Abstract
Introduction:
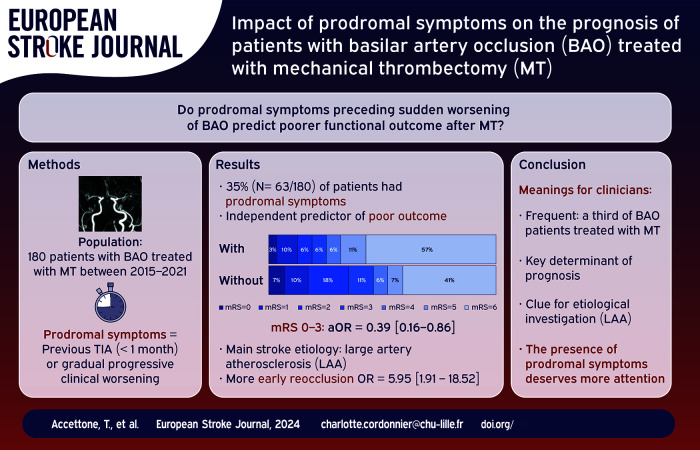
Even with reperfusion therapies, the prognosis of patients with basilar artery occlusion (BAO) related stroke remains poor. We aimed to test the hypothesis that the presence of prodromal symptoms, an easily available anamnestic data, is a key determinant of poor functional outcome.
Patients and methods:
Data from patients with BAO treated in Lille, France, with mechanical thrombectomy (MT) between 2015 and 2021 were prospectively collected. The presence of prodromal symptoms was defined by previous transient neurological deficit or gradual progressive clinical worsening preceding a secondary sudden clinical worsening. We compared the characteristics of patients with and without prodromal symptoms. We built multivariate logistic regression models to study the association between the presence of prodromal symptoms and functional (mRS 0–3 and mortality), and procedural (successful recanalization and early reocclusion) outcomes.
Results:
Among the 180 patients, 63 (35%) had prodromal symptoms, most frequently a vertigo. Large artery atherosclerosis was the predominant cause of stroke (41.3%). The presence of prodromal symptoms was an independent predictor of worse 90-day functional outcome (mRS 0–3: 25.4% vs 47.0%, odds ratio (OR) 0.39; 95% confidence interval (CI) 0.16–0.86) and 90-day mortality (OR 2.17; 95% CI 1.02–4.65). Despite similar successful recanalization rate, the proportion of early basilar artery reocclusion was higher in patients with prodromal symptoms (23.8% vs 5.6%, p = 0.002).
Discussion and conclusion:
More than one third of BAO patients treated with MT had prodromal symptoms, especially patients with large-artery atherosclerosis. Clinicians should systematically screen for prodromal symptoms given the poor related functional outcome and increased risk of early basilar artery reocclusion.
Keywords: Ischemic stroke, basilar artery occlusion, mechanical thrombectomy, prodromal symptoms
Graphical abstract.
Introduction
Acute ischemic stroke due to basilar artery occlusion (BAO) is a rare but severe condition associated with high disability and mortality rates.1,2 The effectiveness of mechanical thrombectomy (MT) in patients with BAO has been uncertain for a couple of reasons. Firstly, these patients were excluded from most trials examining MT for large-artery occlusion ischemic stroke.3–6 Secondly, the initial trials dedicated to BAO failed to provide conclusive evidence.7,8 More recently, the Endovascular Treatment for Acute Basilar Artery Occlusion (ATTENTION) and the Basilar Artery Occlusion Chinese Endovascular (BAOCHE) trials showed a clear benefit of MT in reducing disability and mortality.9,10 However, in these trials, the prognosis of BAO remains poor: among treated patients, more than half had severe residual disabilities, and one third died.
Therefore the ability to predict outcome in this population is a key issue for clinicians. 11 Among other predictors (age, vascular risk factors, initial clinical severity, occlusion site, and extent of ischemic lesions), the onset to treatment time is critical.12–19 In ATTENTION and BAOCHE RCTs, the estimated time of BAO was defined as the time of onset of acute symptoms leading to the clinical diagnosis of BAO. However, determining the exact onset of BAO is often challenging in this condition since a substantial proportion of patients doesn’t experience a sudden onset of symptoms. 2 Some patients with BAO have prodromal symptoms (such as transient neurological deficits or a gradual progressive worsening) preceding sudden worsening. To date, there is no consensual definition of prodromal symptoms, and the literature is scarce: the prevalence ranged from 38% to 78%, without clear time-window between the onset of prodromal symptoms and BAO diagnosis. 20 Various symptoms have been reported but mostly vertigo, nausea, and visual symptoms. In the Basilar Artery International Cooperation Study (BASICS) registry, the presence of prodromal symptoms was associated with a poorer outcome, although only 18% underwent MT. 21
In this large prospective cohort of patients with BAO treated with MT, we aimed to test the hypothesis that the presence of prodromal symptoms, an easily available anamnestic data, is a key determinant of poor functional outcome.
Methods
Ethics
The ethical committee (Comité de protection des personnes Nord-Ouest IV) classified the study as observational on March 9th, 2010, and the committee protecting patient’s personal data approved the study by December 21, 2010 (n° 10.677). Anonymized data supporting the findings of this study are available from the corresponding author on reasonable request.
Design, setting, population and treatments
Between January 1st, 2015, and December 31st, 2021, we retrospectively analyzed data from all consecutive stroke patients included in the Lille reperfusion registry, which is an ongoing observational registry. Details of the registry have been previously reported. 22 For the purpose of this study, we included all consecutive adult patients (no upper age limit) admitted to the Lille University Hospital (Lille, France) with an ischemic stroke related to BAO and treated with MT. The presence of BAO was diagnosed on MRI or CT-scan angiography and confirmed during digital subtracted angiography (DSA). MT was started within 24 h after presumed BAO onset (i.e. acute symptoms or sudden worsening) and selection criteria details are available in the Supplemental Materials.
Data assessments
Definition of prodromal symptoms
In the absence of consensual definition of prodromal symptoms, we defined them by the presence of transient neurological symptoms compatible with transient ischemic attack (TIA) in the posterior circulation occurring within 30 days before BAO diagnosis, or gradual progressive clinical worsening (>1 h) before first medical contact and preceding sudden clinical worsening that led to the stroke alert. We collected the symptoms and the delay between the first symptom and the clinical worsening leading to the diagnosis of BAO. In case of sudden onset of symptoms or early clinical worsening after the first medical contact, patients were classified “without prodromal symptoms.” Patients with wake-up stroke without neurological symptoms in the last month (reported by themselves or their relatives) were also classified “without prodromal symptoms.”
Clinical characteristics
We prospectively collected the following data: demographic characteristics (age, sex), vascular risk factors (arterial hypertension, diabetes mellitus, dyslipidemia, previous or current smoking, excessive alcohol consumption), history of atrial fibrillation, use of antithrombotic agents. We recorded the mRS before stroke. 23 The clinical severity was assessed using the National Institutes of Health Stroke Scale (NIHSS) before reperfusion treatment and 24 h after treatment. Administration of intravenous thrombolysis (IVT) before MT was registered. Time to treatment reported in the current study referred to the time between MT and the onset of acute symptoms leading to the diagnosis of BAO or, in case of “wake-up” stroke, the last time the patient was seen normal. The cause of ischemic stroke was determined at discharge according to the TOAST criteria (Trial of ORG 10172 in Acute Stroke Treatment). 24
Structural brain imaging, acquisition and interpretation
We used the posterior circulation Alberta Stroke Program Early CT Score (pc-ASPECTS) on DWI sequence or on CT-scan to estimate the extent of ischemic lesion. 25 To do so, the pc-ASPECTS was calculated on the first brain imaging by two trained examinators blinded to clinical, procedural, and radiological follow-up. We also noted on first imaging if some part of ischemic lesions were FLAIR-positive. As part of our in-house stroke protocol, all patients underwent follow-up brain imaging (MRI-MRA or CTA) 24 h after treatment or earlier in case of clinical worsening. This follow-up imaging comprised angiography and was used to evaluate the permeability of basilar artery, and the presence of symptomatic intracranial hemorrhage (ECASS2 trial criteria). 26
Angiographic data
Endovascular procedure and analysis of cerebral angiographic images were performed by experienced interventional neuroradiologists (TP, MB) blinded to clinical and MRI or CT data. The site of occlusion was classified on pretreatment angiography into proximal (from the vertebrobasilar junction to the origin of the anterior inferior cerebellar artery), middle (from the origin of the anterior inferior cerebellar artery to the origin of the superior cerebellar artery), and distal occlusion (distal to the origin of the superior cerebellar artery). 27 The type of device (aspiration and/or stent-retrievers) and the use of adjuvant therapeutics (angioplasty and/or stenting of basilar artery) were recorded. We reported the number of passes and the procedure duration. The degree of recanalization after MT was evaluated using the modified Thrombolysis in Cerebral Infarction scale (mTICI).10,28 Successful recanalization was defined as mTICI score ⩾ 2b and complete recanalization as a mTICI score of 3. At the end of the procedure, we recorded the presence or not of residual irregularities of basilar artery.
Outcome
The primary outcome was the percentage of patients who achieved a good 90-day functional outcome, defined as a modified Rankin Score (mRS) of 0–3, or equal to their pre-stroke score if it was > 3. Secondary outcomes included excellent 90-day outcome defined as mRS of 0–2 (or equal to their pre-stroke score if it was > 2), mortality and success of recanalization at the end of the procedure. In addition, in the subgroup of patients with initial successful recanalization, we evaluated the rate of early basilar artery reocclusion on systematic follow-up brain imaging.
Statistical analysis
Categorical variables were expressed as number (percentage). Quantitative variables were expressed as mean ± standard deviation (SD) or median and interquartile range (IQR) according to their distribution. Normality of distributions was assessed using histograms and Shapiro–Wilk test. Comparison of baseline characteristics between the two study groups (patients with and without prodromal symptoms) was performed using Student t-test or Mann–Whitney U test according to the distribution of quantitative variables, and using χ2 test or Fisher exact test for categorical variables. Effect of prodromal symptoms on the prognosis was assessed using a logistic regression model with and without adjustment on the following pre-specified confounders: clinical outcomes were adjusted for age, time to treatment, clinical severity, extent of ischemic lesion, previous IVT, and successful recanalization; the rates of successful and complete recanalization were adjusted for previous IVT, procedure duration, type of device used, and site of occlusion. Various models of adjustment are available in Supplemental Materials. Odds ratio (OR) with its 95% confidence interval (95% CI) was estimated as effect size for the group factor considering patients without prodromal symptoms as reference. All statistical analyses were performed using SAS software version 9.4. The threshold for statistical significance level was set to p < 0.05.
Results
Overall study population
During the study period, 2608 consecutive patients with a large artery occlusion related ischemic stroke were treated with MT in our institution. Among them, 180 patients (6.9%) had BAO and were included in the study. The baseline characteristics of the overall study population are reported in Table 1. In brief, mean age was 65 years (SD 4.8) and 68% were men. Twenty patients (11%) had a pre-stroke mRS > 2. Among them, four were treated despite a mRS 4 (a lack of information during the hyperacute phase). The median NIHSS score was 22 [IQR, 11–38]: 14 patients (7.8%) had a NIHSS score below 6, and 65 patients (36%) were intubated at admission. Sixty-two patients (34.4%) received IVT prior to MT. As part of our in-house care protocol, 89% of our patients underwent MRI as first-line imaging. Regarding outcome, 71 (39.4%) had a 90-day good functional outcome (mRS 0–3 or equal to their prestroke mRS if it was > 3), and 84 (46.7%) died. Regarding procedural outcome, 132 patients (73.3%) achieved a successful recanalization. Among them, 15 (11.4%) experienced an early basilar artery reocclusion. Thirteen of these reoccluded patients (87%) died (Table 2). With the exception of eight patients who underwent acute stenting of the BAO and received per-procedural antiagregation (Aspirin intravenously), patients did not receive antithrombotic agents before follow-up brain imaging at 24 h.
Table 1.
Baseline characteristics of the study population.
| Variable | All patients (n = 180) | With prodromal symptoms (n = 63) | Without prodromal symptoms (n = 117) | p-Value |
|---|---|---|---|---|
| Demographics – Vascular risk factors | ||||
| Age (years), mean (SD) | 64.9 (4.8) | 61.9 (14.2) | 66.5 (14.9) | 0.046 |
| Male, n (%) | 122 (67.78) | 44 (69.8) | 78 (66.7) | 0.66 |
| Hypertension, n (%) | 126 (70) | 41 (65.1) | 85 (72.6) | 0.29 |
| Diabetes, n (%) | 39 (21.7) | 13 (20.6) | 26 (22.2) | 0.81 |
| Dyslipidemia, n (%) | 66 (36.7) | 17 (27.0) | 49 (41.9) | 0.048 |
| Smoking, n (%) | 75 (41.7) | 32 (50.8) | 43 (36.8) | 0.068 |
| Atrial fibrillation, n (%) | 53 (29.4) | 8 (12.7) | 45 (38.5) | <0.001 |
| Previous antiplatelet use, n (%) | 47 (26.1) | 11 (17.5) | 36 (30.8) | 0.053 |
| Previous anticoagulant use, n (%) | 30 (16.7) | 6 (9.5) | 24 (20.5) | 0.059 |
| Stroke etiology | 0.001 | |||
| Large-artery atherosclerosis, n (%) | 55 (30.6) | 26 (41.3) | 29 (24.8) | |
| Cardioembolism, n (%) | 60 (33.3) | 9 (14.3) | 51 (43.6) | |
| Other determined etiology, n (%) | 12 (6.7) | 5 (7.9) | 7 (6.0) | |
| Undetermined etiology, n (%) | 53 (29.4) | 23 (36.5) | 30 (25.6) | |
| Clinical data | ||||
| NIHSS score pre-MT, median (IQR) | 22.0 (11.0;38.0) | 22.0 (12.0;38.0) | 23.0 (11.0;38.0) | 0.96 |
| Wake-up stroke, n (%) | 61 (33.89) | 22 (34.9) | 39 (33.3) | 0.83 |
| Radiological data | ||||
| FLAIR positive infarct a , n (%) | 101 (62.7) | 48 (82.8) | 53 (51.5) | <0.001 |
| Pc-ASPECTS, median (IQR) | 6.0 (5.0;8.0) | 6.0 (5.0;7.0) | 6.0 (5.0;8.0) | 0.52 |
| Procedural data | ||||
| Intravenous thrombolysis, n (%) | 62 (34.4) | 14 (22.2) | 48 (41.0) | 0.011 |
| Time to treatment (min), median (IQR) | 344 (245;615) | 408 (277;648) | 321 (238;520) | 0.22 |
| Catheterism failure, n (%) | 18 (10) | 11 (17.5) | 7 (6.0) | 0.014 |
| Occlusion site, n (%) | 0.011 | |||
| Proximal | 69 (40.1) | 29 (50.9) | 40 (34.8) | |
| Middle | 40 (23.3) | 16 (28.1) | 24 (20.9) | |
| Distal | 63 (36.6) | 12 (21.1) | 51 (44.3) | |
| Device type, n (%) | 0.77 | |||
| Aspiration | 64 (39.75) | 19 (37.3) | 45 (40.9) | |
| Stent retriever | 21 (13.04) | 8 (15.7) | 13 (11.8) | |
| Aspiration and stent retriever | 76 (47.2) | 24 (47.1) | 52 (47.3) | |
| Number of devices passes, median (IQR) | 1.0 (1.0;2.0) | 2.0 (1.0;3.0) | 1.0 (1.0;2.0) | 0.29 |
| Adjuvant angioplasty and/or stenting, n (%) | 16 (8.9) | 11 (17.5) | 5 (4.3) | 0.007 |
| Procedure duration (min), median (IQR) | 31.0 (21.0;57.0) | 40.0 (21.0;80.0) | 29.0 (20.5;50.0) | 0.04 |
| Presence of irregularities of basilar artery at the end of the procedure, n (%) | 62 (37.8) | 31 (57.4) | 31 (28.2) | <0.001 |
SD: standard deviation; IQR: interquartile Range; MT: Mechanical Thrombectomy; NIHSS: National Institutes of Head Stroke Scale; mTICI: modified Treatment in Cerebral Infarction; pc-ASPECTS: Posterior Circulation Alberta Stroke Program Early CT Score.
FLAIR positivity was evaluated in the 161 patients with MRI as first-line imaging (89,4%).
Table 2.
Impact of prodromal symptoms on the prognosis of patients with basilar artery occlusion treated with mechanical thrombectomy.
| Outcomes | All patients | With prodromal symptoms | Without prodromal symptoms | Unadjusted OR (95% CI) | Unadjusted p-value | Adjusted OR (95% CI) | Adjusted p-value |
|---|---|---|---|---|---|---|---|
| Clinical outcomes | |||||||
| 90-day good outcome, n (%) | 71 (39.4) | 16 (25.4) | 55 (47.0) | 0.38 (0.19–0.74) | 0.005 | 0.39 (0.16–0.86) | 0.024 a |
| 90-day excellent outcome, n (%) | 60 (33.3) | 13 (20.6) | 47 (40.2) | 0.39 (0.19–0.79) | 0.009 | 0.38 (0.16–0.93) | 0.033a |
| 90-day all-cause mortality, n (%) | 84 (46.7) | 36 (57.1) | 48 (41.0) | 1.92 (1.03–3.56) | 0.040 | 2.17 (1.02–4.65) | 0.046 a |
| Procedural outcomes | |||||||
| Final mTICI ⩾ 2B, n (%) | 132 (73.3) | 42 (66.7) | 90 (76.9) | 0.60 (0.31–1.18) | 0.140 | 0.84 (0.38–1.84) | 0.664 b |
| Final mTICI = 3, n (%) | 78 (45.9) | 20 (31.8) | 58 (49.6) | 0.47 (0.25–0.90) | 0.022 | 0.58 (0.27–1.22) | 0.151 b |
| Reocclusion at 24 h, n (%) | 15 (11.4) | 10 (23.8) | 5 (5.6) | 5.95 (1.91–18.52) | 0.002 | – | – |
mTICI: modified Treatment in Cerebral Infarction; MT: Mechanical Thrombectomy; IVT: Intravenous Thrombolysis.
90-day good outcome: modified Rankin Score (mRS) of 0–3, or equal to their pre-stroke score if it was > 3.
90-day excellent outcome: modified Rankin Score (mRS) of 0–2, or equal to their pre-stroke score if it was > 2.
Adjusted for age, time to treatment, PC-ASPECT, NIHSS score before MT, successful recanalization (mTICI ⩾ 2B), IVT before MT.
Adjusted for IVT before MT, procedure duration, type of device used, site of occlusion.
Prodromal symptoms
Sixty-three patients (35%) had prodromal symptoms. Among them, 16% (n = 10/63) had a transient neurological symptom, 84% (n = 53/63) had gradual progressive clinical worsening. The most frequently reported symptom was vertigo (n = 28/63; 44.4%), followed by vomiting (n = 16/63; 25.4%), associated headache (n = 15/63; 23.8%), and loss of consciousness (n = 9/63; 14%). The median delay between the first prodromal symptom and the secondary sudden clinical worsening leading to the diagnosis of BAO was 12 (5.5–72) h, and remained unchanged after the exclusion of 10 patients with TIA. Of note, prodromal symptoms had occurred within the last 7 days in 96% of patients (n = 61/63).
Profile of patients with prodromal symptoms
The baseline characteristics of patients with and without prodromal symptoms are reported in Table 1. Despite similar vascular risk factors, large-artery-atherosclerosis was more frequent in patients with prodromal symptoms (41.3% vs 24.8%, p < 0.001). These patients had more often a FLAIR-positive infarct (82.8% vs 51.5%, p < 0.001) and received less frequently IVT (22.2% vs 41%, p = 0.011) than patients without prodromal symptoms. Of note, we observed a high rate of catheterism failure in this population (17.5% vs 6%, p = 0.014). Proximal occlusions were more frequent (50.9% vs 34.8%, p = 0.01). Irregularities of basilar artery at the end of the procedure were more often observed (57.4% vs 28.2%, p < 0.001).
Impact of prodromal symptoms on outcomes
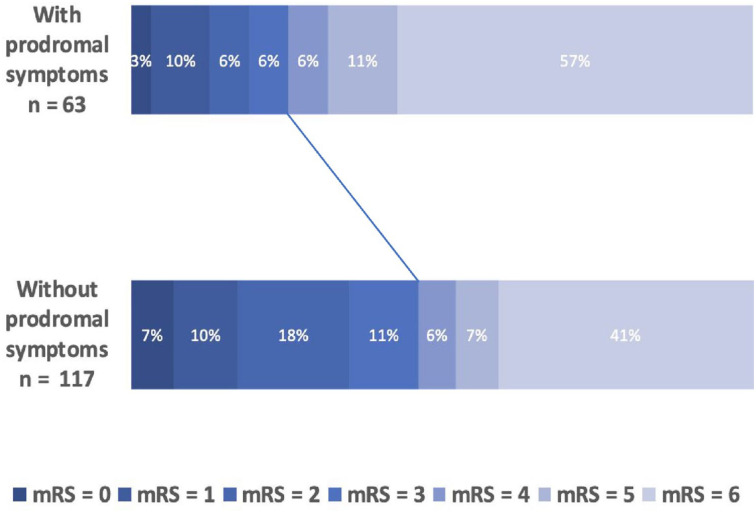
Percentage breakdown of mRS score at 90 days after MT between patients with and without prodromal symptoms is shown in Figure 1. As shown in Table 2, the presence of prodromal symptoms was independently associated with worse 90-day functional outcome (25.4% vs 47.0%; adjusted OR 0.39 [0.16–0.86], p = 0.024) and 90-day all-cause mortality (57.1%vs 41.0%; adjusted OR 2.17 [1.02–4.65], p = 0.046). Given that the a priori definition of confounding factors is debatable, we provide, in Supplemental Table 1, different statistical models with varying degree of adjustment. Whatever the model used, the presence of prodromal symptoms remained an independent predictor of functional outcome. We also repeated the analyses after excluding patients with TIA (n = 10): results remained unchanged (see Supplemental Table 2).
Figure 1.
Percentage breakdown of 90-day mRS score.
mRS scores range from 0 (no disability) to 6 (death). Solid line indicates the gap between the proportion of mRS [0–3] between patients with and without prodromal symptoms. mRS: modified Rankin Scale.
The rate of successful recanalization was not significantly different between patients with or without prodromal symptoms (mTICI ⩾ 2b: 66.7% vs 76.9%; adjusted OR 0.84 [0.38–1.84], p = 0.66), although we observed a slight difference on complete recanalization (mTICI 3: 31.8% vs 49.6%, adjusted OR 0.58 [0.27–1.22], p = 0.15).
Among patients with successful recanalization, the presence of prodromal symptoms increased the risk of early basilar artery reocclusion on follow-up brain imaging (23.8% vs 5.6%, unadjusted OR 5.95 [1.91–18.52], unadjusted p = 0.002). Finally, symptomatic hemorrhage occurred in 13 patients (7.6%), without difference between the two groups (5.1% vs 8.9%, p = 0.55).
Discussion
In 180 consecutive BAO patients treated with MT, more than a third had prodromal symptoms, which was a key determinant of poor prognosis: it was an independent predictor of 90-day poor functional outcome and it doubled mortality. In addition, those patients experienced more frequently early basilar artery reocclusion despite similar initial successful recanalization.
The presence of prodromal symptoms in 35% of our population was an independent and strong predictor of poor prognosis. Patients with prodromal symptoms were 60% less likely to have a good outcome, and had a twofold increased risk of mortality at 3 months. Unlike previously reported predictors, this prodromal status is specific to the BAO population, putting forward its clinical relevance.12–19 Even with the inclusion of 8% of patients with minor strokes, our rate of good outcome among patients with prodromal symptoms was still lower than those reported in the two recent RCTs (ATTENTION and BAOCHE).9,10 It is likely because we included patients with previous disability and elderly patients. Nonetheless, 25% of them had good outcome (and 20% had a mRS 0–2), suggesting that MT may be beneficial for some of these patients too.
Our findings raise several hypotheses for the poor outcome of BAO patients with prodromal symptoms. These patients received less frequently IV thrombolysis, probably because some prodromal symptoms may be associated with minor recent FLAIR positive infarcts leading to a decision to withhold thrombolysis. The proportion of catheterization failure was high among patients with prodromal symptoms, leaving one out of five patients without endovascular treatment. When catheterization was feasible, the procedure duration was longer with higher number of devices passes and the need for adjuvant therapies (angioplasty and/or stenting). These risky procedures also contribute to the poorer prognosis of these patients.29,30 Therefore, we suggest that the interventional neuroradiology team should be informed of the occurrence of prodromal symptoms. Despite similar initial successful recanalization, one out of four patients with prodromal symptoms had an early basilar artery reocclusion and this was another key prognostic determinant leading to a mortality rate of 90%. Although the low number of events hampered deepen statistical investigations, we hypothesize that the presence of basilar artery irregularities at the end of the procedure increased the risk of reocclusion. Whether these patients might benefit from early peri-procedural anti-aggregation to limit the risk of early reocclusion should be investigated in future studies.
Most of these factors contributing to poor prognosis share a common pathophysiological substrate: the over-representation of large-artery atherosclerosis in patients with prodromal symptoms (41% vs 25%) .31,32 This rate was still lower than those reported in recent RCTs (44% in ATTENTION trial, and 66% in BAOCHE trial).9,10 However, these trials included East Asian patients, a population at risk of large-artery atherosclerosis. Several mechanistical issues remain unsolved. For instance, the vascular integrity of the basilar artery at the time of prodromal symptoms is unknown (sub-occlusion or occlusion with initial effective collateral network). It may be suggested that the type of prodromal symptoms (TIA vs progressive worsening) may reflect a distinct underlying pathophysiological process and be associated with different outcomes although our results were unchanged after exclusion of the 10 patients with TIA. Further studies are warranted to better understanding the underlying mechanisms at play and to achieve a consensual definition of prodromal symptoms, including the type and time-window of symptoms. To go further, our results encourage the incorporation of the presence of prodromal symptoms into the design and interpretation of future RCTs.
Our study has several limitations, including the inherent bias of the observational, monocentric study design. Moreover, our population in the North of France is mono-ethnic, mainly represented by Caucasians. Our study focused on BAO patients treated with MT, therefore, we don’t know whether our results may be generalizable to all BAO patients. We arbitrarily classified patients with inaugural coma and no available informant as “without prodromal symptoms.” Moreover, given the lack of consensual definition, we arbitrarily fixed at 30 days the delay between prodromal symptoms and the diagnosis of BAO to limit the memory bias. Although more than 95% of prodromal symptoms occurred within the last 7 days, the proportion of patients with prodromal symptoms could have been underestimated. However, our proportion (35%) was consistent with the findings of the Basilar Artery International Cooperation Study (BASICS) registry: (n = 223/592; 38%), which included all patients with BAO, treated or not with MT. 21
The strengths of our study were the large sample size, with few missing data, and no patient lost to follow-up. We used data obtained from a well-conducted prospective stroke registry, and we included patients with various age, pre-stroke disability, clinical severity, extent of ischemic lesion, and time to treatment.
Conclusion
More than one third of BAO patients treated with MT had prodromal symptoms, especially patients with large-artery atherosclerosis. Clinicians should systematically screen for prodromal symptoms given the poor related functional outcome and increased risk of early basilar artery reocclusion.
Supplemental Material
Supplemental material, sj-docx-1-eso-10.1177_23969873241234844 for Impact of prodromal symptoms on the prognosis of patients with basilar artery occlusion treated with mechanical thrombectomy by Thomas Accettone, Thomas Personnic, Martin Bretzner, Helene Behal, Charlotte Cordonnier, Hilde Henon and Laurent Puy in European Stroke Journal
Supplemental material, sj-pdf-2-eso-10.1177_23969873241234844 for Impact of prodromal symptoms on the prognosis of patients with basilar artery occlusion treated with mechanical thrombectomy by Thomas Accettone, Thomas Personnic, Martin Bretzner, Helene Behal, Charlotte Cordonnier, Hilde Henon and Laurent Puy in European Stroke Journal
Acknowledgments
None
Footnotes
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: C.C. declares the following type of interests: speaker fees (Amgen and Bristol-Myers Squibb); Data And Safety Monitoring Board for Biogen and Bayer; grant funding from the French ministry of health (Programme Hospitalier de Recherche Clinique): A3ICH and TICH-3 Fr studies.
Other authors: none.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical approval and Informed consent: The ethical committee (Comité de protection des personnes Nord-Ouest IV) classified the study as observational on March 9th, 2010, and the committee protecting patient’s personal data approved the study by December 21, 2010 (n° 10.677). Anonymized data supporting the findings of this study are available from the corresponding author on reasonable request.
Guarantor: C.C. is the guarantor of this study.
Contributorship: T.A., L.P and H.H. contributed to the conception of the study; T.A., L.P., T.P., M.B., H.B. and H.H. contributed to the acquisition and analysis of the data. T.A., L.P. drafted the manuscript; H.H., C.C., T.P., M.B. and H.B. provided a review of the manuscript.
ORCID iD: Thomas Accettone  https://orcid.org/0009-0003-9097-721X
https://orcid.org/0009-0003-9097-721X
Supplemental material: Supplemental material for this article is available online.
References
- 1. Sparaco M, Ciolli L, Zini A. Posterior circulation ischaemic stroke-a review part I: anatomy, aetiology and clinical presentations. Neurol Sci 2019; 40: 1995–2006. [DOI] [PubMed] [Google Scholar]
- 2. Mattle HP, Arnold M, Lindsberg PJ, et al. Basilar artery occlusion. Lancet Neurol 2011; 10: 1002–1014. [DOI] [PubMed] [Google Scholar]
- 3. Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet 2016; 387: 1723–1731. [DOI] [PubMed] [Google Scholar]
- 4. Bracard S, Ducrocq X, Mas JL, et al.; THRACE Investigators. Mechanical thrombectomy after intravenous alteplase versus alteplase alone after stroke (THRACE): a randomised controlled trial. Lancet Neurol 2016; 15: 1138–1147. [DOI] [PubMed] [Google Scholar]
- 5. Nogueira R, Jadhav A, Haussen D, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med 2018; 378: 11–21. [DOI] [PubMed] [Google Scholar]
- 6. Albers GW, Marks MP, Kemp S, et al.; DEFUSE 3 Investigators. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med 2018; 378: 708–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liu X, Dai Q, Ye R, et al. Endovascular treatment versus standard medical treatment for vertebrobasilar artery occlusion (BEST): an open-label, randomised controlled trial. Lancet Neurol 2020; 19: 115–122. [DOI] [PubMed] [Google Scholar]
- 8. Langezaal LC, van der Hoeven EJ, Mont’Alverne FJ. Endovascular therapy for stroke due to basilar-artery occlusion. N Engl J Med 2021; 61: 116–1920. [DOI] [PubMed] [Google Scholar]
- 9. Tao C, Nogueira RG, Zhu Y, et al. Trial of endovascular treatment of acute basilar-artery occlusion. N Engl J Med 2022; 387: 1361–1372. [DOI] [PubMed] [Google Scholar]
- 10. Jovin TG, Li C, Wu L, et al.; BAOCHE Investigators. Trial of thrombectomy 6 to 24 hours after stroke due to basilar-artery occlusion. N Engl J Med 2022; 387: 1373–1384. [DOI] [PubMed] [Google Scholar]
- 11. Alemseged F, Nguyen TN, Coutts SB, et al. Endovascular thrombectomy for basilar artery occlusion: translating research findings into clinical practice. Lancet Neurol 2023; 22: 330–337. [DOI] [PubMed] [Google Scholar]
- 12. Singer OC, Berkefeld J, Nolte CH, et al.; ENDOSTROKE Study Group. Mechanical recanalization in basilar artery occlusion: the ENDOSTROKE study. Ann Neurol 2015; 77: 415–424. [DOI] [PubMed] [Google Scholar]
- 13. Yoon W, Kim SK, Heo TW, et al. Predictors of good outcome after stent-retriever thrombectomy in acute basilar artery occlusion. Stroke 2015; 46: 2972–2975. [DOI] [PubMed] [Google Scholar]
- 14. Bouslama M, Haussen DC, Aghaebrahim A, et al. Predictors of good outcome after endovascular therapy for vertebrobasilar occlusion stroke. Stroke 2017; 48: 3252–3257. [DOI] [PubMed] [Google Scholar]
- 15. Gory B, Mazighi M, Labreuche J, et al.; ETIS (Endovascular Treatment in Ischemic Stroke) Investigators. Predictors for mortality after mechanical thrombectomy of acute basilar artery occlusion. Cerebrovasc Dis 2018; 45: 61–67. [DOI] [PubMed] [Google Scholar]
- 16. Greving JP, Schonewille WJ, Wijman CA, et al.; BASICS Study Group. Predicting outcome after acute basilar artery occlusion based on admission characteristics. Neurology 2012; 78: 1058–1063. [DOI] [PubMed] [Google Scholar]
- 17. Strbian D, Sairanen T, Silvennoinen H, et al. Thrombolysis of basilar artery occlusion: impact of baseline ischemia and time. Ann Neurol 2013; 73: 688–694. [DOI] [PubMed] [Google Scholar]
- 18. Kumar G, Shahripour RB, Alexandrov AV. Recanalization of acute basilar artery occlusion improves outcomes: a meta-analysis. J Neurointerv Surg 2015; 7: 868–874. [DOI] [PubMed] [Google Scholar]
- 19. Mokin M, Sonig A, Sivakanthan S, et al. Clinical and procedural predictors of outcomes from the endovascular treatment of posterior circulation strokes. Stroke 2016; 47: 782–788. [DOI] [PubMed] [Google Scholar]
- 20. Ferbert A, Brückmann H, Drummen R. Clinical features of proven basilar artery occlusion. Stroke 1990; 21: 1135–1142. [DOI] [PubMed] [Google Scholar]
- 21. Conforto AB, de Freitas GR, Schonewille WJ, et al.; BASICS Study Group. Prodromal transient ischemic attack or minor stroke and outcome in basilar artery occlusion. J Stroke Cerebrovasc Dis 2015; 24: 2117–2121. [DOI] [PubMed] [Google Scholar]
- 22. Ferrigno M, Bricout N, Leys D, et al. Intravenous recombinant tissue-type plasminogen activator: influence on outcome in anterior circulation ischemic stroke treated by mechanical thrombectomy. Stroke 2018; 49: 1377–1385. [DOI] [PubMed] [Google Scholar]
- 23. van Swieten JC, Koudstaal PJ, Visser MC, et al. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1988; 19: 604–607. [DOI] [PubMed] [Google Scholar]
- 24. Adams HP, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. Toast. Trial of org 10172 in acute stroke treatment. Stroke 1993; 24: 35–41. [DOI] [PubMed] [Google Scholar]
- 25. Tei H, Uchiyama S, Usui T, et al. Posterior circulation ASPECTS on diffusion-weighted MRI can be a powerful marker for predicting functional outcome. J Neurol 2010; 257: 767–773. [DOI] [PubMed] [Google Scholar]
- 26. Hacke W, Kaste M, Fieschi C, et al. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke. The European Cooperative Acute Stroke Study (ECASS). JAMA 1995; 274: 1017–1025. [PubMed] [Google Scholar]
- 27. Archer CR, Horenstein S. Basilar artery occlusion: clinical and radiological correlation. Stroke 1977; 8: 383–390. [DOI] [PubMed] [Google Scholar]
- 28. Tomsick T, Broderick J, Carrozella J, et al.; Interventional Management of Stroke II Investigators. Revascularization results in the Interventional Management of Stroke II trial. AJNR Am J Neuroradiol 2008; 29: 582–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gory B, Mazighi M, Blanc R, et al. Mechanical thrombectomy in basilar artery occlusion: influence of reperfusion on clinical outcome and impact of the first-line strategy (ADAPT vs stent retriever). J Neurosurg 2018; 129: 1482–1491. [DOI] [PubMed] [Google Scholar]
- 30. Zhao C, Hu T, Kong W, et al. First-pass effect in patients with acute basilar artery occlusions undergoing stent retriever thrombectomy. J Neurosurg 2023; 138: 693–700. [DOI] [PubMed] [Google Scholar]
- 31. Mutke MA, Potreck A, Schmitt N, et al. Exact basilar artery occlusion location indicates stroke etiology and recanalization success in patients eligible for endovascular stroke treatment. Clin Neuroradiol 2023; 33: 483–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dhoisne M, Puy L, Bretzner M, et al. Early reocclusion after successful mechanical thrombectomy for large artery occlusion-related stroke. Int J Stroke 2023; 18: 712–719. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-eso-10.1177_23969873241234844 for Impact of prodromal symptoms on the prognosis of patients with basilar artery occlusion treated with mechanical thrombectomy by Thomas Accettone, Thomas Personnic, Martin Bretzner, Helene Behal, Charlotte Cordonnier, Hilde Henon and Laurent Puy in European Stroke Journal
Supplemental material, sj-pdf-2-eso-10.1177_23969873241234844 for Impact of prodromal symptoms on the prognosis of patients with basilar artery occlusion treated with mechanical thrombectomy by Thomas Accettone, Thomas Personnic, Martin Bretzner, Helene Behal, Charlotte Cordonnier, Hilde Henon and Laurent Puy in European Stroke Journal