Abstract
Objectives
This retrospective study aimed to determine the incidence and trends of proteinuria, elevations in serum creatinine and urea, and systolic blood pressure in cats undergoing treatment with toceranib.
Methods
In total, 32 cats treated with toceranib for malignancies were analyzed. Cats were included if urinalysis and urine protein:creatinine ratio (UPC) measurements were available at 28 days (T1) and 56 days (T2) after starting the treatment. Cats with concurrent lower urinary tract disease, including urinary tract malignancy, were excluded. Friedman’s ANOVA compared variables between time points, and the Spearman test assessed the correlation between treatment duration and UPC.
Results
The median starting dose of toceranib was 2.68 mg/kg (range 1.7–3.9). In total, 15 (46.9%) cats received concurrent non-steroidal anti-inflammatory drugs. The most commonly treated tumors were oral squamous cell carcinoma (n = 10) and mast cell tumor (n = 5). None of the 32 cats developed progressive proteinuria or azotemia during the follow-up period (median 56 days; range 56–336). Notably, UPC and serum creatinine were significantly lower at T2 compared with baseline (P = 0.012 and 0.001, respectively). Among the four cats with baseline proteinuria, UPC decreased over time with or without concurrent telmisartan treatment (n = 2). All four of these cats experienced a reduction in tumor size with toceranib concurrently with their decreased UPC. There was no significant correlation between UPC and the duration of toceranib treatment (P = 0.089). Blood pressure was not significantly different over the assessed time points.
Conclusions and relevance
The incidence of proteinuria, renal azotemia and hypertension in cats treated with toceranib for neoplasia appears to be low. Toceranib may be a viable treatment option even in cats with pre-existing proteinuria or renal disease, with careful monitoring of trends recommended.
Keywords: Toceranib, proteinuria, blood pressure, renal, azotemia
Introduction
Toceranib phosphate (Palladia; Zoetis) is a tyrosine kinase inhibitor, originally licensed for the treatment of non-resectable mast cell tumors in dogs. 1 As a result of shared targets in the angiogenesis and tumorigenesis pathways, toceranib is increasingly utilized in cats.2,3 Previous studies have illustrated efficacy in the management of feline malignancies such as mast cell tumor, oral squamous cell carcinoma, pancreatic carcinoma, gastrointestinal stromal cell tumor and chemodectoma, among others.4–9
Preliminary safety and toxicity studies have also demonstrated promising results in that toceranib appears to be generally well tolerated in cats.10,11 Adverse effects may include hepatotoxicity, which is typically mild and occurs in 3–10% of cats treated with toceranib.4,5,10 Gastrointestinal toxicities have also been reported in 22–36% of patients, with the majority recovering uneventfully after dose reductions or temporary breaks in administration.9–11 Myelosuppression appears to be similarly prevalent, with 25–28% of cats reported to be affected in prior studies.6,10,11
Toceranib has been noted to cause renal damage in dogs both directly via glomerulopathy and indirectly via hypertension, though the exact mechanism of both is not completely understood. 12 Renal toxicity associated with toceranib administration in cats is poorly characterized. Azotemia has been intermittently investigated with 11–15% of feline toceranib patients noted to have developed increased serum creatinine and/or urea during administration.10,11 However, these studies did not record concurrent urinalysis results so the association of toceranib with a true renal azotemia in cats is unknown. Similarly, there is a paucity of data regarding urinalysis and the urine protein:creatinine ratio (UPC) in cats receiving toceranib, with only two reports mentioning UPC measurements in a small number of patients.6,13 To the best of our knowledge, the incidence of hypertension has never been reported in cats receiving toceranib, despite the significant influence of hypertension on renal parameters, and the known risk of hypertension development in dogs treated with toceranib. 12 Given the prevalence of renal disease in the senior cat population, this lack of data represents a significant barrier to future toceranib use in feline patients. 14
The purpose of this study was to investigate the incidence and trends of proteinuria, serum creatinine and urea, and systolic blood pressure in cancer-bearing cats being treated with toceranib. Based on clinical observation, we hypothesize that toceranib does not significantly increase the incidence of proteinuria, renal azotemia or hypertension in cats.
Materials and methods
The medical records of feline patients were searched and retrieved from the Western College of Veterinary Medicine Veterinary Medical Centre database between January 2010 and January 2023. The inclusion criteria were limited to cats with either histologically or cytologically confirmed malignancy that was treated with toceranib. Patients were required to have received toceranib for a minimum of 56 days, with urinalysis, UPC, biochemistry and blood pressure measurements available at baseline, and every 4–6 weeks thereafter. The standard protocol at our institution is to conduct complete blood count (CBC), serum biochemistry, urinalysis, UPC and blood pressure measurements at baseline, hereafter referred to as T0. Patients are then assessed with a CBC 14 days after toceranib initiation. This is followed by repeat CBC, biochemistry, urinalysis, UPC and blood pressure measurements at 28 days and 56 days into toceranib therapy, hereafter referred to as T1 and T2, respectively. Cats with no UPC at baseline but no demonstrable proteinuria on urine dipstick analysis were included for descriptive purposes. Urine was routinely collected by cystocentesis, with urinalysis performed at Prairie Diagnostic Services reference laboratory. Urine dipstick analysis was interpreted with an automated, validated analyzer, coupled with a visual assessment by a technologist, while urine sediment was evaluated microscopically by a technologist. Comprehensive urinalysis results were interpreted by a board-certified clinical pathologist. Previous treatment with other modalities, such as surgery, radiation or alternate chemotherapeutics, was permitted for inclusion. Patients were excluded if they had evidence of lower urinary tract disease, such as urinary tract infections or malignancy of the urinary tract. Urinary tract infection was classified based on urine culture results if available, and if not available, patients were characterized based on significant bacteriuria with an active sediment. Malignancy of the urinary tract was determined based on ultrasonographic findings and cytologic diagnosis consistent with neoplasia.
For each patient, sex, neuter status, breed, age, weight, malignancy diagnosis, previous treatments, dose and duration of toceranib treatment, renal-related adverse effects, concurrent medical conditions and concurrent medications were recorded. Patients received toceranib on a Monday–Wednesday–Friday basis, with clinical response and eventual cause of discontinuation noted when available. For the duration of the treatment with toceranib, serial UPC, serum creatinine and urea, and systolic blood pressure were recorded. Blood pressure values were calculated as means of systolic pressures derived from five oscillometric measurements performed in clinic. Significant proteinuria was defined as a UPC consistently ⩾0.4. 15 International Renal Interest Society staging guidelines were used to define renal azotemia as serum creatinine consistently >140 µmol/l, with concurrent inappropriate urine specific gravity at readings <1.035. 16 Hypertension was defined as repeatable systolic blood pressure measurements >160 mmHg. 17
Simple descriptive statistics were used to describe the sex, and the median and range of age, weight and toceranib dose of the included patients. Friedman’s ANOVA test was performed to analyze any significant changes in UPC, serum urea, serum creatinine or blood pressure at different time points (T0, T1 and T2). Normality was assessed using the Shapiro–Wilk test. To assess the relationship between UPC at last measurement and the duration of toceranib exposure, Spearman’s correlation analysis was performed. Statistical significance was set at P <0.05. The collected data were analyzed using commercially available statistical software (SPSS version 28; IBM).
Results
A total of 33 cats receiving toceranib met the inclusion criteria. One cat treated with toceranib was excluded because of the presence of urothelial carcinoma. Of the included patients, 20 were castrated males and the remaining 12 were spayed females, with their median ages and weights at diagnosis described in Table 1. Breed was described as domestic shorthair or domestic longhair in all patients apart from one Himalayan and one Siamese. In total, 23 patients were receiving toceranib for the management of macroscopic (gross) disease, with the remaining nine cats receiving toceranib in the microscopic (postoperative with incomplete margins) disease setting. The most commonly reported malignancy treated was oral squamous cell carcinoma, followed by mast cell tumor (Table 2). Nine patients had previously been treated surgically, and five patients had previously received chemotherapy (carboplatin n = 3, lomustine n = 1, vinblastine n = 1). No patients received prior radiation therapy. Pertinent concurrent medications included robenacoxib (n = 4), prednisolone (n = 2), methimazole (n = 2) and telmisartan (n = 2). Meloxicam was administered to 11 cats at a dose of 0.05 mg/kg once daily, apart from one cat that received a dose of 0.02 mg/kg.
Table 1.
Characteristics of cancer-bearing cats at the time of initiating toceranib treatment
| Characteristic | Median | Range | n |
|---|---|---|---|
| Age (years) | 13 | 2–19 | 32 |
| Weight (kg) | 4.48 | 2.55–8.27 | 32 |
| Mean systolic blood pressure (mmHg) | 160 | 102–198 | 32 |
| Urine specific gravity | 1.031 | 1.013–1.060 | 32 |
| Serum urea (mmol/l)* | 8.90 | 7–17.6 | 32 |
| Serum creatinine (µmol/l) † | 146 | 79–219 | 32 |
| Urine protein:creatinine ratio | 0.18 | 0.05–2 | 21 |
| Toceranib dose (mg/kg) | 2.68 | 1.7–3.9 | 32 |
Reference interval 6–11.4 mmol/l
Reference interval 78–178 µmol/l
Table 2.
Tumor types in 32 cats treated with toceranib
| Tumor type | n |
|---|---|
| Oral squamous cell carcinoma | 10 |
| Mast cell tumor | 5 |
| Pancreatic carcinoma | 3 |
| Carcinomatosis | 3 |
| Mammary carcinoma | 2 |
| Pulmonary carcinoma | 2 |
| Nasal carcinoma | 2 |
| Salivary adenocarcinoma | 2 |
| Colonic carcinoma | 1 |
| Ceruminous adenocarcinoma | 1 |
| Apocrine adenocarcinoma | 1 |
At the time of toceranib initiation, 4/32 (12.5%) patients had pre-existing proteinuria, 3/32 (9.4%) were hypertensive, 5/32 (15.6%) had renal azotemia and 2/32 (6.25%) were receiving medical therapy for hyperthyroidism, with the remaining patient characteristics described in Table 1. The median duration of toceranib treatment was 56 days (range 56–336). The most common cause for toceranib discontinuation was progressive disease (n = 21), followed by a new unrelated malignant diagnosis (n = 2), difficulty with medication administration (n = 2), gastrointestinal side effects (n = 2), unrelated death from trauma (n = 2) and lack of recurrence or metastasis after surgical resection (n = 3). Notably, no patients required dose reductions or discontinuation of toceranib due to renal-related adverse effects.
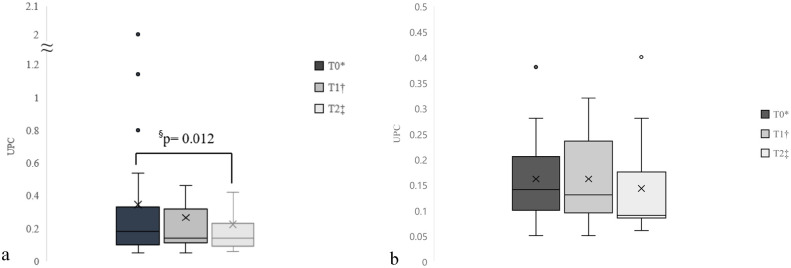
When UPC was compared between the three time points in the 21 cats with available data at T0, T1 and T2, the mean UPC of cats receiving toceranib was significantly lower at T2 (mean 0.22, 95% confidence interval [CI] 0.06–1.46) compared with T0 (mean 0.34, 95% CI 0.05–2.0; P = 0.012; n = 21) (Figure 1a). However, when the four cats with pre-existing proteinuria were excluded, no significant difference was detected in UPC over three time points (Figure 1b). Only 2/4 cats with pre-existing proteinuria and hypertension received specific treatment for these conditions with telmisartan. Of these four cases with existing proteinuria, all patients were being treated with toceranib in the gross disease setting for pulmonary carcinoma (n = 1), carcinomatosis (n = 1) and oral squamous cell carcinoma (n = 2). Improvement in UPC (n = 2) or resolution of proteinuria (n = 2) was observed concurrently with partial response of the tumors to toceranib (based on imaging or physical measurements) based on Response Evaluation Criteria in Solid Tumors guidelines. 18 UPC measurements at the time of disease progression were not available for these four cases.
Figure 1.
Box and whisker plots showing (a) urine protein:creatinine ratio (UPC) at three time points in 21 cats treated with toceranib and (b) UPC measurements at three time points in 17 cats without pre-existing proteinuria treated with toceranib. The boxes extend from the first to the third quartile, x denotes the mean and the horizontal black lines within each box denote median values. The whiskers denote minimum and maximum values; dots denote outliers. *T0 defined as baseline before toceranib administration. †T1 defined as 28 days after toceranib administration. ‡T2 defined as 56 days after toceranib administration. §denotes a statistically significant difference in UPC. ≈ represents a break in the graph axis
When other parameters were compared between the time points, no significant difference was detected in serum urea (n = 30) over time (P = 0.061). However, mean serum creatinine did demonstrate a significant decrease from 142 µmol/l (95% CI 79–219) at T0 to 131.6 µmol/l at T2 (95% CI 56–206; P = 0.001). No significant difference was observed for systolic blood pressure over any time point (P = 0.021, where P <0.017 is considered significant as per the Bonferroni adjustment) (Table 3).
Table 3.
Mean urine protein:creatinine ratio (UPC), systolic blood pressure, serum creatinine and serum urea in 21 cats treated with toceranib for at least 56 days
| Mean T0* | Mean at T1 † | Mean at T2 ‡ | P value | |
|---|---|---|---|---|
| UPC | 0.34 (0.05–2.0) | 0.26 (0.05–1.5) | 0.22 (0.06–1.46) | 0.012 |
| Serum urea (mmol/l) | 9.87 (7–18) | 9.40 (4–16) | 8.79 (5–14) | 0.061 |
| Serum creatinine (µmol/l) | 142 (79–219) | 131 (62–213) | 132 (56–206) | 0.001 |
| Systolic blood pressure (mmHg) | 158 (102–198) | 155 (100–190) | 152 (111–187) | 0.021 |
Data are mean (95% confidence interval). Significant differences are indicated in bold type.
T0 defined as baseline before toceranib administration
T1 defined as 28 days after toceranib initiation
T2 defined as 56 days after toceranib initiation
Spearman’s analysis was used to assess for correlation between the length of toceranib administration and UPC value. No significant correlation was found (P = 0.089, rs = −0.334).
Discussion
Similar to previous reports, our study indicates that toceranib seems to be generally well tolerated in cats with various malignancies.10,11 However, no previous studies specifically describe trends in proteinuria in cats receiving toceranib. In assessments of toceranib in the management of oral squamous cell carcinoma and injection site sarcoma in cats, the incidence of proteinuria was reported as 0% and 5%, respectively, though neither study reliably assessed UPC over time in all patients.5,13 In the current population, no cats developed proteinuria in association with toceranib administration. This is in marked contrast with toceranib use in dogs, where the incidence of proteinuria development has been reported at 21–24% in previous studies.12,19 Although it is possible that with a greater number of patients receiving toceranib over a longer period of time the incidence of proteinuria development may be greater, it is worth noting that previous work in dogs showed that affected patients developed proteinuria at a median of 49 days after starting toceranib therapy. 19 Given that the median length of administration (56 days) in our cohort is similar to 49 days and the lack of correlation between UPC value and length of administration, the overall risk for proteinuria development in cats receiving toceranib is still suspected to be low.
Notably, while the sample size is small, four cats with pre-existing proteinuria experienced a significant improvement in their UPC over the course of their treatment with toceranib. In the four affected patients, their proteinuria was suspected to be related to their neoplasia. This assumption is based on the exclusion of other causes based on available diagnostics, though it should be noted that renal histopathology was not available for definitive confirmation. While the incidence of neoplasia-associated proteinuria in cats is not well characterized, proteinuria may be prevalent in cancer-bearing cats similar to dogs, where the incidence of proteinuria in cancer-bearing dogs is reportedly in the range of 10–51%.12,19,20 This is also supported by our finding that 2/4 cases experienced significant decreases in their UPC along with tumor response without the use of telmisartan. Our results do not preclude the use of toceranib in cats with pre-existing proteinuria suspected to be caused by neoplasia. Further investigation is recommended not only in cats with severe pre-existing proteinuria, but also in those with mild to moderate proteinuria as the number of cats with pre-existing proteinuria in the present study is low. This is likely because of selection bias, where cats with more severe pre-existing proteinuria may have not been offered toceranib owing to a paucity of literature at the time. In addition, there would be a benefit in future studies to assessing UPC at the time of tumor progression to determine if UPC increases again in association with increasing tumor burden, as these data were not available for our subjects.
The development of new renal azotemia was not observed in the current investigation. This is contrary to previous literature, which reported an incidence of new azotemia in 11–15% of cats treated with toceranib.10,11 However, in contrast with the current study, previous reports lack consistent urinalysis data, so the development of a true renal azotemia is uncertain.10,11 Another consideration mentioned in previous literature is that the chronic nature of toceranib administration may permit unrelated chronic renal disease to develop concurrently during toceranib treatment. 4 This could be influential in the higher incidence of azotemia observed in the study by Merrick et al, 10 as their reported median duration of treatment was 100 days compared with 56 days in the present study.
The cause of the decrease in serum creatinine values over time in the current study is unclear and is likely influenced by limited power. We postulate that since progressive disease was the most common cause of toceranib discontinuation, loss of lean body mass or cachexia may have contributed to decreased serum creatinine. Unfortunately, muscle condition scoring was not routinely performed and recorded, which limits the ability to assess muscle loss in relation to this observation. Pathophysiologic phenomena that increase renal elimination of creatinine could also be considered, but were not readily identified in this population and have not been previously observed with tyrosine kinase inhibitor use in other species. Interestingly, tyrosine kinase inhibitors have been observed to increase serum creatinine concentrations through multiple renal-related mechanisms in humans; therefore, further studies may help elucidate species-specific differences in cats. 21
The development of hypertension during toceranib therapy also did not appear to be of significant concern in the present study. Although there is no feline literature available for comparison, this differs significantly from canine data, which found that 37% of dogs treated with toceranib developed hypertension. 12 The reason for this marked contrast between species is unclear, though reliable blood pressure readings between species may be influential. Previous work has illustrated that oscillometric measurement devices (which were used in this study) may under estimate blood pressure in conscious cats experiencing hypertension. 22 In addition, cats seem to experience greater variability in situational hypertension during in-clinic visits than dogs, whose blood pressure measurements tend to be more consistent throughout a clinic visit.23,24 These factors, in addition to potential undescribed differences in vascular and renal responses to toceranib between dogs and cats, may explain why hypertension development was not observed in the current study. Notably, only two cats were documented to have pre-existing hypertension before toceranib administration; therefore, additional studies would be required to further describe toceranib use in these patients.
Although not a primary directive of this study, it is also worth noting that nearly half of all included patients (15/32) were receiving concurrent non-steroidal anti-inflammatory drugs during therapy with toceranib. Similar to a recent study on the use of toceranib with low-dose meloxicam, this combination appears to be well tolerated without an obvious increase in renal toxicity in the current investigation. 25 Notably, the dose of meloxicam used in the current study (0.02–0.05 mg/kg) was higher than the dose reported by Keepman and Pellin (0.01–0.02 mg/kg). 25 Further conclusions regarding the influence of steroid use and methimazole on UPC, serum creatinine and urea, and systolic blood pressure would require additional investigation owing to the limited number of affected patients in the current study.
The limitations of the study are related to its retrospective nature, including the small sample size, and limited long-term data to assess for more chronic effects of toceranib administration in cats. Diagnostic testing was generally not performed at the time of toceranib discontinuation, which limits the interpretation of the observed trends at the time of disease progression. Similarly, potential confounders and influential factors, such as concurrent medications, comorbidities and muscle/body condition scoring, were not able to be analyzed owing to limited data. Selection bias may have occurred, as cats with existing renal azotemia, hypertension or proteinuria may not have been historically offered toceranib as a treatment option.
Conclusions
The results of the present study suggest that toceranib appears to pose a low risk for renal toxicity in cancer-bearing cats during a short-term treatment period. Specifically, no new proteinuria, renal azotemia or hypertension developed during toceranib therapy of a median duration of 8 weeks in a group of 32 cats. Although the study’s data are limited by its short follow-up duration, we consider the results clinically relevant, especially considering that the majority of feline cancer patients undergo toceranib treatment only for brief periods primarily because of tumor progression. Therefore, routine screening such as CBC, biochemistry and urinalysis with UPC is recommended before initiating toceranib therapy but rechecking UPC measurements may not be necessary in patients without pre-existing proteinuria for the first 2 months. In the few patients with pre-existing proteinuria, their UPC improved during their course of treatment with toceranib, indicating that toceranib may not be contraindicated, but would require further investigation.
Footnotes
Accepted: 17 June 2024
Author note: A portion of this study was presented as an abstract at the 2023 Virtual Institute for Comparative Cancer Investigation Research Symposium.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Ethical approval: The work described in this manuscript involved the use of non-experimental (owned or unowned) animals. Established internationally recognized high standards (‘best practice’) of veterinary clinical care for the individual patient were always followed and/or this work involved the use of cadavers. Ethical approval from a committee was therefore not specifically required for publication in JFMS. Although not required, where ethical approval was still obtained, it is stated in the manuscript.
Informed consent: Informed consent (verbal or written) was obtained from the owner or legal custodian of all animals described in this work (experimental or non-experimental animals, including cadavers) for all procedures undertaken (prospective or retrospective studies). No animals or people are identifiable within this publication, and therefore additional informed consent for publication was not required.
ORCID iD: Kimberly Williams  https://orcid.org/0009-0006-8388-8181
https://orcid.org/0009-0006-8388-8181
Valerie MacDonald-Dickinson  https://orcid.org/0009-0005-8255-9194
https://orcid.org/0009-0005-8255-9194
Arata Matsuyama  https://orcid.org/0000-0002-8314-5656
https://orcid.org/0000-0002-8314-5656
References
- 1. London C, Malpas P, Wood-Follis S, et al. Multi-center, placebo-controlled, double-blind, randomized study of oral toceranib phosphate (SU11654), a receptor tyrosine kinase inhibitor, for the treatment of dogs with recurrent (either local or distant) mast cell tumor following surgical excision. Clin Cancer Res 2009; 15: 3856–3865. [DOI] [PubMed] [Google Scholar]
- 2. Sabattini S, Frizzon MG, Gentilini F, et al. Prognostic significance of kit receptor tyrosine kinase dysregulations in feline cutaneous mast cell tumors. Vet Pathol 2013; 50: 797–805. [DOI] [PubMed] [Google Scholar]
- 3. Isotani M, Yamada O, Lachowicz JL, et al. Mutations in the fifth immunoglobulin-like domain of kit are common and potentially sensitive to imatinib mesylate in feline mast cell tumours. Br J Haematol 2010; 148: 144–153. [DOI] [PubMed] [Google Scholar]
- 4. Berger EP, Johannes CM, Post GS, et al. Retrospective evaluation of toceranib phosphate (Palladia) use in cats with mast cell neoplasia. J Feline Med Surg 2018; 20: 95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Olmsted G, Farrelly J, Post G, et al. Tolerability of toceranib phosphate (Palladia) when used in conjunction with other therapies in 35 cats with feline oral squamous cell carcinoma: 2009–2013. J Feline Med Surg 2017; 19: 568–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wiles V, Hohenhaus A, Lamb K, et al. Retrospective evaluation of toceranib phosphate (Palladia) in cats with oral squamous cell carcinoma. J Feline Med Surg 2017; 19: 185–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Todd J, Nguyen S. Long-term survival in a cat with pancreatic adenocarcinoma treated with surgical resection and toceranib phosphate. JFMS Open Rep 2020; 6. DOI: 10.1177/2055116920924911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McGregor O, Mooreb AS, Yeomansc S. Management of a feline gastric stromal cell tumour with toceranib phosphate: a case study. Aust Vet J 2020; 98: 181–184. [DOI] [PubMed] [Google Scholar]
- 9. Martinez I, Brockman D, Purzycka K. Caval chemodectoma in a cat. JFMS Open Rep 2022; 8. DOI: 10.1177/20551169221106990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Merrick CH, Pierro J, Schleis S, et al. Retrospective evaluation of toceranib phosphate (Palladia®) toxicity in cats. Vet Comp Oncol 2017; 15: 710–717. [DOI] [PubMed] [Google Scholar]
- 11. Harper A, Blackwood L. Toxicity and response in cats with neoplasia treated with toceranib phosphate. J Feline Med Surg 2017; 19: 619–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tjostheim SS, Stepien RL, Markovic LE, et al. Effects of toceranib phosphate on systolic blood pressure and proteinuria in dogs. J Vet Intern Med 2016; 30: 951–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Holtermann N, Kiupel M, Hirschberger J. The tyrosine kinase inhibitor toceranib in feline injection site sarcoma: efficacy and side effects. Vet Comp Oncol 2017; 15: 632–640. [DOI] [PubMed] [Google Scholar]
- 14. Marino CL, Lascelles BD, Vaden SL, et al. Prevalence and classification of chronic kidney disease in cats randomly selected from four age groups and in cats recruited for degenerative joint disease studies. J Feline Med Surg 2014; 16: 465–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lees GE, Brown SA, Elliot J, et al. Assessment and management of proteinuria in dogs and cats: 2004 ACVIM forum consensus statement (small animal). J Vet Intern Med 2005; 19: 377–385. [DOI] [PubMed] [Google Scholar]
- 16. Palm C, Syme H, Jepson R. Blood urea nitrogen and creatinine and clinical approach and laboratory evaluation of renal disease. In: Ettinger SJ, Feldman EC, Côté E. (eds). Textbook of veterinary internal medicine: diseases of the dog and cat. 8th ed. St. Louis: Elsevier, 2017, pp 777–779 and 4618. [Google Scholar]
- 17. Acierno MJ, Brown S, Coleman AE, et al. ACVIM consensus statement: guidelines for the identification, evaluation, and management of systemic hypertension in dogs and cats. J Vet Intern Med 2018; 32: 1803–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumors: revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45: 228–247. [DOI] [PubMed] [Google Scholar]
- 19. Piscoya SL, Hume KR, Balkman CE. A retrospective study of proteinuria in dogs receiving toceranib phosphate. Can Vet J 2018; 59: 611–616. [PMC free article] [PubMed] [Google Scholar]
- 20. Prudic RA, Saba CF, Lourenco BN, et al. Prevalence of proteinuria in a canine oncology population. J Small Anim Pract 2018; 59: 496–500. [DOI] [PubMed] [Google Scholar]
- 21. Omote S, Matsuoka N, Arakawa H, et al. Effect of tyrosine kinase inhibitors on renal handling of creatinine by MATE1. Sci Rep 2018; 8: 9237. DOI: 10.1038/s41598-018-27672-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jepson RE, Hartley V, Mendl M, et al. A comparison of CAT Doppler and oscillometric Memoprint machines for non-invasive blood pressure measurement in conscious cats. J Feline Med Surg 2005; 7: 147–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lyberg M, Ljungvall I, Häggström J, et al. Impact of equipment and handling on systolic blood pressure measurements in conscious dogs in an animal hospital environment. J Vet Intern Med 2021; 35: 739–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Belew AM, Barlett T, Brown SA. Evaluation of the white-coat effect in cats. J Vet Intern Med 1999; 13: 134–142. [DOI] [PubMed] [Google Scholar]
- 25. Keepman SJ, Pellin MA. Low dose meloxicam is safe and tolerable when combined with toceranib phosphate in cancer-bearing cats. J Feline Med Surg 2022; 24: 1187–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]