Abstract
Study Design
Retrospective cohort study.
Objective
Determine impact of standard/novel spinopelvic parameters on global sagittal imbalance, health-related quality of life (HRQoL) scores, and clinical outcomes in patients with multi-level, tandem degenerative spondylolisthesis (TDS).
Methods
Single institution analysis; 49 patients with TDS. Demographics, PROMIS and ODI scores collected. Radiographic measurements—sagittal vertical axis (SVA), pelvic incidence (PI), lumbar lordosis (LL), PI-LL mismatch, sagittal L3 flexion angle (L3FA) and L3 sagittal distance (L3SD). Stepwise linear multivariate regression performed using full length cassettes to identify demographic and radiographic factors predictive of aberrant SVA (≥5 cm). Receiver operative curve (ROC) analysis used to identify cutoffs for lumbar radiographic values independently predictive of SVA ≥5 cm. Univariate comparisons of patient demographics, (HRQoL) scores and surgical indication were performed around this cutoff using two-way Student’s t-tests and Fisher’s exact test for continuous and categorical variables, respectively.
Results
Patients with increased L3FA had worse ODI (P = .006) and increased rate of failing non-operative management (P = .02). L3FA (OR 1.4, 95% CI) independently predicted of SVA ≥5 cm (sensitivity and specifity of 93% and 92%). Patients with SVA ≥5 cm had lower LL (48.7 ± 19.5 vs 63.3 ± 6.9 mm, P < .021), higher L3SD (49.3 ± 12.9 vs 28.8 ± 9.2, P < .001) and L3FA (11.6 ± 7.9 vs −3.2 ± 6.1, P < .001) compared to patients with SVA ≤5 cm.
Conclusions
Increased flexion of L3, which is easily measured by the novel lumbar parameter L3FA, predicts global sagittal imbalance in TDS patients. Increased L3FA is associated with worse performance on ODI, and failure of non-operative management in patients with TDS.
Keywords: spine, spondylolisthesis, health-related quality of life, global sagittal imbalance
Introduction
Degenerative spondylolisthesis (DS) occurs in 19.1 to 43.1% of elderly patients and is a common cause of spinal stenosis.1,2 DS of the lumbar spine most commonly involves a single level, 3 however, multi-level degenerative spondylolisthesis, or tandem spondylolisthesis (TDS), is relatively uncommon, representing only 5-12% of all degenerative spondylolistheses.4-6 Most commonly DS occurs at the L4-L5 level, and the literature focuses predominantly on single level disease. 5
In addition to classically cited risk factors such as elevated BMI, advanced age and female gender, there is a growing body of literature implicating aberrant sagittal radiographic parameters in the pathogenesis of DS. 5 In particular, a high pelvic incidence (PI) has been associated with an increased risk of DS.7-9 Additionally, whether or not a patient with DS presents with global, sagittal anterior malalignment has been speculated to be related to various compensatory mechanisms including pelvic retroversion, thoracic flattening, and lower limb responses.5,9 Despite this growing body of work, the relationship between focal degenerative changes associated with DS and more global sagittal deformity has not been well defined. A prior work that compared TDS to single-level degenerative spondylolisthesis found that patients with TDS had a significantly greater pelvic incidence, C7 tilt, pelvic tilt (PT), and PI-LL mismatch than those with single-level DS. 10 These findings suggest that TDS may be a distinct clinical entity from single-level DS and may represent a significant, and possibly underappreciated, source of severe global sagittal imbalance. However, there is a paucity of data that evaluates what radiographic parameters may impact the clinical outcomes of patients with TDS.
The purpose of this study was to correlate the novel lumbar radiographic parameters L3 lumbar flexion angle (L3FA) and L3 sagittal distance (L3SD) with global sagittal alignment parameters, patient reported outcomes, and ultimately failure of non-operative treatment in patients with TDS. The hypothesis of this study was that L3FA and L3SD would correlate with SVA and that elevated L3FA and L3SD would correlate with poorer patient reported outcome scores and consequently an increased likelihood of patients with TDS failing non-operative management and requiring surgical intervention.
Methods
This study was institutional review board (IRB) approved with IRB number STUDY20040115 and was exempt from obtaining informed consent. This study was a retrospective analysis of a prospectively collected database of patients with low back pain or extremity symptoms in the setting of TDS at a single institution from 2016 to 2020. Inclusion criteria were patients with TDS and adequate standing, anterior-posterior (AP) and lateral radiographs of the lumbar spine. Adequate standing lumbar spine radiographs for analysis of spinopelvic parameters have previously been defined as radiographs that include the upper end plate of the L1 vertebra, the sacral dome, and both femoral heads. 11 Exclusion criteria were patients with high grade DS, a history of lumbar spine trauma, lumbar spine tumors, any symptoms concerning for cauda equina, conus medullaris, or other reasons to proceed with urgent surgery after the initial clinic visit, prior lumbar spine surgery (all patients with iatrogenic spondylolisthesis were excluded) or abdominal surgery, low-quality radiographic data, congenital malformations of the lumbar spine, or a history of a spine infections. High grade DS was defined according to the Meyerding classification as a ratio of overhang from the superior vertebral body to the anteroposterior length of the adjacent inferior vertebral body of greater than 50% (above Meyerding Grade 2). 12
For the purposes of this study, TDS was defined as anterolisthesis of at least 3 mm at two levels of the lumbar spine (L1-S1), which is a definition that has been used in prior work related to TDS. 1 Two non-contiguous, anterior spondylolistheses were still considered TDS (this pattern was only encountered in one patient in this study). 1 This study focused on TDS resulting in anterolisthesis due to posterior TDS being exceptionally rare (no patients with posterior TDS were identified in this study). 13 SVA was measured on 36” standing full-length spine plain radiographs. LL, PI, L3SD and L3FA were measured on lumbar spine plain radiographs. L3 was chosen as the center of measurements because L3-5 is the most common presentation of TDS and because the apex of physiologic lumbar lordosis is typically near the inferior aspect of L3 (Figure 1). 14 All radiographic measurements were performed manually by two senior orthopaedic surgery residents and subsequently averaged together to obtain the final value. An intra-class correlation coefficient (ICC) was calculated using R statistics software in order to assess intra-rater reliability for all lumbar spinopelvic parameters, and for the novel parameters L3SD and L3FA. All ICC calculations were noted to be excellent (>.9) between the two observers. 15 Philips DICOM Viewer software (Koninklijke Philips N.V.) was used to view radiographs and perform measurements. An inter-rater correlation coefficient was then calculated to determine reliability.
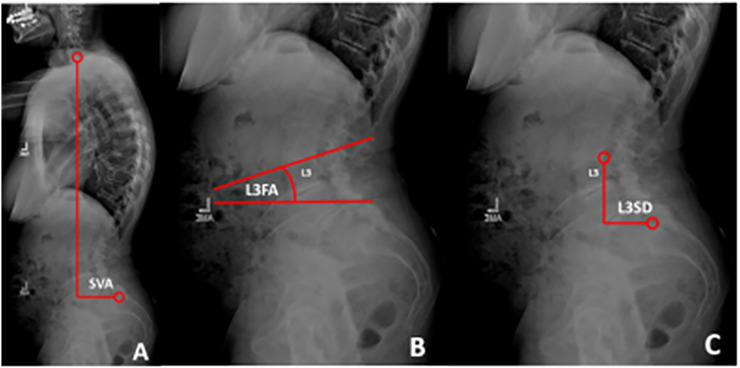
Figure 1.
A. SVA was measured as the angle between the posterior-superior corner of S1 to a vertical plumbline drawn from the center of the C7 vertebral body B. L3FA was measured as the angle between the superior endplate of L3 and a horizontal reference line. C. L3SD was measured as the horizontal distance between a vertical reference from the posterior-superior Corner of L3 vertebral body to the posterior-superior corner of S1.
The electronic medical record of included patients was retrospectively reviewed to determine if the patient had failed non-operative treatment and ultimately required surgery at any point during clinical follow-up. All patients initially presented to the same clinical practice for low back and/or extremity pain. All patients were treated with the same treatment algorithm, which includes standing lumbar radiographs at first visit, and a multi-tiered trial of non-operative treatment. First-line nonoperative treatment included physical therapy, non-steroidal anti-inflammatory drugs, and the addition of a Medrol dosepak if the onset of pain was relatively more recent. Second-line treatment was initiated if patients represented with continued pain complaints and included magnetic resonance imaging to identify areas of stenosis for consideration of epidural steroid injection. If patients represented with failure of both first-line and second-line treatments, they were offered repeat steroid injections and discussed surgery with the attending surgeon. Failure of non-operative treatment was defined as patients continuing to have pain and/or neurological complaints after first-line and second-line treatments and deciding to proceed with surgery, rather than re-attempt a second-line treatment. Additional collected demographic and clinical data included age, sex, BMI, co-morbidities as measured with the Age-Adjusted Charlson Comorbidity Index (ACCI), which has been used previously in orthopaedic spine literature. 16 Health-related quality of life (HRQoL) scores collected at the initial clinical visit included Oswestry Disability Index (ODI) and the PROMIS Global-10 physical function and mental health sub-scores.
Statistical Testing
Statistical analysis was performed using SPSS 28 (IBM, Armonk, NY, USA). Missing values were inputed 5 times to permit adequate pooled analysis. 17 A stepwise linear multivariate regression was performed using those patients with full length cassettes to identify demographic and radiographic factors independently predictive of SVA ≥5 cm. 18 A Receiver operative curve (ROC) analysis was used to identify an ideal cutoff for lumbar radiographic values independently predictive of SVA ≥5 cm. Univariate comparisons of patient demographics, HRQoL scores and surgical indication were then performed around this cutoff using two-way Student’s t-tests for continuous variables and Fisher’s exact test for categorical variables.
Significance was defined as P < .05 in all cases. A post-hoc power analysis was performed for both the global sagittal malalignment and clinical outcome cohort cohorts. Power was found to be >95% and >80% for detecting statistically significant differences in the global sagittal malalignment cohort’s average SVA and the clinical outcome cohort’s average L3FA, respectively.
Results
A total of 49 patients met our inclusion criteria, of whom 26 had 36” full length cassettes. Mean age was 70.1 ± 7.4 years, 43/49 (87.8%) were female, mean BMI was 29.9 ± 6.1 and mean ACCI was 3.9 ± 1.9. Mean ODI at presentation was 41.8 ± 14.1, and the mean PROMIS physical and mental sub-scores were 12.1 ± 3.0 and 13.5 ± 3.6, respectively. Mean SVA was 5.1 ± 2.1 cm, mean PI was 68.1 ± 11.9, mean PT was 27.6 ± 9.6 and mean LL 54.4 ± 15.8 (mean PI-LL mismatch 13.7 ± 15.9). Mean L3SD was 36.6 ± 18.7 mm while mean L3FA was 4.7 ± 9.9. The majority of patients had either L3-L5 (26/49, 53.1%) or L4-S1 (18/49, 36.7%) TDS. Other presentations included L2-L4 (2/49, 4.1%) and L3-L4 + L5-S1 non-contiguous TDS (3/49, 6.1%). Meyerding grading was performed for all levels. 12 For L2-L3, 2/2 (100%) of patients were grade 1. For L3-L4, the average grade was 1.1 ± .3 (28/31 (90.3%) of patients were grade 1, and 3/31 (9.7%) were grade 2). For L4-L5, the average grade was 1.1 ± .4 (38/44 (86.4%) of patients were grade 1, and 6/44 (13.6%) were grade 2). For L5-S1, 21/21 (100%) of patients were grade 1.
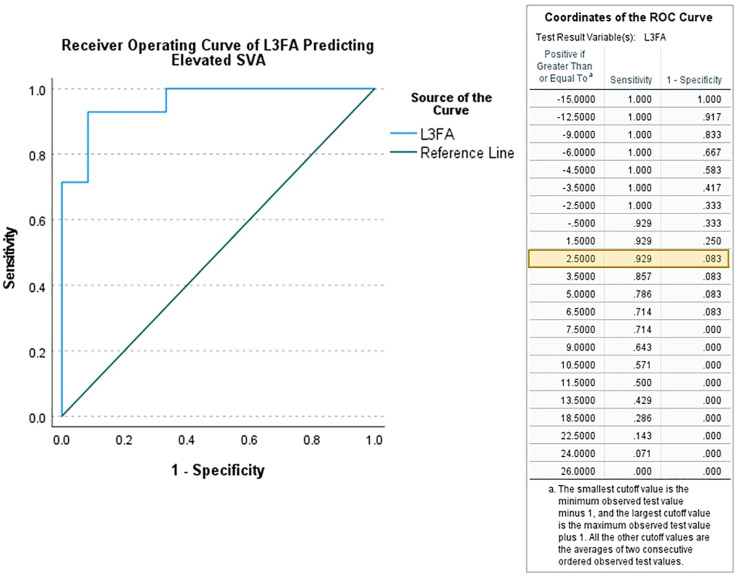
In a stepwise multivariate logistic regression of those patients with full length cassettes (n = 26), only L3FA (OR 1.4, 95% CI) was independently predictive of SVA ≥5 cm (area under the curve = .96). ROC analysis indicated a cutoff of L3FA cutoff of ≥2.5 was optimally predictive of SVA ≥5 cm (Figure 2). When patients with a pre-operative SVA above and below 5 cm were compared, standing PI, SS, and PT were equivalent between groups. The LL of the elevated SVA group was significantly lower than in the normal SVA group (48.7 ± 19.5 vs 63.3 ± 6.9 mm, P < .021). L3SD was significantly higher in the elevated SVA group than in the normal SVA group (49.3 ± 12.9 vs 28.8 ± 9.2, P < .001), as was L3FA (11.6 ± 7.9 vs −3.2 ± 6.1, P < .001). Sensitivity and specificity analyses demonstrated that an L3FA threshold greater than 2 degrees yielded a sensitivity and specificity for predicting an SVA >5 cm of 93% and 92%, respectively. When comparing the subgroup of patients with full length cassettes to the entire clinical cohort by demographics, radiographic parameters, and level of spondylolisthesis, there were no significant differences between the two groups (Table 1).
Figure 2.
Receiver operating curve analysis demonstrating the ideal cutoff value of 2.5 degrees for lumbar radiographic values independently predictive of SVA ≥ 5 cm Legend: SVA = Sagittal Vertical Axis L3FA = Flexion angle of the L3 vertebral body.
Table 1.
Comparison of demographic, radiographic and HRQoL parameters of patients with lower vs elevated L3FA.
| Lower L3FA (N = 21) | Elevated L3FA (N = 28) | Univariate P-value | |
|---|---|---|---|
| Patient demographics | |||
| Age | 72.7 ± 6.9 | 68.2 ± 7.3 | 0.03 |
| Sex (% female) | 20 / 21 | 23 / 28 | 0.2 |
| BMI | 29.1 ± 5.6 | 30.5 ± 6.5 | 0.4 |
| Age-adjusted | 4.0 ± 1.7 | 3.9 ± 2.1 | 0.9 |
| Charlson score | |||
| Radiographic parameters | |||
| Pi | 65.1 ± 9.1 | 70.3 ± 13.4 | 0.1 |
| Pelvic tilt | 26.3 ± 10.8 | 28.4 ± 8.6 | 0.4 |
| Sacral slope | 39.8 ± 9.8 | 41.5 ± 12.3 | 0.6 |
| PI-ll mismatch | 5.0 ± 12.1 | 20.1 ± 15.4 | < 0.001 |
| L3 SD | 21.7 ± 11.6 | 47.7 ± 14.7 | < 0.001 |
| Patient reported outcomes | |||
| ODI | 33.2 ± 13.1 | 44.1 ± 13.0 | 0.006 |
| Promis physical subscore | 12.8 ± 2.6 | 11.5 ± 2.9 | 0.1 |
| Promis mental subscore | 13.7 ± 3.8 | 13.7 ± 3.6 | 0.5 |
| Surgical indication | |||
| Offered surgery | 7 / 21 (33.3%) | 19 / 28 (67.9%) | 0.02 |
In a univariate analysis of the entire cohort (n = 49), among patient factors only younger age was associated with an increased L3FA (68.2 ± 7.3 years vs 72.7 ± 6.9 years in low L3FA group, P = .03). Increased L3SD (47.4 ± 14.7 mm vs 21.7 ± 11.6 mm, P < .001), decreased LL (50.1 ± 18.2° vs 60.1 ± 9.6, P = .03) and increased PI-LL mismatch (20.1 ± 15.4 vs 5.0 ± 12., P < .001) were also associated with increased L3FA. While PROMIS scores were equivalent between high vs low L3FA groups, an increased L3FA was associated with an elevated ODI (44.1 ± 13.0 vs 33.2 ± 13.1, P = .006, Table 1).
The influence of L3FA on failure of non-operative treatment was evaluated. A significantly larger number of patients with elevated L3FA angles (19/28, 67.9%) were offered surgery than those with lower L3FA angles (7/21, 33.3%, P = .02).
Discussion
TDS is an uncommon multi-level spondylolisthesis with unclear sagittal alignment and clinical severity implications. In a retrospective analysis of 49 patients with TDS managed over a 4-year period, we found that increased L3FA, or downward flexion of the L3 vertebral body, was independently associated with elevated SVA. A L3FA cutoff of ≥2.5 was predictive of a SVA ≥5 cm. Patients in the present study with TDS had a mean SVA of 51.3 ± 38.8 mm (range: −12.8-135.8 mm), which is markedly greater than that of 22.0 ± 8.0 mm previously measured in patients with single-level DS. 19 Patients above the L3FA cutoff had an increased PI-LL mismatch, elevated ODI scores and were more likely to fail nonoperative treatment. Findings suggest that the relative flexion of the L3 vertebra in the setting of TDS may be associated with global spinal balance and patient reported outcomes.
Native spinopelvic morphology is thought to play a significant role in dictating mechanical stresses at the lumbo-sacral junction, thereby predisposing certain individuals to the development of DS. 20 It has been previously hypothesized that a higher PI requires increased LL to maintain a neutral sagittal alignment, thereby placing higher forces on the posterior articular joints and excess mechanical stresses on the posterior facets.8,21,22 The resulting accelerated posterior arthritis, in conjunction with increased baseline inclination of the vertebral endplate of L5 due to increased PI, has been postulated to be a significant predisposing factor to vertebral slippage.23,24 All of these parameters have been noted in prior research to be more severely aberrant in patients with TDS compared with single-level DS. 10 Thus, it is reasonable to speculate that TDS may occur due to more severely abnormal native spinopelvic morphology, more severe degenerative facet changes and vertebral slippage and, ultimately, compensatory flattening of LL and elevated SVA. Roussouly et al. 21 classified the normal spine into four morphotypes based on increasing PI and sacral slope (SS). It has been previously speculated that Roussouly morphotype 4, which includes a SS of >45 degrees with hyperlordosis and a high PI, may predispose to posterior arthritis and degenerative spondylolisthesis. 25 Interestingly, the apex of lumbar curvature of Roussouly Type 4 spines has been reported to be most typically centered at L3, which is more proximal than the average apex of lumbar lordosis in the general population. 21 Another recent work concurred with Roussouly regarding the proximal migration of the apex towards L3 in high PI individuals. 26 However, the authors posited that this finding was due to a higher PI requiring the recruitment of more proximal lumbar segments to contribute a large proportion of the lumbar spine’s total lordosis, which ultimately drove the apex proximally. 26 This finding is somewhat in contrast to Roussouly, because it emphasizes the importance of the proximal lordotic segments, rather than the lower lumbar arc, in terms of determining the shape of the global lumbar lordosis.21,26 These works indicate a connection between a high PI and a more proximal lumbar apex, namely at L3. As previously mentioned, a high PI has also been associated in prior work with TDS. It is difficult to draw mechanistic conclusions between increased L3FA and worse clinical outcomes amongst TDS patients noted in the present work. However, it is possible that the loss of the natural L3 apex via increased L3FA (downward flexion) reflects deterioration of a crucial structural element of the lumbar spine, which both drives the apex further proximally and places further lordotic demand on the proximal lumbar spine until it is unable to compensate further. This specific pathologic cascade, detected via increased L3FA, may be linked to deterioration of both the harmonious balance of the lumbar spine and global sagittal balance in patients predisposed to TDS.
The tendency toward poor global spinal balance has been more commonly noted in patients with high-grade DS vs low-grade DS.27,28 Mechanistic factors that have been proposed for the association between higher grade DS and poor global spinal balance include a pathologic cascade that involves more severe anterior vertebral slippage, which leads to flattening of the lumbar spine via decreased LL. 8 Given that PI is an anatomic feature and thus fixed after birth, decreased LL leads to increased PI-LL mismatch and ultimately results in a flexion moment of the lumbar spine and the anterior displacement of SVA.8,29 This emphasis on the important relationship between PI-LL and elevated SVA is consistent with the present work, which found an SVA >5 cm in 54% of TDS patients and increased PI-LL mismatch in patients above the L3FA cutoff. However, it should be noted that an increased SVA may also be representative of the severe degree of stenosis in patients with TDS. Shin et al. reported that patients with increased SVA and PI-LL mismatch in the setting of spinal stenosis often have improved alignment following decompressive surgery because they no longer need to lean forward to unbuckle their ligamentum flavum and decrease their stenosis symptoms. 30 This concept of spinal alignment improving by virtue of decompression alone is well established in the adult spinal deformity literature, in which patients with sagittal malalignment and a flexible spine may achieve improved alignment with a decompression alone rather than a multilevel fusion.31,32 It is therefore possible that the SVA in TDS patients with an elevated L3FA is inflated by the patient’s response to stenosis rather than a purely mechanical problem. This can be better understood by comparing pre- and post-operative imaging, which was not analyzed by the present work.
The preliminary clinical findings of this work suggest that a relatively flexed L3 in patients with TDS is correlated with both elevated SVA and worse ODI scores. This association is not surprising given that prior work has commented on the association between SVA above 4.7 cm and the presence of severe disability as measured by ODI above 40 in the setting of adult spinal deformity. 33 It may be speculated that ODI is a sensitive tool for assessing relative disability within the TDS population and that L3FA may be a primary driver behind poor ODI scores.
Associating TDS with more severe SVA elevation and predicting poor global sagittal balance via L3FA in the setting of TDS is important for a number of reasons. In comparison with single-level DS, TDS may be best seen as existing more commonly in the category of true adult spinal deformity, rather than as a focal, lumbar degenerative spinal pathology. Surgical treatment of TDS is often targeted at restoring LL and sagittal balance, and frequently requires much more extensive instrumentation and more frequent use of osteotomies compared with single-level DS. Surgical intervention for TDS thus incurs risks specific to adult spinal deformity. Elevated SVA is a significant risk factor for proximal junctional kyphosis and proximal junctional failure in adult spinal deformity patients after fusion.34,35 An unrecognized elevated SVA prior to a fusion can lead to a locked position of fused lumbar vertebrae and subsequent fixed forward inclination of the trunk.36-39 This alteration in sagittal alignment parameters and spinopelvic angulation have been shown to be related to significantly worse postoperative back and leg pain, increased rates of radicular symptoms, and a higher rate of adjacent segment degeneration.40-43 L3FA appears to reliably correlate with elevated SVA in the TDS population and can be measured on lumbar films, which avoids the need for 36-inch full length cassette films that are both expensive, and inconsistently available in the community setting.44-46
The retrospective nature of this work creates a limitation in terms of assessing how L3FA and L3SD may change or improve after successful non-operative treatment, because routine follow-up imaging is not routinely obtained in these patients. Future work may prospectively assess how L3FA and L3SD change or improve over time in patients who are successfully treated non-operatively. This work has several limitations beyond those intrinsic to retrospective studies. A critical limitation of this work is its small sample size. Given the relative rarity of TDS in the general population, the number of patients (N = 49) available from a single institution was proportionately similar to that of a prior multicenter (13 institutions) study of TDS patients (n = 78). 10 Additionally, a post-hoc power analysis demonstrated that this study was adequately powered. Another limitation is the lack of full length 36” cassettes for all patients. This is the result of recent increased utilization of full length imaging as routine standard of practice at our institution due to the availability of full length imaging (EOS). More consistent imaging availability would be preferred in future work. Additional future work may include in vivo biomechanical studies to establish a causal link between L3 deformity and global sagittal malalignment. We also seek to understand the utility of L3FA in patients with single-level spondylolisthesis, as the present work sought to initially evaluate this metric with patients with TDS alone due to the relative severity of this group’s pathology. Finally, it is important to note that we are only discussing patients with TDS who have symptomatic spinal stenosis. These were only TDS patients whose pathology was severe enough to warrant surgery. This highlights that we are not describing TDS as a singular pathology, but only TDS within the surgical stenosis population. Describing TDS more fully would likely require a large multi-institutional prospective study.
In conclusion, L3FA ≥2.5 in patients with TDS can serve as a surrogate for SVA ≥5.0 cm and is predictive of poor patient reported outcome scores and the failure of non-operative management. L3FA may be a rapid way to evaluate the clinical impact of TDS on these potentially vulnerable patients as well as a target for surgical correction in the future.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
IRB Approval: The manuscript submitted does not contain information about medical device(s)/drug(s).
ORCID iDs
Jonathan F. Dalton https://orcid.org/0000-0002-7452-2712
Mitchell S. Fourman https://orcid.org/0000-0001-5886-546X
Landon Cluts https://orcid.org/0000-0001-6042-4380
References
- 1.Iguchi T, Wakami T, Kurihara A, Kasahara K, Yoshiya S, Nishida K. Lumbar multilevel degenerative spondylolisthesis: radiological evaluation and factors related to anterolisthesis and retrolisthesis. J Spinal Disord Tech. 2002;15:93-99. doi: 10.1097/00024720-200204000-00001 [DOI] [PubMed] [Google Scholar]
- 2.Wang YXJ, Káplár Z, Deng M, Leung JCS. Lumbar degenerative spondylolisthesis epidemiology: A systematic review with a focus on gender-specific and age-specific prevalence. J Orthop Translat. 2017;11:39-52. doi: 10.1016/j.jot.2016.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park DK, An HS, Lurie JD, et al. Does multilevel lumbar stenosis lead to poorer outcomes?: a subanalysis of the Spine Patient Outcomes Research Trial (SPORT) lumbar stenosis study. Spine 1976) 35, 439-446, doi: 10.1097/BRS.0b013e3181bdafb9 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenberg NJ. Degenerative spondylolisthesis. Predisposing factors. J Bone Joint Surg Am. 1975;57:467-474. [PubMed] [Google Scholar]
- 5.Ferrero E, Ould-Slimane M, Gille O, Guigui P, French Spine Society SFCR . Sagittal spinopelvic alignment in 654 degenerative spondylolisthesis. Eur Spine J. 2015;24:1219-1227. doi: 10.1007/s00586-015-3778-4 [DOI] [PubMed] [Google Scholar]
- 6.Fitzgerald JA, Newman PH. Degenerative spondylolisthesis. J Bone Joint Surg Br. 1976;58:184-192. doi: 10.1302/0301-620x.58b2.932080 [DOI] [PubMed] [Google Scholar]
- 7.Morel E, Ilharreborde B, Lenoir T, et al. [Sagittal balance of the spine and degenerative spondylolisthesis]. Revue de Chirurgie Orthopedique et Reparatrice de l'Appareil Moteur. 2005;91:615-626. doi: 10.1016/s0035-1040(05)84465-4 [DOI] [PubMed] [Google Scholar]
- 8.Barrey C, Jund J, Noseda O, Roussouly P. Sagittal balance of the pelvis-spine complex and lumbar degenerative diseases. A comparative study about 85 cases. Eur Spine J. 2007;16:1459-1467. doi: 10.1007/s00586-006-0294-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Funao H, Tsuji T, Hosogane N, et al. Comparative study of spinopelvic sagittal alignment between patients with and without degenerative spondylolisthesis. Eur Spine J. 2012;21:2181-2187. doi: 10.1007/s00586-012-2374-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferrero E, Simon AL, Magrino B, Ould-Slimane M, Guigui P. Double-level degenerative spondylolisthesis: What is different in the sagittal plane? Eur Spine J. 2016;25:2546-2552. doi: 10.1007/s00586-016-4384-9 [DOI] [PubMed] [Google Scholar]
- 11.Chung NS, Jeon CH, Lee HD, Won SH. Measurement of Spinopelvic Parameters on Standing Lateral Lumbar Radiographs: Validity and Reliability. Clin Spine Surg. 2017;30:E119-e123. doi: 10.1097/bsd.0000000000000448 [DOI] [PubMed] [Google Scholar]
- 12.Koslosky E, Gendelberg D. Classification in Brief: The Meyerding Classification System of Spondylolisthesis. Clin Orthop Relat Res. 2020;478:1125-1130. doi: 10.1097/corr.0000000000001153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang GZ, Deng YJ, He XG, et al. Different Types of Double-Level Degenerative Lumber Spondylolisthesis: What Is Different in the Sagittal Plane? World Neurosurg. 2021;150:e127-e134. doi: 10.1016/j.wneu.2021.02.125 [DOI] [PubMed] [Google Scholar]
- 14.Zhang S, Ye C, Lai Q, et al. Double-level lumbar spondylolysis and spondylolisthesis: A retrospective study. J Orthop Surg Res. 2018;13:55. doi: 10.1186/s13018-018-0723-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koo TK, Li MY. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J Chiropr Med. 2016;15:155-163. doi: 10.1016/j.jcm.2016.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fourman MS, Shaw JD, Nwasike CO, et al. Use of Fondaparinux Following Elective Lumbar Spine Surgery Is Associated With a Reduction in Symptomatic Venous Thromboembolism. Global Spine J. 2020;10:844-850. doi: 10.1177/2192568219878418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sinharay S, Stern HS, Russell D. The use of multiple imputation for the analysis of missing data. Psychol Methods. 2001;6:317-329. [PubMed] [Google Scholar]
- 18.Vedantam R, Lenke LG, Keeney JA, Bridwell KH. Comparison of standing sagittal spinal alignment in asymptomatic adolescents and adults. Spine 1976) 23, 211-215, doi: 10.1097/00007632-199801150-00012 (1998). [DOI] [PubMed] [Google Scholar]
- 19.Wang T, Wang H, Liu H, Ma L, Liu FY, Ding WY. Sagittal spinopelvic parameters in 2-level lumbar degenerative spondylolisthesis: A retrospective study. Medicine (Baltimore). 2016;95:e5417. doi: 10.1097/md.0000000000005417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Labelle H, Roussouly P, Chopin D, Berthonnaud E, Hresko T, O'Brien M. Spino-pelvic alignment after surgical correction for developmental spondylolisthesis. Eur Spine J. 2008;17:1170-1176. doi: 10.1007/s00586-008-0713-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roussouly P, Gollogly S, Berthonnaud E, Dimnet J. Classification of the normal variation in the sagittal alignment of the human lumbar spine and pelvis in the standing position. Spine 1976) 30, 346-353, doi: 10.1097/01.brs.0000152379.54463.65 (2005). [DOI] [PubMed] [Google Scholar]
- 22.Labelle H, Roussouly P, Berthonnaud E, et al. Spondylolisthesis, pelvic incidence, and spinopelvic balance: a correlation study. Spine 2004;29:2049-2054, doi: 10.1097/01.brs.0000138279.53439.cc [DOI] [PubMed] [Google Scholar]
- 23.Berlemann U, Jeszenszky DJ, Bühler DW, Harms J. Facet joint remodeling in degenerative spondylolisthesis: An investigation of joint orientation and tropism. Eur Spine J. 1998;7:376-380. doi: 10.1007/s005860050093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsunaga S, Sakou T, Morizono Y, Masuda A, Demirtas AM. Natural history of degenerative spondylolisthesis. Pathogenesis and natural course of the slippage. Spine 1976) 15, 1204-1210, doi: 10.1097/00007632-199011010-00021 (1990). [DOI] [PubMed] [Google Scholar]
- 25.Roussouly P, Pinheiro-Franco JL. Biomechanical analysis of the spino-pelvic organization and adaptation in pathology. Eur Spine J. 2011;20(Suppl 5):609-618. doi: 10.1007/s00586-011-1928-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pesenti S, Lafage R, Stein D, et al. The Amount of Proximal Lumbar Lordosis Is Related to Pelvic Incidence. Clin Orthop Relat Res. 2018;476:1603-1611. doi: 10.1097/corr.0000000000000380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hresko MT, Labelle H, Roussouly P, Berthonnaud E. Classification of high-grade spondylolistheses based on pelvic version and spine balance: possible rationale for reduction. Spine 2007;32:2208-2213, doi: 10.1097/BRS.0b013e31814b2cee [DOI] [PubMed] [Google Scholar]
- 28.Mac-Thiong JM, Wang Z, de Guise JA, Labelle H. Postural model of sagittal spino-pelvic alignment and its relevance for lumbosacral developmental spondylolisthesis. Spine 2008;33:2316-2325, doi: 10.1097/BRS.0b013e318186b236 [DOI] [PubMed] [Google Scholar]
- 29.Le Huec JC, Aunoble S, Philippe L, Nicolas P. Pelvic parameters: origin and significance. Eur Spine J. 2011;20(Suppl 5):564-571. doi: 10.1007/s00586-011-1940-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shin EK, Kim CH, Chung CK, et al. Sagittal imbalance in patients with lumbar spinal stenosis and outcomes after simple decompression surgery. Spine J. 2017;17:175-182. doi: 10.1016/j.spinee.2016.08.023 [DOI] [PubMed] [Google Scholar]
- 31.Fourman MS, Yates AJ, Kim HJ. Clinical faceoff: Hip osteoarthritis in the setting of adult spinal deformity. Clin Orthop Relat Res. 2023;481:32-38. doi: 10.1097/corr.0000000000002485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hikata T, Watanabe K, Fujita N, et al. Impact of sagittal spinopelvic alignment on clinical outcomes after decompression surgery for lumbar spinal canal stenosis without coronal imbalance. J Neurosurg Spine. 2015;23:451-458. doi: 10.3171/2015.1.Spine14642 [DOI] [PubMed] [Google Scholar]
- 33.Schwab FJ, Blondel B, Bess S, et al. Radiographical spinopelvic parameters and disability in the setting of adult spinal deformity: A prospective multicenter analysis. Spine 2013;38:E803-E812, doi: 10.1097/BRS.0b013e318292b7b9 [DOI] [PubMed] [Google Scholar]
- 34.Zou L, Liu J, Lu H. Characteristics and risk factors for proximal junctional kyphosis in adult spinal deformity after correction surgery: a systematic review and meta-analysis. Neurosurg Rev. 2019;42:671-682. doi: 10.1007/s10143-018-1004-7 [DOI] [PubMed] [Google Scholar]
- 35.Park SJ, Lee CS, Chung SS, Lee JY, Kang SS, Park SH. Different risk factors of proximal junctional kyphosis and proximal junctional failure following long instrumented fusion to the sacrum for adult spinal deformity: survivorship analysis of 160 patients. Neurosurgery. 2017;80:279-286. doi: 10.1227/neu.0000000000001240 [DOI] [PubMed] [Google Scholar]
- 36.Gödde S, Fritsch E, Dienst M, Kohn D. Influence of cage geometry on sagittal alignment in instrumented posterior lumbar interbody fusion. Spine 1976) 28, 1693-1699, doi: 10.1097/01.Brs.0000083167.78853.D5 (2003). [DOI] [PubMed] [Google Scholar]
- 37.Goldstein JA, Macenski MJ, Griffith SL, McAfee PC. Lumbar sagittal alignment after fusion with a threaded interbody cage. Spine 2001;26:1137-1142, doi: 10.1097/00007632-200105150-00009 [DOI] [PubMed] [Google Scholar]
- 38.Stephens GC, Yoo JU, Wilbur G. Comparison of lumbar sagittal alignment produced by different operative positions. Spine 1996;21:1802-1806, doi: 10.1097/00007632-199608010-00016. [DOI] [PubMed] [Google Scholar]
- 39.Tribus CB, Belanger TA, Zdeblick TA. The effect of operative position and short-segment fusion on maintenance of sagittal alignment of the lumbar spine. Spine 1999;24, 58-61, doi: 10.1097/00007632-199901010-00014 [DOI] [PubMed] [Google Scholar]
- 40.Lazennec JY, Ramare S, Arafati N, et al. Sagittal alignment in lumbosacral fusion: relations between radiological parameters and pain. Eur Spine J. 2000;9:47-55. doi: 10.1007/s005860050008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kumar MN, Baklanov A, Chopin D. Correlation between sagittal plane changes and adjacent segment degeneration following lumbar spine fusion. Eur Spine J. 2001;10:314-319. doi: 10.1007/s005860000239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Le Huec JC, Faundez A, Dominguez D, Hoffmeyer P, Aunoble S. Evidence showing the relationship between sagittal balance and clinical outcomes in surgical treatment of degenerative spinal diseases: a literature review. Int Orthop. 2015;39:87-95. doi: 10.1007/s00264-014-2516-6 [DOI] [PubMed] [Google Scholar]
- 43.Aoki Y, Nakajima A, Takahashi H, et al. Influence of pelvic incidence-lumbar lordosis mismatch on surgical outcomes of short-segment transforaminal lumbar interbody fusion. BMC Musculoskelet Disord. 2015;16:213. doi: 10.1186/s12891-015-0676-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Parks RD, Menger RP, Zhang A, Sin A. Value Proposition to a Hospital of Obtaining 36-inch Standing Scoliosis Film Technology. Cureus. 2018;10:e3044. doi: 10.7759/cureus.3044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Angevine PD, Kaiser MG. Radiographic measurement techniques. Neurosurgery. 2008;63:40-45. doi: 10.1227/01.Neu.0000320425.55569.21 [DOI] [PubMed] [Google Scholar]
- 46.Bess S, Protopsaltis TS, Lafage V, et al. Clinical and Radiographic Evaluation of Adult Spinal Deformity. Clin Spine Surg. 2016;29:6-16. doi: 10.1097/bsd.0000000000000352 [DOI] [PubMed] [Google Scholar]