotta Cariddi, Simone Vidale, Maurizio Paciaroni, Cristiano Azzini, Marina Padroni, Massimo Gamba, Mauro Magoni, Massimo Del Sette, Rossana Tassi, Ivo Giuseppe De Franco, Anna Cavallini, Rocco Salvatore Calabrò, Manuel Cappellari, Elisa Giorli, Giacomo Giacalone, Corrado Lodigiani, Mara Zenorini, Francesco Valletta, Gianni Cutillo, Guido Bonelli, Giorgia Abrignani, Paola Castellini, Antonio Genovese, Lilia Latte, Maria Claudia Trapasso, Chiara Ferraro, Francesco Piancatelli, Rosario Pascarella, Ilaria Grisendi, Federica Assenza, Manuela Napoli, Claudio Moratti, Maurizio Acampa and Mario Grassi in European Stroke Journal
Supplemental material, sj-docx-2-eso-10.1177_23969873241247745 for Early seizures and risk of epilepsy and death after intracerebral haemorrhage: The MUCH Italy by Alessandro Pezzini, Barbara Tarantino, Marialuisa Zedde, Simona Marcheselli, Giorgio Silvestrelli, Alfonso Ciccone, Maria Luisa DeLodovici, Lucia Princiotta Cariddi, Simone Vidale, Maurizio Paciaroni, Cristiano Azzini, Marina Padroni, Massimo Gamba, Mauro Magoni, Massimo Del Sette, Rossana Tassi, Ivo Giuseppe De Franco, Anna Cavallini, Rocco Salvatore Calabrò, Manuel Cappellari, Elisa Giorli, Giacomo Giacalone, Corrado Lodigiani, Mara Zenorini, Francesco Valletta, Gianni Cutillo, Guido Bonelli, Giorgia Abrignani, Paola Castellini, Antonio Genovese, Lilia Latte, Maria Claudia Trapasso, Chiara Ferraro, Francesco Piancatelli, Rosario Pascarella, Ilaria Grisendi, Federica Assenza, Manuela Napoli, Claudio Moratti, Maurizio Acampa and Mario Grassi in European Stroke Journal