Abstract
BACKGROUND
Rathke’s cleft cysts (RCCs) are benign fluid-filled cysts that develop in the pituitary gland because of the abnormal embryological development of Rathke’s pouch. Most RCCs are small and asymptomatic; however, they can present with symptoms or documented growth, sometimes prompting surgical treatment. For smaller asymptomatic lesions, an unknown proportion demonstrates regression over time. This study describes 3 cases of spontaneous RCC regression.
OBSERVATIONS
Three patients with a diagnosis of RCC demonstrated an average decrease of 78% in cyst volume over a mean interval of 3.7 months. One patient experienced the resolution of chronic headaches, whereas the other 2 patients had persistent headaches and endocrinopathies at follow-up. A systematic review included 9 studies that reported results from observational cohorts of patients with RCC, totaling 619 observed patients, with 158 (25.5%) patients demonstrating spontaneous cyst regression. In the patients with cyst regression, the majority had a resolution of symptoms.
LESSONS
A substantial proportion of RCC patients managed nonsurgically demonstrated spontaneous regression. There is a role for the conservative management of RCCs in patients without significant symptoms, and surveillance should continue for a minimum of 5 years to confirm cyst stability. For patients undergoing planned surgery, same-day or recent imaging is recommended to prevent operating on involuted RCCs.
Keywords: Rathke’s cleft cyst, pituitary cyst, spontaneous regression, conservative management, case report
ABBREVIATIONS: MRI = magnetic resonance imaging, NSAID = nonsteroidal anti-inflammatory drug, RCC = Rathke’s cleft cyst, TSS = transsphenoidal surgery.
Rathke’s cleft cysts (RCCs) are benign fluid-filled cysts that develop in the region of the pars intermedia between the anterior and posterior pituitary gland.1 RCCs are posited to be the result of abnormal embryological development of Rathke’s pouch.2 The majority of RCCs are small and asymptomatic, and a study of pituitary lesions in 1000 unselected autopsies demonstrated RCCs in 22% of specimens, making them the most common sellar pathology.3 However, a minority of RCCs can cause symptoms including headache, visual disturbance, and endocrinopathies.4 Once they are symptomatic, the standard treatment of RCCs is typically transsphenoidal cyst fenestration and drainage; however, the management of incidental and asymptomatic cysts remains controversial.
Several studies monitoring outcomes of RCCs managed conservatively have demonstrated that the majority (67%–72%) show static disease or even regression on magnetic resonance imaging (MRI) follow-up.5, 6 The case for conservative management of asymptomatic RCCs becomes stronger when considering the risks of transsphenoidal surgery, which include nontrivial rates of cerebrospinal fluid leakage and endocrinopathy, especially diabetes insipidus, following RCC surgery.7–9
To date, there have been limited reports of the spontaneous regression of RCCs. Here, we report a case series of 3 patients treated at our institution who demonstrated spontaneous regression of RCCs over periods of 2–5 months, as well as the results of a systematic literature review.
Study Description
A systematic review of the literature was performed. The PubMed database was queried using the keywords “Rathke’s cleft cyst” and “regression” or “involution.” Studies were included if they reported on patients with a presumed diagnosis of RCC based on imaging, who were managed nonsurgically, and with regression of the cyst over time. Case reports, case series, and observational cohort studies were included. Studies were excluded if the pathology was not consistent with RCC or if patients underwent surgery during the observation period. The bibliographies of included studies were analyzed to identify publications missed in the initial search. Two reviewers independently checked all studies for information on patient demographics, time to regression, and regression volume.
In addition to conducting a systematic review, we compiled a case series of patients with an imaging-based diagnosis of RCC whose cyst demonstrated regression prior to surgical intervention. Volumetric analysis of the cyst before and after regression was performed to calculate the change in volume over time.
Case Series
Case 1
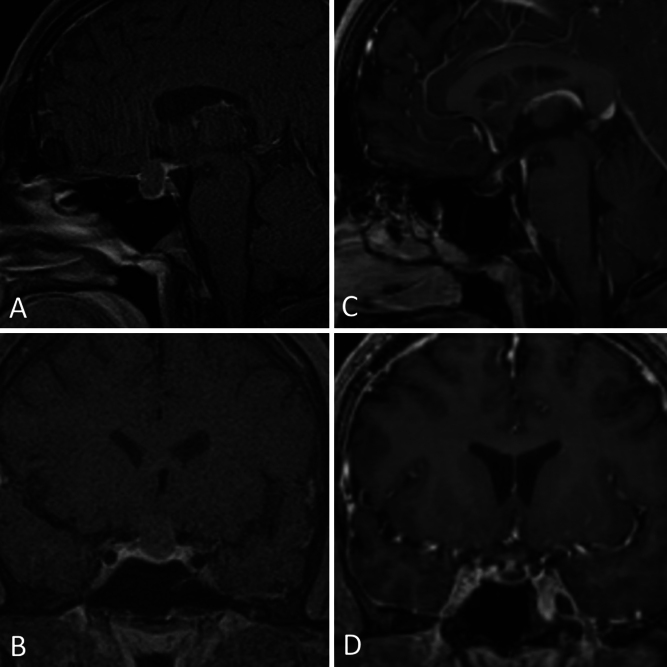
A 31-year-old man presented to the emergency department with severe headaches that had been progressively worsening over several months. Further history also revealed decreased libido over the same period. Imaging demonstrated a 1.6-cm cystic sellar mass with suprasellar extension (Fig. 1), presumed to be an RCC. The ophthalmological exam revealed no visual field deficit. Laboratory values showed decreased serum testosterone. He was scheduled for elective endonasal transsphenoidal fenestration of the mass. Neuronavigation MRI performed the morning of the planned operation revealed a 79% volumetric decrease of the cyst with a normal-appearing pituitary gland seated in the sella. The patient was still having headaches; however, due to the substantial decrease in cyst size, his surgery was deferred, and he was scheduled for routine outpatient surveillance and follow-up with endocrinology for testosterone replacement. At the latest follow-up, his headaches were mildly improved and he continued to receive testosterone replacement.
FIG. 1.
Case 1. Brain MRI in a 31-year-old male with a cystic sellar mass (AandB) demonstrating a significant decrease in cyst volume (CandD).
Case 2
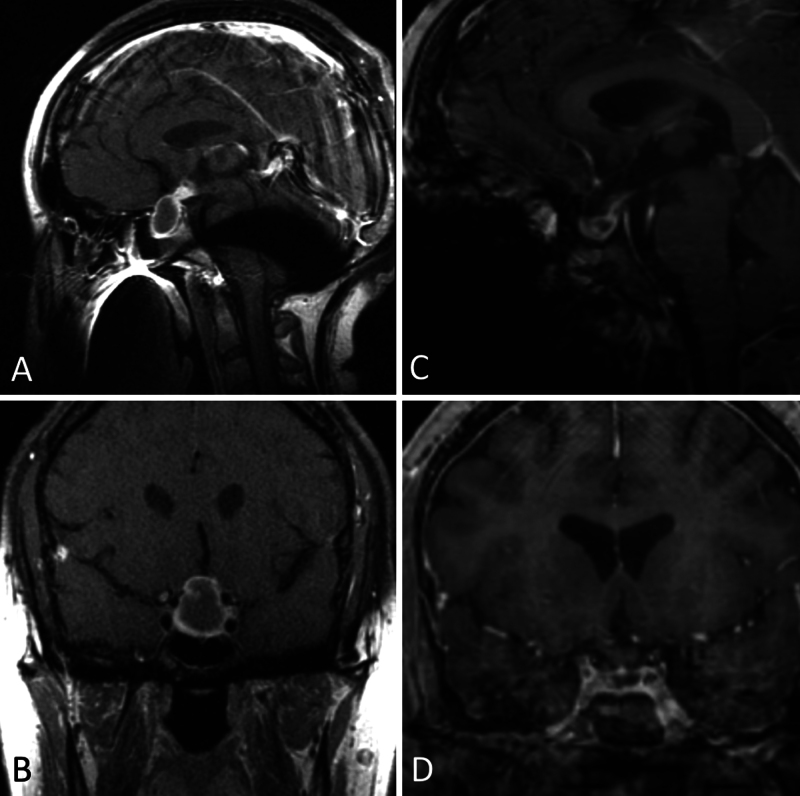
A 23-year-old woman was admitted with hallucinations, prompting MRI of the brain that revealed an incidental 2.8-cm cystic sellar mass with suprasellar extension (Fig. 2). When interviewed, she described recent weight gain, blurred vision, and intermittent headaches. She had no visual field deficit. Laboratory values were significant for panhypopituitarism, and the patient was started on desmopressin, hydrocortisone, and levothyroxine. She was scheduled for elective resection of the mass; however, she presented prior to her appointment with worsening urinary frequency and dehydration. Repeat imaging showed a marked 61% volumetric decrease of the cystic mass. Surgery was deferred in favor of surveillance, with the patient continuing hormone replacement for persistent central diabetes insipidus, adrenal insufficiency, and hypothyroidism despite the decrease in mass volume. Six-month follow-up MRI demonstrated a stable cyst size.
FIG. 2.
Case 2. In a 23-year-old female with panhypopituitarism, MRI demonstrated a cystic sellar mass with suprasellar extension (AandB) that demonstrated spontaneous regression (CandD).
Case 3
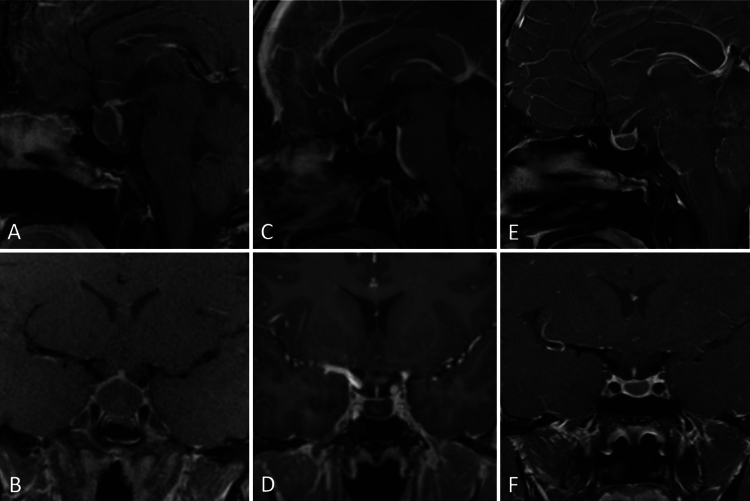
A 29-year-old man underwent an outpatient workup for persistent headaches, nausea, and vomiting. The headaches were new and occurred nearly daily. He also reported diminished libido and fatigue. MRI showed a 1.4-cm cystic sellar mass with suprasellar extension (Fig. 3). He had no visual field deficit, and laboratory values showed normal pituitary function. He was scheduled for elective resection of the mass. Neuronavigation MRI obtained the morning of the surgery showed a 94% volumetric decrease of the cyst with interval evolution of intralesional hemorrhage. The surgery was canceled in favor of serial observation. Surveillance MRI performed 1 year later showed a stable size of the cyst with no progression. He had no symptoms at the time of the most recent follow-up.
FIG. 3.
Case 3. MRI in a 29-year-old male with headaches and a cystic sellar mass (AandB) that regressed spontaneously (CandD). Cyst size remained stable 1 year later with no evidence of new growth (EandF).
Systematic Review
A literature search was conducted on the PubMed database using the key terms “Rathke’s cleft cyst” and “regression” or “involution,” identifying 129 unique publications. Titles were screened to identify potentially eligible articles, and 39 articles were retrieved for abstract evaluation. After abstract review, the full text of 28 articles was assessed for eligibility. Bibliographies of the included articles were screened to find additional publications.
A total of 21 studies reporting spontaneous RCC regression met the inclusion criteria and were included in our qualitative analyses (Table 1). These studies were published between 1999 and 2022 and comprised 188 patients with RCC showing regression. In this regression cohort, patient ages ranged from 5 to 70 years old, and 40.8% of patients were male; however, not all included studies reported complete demographic data (Table 1). Data on mean time to regression, mean regression volume, presence of endocrine dysfunction, visual dysfunction, chronic/acute headache, and mean follow-up were recorded when available. Nine observational cohort studies were included in our quantitative analyses assessing the rate of RCC regression in observed patients. These studies comprised 619 patients, with 158 (25.5%) patients experiencing regression (Table 2).
TABLE 1.
Results of systematic review of publications describing patients with RCCs managed conservatively and demonstrating regression
| Authors & Year | No. of Patients w/ RCC Regression | Median Age, Yrs (range) | No. of Males (%) | Mean Time to Regression, Mos (range) | Mean Decrease in Volume | Mean Follow-up (mos) |
|---|---|---|---|---|---|---|
| Igarashi et al., 199928 | 4 | 54 (26–67) | 4 (100) | — | — | 48 |
| Saeki et al., 199929 | 3 | 30 (25–58) | 1 (33) | — | 47.60% | — |
| Terao et al., 200130 | 1 | 67 | 1 (100) | 60 | 100% | 60 |
| Nishio et al., 200125 | 2 | 22.5 (14–31) | 1 (50) | 4.5 | — | 24 |
| Sanno et al., 200331 | 15 | 42.1 | — | 31.3 (2–72) | — | 38.9 |
| Amhaz et al., 201010 | 9 | 18 (5–57) | 4 (44) | 31 (5–21) | — | 16 |
| Munich & Leonardo, 201223 | 1 | 8.5 | 0 | 6 | 100% | 27 |
| Maniec et al., 201132 | 1 | 59 | 1 (100) | 1 | 100% | 1 |
| Al Safatli et al., 201522 | 1 | 70 | 1 (100) | 12 | 100% | 24 |
| Culver et al., 20156 | 11 | — | — | — | — | 24 |
| Rasmussen et al., 201624 | 1 | 70 | 0 | 24 | 100% | 24 |
| Kim et al., 20167 | 14 | 32 (5–67) | 8 (57.1) | 7.3 (5.7–42.8) | 78% | 7.3 |
| Cheng et al., 201621 | 1 | 50 | 0 | 10 | — | 26 |
| Shepard et al., 201833 | 6 | 8.5 | 3 (50) | 23.5 | — | 50 |
| Sala et al., 201811 | 2 | — | — | — | — | 41 |
| Barkhoudarian et al., 20194 | 3 | — | — | — | — | 13 |
| Lee & Park, 201914 | 2 | 43 (34–52) | 0 (0) | 9 (6–12) | 89% | 12 |
| Salle et al., 202120, 34 | 1 | 27 | 0 (0) | 6 | 100% | 6 |
| Petersson et al., 202220 | 35 | — | — | — | 34.30% | — |
| Truong et al., 202227 | 2 | 49 (46–52) | 2 (100) | 24 | — | — |
| Kinoshita et al, 202213 | 73 | 39 (25.5–55) | 23 (31.5) | 11.4 (3.2–38.9) | — | 58.7 |
| Present study | 3 | 29 (23–31) | 2 (66.7) | 3.7 | 74% | 7 |
— = data not available.
TABLE 2.
Qualitative review results of RCC regression rates across studies reporting data for observational cohorts of conservatively managed patients with RCCs
| Authors & Year | No. of Patients | Regression | Rate |
|---|---|---|---|
| Igarashi et al., 199928 | 10 | 4 | 40% |
| Sanno et al., 200331 | 94 | 15 | 16% |
| Amhaz et al., 201010 | 29 | 9 | 31% |
| Culver et al., 20156 | 75 | 11 | 14.7% |
| Shepard et al., 201833 | 17 | 6 | 35% |
| Sala et al., 201811 | 62 | 2 | 3.2% |
| Barkhoudarian et al., 20194 | 30 | 3 | 10% |
| Petersson et al., 202220 | 73 | 35 | 48% |
| Kinoshita et al, 202213 | 229 | 73 | 31.9% |
| Total | 619 | 158 | 25.5% |
Presenting symptoms were reported in 143 patients included in the analysis. The most common symptoms were chronic or acute headaches (29% and 27%, respectively). Endocrine dysfunction was present in 16% of patients. Apoplexy and visual dysfunction were experienced by 6% of patients each.
A meta-analysis of individual case reports included symptom and cyst characteristic details for 41 patients. Headache was the most common presenting symptom, and 89% of patients experienced resolution of headaches after cyst regression. Endocrinopathies resolved in 50% of patients, with hypercortisolemia, hyponatremia, and hypogonadism showing the highest rates of improvement. Visual symptoms improved in 70% of patients presenting with either decreased acuity or visual field defects.
The average cyst volume at the time of diagnosis was 1.46 ml, with a follow-up average of 0.27 ml, representing an overall 82% decrease in volume with regression. The average initial maximum cyst diameter was 16.1 mm, with an overall decrease of 48% to 8.3 mm at follow-up.
Patient Informed Consent
The necessary patient informed consent was obtained in this study.
Discussion
In this study, we present a case series of 3 patients treated at our hospital who experienced spontaneous RCC regression. The systematic review of the literature revealed that up to one-quarter of serially observed RCCs can undergo spontaneous regression, prompting us to conclude that same-day or recent neuroimaging is critical when considering planned surgical treatment of RCCs to avoid operating on involuted or shrinking RCCs.
RCCs are typically diagnosed as incidental lesions on MRI or following nonspecific symptoms such as headache, which often, but not always, resolve after resection.10 Visual field deficits are also common, occurring in up to 58% of patients.5 In addition, patients can present with hormonal imbalances including hypogonadism, growth hormone deficiency, hypocortisolemia, hyperprolactinemia, or diabetes insipidus. However, rates of hypopituitarism vary widely in the literature from 0% to 81%.5, 11 RCCs have a female/male preponderance of 2:1 and can be diagnosed in all age groups, although they have a peak incidence in the 4th–5th decade.12
Observations
Our systematic review revealed RCC regression rates ranging from 3% to 48% in adults and as high as 38% in the pediatric population.5 Amhaz et al.10 speculated that spontaneous regression of RCCs may be a common but underreported phenomenon because patients often remain asymptomatic and undiagnosed. In one autopsy study, 22% of specimens were found to have previously undiagnosed RCCs.3 With the increased use of MRI in the general population contributing to a higher incidental diagnosis of RCC, the natural history of RCCs has become an area of interest. While one-quarter of RCCs demonstrated regression in our analysis, the previous literature has reported that roughly half of RCCs remain stable and another quarter demonstrate progression over time when managed conservatively.4, 6, 13
Because the majority of RCCs are small and asymptomatic, most can be managed conservatively with serial imaging.14 However, a minority of patients with RCC present with symptoms including visual disturbances and endocrinopathies such as hypopituitarism, likely caused by mass effect, in which case surgery is indicated. Trifanescu et al. propose that there is a positive correlation between cyst size and the likelihood of recurrence, but no clear relationship exists between cyst size and the likelihood of presenting with symptoms.15
In our meta-analysis of case reports, we found significant improvement in headaches, which exceeds the rate of headache improvement following transsphenoidal surgery (TSS) for RCC.16 One possible reason for this may be that severe or debilitating headaches are a relative indication for surgery and that patients with less severe headaches are more likely to be managed conservatively. The moderate improvement in endocrinopathies following cyst regression closely mirrors the rate of improvement after TSS.17, 18 Hypothyroidism, growth hormone deficiency, and hyponatremia showed the highest rate of improvement, whereas diabetes insipidus did not resolve in any patients included in the analysis. However, the sample size for this study is too small to make clear inferences on specific axes of pituitary dysfunction, a topic lacking data in the current literature. Resolution of visual dysfunction was seen at a similar rate as resolution after TSS reported in the literature.18, 19
Prior studies have had mixed conclusions regarding the predictive value of cyst size on behavior, with no distinct correlation between maximum diameter and the likelihood of progression. The number of cases included in our meta-analysis was too low to analyze the relationship between initial cyst size and overall change in size. In a Swedish cohort study of the natural history of RCCs, cysts < 10 mm remained stable, and cysts ≥ 10 mm tended to decrease in size over time without surgical intervention.20 In our analysis, we were unable to determine a relationship between cyst size and the likelihood of spontaneous regression. We did, however, observe an average volume shrinkage of 82% in patients with regression, for whom volumetric data were available, as well as a volume decrease ranging from 34% to 100% reported in the cohorts.
In about one-quarter of the patients, RCCs managed conservatively through regular imaging follow-up have been shown to spontaneously regress. Regression of RCCs in the literature occured at 0.5–27 months postdiagnosis.21–24 The case series we presented here demonstrated significant regression over a period of 2–5 months. The potential for spontaneous regression and the low rate of progression support a conservative approach to RCC management, although follow-up imaging is important to rule out growth.6, 14 While the ideal surveillance period has not been determined, for asymptomatic patients without optic chiasm compression, initial follow-up MRI at 1 year or at the onset of new symptoms together with continued surveillance for at least 5 years has been proposed.6, 13
Spontaneous RCC regression is hypothesized to take place through several possible mechanisms: 1) cystic fluid reabsorption into the surrounding blood vessels, 2) cyst rupture and collapse, and 3) glucocorticoid therapy via the immunosuppressive effect of the steroid.21 Apoplexy is also hypothesized to be a rare mechanism of RCC involution, with a few case reports of patients with known RCC presenting with new acute headache and evidence of hemorrhage on imaging preceding cyst involution.13, 25–27 There have been no studies looking at the effects of lifestyle modifications such as weight loss, nonsteroidal anti-inflammatory drug (NSAID) use, or nutrition on RCC behavior.
Recognizing the possibility of cyst regression is critical, especially in patients scheduled for surgery. In all three patients in our cohort, the regression was noted on neuronavigation imaging performed the morning of the scheduled surgery, prompting cancellation of the case and outpatient follow-up. Therefore, we advocate performing updated imaging immediately preceding surgical intervention, especially in patients in whom symptoms have shown signs of improvement.
This study is limited by the retrospective nature of the systematic review and the low number of patients and events reported. Details regarding cyst characteristics and patient symptoms were sparse, leading to minimal analysis of these factors in the context of RCC regression. In addition, most of the reported literature comprised case reports or small case series, with few large observational cohorts available for analysis. Selection and publication bias are other potential limitations of this work. Volumetric analysis may prove useful in future studies on the natural history of RCCs to determine whether there is a correlation between cyst size and the likelihood of regression, stability, or progression. Other factors such as lifestyle modifications and the use of steroids or NSAIDs that may play a role in RCC behavior should also be considered in future cohorts. While there are many reports describing symptoms associated with RCCs, there is a paucity of data on the resolution of symptoms following RCC regression, especially regarding hormonal function.
Lessons
More than one-quarter of patients with conservatively managed RCCs will experience spontaneous regression of the cyst at follow-up. The presence of headaches should not preclude conservative management for small RCCs without visual disturbance or endocrinopathy due to mass effect. Because the majority of RCCs will remain stable or shrink over time, patients without compressive symptoms or growing lesions can be monitored with serial imaging for a period of at least 5 years unless new symptoms or growth occur.
Disclosures
Dr. Carmichael reported serving as a consultant on advisory boards for Camurus, Xeris, and Novo Nordisk and as a principal investigator for research studies with Novo Nordisk and Crinetics.
Author Contributions
Conception and design: Peterson, Shiroishi, Zada. Acquisition of data: Peterson, Fixman, Carmichael, Zada. Analysis and interpretation of data: Peterson, Fixman, Shiroishi. Drafting the article: Peterson, Fixman. Critically revising the article: all authors. Reviewed submitted version of manuscript: Peterson, Fixman, Shiroishi, Zada. Approved the final version of the manuscript on behalf of all authors: Peterson. Statistical analysis: Fixman. Study supervision: Zada.
Supplemental Information
Previous Presentations
Portions of this work were presented at the Annual Meeting of the North American Skull Base Society, Tampa, FL, February 17–19, 2023.
Correspondence
Racheal Peterson: Keck School of Medicine, University of Southern California, Los Angeles, CA. rachealw09@gmail.com.
References
- 1.Naik VD, Thakore NR. A case of symptomatic Rathke’s cyst. BMJ Case Rep. 2013;2013:bcr2012006943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zada G. Rathke cleft cysts: a review of clinical and surgical management. Neurosurg Focus. 2011;31(1):E1. [DOI] [PubMed] [Google Scholar]
- 3.Teramoto A, Hirakawa K, Sanno N, Osamura Y. Incidental pituitary lesions in 1,000 unselected autopsy specimens. Radiology. 1994;193(1):161-164. [DOI] [PubMed] [Google Scholar]
- 4.Barkhoudarian G, Palejwala SK, Ansari S, et al. Rathke’s cleft cysts: a 6-year experience of surgery vs. observation with comparative volumetric analysis. Pituitary. 2019;22(4):362-371. [DOI] [PubMed] [Google Scholar]
- 5.Chong GYC, Tan KCB, Lau EYF, et al. A study on clinical outcomes of Rathke’s cleft cyst in patients managed conservatively. Pituitary. 2022;25(2):258-266. [DOI] [PubMed] [Google Scholar]
- 6.Culver SA, Grober Y, Ornan DA, et al. A case for conservative management: characterizing the natural history of radiographically diagnosed Rathke cleft cysts. J Clin Endocrinol Metab. 2015;100(10):3943-3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim CW, Hwang K, Joo JD, Kim YH, Han JH, Kim CY. Spontaneous involution of Rathke’s cleft cysts without visual symptoms. Brain Tumor Res Treat. 2016;4(2):58-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ciric I, Ragin A, Baumgartner C, Pierce D. Complications of transsphenoidal surgery: results of a national survey, review of the literature, and personal experience. Neurosurgery. 1997;40(2):225-236. [DOI] [PubMed] [Google Scholar]
- 9.Zada G, Kelly DF, Cohan P, Wang C, Swerdloff R. Endonasal transsphenoidal approach to treat pituitary adenomas and other sellar lesions: an assessment of efficacy, safety, and patient impressions of the surgery. J Neurosurg. 2003;98(2):350-358. [DOI] [PubMed] [Google Scholar]
- 10.Amhaz HH, Chamoun RB, Waguespack SG, Shah K, McCutcheon IE. Spontaneous involution of Rathke cleft cysts: is it rare or just underreported?. J Neurosurg. 2010;112(6):1327-1332. [DOI] [PubMed] [Google Scholar]
- 11.Sala E, Moore JM, Amorin A, et al. Natural history of Rathke’s cleft cysts: a retrospective analysis of a two centres experience. Clin Endocrinol (Oxf). 2018;89(2):178-186. [DOI] [PubMed] [Google Scholar]
- 12.Sade B, Albrecht S, Assimakopoulos P, Vézina JL, Mohr G. Management of Rathke’s cleft cysts. Surg Neurol. 2005;63(5):459-466. [DOI] [PubMed] [Google Scholar]
- 13.Kinoshita Y, Taguchi A, Yamasaki F, Tominaga A, Arita K, Horie N. Natural course of Rathke’s cleft cysts and risk factors for progression. J Neurosurg. 2022;138(5):1426-1432. [DOI] [PubMed] [Google Scholar]
- 14.Lee C, Park SH. Spontaneously regressed Rathke’s cleft cyst. J Korean Neurosurg Soc. 2019;62(6):723-726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trifanescu R, Ansorge O, Wass JAH, Grossman AB, Karavitaki N. Rathke’s cleft cysts. Clin Endocrinol (Oxf). 2012;76(2):151-160. [DOI] [PubMed] [Google Scholar]
- 16.Altuwaijri N, Cote DJ, Lamba N, et al. Headache resolution after Rathke cleft cyst resection: a meta-analysis. World Neurosurg. 2018;111:e764-e772. [DOI] [PubMed] [Google Scholar]
- 17.Park JK, Lee EJ, Kim SH. Optimal surgical approaches for Rathke cleft cyst with consideration of endocrine function. Neurosurgery. 2012;70(2 Suppl Operative):250-256. [DOI] [PubMed] [Google Scholar]
- 18.Wait SD, Garrett MP, Little AS, Killory BD, White WL. Endocrinopathy, vision, headache, and recurrence after transsphenoidal surgery for Rathke cleft cysts. Neurosurgery. 2010;67(3):837-843. [DOI] [PubMed] [Google Scholar]
- 19.Aho CJ, Liu C, Zelman V, Couldwell WT, Weiss MH. Surgical outcomes in 118 patients with Rathke cleft cysts. J Neurosurg. 2005;102(2):189-193. [DOI] [PubMed] [Google Scholar]
- 20.Petersson M, Berinder K, Eden Engström B, et al. Natural history and surgical outcome of Rathke’s cleft cysts–a study from the Swedish Pituitary Registry. Clin Endocrinol (Oxf). 2022;96(1):54-61. [DOI] [PubMed] [Google Scholar]
- 21.Cheng L, Guo P, Jin P, Li H, Fan M, Cai E. Spontaneous involution of a Rathke cleft cyst. J Craniofac Surg. 2016;27(8):e791-e793. [DOI] [PubMed] [Google Scholar]
- 22.Al Safatli D, Kalff R, Waschke A. Spontaneous involution of a presumably Rathke’s cleft cyst in a patient with slight subclinical hypopituitarism: a case report and review of the literature. Case Rep Surg. 2015;2015:971364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Munich SA, Leonardo J. Spontaneous involution of a Rathke’s cleft cyst in a patient with normal cortisol secretion. Surg Neurol Int. 2012;3:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rasmussen Z, Abode-Iyamah KO, Kirby P, Greenlee JDW. Rathke’s cleft cyst: a case report of recurrence and spontaneous involution. J Clin Neurosci. 2016;32:122-125. [DOI] [PubMed] [Google Scholar]
- 25.Nishio S, Morioka T, Suzuki S, Fukui M. Spontaneous regression of a pituitary cyst: report of two cases. Clin Imaging. 2001;25(1):15-17. [DOI] [PubMed] [Google Scholar]
- 26.Onesti ST, Wisniewski T, Post KD. Pituitary hemorrhage into a Rathke’s cleft cyst. Neurosurgery. 1990;27(4):644-646. [DOI] [PubMed] [Google Scholar]
- 27.Truong LUF, Marlier B, Decoudier B, Litré CF, Barraud S. Vanishing Rathke’s cleft cyst. Ann Endocrinol (Paris). 2022;83(4):260-262. [DOI] [PubMed] [Google Scholar]
- 28.Igarashi T, Saeki N, Yamaura A. Long-term magnetic resonance imaging follow-up of asymptomatic sellar tumors–their natural history and surgical indications. Neurol Med Chir (Tokyo). 1999;39(8):592-599. [DOI] [PubMed] [Google Scholar]
- 29.Saeki N, Kubota M, Yamaura A, Ishige N. Fluctuating visual field defects in Rathke’s cleft cysts: MRI analysis. J Clin Neurosci. 1999;6(6):524-527. [DOI] [PubMed] [Google Scholar]
- 30.Terao T, Sawauchi S, Hashimoto T, Miyazaki Y, Akiba Y, Abe T. A case of spontaneous rupture of a suprasellar cystic mass [Article in Japanese]. No Shinkei Geka Neurol Surg. 2001;29(8):755-758. [PubMed] [Google Scholar]
- 31.Sanno N, Oyama K, Tahara S, Teramoto A, Kato Y. A survey of pituitary incidentaloma in Japan. Eur J Endocrinol. 2003;149(2):123-127. [DOI] [PubMed] [Google Scholar]
- 32.Maniec K, Watson JC. Spontaneous rupture, disappearance, and reaccumulation of a Rathke’s cleft cyst. Case Rep Endocrinol. 2011;2011:549262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shepard MJ, Elzoghby MA, Kiehna EN, Payne SC, Jane JA. Presentation and outcomes in surgically and conservatively managed pediatric Rathke cleft cysts. J Neurosurg Pediatr. 2018;21(3):308-314. [DOI] [PubMed] [Google Scholar]
- 34.Salle L, Teissier-Clément MP, Mas R, Boncoeur-Martel MP, Salle H. Spontaneous involution of a Rathke cleft cyst. Ann Endocrinol (Paris). 2021;82(6):626-628. [DOI] [PubMed] [Google Scholar]