Abstract
BACKGROUND
Approximately 15% of Lyme disease cases involve the nervous system and are termed “neuroborreliosis.” A rare complication of neuroborreliosis is idiopathic intracranial hypertension with resulting neurological deterioration. There are very few reports of this in the literature, most of which consist of case reports and small case series. Neurosurgical intervention is exceedingly rare but may be needed in select cases.
OBSERVATIONS
The authors present the case of a 13-year-old male with Lyme disease and concurrent babesiosis with progressive headache, meningismus, emesis, and visual loss over several weeks. Serum and cerebrospinal fluid (CSF) testing confirmed a diagnosis of neuroborreliosis. Despite antimicrobial therapy and acetazolamide, visual loss worsened. An external ventricular drain (EVD) was urgently placed for CSF diversion. The use of CSF diversion, antimicrobial therapy, and acetazolamide led to significant improvement in the patient’s symptoms with nearly complete resolution. The EVD could be weaned, and permanent CSF diversion was not needed.
LESSONS
This case highlights a rare but significant complication of neuroborreliosis. Intracranial hypertension with resulting neurological deterioration, while uncommon, can occur in patients with Lyme disease. Management is most often medical, consisting of intravenous antibiotics and acetazolamide to reduce CSF production. In rare cases, temporary CSF diversion is necessary and can provide significant benefits to select patients.
Keywords: Lyme disease, neuroborreliosis, external ventricular drain, ventriculostomy, intracranial hypertension, case report
ABBREVIATIONS: CSF = cerebrospinal fluid, EVD = external ventricular drain, ICP = intracranial pressure, IIH = idiopathic intracranial hypertension, LP = lumbar puncture, MRI = magnetic resonance imaging, MRV = magnetic resonance venography, NPH = normal pressure hydrocephalus, VP = ventriculoperitoneal.
Lyme disease is an increasingly prevalent illness in the United States.1 The causative organism is the spirochete Borrelia burgdorferi transmitted by the vector Ixodes scapularis, commonly referred to as the “black-legged tick.”2 In approximately 15% of cases, infection, termed “neuroborreliosis,” involves the nervous system.3 The most common neurological complications of neuroborreliosis include cranial neuritis, meningitis, radiculoneuritis, and mononeuropathy multiplex.2 Rarely, cases of intracranial hypertension have been reported, most commonly in pediatric patients.4–8
Here, we present a rare case of rapidly progressive intracranial hypertension and subsequent severe visual loss due to neuroborreliosis in a pediatric patient. The purpose of this case presentation is to highlight a rare but noteworthy complication of Lyme disease requiring urgent neurosurgical intervention to prevent further neurological deterioration. While neurosurgical intervention in cases of Lyme disease is uncommon, it is important for neurosurgeons to be aware of disease complications that may require neurosurgical expertise.
Illustrative Case
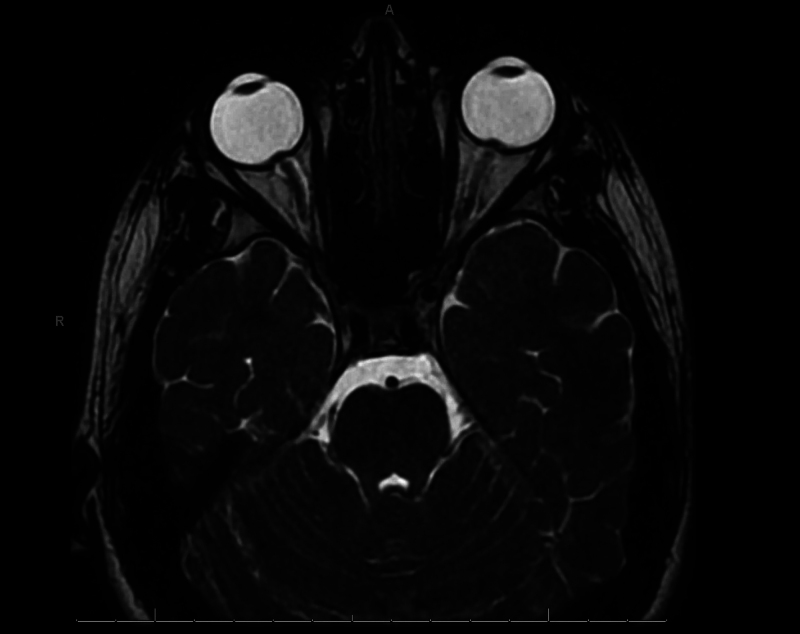
A previously healthy 13-year-old male presented to the emergency department with approximately 4 weeks of headache, neck stiffness, nausea, vomiting, and abdominal pain and 1 week of progressive visual loss. Two weeks prior to his presentation, he sought care at another facility where he tested positive for Lyme disease and was started on doxycycline. One week prior to his presentation, he was additionally informed that he had tested positive for Babesia and was started on atovaquone and azithromycin. His symptoms were not improving despite antimicrobial therapy, so outpatient magnetic resonance imaging (MRI) of the brain was performed, which demonstrated bilateral posterior scleral flattening, prominent optic papillae at the site of optic nerve insertion, tortuosity of the intraorbital optic nerves, and prominence of the optic nerve sheath complex suggestive of papilledema and increased intracranial pressure (ICP; Fig. 1). These MRI findings prompted urgent referral to the emergency department.
FIG. 1.
Axial T2 three-dimensional fast spin echo orbit sequence demonstrating bilateral posterior scleral flattening, prominent optic papillae at the site of optic nerve insertion, tortuosity of the intraorbital optic nerves, and prominence of the optic nerve sheath complex suggestive of papilledema and increased ICP.
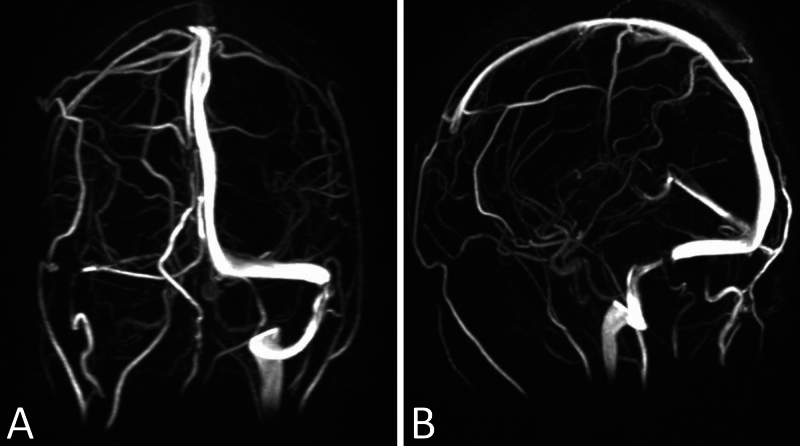
Initial laboratory studies were remarkable for a mild elevation in the serum erythrocyte sedimentation rate and C-reactive protein. No fever or leukocytosis was noted. Lumbar puncture (LP) was performed, demonstrating an opening pressure of 47 mm Hg. Lymphocytic pleocytosis was noted, with a white blood cell count of 39/µL, 96% of which were lymphocytes, and a red blood cell count of 2000/µL. Cerebrospinal fluid (CSF) protein was mildly elevated at 60 mg/dL. CSF glucose was normal at 56 mg/dL. Serum and CSF Lyme immunoglobulin M (IgM) and IgG were positive, as well as Lyme Western blot testing for Borrelia burgdorferi. A peripheral smear to identify Babesia species was negative on two separate studies. MRI, magnetic resonance angiography, and magnetic resonance venography (MRV) demonstrated persistent papilledema, no dural or cranial nerve enhancement, and distal severe stenosis of the left transverse sinus with a hypoplastic right transverse sinus (Fig. 2). Doxycycline, atovaquone, and azithromycin were discontinued, and the patient was started on intravenous ceftriaxone. He was evaluated by the ophthalmology team, who identified 360° blurring of the optic disc margins, obscuration of major and minor vessels, retinal hemorrhages, and cotton wool spots suggestive of papilledema in both eyes and a left afferent pupillary defect. Visual acuity was 20/200 in both eyes. Given concern for idiopathic intracranial hypertension (IIH), he was also started on acetazolamide. Shortly after admission, he began having near syncopal and syncopal episodes.
FIG. 2.
Coronal (A) and sagittal (B) three-dimensional reconstruction magnetic resonance venograms demonstrating severe distal left transverse sinus stenosis and a hypoplastic right transverse sinus.
Because of his rapidly progressive visual symptoms, the neurosurgery team was consulted for CSF diversion. A repeat LP was performed, again demonstrating an elevated opening pressure of 55 mm Hg. Ventriculoperitoneal (VP) shunting was deferred because of his positive CSF result for Lyme. A right frontal external ventricular drain (EVD) was placed for urgent CSF diversion. ICP was initially elevated at 33 mm H2O but improved after the first 24 hours.
The EVD remained in place for 12 days for CSF drainage. Output was initially greater than 200 mL per day but progressively decreased to less than 100 mL per day. The patient reported slow improvement in his vision with each passing day. The EVD was challenged by raising the level over 2 days and clamping for 24 hours, with no change in the neurological examination or vision. The EVD was removed 15 days after initial placement.
The patient remained an inpatient for 19 days. On the day of discharge, an ophthalmological examination demonstrated improvement in blurring of the optic disc margins, no significant obscuration of major or minor vessels, resolving retinal hemorrhages, and visual acuity of 20/20. He reported resolution of his headaches, neck stiffness, nausea, and vomiting. He received a complete 3-week course of intravenous ceftriaxone. He was discharged home with no symptom recurrence.
Three months posthospitalization, he remained without a recurrence of Lyme disease or intracranial hypertension symptoms. Ophthalmological examination demonstrated no evidence of elevated ICP, and he reported that his vision was back to near baseline.
Patient Informed Consent
The necessary patient informed consent was obtained in this study.
Discussion
Observations
We present a rare case of intracranial hypertension due to neuroborreliosis in a pediatric patient treated with EVD placement for CSF diversion and intravenous ceftriaxone, resulting in a significant improvement in symptoms. Intracranial hypertension has rarely been described as a complication of neuroborreliosis, and most reports have consisted of case reports and small case series.4, 6, 7, 9–16 Most cases present with combinations of headache, meningismus, papilledema, or abducens nerve palsies;4, 6, 7, 9, 10 however, there are few reports of severe intracranial hypertension with resulting visual loss.4
Reported cases of neuroborreliosis with intracranial hypertension have been mostly treated with intravenous antibiotics and acetazolamide with success.4–7, 9–11, 17 Rapidly progressive or refractory cases have required neurosurgical intervention in a few reports (Table 1).4, 12–16, 18 Rogers et al.4 reported a series of 11 pediatric patients with neuroborreliosis and intracranial hypertension, 2 of whom developed fulminant intracranial hypertension with visual loss requiring temporary lumbar drain placement in addition to acetazolamide and antibiotics. Lumbar drain placement with acetazolamide resulted in an improvement in visual acuity back to 20/20 in both cases. Neither patient required permanent CSF diversion.
TABLE 1.
Characteristics, symptomatology, and management of patients with intracranial hypertension secondary to neuroborreliosis treated surgically
| Authors & Year | Patient Age (yrs) | Patient Sex | Symptoms & Physical Findings | Surgical Treatment(s) | Medical Treatment(s) | Outcome(s) |
|---|---|---|---|---|---|---|
| Lochhead & Thompson, 200112 | 7 | F | Bilat papilledema, visual loss, headache, back pain, emesis, rt cranial nerve VII palsy | Serial LPs | Ceftriaxone, methylprednisolone, acetazolamide | Complete resolution of papilledema, partial improvement of visual acuity, remaining optic nerve atrophy |
| Nord & Karter, 200313 | 37 | M | Headache, myalgias, fever, fatigue | Serial LPs | Doxycycline | Complete symptom resolution after 3 LPs over 10 days |
| Moussawi et al., 201614 | 20 | M | Bilat papilledema, diplopia, cranial nerve VI palsy, meningismus | Serial LPs | Ceftriaxone, acetazolamide | Complete symptom resolution in 1 mo |
| Rogers et al., 20234 | 5 | F | Bilat papilledema, diplopia, headache, emesis | Lumbar drain | Amoxicillin, ceftriaxone, furosemide, acetazolamide | Resolution of papilledema in 55 days, remaining retinal nerve atrophy |
| Rogers et al., 20234 | 6 | M | Bilat papilledema, diplopia, cranial nerve VI palsy, headache | Lumbar drain | Cefepime, doxycyline, ceftriaxone, methylprednisolone, furosemide, acetazolamide | Complete symptom resolution by 61 days, recurrence of asymptomatic papilledema at follow-up, acetazolamide led to complete resolution again at 56 days postrecurrence |
| Nørreslet Gimsing & Lunde Larsen, 202018 | 51 | F | Bilat papilledema, visual loss, headache, emesis, dizziness, tinnitus | Serial LPs, VP shunt | Ceftriaxone, acetazolamide | Serial LPs provided relief w/ recurrence after 3 wks, shunting halted symptom progression w/ resolution by 6 mos, remaining optic nerve atrophy, central scotoma, & loss of color vision |
| Rothermel et al., 200116 | 11 | M | Bilat papilledema, optic neuritis, diplopia, cranial nerve VI palsy, cranial nerve VII palsy, meningismus, hyporeflexia | VP shunt | Doxycycline, ceftriaxone, methylprednisolone | Resolution of papilledema by 6 mos, remained blind in both eyes |
IIH and communicating hydrocephalus mistaken for normal pressure hydrocephalus (NPH) have been reported as chronic sequelae of neuroborreliosis.9, 15, 19 Patients developing IIH, thought to be secondary to prior neuroborreliosis, have been treated with VP shunt placement and an appropriate antibiotic course.9, 15 Gimsing and Hejl15 reported the case of a 67-year-old male who presented with chronic neurological deterioration. Neuroimaging demonstrated communicating hydrocephalus concerning for NPH; however, CSF analysis demonstrated neuroborreliosis. The patient was treated with intravenous antibiotics, and the ventricular size normalized. The patient did not require VP shunt placement, and his symptoms had resolved by the 1-year follow-up. The authors additionally reviewed the literature and found 6 other patients with communicating hydrocephalus thought to be secondary to neuroborreliosis, with successful medical treatment and avoiding VP shunt placement. Topakian et al.20 reported a similar case in which communicating hydrocephalus mistaken for NPH was found to be neuroborreliosis and responded dramatically to intravenous antibiotic therapy.
In published reports to date, refractory intracranial hypertension secondary to neuroborreliosis has been managed with serial LPs, lumbar drain placement, or VP shunt placement (Table 1).4, 12–16 In our report, ventriculostomy was chosen over lumbar drain placement or serial LPs for several reasons. The patient’s ventricles had a normal structure and were not slit, making ventriculostomy catheter placement possible. The basal cisterns were slightly crowded; therefore, the theoretical risk of transtentorial or tonsillar herniation with continuous or repeated lumbar CSF drainage was considered.21, 22 If VP shunting was required, the proximal catheter could be placed using the same catheter tract previously occupied by the ventriculostomy catheter, under direct visualization through neuroendoscopy.23 VP shunting was not pursued acutely to allow time for infection clearance with antibiotic therapy. Ventriculostomy weaning was conducted, accounting for patient symptoms, neurological examination, drain output, and ventricle size on neuroimaging. When the patient’s visual symptoms, headache, and nausea had persistently improved and drain output was decreasing, the ventriculostomy bag height above the tragus was progressively raised by 5 cm H2O daily until 20 cm H2O above the tragus was reached. The drain was then clamped, and ICP was monitored for 48 hours. A head computed tomography scan was obtained, demonstrating a stable ventricle size; therefore, the ventriculostomy was believed to be safe to remove. Throughout the weaning process, the patient’s symptoms remained improved and no ICP issues were encountered.
Three factors confound this patient’s case and are worthy of discussion. First is the well-described association of tetracycline antibiotics with the development of IIH.24 Two weeks prior to presentation, the patient initially tested positive for Lyme disease and was started on oral doxycycline. It is unclear how this might have affected the development and severity of his intracranial hypertension. Second, MRV demonstrated distal severe stenosis of the left transverse sinus with a hypoplastic right transverse sinus. Venous sinus stenosis is well described in IIH, and impaired venous outflow is a component of the proposed pathophysiology of IIH.25 The patient did not undergo venous manometry; therefore, the significance of this finding and the development of intracranial hypertension are uncertain. Lastly, prior to presentation, the patient was also diagnosed with babesiosis and was started on atovaquone and azithromycin. Repeat testing in the emergency department was negative for Babesia infection; however, he had already received antimicrobial therapy for 7 days. Neurological manifestations of babesiosis are uncommon but have been reported.26–28 These include headache, lethargy, cognitive impairment, and, much less commonly, decreased level of consciousness and coma. Intracranial hypertension secondary to babesiosis has never been reported; however, patients with resulting coma were not assessed for ICP.27 While it is possible that the described patient’s presentation was confounded by these factors, neuroborreliosis was believed to be the causative etiology. The lymphocytic pleocytosis and Borrelia burgdorferi serology and DNA identified in the CSF suggest an active neuroborreliosis infection alongside the development and progression of symptoms. The patient’s intracranial hypertension symptoms progressed despite the discontinuation of doxycycline, making tetracycline-induced IIH less likely. Babesia infection was believed to be a less likely contributing factor as well, as two separate peripheral smear studies after presentation failed to identify infection.
Additional infectious encephalopathies can lead to acute and/or fulminant intracranial hypertension requiring neurosurgical evaluation and are worthy of discussion. Acute bacterial meningitis can cause intracranial hypertension and result in significant morbidity and death.29, 30 The most prevalent pathogens for community-acquired bacterial meningitis are Streptococcus pneumoniae, Neisseria meningitidis, and Haemophilus influenzae, with additional organisms affecting specific patient groups such as newborns, the elderly, or immunocompromised patients.29 ICP monitoring has been utilized to guide medical management, and ventriculostomies have been utilized for therapeutic CSF diversion, especially if ventriculitis and/or hydrocephalus are present.29–32 A recently published systematic review by El-Hajj et al. reported improved outcomes in patients with bacterial meningitis treated with ICP monitoring and therapeutic CSF drainage; however, there was weak evidence certainty given the heterogeneity of patient management.29 Acute viral encephalitis can also cause intracranial hypertension, with resulting significant morbidity and death, most often due to the development of cerebral edema.33, 34 The most common causative pathogen is herpes simplex virus.34 ICP monitoring and treatment have rarely been described in viral encephalitis patients, mostly in cases with herpes simplex virus infections or secondary status epilepticus.33,35
Lessons
Our case highlights a rare but significant complication of neuroborreliosis. Intracranial hypertension with resulting neurological deterioration, while uncommon, can occur in patients with Lyme disease. Management is most often medical, consisting of intravenous antibiotics and acetazolamide to reduce CSF production. In rare cases, neurosurgical intervention is necessary and can provide significant benefit to select patients.4, 9 Both EVD and lumbar drain4 placement for temporary CSF diversion represent viable options for the treatment of refractory severe intracranial hypertension in patients with neuroborreliosis. Permanent CSF shunt placement can often be avoided, and most patients will recover substantially.4, 15 These conclusions should be interpreted with caution, as the very small sample of patients in the literature with intracranial hypertension secondary to neuroborreliosis is a significant limitation. Reports are limited to case reports and small case series. Further study is needed to enhance understanding, diagnosis, and optimal management of intracranial hypertension secondary to neuroborreliosis.
Disclosures
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Author Contributions
Conception and design: Sweeney, Adamo. Acquisition of data: Sweeney, Ku, Terry, Prabhala. Analysis and interpretation of data: Sweeney, Ku, Adamo. Drafting the article: Sweeney, Ku, Bheemireddy. Critically revising the article: all authors. Reviewed submitted version of manuscript: all authors. Approved the final version of the manuscript on behalf of all authors: Sweeney. Statistical analysis: Sweeney. Administrative/technical/material support: Prabhala. Study supervision: Adamo.
Correspondence
Jared F. Sweeney: Albany Medical Center, Albany, NY. sweenej2@amc.edu.
References
- 1.Halperin JJ. Lyme neuroborreliosis. Curr Opin Infect Dis. 2019;32(3):259-264. [DOI] [PubMed] [Google Scholar]
- 2.Roos KL. Neurologic complications of Lyme disease. Continuum (Minneap Minn). 2021;27(4):1040-1050. [DOI] [PubMed] [Google Scholar]
- 3.Halperin JJ. Nervous system Lyme disease–facts and fallacies. Infect Dis Clin North Am. 2022;36(3):579-592. [DOI] [PubMed] [Google Scholar]
- 4.Rogers JD, Jordan CO, Inger HE, Aylward SC. Secondary intracranial hypertension in pediatric Lyme meningitis. J Child Neurol. 2023;38(10-12):611-616. [DOI] [PubMed] [Google Scholar]
- 5.Bruinsma RA, Zomer TP, Skogman BH, van Hensbroek MB, Hovius JW. Clinical manifestations of Lyme neuroborreliosis in children: a review. Eur J Pediatr. 2023;182(5):1965-1976. [DOI] [PubMed] [Google Scholar]
- 6.Chhajed M, Jain A, Gunasekaran PK, Dhaliwal N, Saini L. Lyme neuroborreliosis with intracranial hypertension and erythema multiforme: a rare presentation. J Trop Pediatr. 2022;68(4):fmac060. [DOI] [PubMed] [Google Scholar]
- 7.Vaysbrot EE, Bannuru RR, Christopher MC, Osani MC, Halperin JJ. Papilledema secondary to neurologic Lyme borreliosis: a meta-case series. J Neuroophthalmol. 2021;41(4):e498-e508. [DOI] [PubMed] [Google Scholar]
- 8.Sood SK. Lyme disease in children. Infect Dis Clin North Am. 2015;29(2):281-294. [DOI] [PubMed] [Google Scholar]
- 9.Ezequiel M, Teixeira AT, Brito MJ, Luís C. Pseudotumor cerebri as the presentation of Lyme disease in a non-endemic area. BMJ Case Rep. 2018;2018:bcr2017222976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Härtel C, Schilling S, Neppert B, Tiemer B, Sperner J. Intracranial hypertension in neuroborreliosis. Dev Med Child Neurol. 2002;44(9):641-642. [DOI] [PubMed] [Google Scholar]
- 11.Ramgopal S, Obeid R, Zuccoli G, Cleves-Bayon C, Nowalk A. Lyme disease-related intracranial hypertension in children: clinical and imaging findings. J Neurol. 2016;263(3):500-507. [DOI] [PubMed] [Google Scholar]
- 12.Lochhead J, Thompson GM. Bilateral papilloedema with concomitant neuroretinitis in a 7-year-old girl with Lyme disease. Eye (Lond). 2001;15(6):799-801. [DOI] [PubMed] [Google Scholar]
- 13.Nord JA, Karter D. Lyme disease complicated with pseudotumor cerebri. Clin Infect Dis. 2003;37(2):e25-e26. [DOI] [PubMed] [Google Scholar]
- 14.Moussawi K, Gupta A, Reda H. Clinical reasoning: a 20-year-old man with headache and double vision. Neurology. 2016;87(15):e162-e167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gimsing LN, Hejl AM. Normal pressure hydrocephalus secondary to Lyme disease, a case report and review of seven reported cases. BMC Neurol. 2020;20(1):347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rothermel H, Hedges TR, III, Steere AC. Optic neuropathy in children with Lyme disease. Pediatrics. 2001;108(2):477-481. [DOI] [PubMed] [Google Scholar]
- 17.Silverstein M, Jordan CO, Aylward SC, Inger H. Yearly occurrence and seasonality of neuro-ophthalmic manifestations of pediatric Lyme disease. J Pediatr Ophthalmol Strabismus. 2024;61(3):179-182. [DOI] [PubMed] [Google Scholar]
- 18.Nørreslet Gimsing L, Lunde Larsen LS. A rare case of pseudotumor cerebri in adult Lyme disease. Clin Case Rep. 2020;8(1):116-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Şahin B, İncecik F, Hergüner ÖM, Alabaz D, Beşen Ş. A presentation of Lyme disease: pseudotumor cerebri. Turk J Pediatr. 2015;57(5):522-524. [PubMed] [Google Scholar]
- 20.Topakian R, Artemian H, Metschitzer B, Lugmayr H, Kühr T, Pischinger B. Dramatic response to a 3-week course of ceftriaxone in late neuroborreliosis mimicking atypical dementia and normal pressure hydrocephalus. J Neurol Sci. 2016;366:146-148. [DOI] [PubMed] [Google Scholar]
- 21.Badhiwala J, Lumba-Brown A, Hawryluk GWJ, Ghajar J. External lumbar drainage following traumatic intracranial hypertension: a systematic review and meta-analysis. Neurosurgery. 2021;89(3):395-405. [DOI] [PubMed] [Google Scholar]
- 22.Joffe AR. Lumbar puncture and brain herniation in acute bacterial meningitis: a review. J Intensive Care Med. 2007;22(4):194-207. [DOI] [PubMed] [Google Scholar]
- 23.Yim B, Reid Gooch M, Dalfino JC, Adamo MA, Kenning TJ. Optimizing ventriculoperitoneal shunt placement in the treatment of idiopathic intracranial hypertension: an analysis of neuroendoscopy, frameless stereotaxy, and intraoperative CT. Neurosurg Focus. 2016;40(3):E12. [DOI] [PubMed] [Google Scholar]
- 24.Passi SF, Butcher R, Orme DR, et al. Increased incidence of pseudotumor cerebri syndrome among users of tetracycline antibiotics. J Neuroophthalmol. 2022;42(3):323-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao K, Gu W, Liu C, et al. Advances in the understanding of the complex role of venous sinus stenosis in idiopathic intracranial hypertension. J Magn Reson Imaging. 2022;56(3):645-654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Waked R, Krause PJ. Human babesiosis. Infect Dis Clin North Am. 2022;36(3):655-670. [DOI] [PubMed] [Google Scholar]
- 27.Usmani-Brown S, Halperin JJ, Krause PJ. Neurological manifestations of human babesiosis. Handb Clin Neurol. 2013;114:199-203. [DOI] [PubMed] [Google Scholar]
- 28.Venigalla T, Adekayode C, Doreswamy S, Al-Sudani H, Sekhar S. Atypical presentation of babesiosis with neurological manifestations as well as hematological manifestations. Cureus. 2022;14(7):e26811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.El-Hajj VG, Pettersson I, Gharios M, et al. Detection and management of elevated intracranial pressure in the treatment of acute community -acquired bacterial meningitis: a systematic review. Neurocrit Care. Published online February 14, 2024. doi: 10.1007/s12028-023-01937-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Larsen L, Poulsen FR, Nielsen TH, Nordström CH, Schulz MK, Andersen ÅB. Use of intracranial pressure monitoring in bacterial meningitis: a 10-year follow up on outcome and intracranial pressure versus head CT scans. Infect Dis (Lond). 2017;49(5):356-364. [DOI] [PubMed] [Google Scholar]
- 31.Svedung Wettervik T, Howells T, Ljunghill Hedberg A, Lewén A, Enblad P. Intracranial pressure dynamics and cerebral vasomotor reactivity in community-acquired bacterial meningitis during neurointensive care. J Neurosurg. 2022;136(3):831-839. [DOI] [PubMed] [Google Scholar]
- 32.Tariq A, Aguilar-Salinas P, Hanel RA, Naval N, Chmayssani M. The role of ICP monitoring in meningitis. Neurosurg Focus. 2017;43(5):E7. [DOI] [PubMed] [Google Scholar]
- 33.Kumar G, Kalita J, Misra UK. Raised intracranial pressure in acute viral encephalitis. Clin Neurol Neurosurg. 2009;111(5):399-406. [DOI] [PubMed] [Google Scholar]
- 34.Tyler KL. Acute viral encephalitis. N Engl J Med. 2018;379(6):557-566. [DOI] [PubMed] [Google Scholar]