Abstract
While baculovirus expression of Gag proteins from numerous retroviruses has led reliably to production of virus-like particles (VLPs), we observed that expression of Rous sarcoma virus Gag failed to produce VLPs. Transmission and scanning electron microscopy analysis revealed that the Gag protein reached the plasma membrane but was unable to correctly form particles. Addition of a myristylation signal had no effect on the budding defect, but deletion of the PR domain of Gag restored normal budding. The resulting VLPs were morphologically distinct from human immunodeficiency virus type 1 VLPs expressed in parallel.
The baculovirus expression system has been widely used in the study of retrovirus assembly, with examples including human immunodeficiency virus (HIV) types 1 and 2; simian, bovine, and feline immunodeficiency viruses; Mason-Pfizer monkey virus, and human T-cell lymphotropic virus type 2 as well as the retrotransposon TED and the human endogenous retrovirus HERV-K (2, 4, 9, 11, 12, 16, 22, 26, 27). In each case, expression of the gag gene led to the production of regular virus-like particles (VLPs). In contrast, we have found that the Rous sarcoma virus (RSV) Gag protein is an exception; overexpression of the gag gene yielded high levels of protein but negligible production of extracellular VLPs.
Two aspects of RSV Gag distinguish it from the other retroviral Gag proteins. First, RSV Gag is not myristylated at its N terminus (13). Myristylation is known to be required for budding of most of the well-studied retroviruses (17, 18). Similarly, when a single amino acid change to abolish myristylation was introduced into baculovirus-produced SIV or HIV-1 Gag, intracellular VLPs were still produced, but they were unable to exit the cells (2, 4). Hence, it seemed possible that the budding defect of RSV is due to lack of myristylation. Second, unlike most other retroviruses, RSV encodes the viral protease (PR) as part of Gag. Retroviral PR domains cause difficulties in overexpression systems at two different levels. Proteins encoding an active PR domain inevitably are poorly produced in the baculovirus system (7), presumably because the PR enzyme is cytotoxic. For some purposes this difficulty can be overcome by using an active-site mutant of PR. In addition, retroviral PR domains are extremely insoluble (6, 10, 21). Expression of an HIV-1 Gag protein with an inactivated PR domain at its C terminus (creating a molecule equivalent to RSV Gag) resulted in a reduction of extracellular VLP production by 5- to 10-fold compared with wild-type Gag, and the particles produced had a lower density and were irregular in shape (19). These data suggest that the PR domain might lead to unnatural aggregation or precipitation of RSV Gag in the baculovirus overexpression system, in effect preventing proper budding.
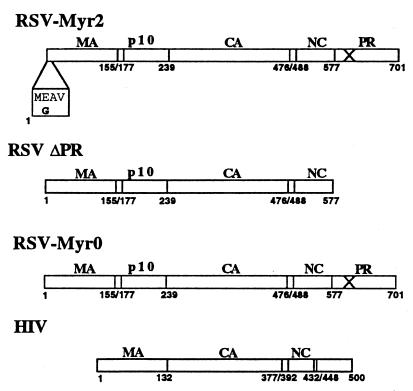
To investigate the possible causes of the RSV-specific budding defect in insect cells, we created three recombinant baculoviruses (Fig. 1). The first expresses the Gag mutant Myr2, which carries an artificially introduced N-terminal myristylation signal. This mutant is infectious when introduced into an RSV proviral construct (14). The second recombinant baculovirus, ΔPR, expresses an RSV Gag mutant in which the first amino acid of the PR domain is replaced by a stop codon, effectively deleting the PR domain. The third baculovirus, Myr0, expresses RSV Gag with a wild-type membrane-binding domain. The Myr2, Myr0, and ΔPR constructs were originally subcloned into the baculovirus transfer vector pFastBac1 (Life Technologies) from plasmids pSV.Myr2 (14), pΔSV.Myr0 (29), and pET3xcGagΔPR (1a), respectively, using standard cloning techniques (Fig. 1). Constructs then were shuttled into baculovirus according to the Bac-to-Bac baculovirus expression system protocol (Life Technologies). In initial experiments, Gag constructs which contained active PR were used, but it was found that the protein yield from these constructs was extraordinarily low, presumably due to PR-associated toxicity (data not shown). To avoid this PR-associated toxicity, the PR domains of Myr0 and Myr2 were exchanged with the inactivated PR domain from plasmid pgag.neo.D37N (25). The recombinant baculovirus Gag12myr (20) expressing wild-type HIV-1 Gag was used as a positive control for assembly and budding.
FIG. 1.
Baculovirus clones used in this study. Boxed amino acid sequence represents wild-type sequence (top) and altered Myr2 sequence (bottom). The X through the PR domain denotes an inactive protease. Numbers represent amino acid positions of domain borders.
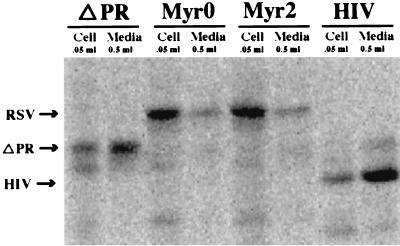
To determine the optimal time of protein expression, single flasks of Sf9 cells growing in suspension culture were infected at a multiplicity of infection of 0.1, and samples were harvested each day for 5 days. With each virus, the total Gag protein both inside the cells and in the medium increased progressively until day 3. After that, the level of extracellular Gag stabilized, while intracellular Gag decreased dramatically, presumably due to protein degradation (data not shown). For this reason, all budding assays were performed by infecting at the same low multiplicity of infection and collecting virus 2 to 3 days postinfection. Gag protein from each baculovirus clone was initially characterized by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis. Infected Sf9 cells were steady-state labeled with [35S] methionine (2 μCi/ml) in methionine-reduced medium (0.1 mM methionine) from 1 day postinfection until collection at 3 days postinfection. VLPs were collected from culture medium by centrifugation through a 10% sucrose cushion at 100,000 × g for 90 min. Proteins were separated by SDS–15% PAGE and detected by PhosphorImager analysis of fixed, dried gels (Fig. 2). In order to highlight the differences among the viruses, 10 times more of the VLP pellet than of the cell lysate (as a percentage of the total) was loaded. The results from several experiments showed that less than 5% of Myr0 and Myr2 protein was released into the medium in particulate form, whereas 15 to 25% of ΔPR protein and 30 to 50% of HIV-1 protein was released. In the particular experiment shown, the percentage of total viral protein detected in the medium was 40, 20, 2, and 2% for HIV, ΔPR, Myr2, and Myr0, respectively.
FIG. 2.
SDS-PAGE analysis of VLP production. (A) 35S detection. (B) Western blots. The amount of cells or medium loaded on the gel is shown above each well.
Compared with the ΔPR and HIV-1 Gag proteins, the Myr0 and Myr2 proteins in cell lysates differed markedly in solubility. For example, in a pulse-chase experiment, baculovirus-infected cells were labeled for 20 min with [35S]methionine, and then lysates were prepared in RIPA buffer (150 mM NaCl, 1% NP-40, 0.5% deoxycholate, 0.1% SDS, 50 mM Tris[pH 7.0]). While over 80% of the newly synthesized ΔPR and HIV-1 Gag was soluble in these conditions, over 60% of the Myr2 protein was insoluble, as evidenced by ability to be collected by centrifugation for 5 min at 14,000 × g (data not shown). Similar results were obtained after different chase times. Myr0 Gag was not analyzed by pulse-chase but was identical to Myr2 in other solubility experiments. This information led us to hypothesize that Myr0 and Myr2 proteins do not bud properly because they are shuttled into inclusion bodies and consequently do not reach the plasma membrane. To test this hypothesis, infected cells as well as VLP pellets from each virus were thin sectioned and viewed by transmission electron microscopy (TEM). For TEM analysis, infected cells and VLPs were harvested 3 days postinfection, fixed with 2.5% glutaraldehyde, postfixed with 1% osmium tetroxide, dehydrated through a graded series of ethanol and ethanol-Spurr (23) washes, and embedded in pure Spurr. Embedded samples were thin sectioned and viewed on a Phillips 301 transmission electron microscope.
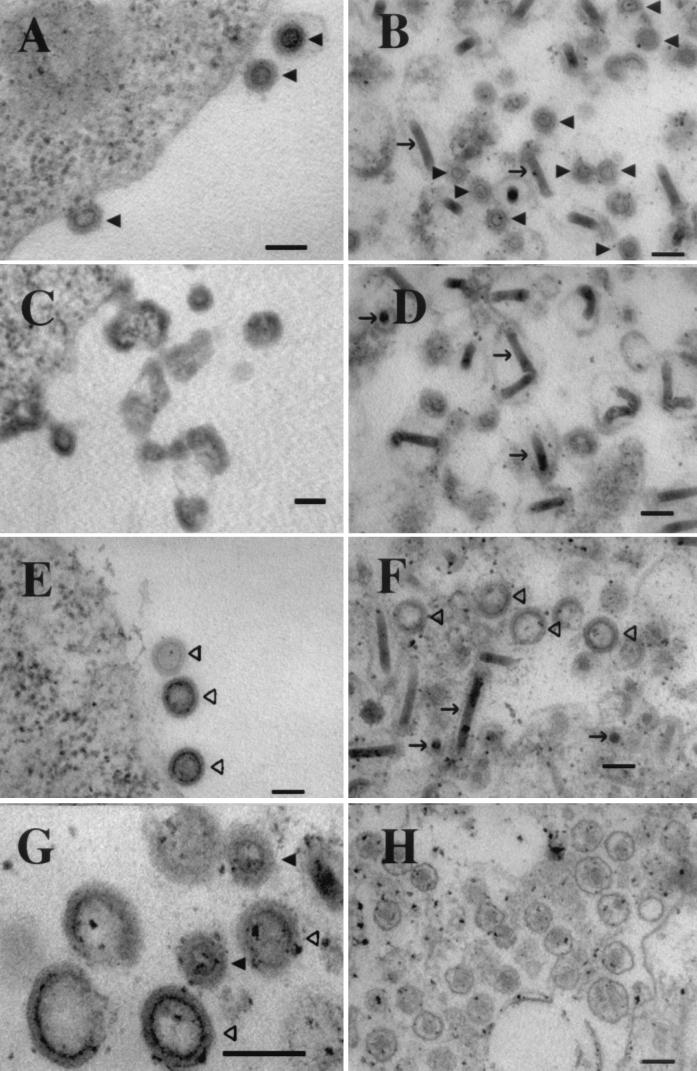
No occluded masses of proteins or clusters of assembled particles were detected by TEM within cells infected with Myr0 or Myr2 (not shown). However, thin sections at the plasma membrane showed abnormal structures for Myr0 and Myr2. While ΔPR-infected and HIV-1-infected cells displayed regular retrovirus-like particles budding at the plasma membrane (Fig. 3A and E, respectively), Myr0-infected and Myr2-infected cells displayed dense aggregates at the plasma membrane that vaguely resembled incompletely formed particles (shown for Myr2 in Fig. 3C). Immunofluorescence analysis by confocal microscopy confirmed that each of the RSV proteins resided predominantly at the plasma membrane (not shown). Images of thin-sectioned viral pellets collected from the medium reflect what was seen at the plasma membrane (Fig. 3B, D, and F). While all pellets contained regular, rod-shaped baculovirus particles, only HIV-1 and ΔPR pellets showed evidence of regular VLPs resembling immature retroviruses. The Myr0 and Myr2 pellets contained what appear to be irregular or broken particles. In conclusion, although each of the RSV Gag proteins was able to reach the plasma membrane, ΔPR was the only RSV protein able to properly assemble into viral particles. We take these observations as evidence that membrane targeting is not compromised in Myr0 or Myr2.
FIG. 3.
Thin-section TEM. (A, C, and E) Plasma membrane of Sf9 cells infected with ΔPR, Myr2, and HIV-1, respectively. (B, D, and F) VLP pellets from growth medium of cells infected with ΔPR, Myr2, and HIV-1, respectively. (G) Mixed pellet containing both ΔPR and HIV-1 VLPs. (H) Infectious RSV particles. The RSV is infectious virus of the RCAS strain (15). Solid triangles indicate RSV VLPs, open triangles show HIV-1 VLPs, and arrows show baculovirus particles. Bars, 100 nm.
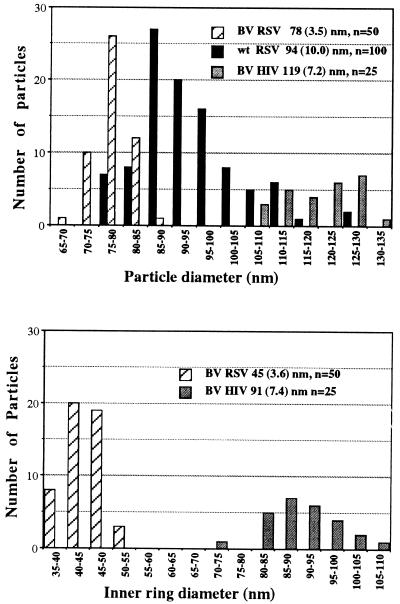
VLPs produced by the ΔPR construct exhibit a morphology distinct from other VLPs. All baculovirus-produced retroviral VLPs that have been reported are similar to HIV-1 VLPs, exhibiting a dense inner ring very near the surface of the particle (2, 4, 11, 12, 16, 22, 26). The inner ring observed in ΔPR particles, however, was distinctly closer to the center. To highlight the difference between the two types of VLPs, a mixed pellet of HIV-1 and ΔPR particles was sectioned and viewed by TEM (Fig. 3G). In addition to the difference in morphology, the ΔPR particles were noticeably smaller than HIV-1 VLPs. The size distributions of VLPs and of the dark inner rings of RSV ΔPR, HIV-1, and authentic infectious RSV (Fig. 3H) collected from infected turkey cells are shown in Fig. 4. ΔPR VLPs were relatively homogenous in size but were approximately 10 to 15 nm smaller in diameter than mature infectious RSV (RCAS strain), and approximately 40 nm smaller than HIV-1 VLPs. The diameter of the dark inner ring of ΔPR particles was approximately half the diameter of the ring from HIV-1 particles.
FIG. 4.
Size distribution of particles and inner rings. BV RSV and BV HIV-1 are baculovirus produced ΔPR and HIV-1 VLPs, respectively. Numbers in parentheses are the standard deviations of the particle sizes. n, number of particles counted.
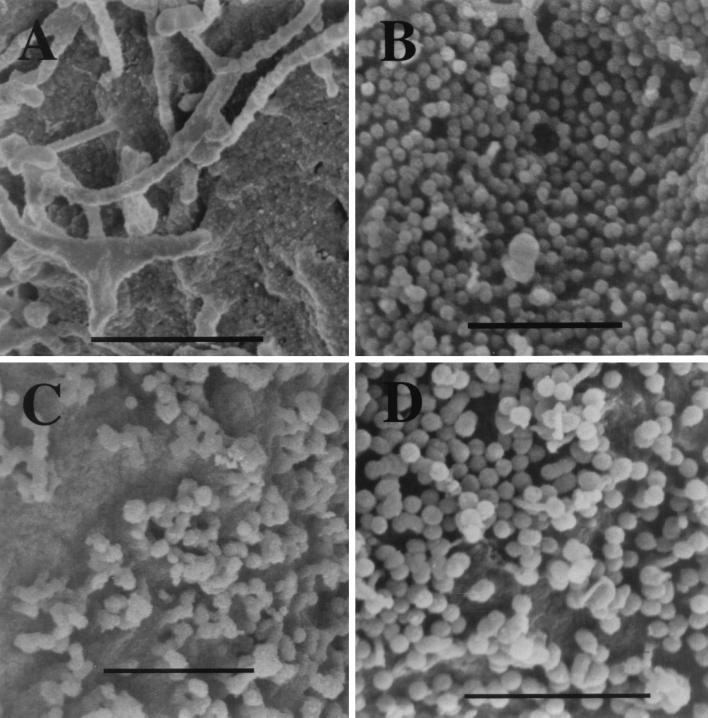
To provide a better overview of the altered budding structures, infected cells were also examined by scanning electron microscopy (SEM) (Fig. 5). Cells and VLPs were harvested at 3 days postinfection, fixed with 2.5% glutaraldehyde, postfixed with 1% osmium tetroxide, dehydrated through a graded series of ethanol washes, critical point dried, mounted onto aluminum stubs, and viewed on a Hitachi 4500 SEM. The surface of cells infected with baculoviruses expressing ΔPR or HIV-1 (Fig. 5B and D, respectively) was covered with clusters of perfectly round VLPs. In contrast, the surface of cells expressing Myr0 or Myr2 showed irregular, lumpy, or imperfectly spherical budding structures (Fig. 5C).
FIG. 5.
SEM analysis of baculovirus-infected and uninfected Sf9 cells. (A) Uninfected Sf9 cell. (B) ΔPR-infected cell. (C) Myr0-infected cell. (D) HIV-1-infected cell. Bars, 1 μm.
An important conclusion from this work is that the RSV Gag protein is defective for budding when highly expressed in insect cells. A similar finding has recently been reported for the Gag protein of human T-cell leukemia virus (1). The RSV defect is caused by the presence of the PR domain and not by a failure of the protein to reach the plasma membrane. Since RSV Gag buds properly from both mammalian and avian cells (8, 24), the budding defect is either due to the cell type or peculiar to the baculovirus expression system. We speculate that a chaperone activity in vertebrate cells promotes proper folding and assembly of PR-containing proteins and that this activity is not present in invertebrate cells or is quantitatively insufficient in the baculovirus system. Other differences between insect and vertebrate cells also might contribute to or be responsible for the budding defect, for example, growth temperature and membrane composition.
A further conclusion is that baculovirus-produced ΔPR particles viewed by thin-section TEM are 10 to 15 nm smaller in average diameter than infectious RSV particles collected from avian cells. Although this difference is distinct, the size distributions of the two viruses do overlap. Several explanations might account for this difference. First, the membrane of infectious RSV is studded with viral envelope proteins, possibly making the membrane and therefore the particle diameter appear thicker than the membrane of the baculovirus-produced particles. Second, because the ΔPR protein is 124 amino acids smaller than wild-type Gag, it could pack differently, yielding a particle with a smaller radius of curvature. Third, the maturation triggered by proteolytic processing of wild-type Gag conceivably could lead to expansion of the diameter of the virion. This explanation seems the least likely, as protease-deficient and wild-type murine leukemia virus particles measured by cryo-EM have identical diameters (30).
Finally, ΔPR VLPs are distinct from HIV-1 VLPs in both size and morphology. The larger size of the HIV-1 VLPs is striking, with an observed 40-nm-larger particle diameter, implying about a twofold difference in volume. The size difference does not correlate with genome lengths, which are similar for RSV and HIV-1. The measurements by thin-section TEM are approximately 20% smaller than has been reported for RSV and HIV-1 particles analyzed more accurately by cryo-EM (3, 5; R. L. Kingston, personal communication). This degree of shrinkage commonly occurs during the fixation and dehydration procedures. The ΔPR particles are morphologically distinct from HIV-1 particles in that the dark inner rings of ΔPR particles are both smaller, approximately half the diameter, and more central than the rings in HIV-1 particles. The difference in the organization of the Gag domains of the ΔPR and HIV-1 Gag proteins in part may account for these observed differences. The N terminus of RSV CA begins at amino acid residue 239, while that of HIV-1 CA begins at residue 132. Overall, the differences between the baculovirus-expressed RSV ΔPR and HIV-1 Gag particles imply a different Gag stoichiometry. The estimated number of Gag molecules in wild-type RSV is about 1,500 (28), and in particles assembled in vitro it is about 1,200 (31). Similar measurements have not been reported for HIV-1.
Acknowledgments
We thank John Wills for the RSV constructs pSV,Myr2.T14K and pΔSV.Myr0 and Pierre Boulanger for the baculovirus Gag12myr. We credit Thomas Wilk for originally observing that regular VLPs could not be found in the medium of baculovirus-infected insect cells expressing a PR-defective RSV Gag protein.
REFERENCES
- 1.Bouamr F, Garnier L, Rayne F, Verna A, Rebeyrotte N, Cerutti M, Mamoun R Z. Differential budding efficiencies of human T-cell leukemia virus type I (HTLV-I) Gag and Gag-Pro polyproteins from insect and mammalian cells. Virology. 2000;278:597–609. doi: 10.1006/viro.2000.0663. [DOI] [PubMed] [Google Scholar]
- 1a.Campbell S, Vogt V M. In vitro assembly of virus-like particles with Rous sarcoma virus Gag deletion mutants: identification of the p10 domain as a morphological determinant in the formation of spherical particles. J Virol. 1997;71:4425–4435. doi: 10.1128/jvi.71.6.4425-4435.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Delchambre M, Gheysen D, Thines D, Thiriart C, Jacobs E, Verdin E, Horth M, Burny A, Bex F. The GAG precursor of simian immunodeficiency virus assembles into virus-like particles. EMBO J. 1989;8:2653–2660. doi: 10.1002/j.1460-2075.1989.tb08405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fuller S D, Wilk T, Gowen B E, Krausslich H G, Vogt V M. Cryo-electron microscopy reveals ordered domains in the immature HIV-1 particle. Curr Biol. 1997;7:729–738. doi: 10.1016/s0960-9822(06)00331-9. [DOI] [PubMed] [Google Scholar]
- 4.Gheysen D, Jacobs E, de Foresta F, Thiriart C, Francotte M, Thines D, De Wilde M. Assembly and release of HIV-1 precursor Pr55gag virus-like particles from recombinant baculovirus-infected insect cells. Cell. 1989;59:103–112. doi: 10.1016/0092-8674(89)90873-8. [DOI] [PubMed] [Google Scholar]
- 5.Gross I, Hohenberg H, Wilk T, Wiegers K, Grattinger M, Muller B, Fuller S, Krausslich H G. A conformational switch controlling HIV-1 morphogenesis. EMBO J. 2000;19:103–113. doi: 10.1093/emboj/19.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hruskova-Heidingsfeldova O, Andreansky M, Fabry M, Blaha I, Strop P, Hunter E. Cloning, bacterial expression, and characterization of the Mason-Pfizer monkey virus proteinase. J Biol Chem. 1995;270:15053–15058. doi: 10.1074/jbc.270.25.15053. [DOI] [PubMed] [Google Scholar]
- 7.Hu Y W, Kang C Y. Enzyme activities in four different forms of human immunodeficiency virus 1 pol gene products. Proc Natl Acad Sci USA. 1991;88:4596–4600. doi: 10.1073/pnas.88.11.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krishna N K, Campbell S, Vogt V M, Wills J W. Genetic determinants of Rous sarcoma virus particle size. J Virol. 1998;72:564–577. doi: 10.1128/jvi.72.1.564-577.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lerch R A, Friesen P D. The baculovirus-integrated retrotransposon TED encodes Gag and Pol proteins that assemble into viruslike particles with reverse transcriptase. J Virol. 1992;66:1590–1601. doi: 10.1128/jvi.66.3.1590-1601.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leuthardt A, Roesel J L. Cloning, expression and purification of a recombinant poly-histidine-linked HIV-1 protease. FEBS Lett. 1993;326:275–280. doi: 10.1016/0014-5793(93)81807-c. [DOI] [PubMed] [Google Scholar]
- 11.Luo L, Li Y, Kang C Y. Expression of gag precursor protein and secretion of virus-like gag particles of HIV-2 from recombinant baculovirus-infected insect cells. Virology. 1990;179:874–880. doi: 10.1016/0042-6822(90)90159-o. [DOI] [PubMed] [Google Scholar]
- 12.Morikawa S, Booth T F, Bishop D H. Analyses of the requirements for the synthesis of virus-like particles by feline immunodeficiency virus gag using baculovirus vectors. Virology. 1991;183:288–297. doi: 10.1016/0042-6822(91)90141-w. [DOI] [PubMed] [Google Scholar]
- 13.Palmiter R D, Gagnon J, Vogt V M, Ripley S, Eisenman R N. The NH2-terminal sequence of the avian oncovirus gag precursor polyprotein (Pr76gag) Virology. 1978;91:423–433. doi: 10.1016/0042-6822(78)90388-4. [DOI] [PubMed] [Google Scholar]
- 14.Parent L J, Wilson C B, Resh M D, Wills J W. Evidence for a second function of the MA sequence in the Rous sarcoma virus Gag protein. J Virol. 1996;70:1016–1026. doi: 10.1128/jvi.70.2.1016-1026.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petropoulos C J, Hughes S H. Replication-competent retrovirus vectors for the transfer and expression of gene cassettes in avian cells. J Virol. 1991;65:3728–3737. doi: 10.1128/jvi.65.7.3728-3737.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rasmussen L, Battles J K, Ennis W H, Nagashima K, Gonda M A. Characterization of virus-like particles produced by a recombinant baculovirus containing the gag gene of the bovine immunodeficiency-like virus. Virology. 1990;178:435–451. doi: 10.1016/0042-6822(90)90341-n. [DOI] [PubMed] [Google Scholar]
- 17.Rein A, McClure M R, Rice N R, Luftig R B, Schultz A M. Myristylation site in Pr65gag is essential for virus particle formation by Moloney murine leukemia virus. Proc Natl Acad Sci USA. 1986;83:7246–7250. doi: 10.1073/pnas.83.19.7246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rhee S S, Hunter E. Myristylation is required for intracellular transport but not for assembly of D-type retrovirus capsids. J Virol. 1987;61:1045–1053. doi: 10.1128/jvi.61.4.1045-1053.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Royer M, Bardy M, Gay B, Tournier J, Boulanger P. Proteolytic activity in vivo and encapsidation of recombinant human immunodeficiency virus type 1 proteinase expressed in baculovirus-infected cells. J Gen Virol. 1992;78:131–142. doi: 10.1099/0022-1317-78-1-131. [DOI] [PubMed] [Google Scholar]
- 20.Royer M, Hong S S, Gay B, Cerutti M, Boulanger P. Expression and extracellular release of human immunodeficiency virus type 1 Gag precursors by recombinant baculovirus-infected cells. J Virol. 1992;66:3230–3235. doi: 10.1128/jvi.66.5.3230-3235.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sellos-Moura M, Vogt V M. Proteolytic activity of purified avian sarcoma and leukemia virus NC-PR protein expressed in Escherichia coli. Virology. 1996;221:335–345. doi: 10.1006/viro.1996.0383. [DOI] [PubMed] [Google Scholar]
- 22.Sommerfelt M A, Roberts C R, Hunter E. Expression of simian type D retroviral (Mason-Pfizer monkey virus) capsids in insect cells using recombinant baculovirus. Virology. 1993;192:298–306. doi: 10.1006/viro.1993.1033. [DOI] [PubMed] [Google Scholar]
- 23.Spurr A R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969;26:31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- 24.Stewart L, Schatz G, Vogt V M. Properties of avian retrovirus particles defective in viral protease. J Virol. 1990;64:5076–5092. doi: 10.1128/jvi.64.10.5076-5092.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stewart L, Vogt V M. trans-Acting viral protease is necessary and sufficient for activation of avian leukosis virus reverse transcriptase. J Virol. 1991;65:6218–6231. doi: 10.1128/jvi.65.11.6218-6231.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takahashi R H, Nagashima K, Kurata T, Takahashi H. Analysis of human lymphotropic T-cell virus type II-like particle production by recombinant baculovirus-infected insect cells. Virology. 1999;256:371–380. doi: 10.1006/viro.1999.9655. [DOI] [PubMed] [Google Scholar]
- 27.Tonjes R R, Boller K, Limbach C, Lugert R, Kurth R. Characterization of human endogenous retrovirus type K virus-like particles generated from recombinant baculoviruses. Virology. 1997;233:280–291. doi: 10.1006/viro.1997.8614. [DOI] [PubMed] [Google Scholar]
- 28.Vogt V M, Simon M N. Mass determination of Rous sarcoma virus virions by scanning transmission electron microscopy. J Virol. 1999;73:7050–7055. doi: 10.1128/jvi.73.8.7050-7055.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wills J W, Craven R C, Achacoso J A. Creation and expression of myristylated forms of Rous sarcoma virus Gag protein in mammalian cells. J Virol. 1989;63:4331–4343. doi: 10.1128/jvi.63.10.4331-4343.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yeager M, Wilson-Kubalek E M, Weiner S G, Brown P O, Rein A. Supramolecular organization of immature and mature murine leukemia virus revealed by electron cryo-microscopy: implications for retroviral assembly mechanisms. Proc Natl Acad Sci USA. 1998;95:7299–7304. doi: 10.1073/pnas.95.13.7299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu F, Joshi S M, Ma Y M, Kingston R L, Simon M N, Vogt V M. Characterization of Rous sarcoma virus Gag particles assembled in vitro. J Virol. 2001;75:2753–2764. doi: 10.1128/JVI.75.6.2753-2764.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]