Abstract
Background:
Acute kidney injury (AKI) is prevalent in patients with acute stroke. Although AKI is linked to poor clinical outcomes, data about its incidence and effect on stroke outcomes is limited.
Methods:
This was a prospective observational study carried out at a single tertiary care center that analyzed the data of 204 consecutive subjects with acute ischemic stroke and intracerebral hemorrhage. Considering serum creatinine at admission as the baseline, AKI was defined as a rise in serum creatinine value of 0.3 mg/dl over 48 h or a percentage increase of at least 50% from baseline over 7 days during hospitalization. The primary outcome was to measure the prevalence of AKI in patients with acute stroke. Secondary outcome measures were all-cause mortality, duration of hospital stay, need for dialysis, and comparison of outcomes in ischemic and hemorrhagic stroke. For both the stroke subtypes, we employed a multivariate logistic regression model, with AKI and hospital mortality being the outcomes. Covariates included gender, age, ventilatory requirement, duration of hospital stay, and National Institutes of Health Stroke Scale score at admission.
Results:
There were 144 cases of ischemic stroke with 12 deaths (8.3%) and 60 cases of intracranial hemorrhage (ICH) with 22 deaths (36.7%). The mean age was 55 years, 72.6% were males, and AKI complicated 34% of ischemic stroke and 66.7% of ICH hospitalizations. AKI was linked to increased hospital mortality from ischemic stroke (odds ratio [OR] 27.21, 95% CI 3.39–218.13) and hemorrhagic stroke (OR 5.12, 95% CI 1.29–20.28) in multivariate analysis stratified by stroke type.
Conclusions:
AKI complicates stroke frequently and increases hospital mortality. Additional studies are required to assess if the association is causal and if remedies to prevent AKI would decrease mortality.
Keywords: Acute kidney injury, intracerebral hemorrhage, ischemic stroke, kidney disease
INTRODUCTION
Stroke is the second leading cause of death and a leading cause of disability.[1,2,3] The frequency of stroke due to ischemia is 68%, and the frequency of hemorrhagic stroke is 32%.[1,2] The overall rate of stroke-related mortality is decreasing in high- and low-income countries. However, the number of people with stroke, stroke survivors, and the global burden of stroke-related disability is increasing.[4] The cumulative incidence of stroke in India is 105–152/100,000 persons per year.[5] Acute kidney injury (AKI) is prevalent in hospitalized individuals, particularly critically ill patients with predisposing medical conditions.[6,7] AKI is common in patients with acute stroke, and though it is associated with poor clinical outcomes, the data is sparse.[8,9] Suboptimal fluid intake, sepsis, drugs, sympathetic nervous system overactivation, and contrast-mediated diagnostic studies contribute to AKI in vulnerable patients.[10] AKI adversely affects the prognosis in stroke patients, independent of the clinical setting.[8,11] The severity of renal dysfunction in stroke patients may be a marker of end organ damage due to atherosclerosis and its associated vascular risk factors (aging, smoking, hypertension, diabetes mellitus, and cardiovascular diseases).[6,12] The exact mechanism underlying AKI with stroke is unknown and has to be explored.[12] There is growing evidence of the role of cardiovascular and cerebrovascular diseases in renal dysfunction, given the similarities between the vascular beds of the kidney, heart, and brain.[13,14,15] The association between two disease states could be due to a chance association, a bidirectional causal association,[16] a shared risk factor for the two disease states, or a standard biology.[17] Brain injury can affect the kidney function by several mechanisms such as neuroinflammation, increased neurosympathetic activity, and through the hypothalamic–pituitary axis. Cells of the central nervous system (CNS) are an abundant source of inflammatory mediators, and CNS expression of proinflammatory cytokines and complement components leads to the recruitment of neutrophils and monocytes (macrophages) across the blood–brain barrier and enhancement of the established neuroinflammation.[16,18] Neuroendocrine pathways, such as the hypothalamic–pituitary–adrenal axis and the autonomic nervous system, also mediate the systemic effects of neuroinflammation.[19,20,21] Recent studies have shown that AKI is an independent predictor of short-term mortality after acute stroke.[8,21,22,23] Serum creatinine (SCr) is an independent predictor of mortality even after adjustment among stroke survivors.[24,25,26] The estimated overall incidence of AKI in stroke in various studies is 4.63%. The incidence of AKI is higher in hemorrhagic stroke (19.17%) than in ischemic stroke (9.62%).[10,25] Studies reported that AKI was associated with an increased hospital stay ranging from 2 to 18 extra days, which causes a huge financial burden to the patient and the state.[22,26,27] Thus, the present study aims to assess the prevalence of AKI in patients with acute stroke, determine the risk factors for AKI in acute stroke patients, and compare outcomes in acute stroke patients with and without AKI.
MATERIALS AND METHODS
Study population
This prospective observational study was conducted on 204 adult patients with acute stroke reporting to the hospital at a single tertiary care center for 16 months. We conducted the study as per the Declaration of Helsinki and the principles of Good Clinical Practice, and the Institutional Ethics Committee approved the study protocol. We took written informed consent from participants or their guardians. We included all consecutive acute stroke patients over 18 years of age presenting within 48 h of symptom onset. We excluded patients with baseline chronic kidney disease (CKD), cerebral venous thrombosis (CVT), known malignancy, and who were on nephrotoxic medication for other illnesses.
Definition of AKI
In 2012, AKI was defined according to the Kidney Disease Improving Global Outcomes as follows: increase in SCr level by ≥0.3 mg/dl (≥26.5 µmol/l) within 48 h or rise in SCr level to ≥1.5 times of the baseline, which is known or presumed to occur within the previous 7 days, or decrease in urine volume to <0.5 ml/kg/h for 6 h.[28,29] The acute insult and background morbidity contribute to the risk of AKI. Acute insult may be because of various causes such as sepsis, hypovolemia, drug toxicity, obstruction, or parenchymal kidney disease. Background morbidities in the form of old age, CKD, cardiac failure, liver failure, diabetes mellitus, vascular disease, and nephrotoxic medication also contribute to insult. In Acute Kidney Injury Network (AKIN) stage 1, risk increase in SCr ≥1.5 × baseline or decrease in glomerular filtration rate (GFR) ≥ 25% Urine Output (UO) <0.5 ml/kg/h × 6 h plus increase in SCr ≥0.3 mg/dl (≥26.4 µmol/l) within 48 h. In AKIN stage 2, increase in SCr ≥2.0 × baseline or decrease in GFR ≥50% UO <0.5 ml/kg/h × 12 h. In AKIN stage 3, failure increase in SCr ≥3.0 × baseline or SCr ≥4.0 mg/dl (354 µmol/l) or decrease in GFR ≥ 75% UO < 0.3 ml/kg/h × 24 h or anuria × 12 h plus initiation of Renal replacement therapy (RRT).[28]
Data acquisition and definitions
The primary outcome was to ascertain the prevalence of AKI in patients with acute stroke within 48 h of symptom onset. Secondary outcome measures were all-cause mortality, duration of hospital stay, need for dialysis, and comparison of outcomes between ischemic and hemorrhagic strokes. We evaluated the risk factors causing AKI and compared outcomes in patients with and without AKI. We explained the study’s purpose and obtained informed consent from the patients or caregivers. At the time of presentation, we performed the National Institutes of Health Stroke Scale (NIHSS), modified Rankin Scale, a noncontrast computed tomography of the head, and, if required, computed tomography (CT) angiography of the brain and neck vessels for all patients. We treated all the patients with the standard of care. We did interventions such as thrombolysis, mechanical thrombectomy, or decompressive hemicraniectomy for patients with ischemic stroke and hematoma evacuation, extraventricular drain, or decompressive craniectomy in cases of hemorrhagic stroke or conservative treatment as per the latest guidelines. We did baseline investigations, including complete hemogram, renal function tests, liver function tests, and urinalysis for all patients. Clinical variables, including age, gender, stroke subtype, stroke severity (NIHSS at admission), Glasgow Coma Scale (GCS), diabetes mellitus, hypertension, and use of CT angiography, were recorded. We followed the patients during their hospital stay and recorded their daily parameters, including blood parameters, temperature, and input/output records. We divided the patients into two groups, with AKI and without AKI, and outcomes were measured between the two groups. We made further categorizations into ischemic and hemorrhagic stroke, and the outcomes between the two groups were measured.
Statistical analysis
We analyzed the study data using Statistical Package for the Social Sciences software version 14. Data was expressed as mean ± standard deviation or number and percentage as appropriate for quantitative and qualitative variables. We tested the data for normality by the Kolmogorov–Smirnov test. We used the Student’s t-test to compare the parametric values, whereas the Mann–Whitney U test was used to compare nonparametric values. We used the Chi-square/Fisher’s exact test to compare categorical data. Multivariable regression analysis was performed using logistic regression. A stepwise approach was used to estimate the risk and relative 95% confidence interval for each covariate. A P-value <0.05 was considered statistically significant.
RESULTS
Our study included 204 consecutive stroke patients (144 ischemic and 60 hemorrhagic). Baseline characteristics are shown in Table 1 and Supplementary Table 1. Of the subjects, 72.6% were males, and the male-to-female ratio was 2.64:1. Median systolic blood pressure and diastolic blood pressure were higher in hemorrhagic stroke patients (P = 0.001). Hypertension and heart disease were more common in the ischemic group (P = 0.04 and 0.001, respectively). The median GCS at presentation was lower, and more patients required tracheostomy and ventilators in the hemorrhagic group (P = 0.001 for each). Patients in the hemorrhagic group presented late (11.5 vs. 6 h, P = 0.001), had higher median NIHSS scores at presentation (22 vs. 9, P = 0.001), and had a more extended hospital stay (mean stay 12 vs. 6 days, P = 0.001). In baseline investigations, the hemorrhagic group had higher TLC and blood urea levels (P = 0.001 for each). In addition, 66.7% of patients had AKI in the hemorrhagic group compared to 34% in the ischemic group, and the difference was significant (P = 0.001).
Table 1.
Baseline characteristics of ischemic and hemorrhagic stroke patients
| Characteristics | Ischemic (n=144) | Hemorrhagic (n=60) | P |
|---|---|---|---|
| Age, mean±SD | 54.74±14.67 | 57.4±12.72 | 0.225 |
| Males, n (%) | 104 (72.2) | 44 (73.3) | 0.871 |
| eGFR (ml/min/1.73 m2), mean±SD | 98.24±16.70 | 93.32±16.48 | 0.799 |
| BP (mmHg), median (IQR) | |||
| SBP | 144 (140.4-159.0) | 180 (165.4-181.9) | 0.001 |
| DBP | 84 (81.9-92.8) | 100 (96.8-105.1) | 0.001 |
| Risk factors prevalence, n (%) | |||
| Hypertension | 76 (52.8) | 41 (68.3) | 0.04 |
| Diabetes mellitus | 43 (29.9) | 11 (18.3) | 0.089 |
| Smoking | 41 (28.5) | 14 (23.3) | 0.451 |
| Alcohol intake | 23 (16) | 9 (15.3) | 0.899 |
| Dyslipidemia | 11 (7.6) | 3 (5) | 0.762 |
| Heart disease | 41 (28.5) | 2 (3.3) | 0.001 |
| Previous stroke | 11 (7.6) | 3 (5) | 0.367 |
| Family history | 2 (1.4) | 3 (5) | 0.153 |
| Use of antiplatelets | 17 (11.8) | 5 (8.3) | 0.466 |
| Contrast used | 136 (94.4) | 5 (8.3) | 0.001 |
| Ventilatory requirement | 22 (15.3) | 41 (68.3) | 0.001 |
| Tracheostomy | 10 (6.9) | 24 (40) | 0.001 |
| GCS at presentation, median (IQR) | 15 (10.2-15.0) | 9.5 (8.6-12.0) | 0.001 |
| GCS <8, n (%) | 9 (27.3) | 24 (72.7) | 0.001 |
| GCS 9-15, n (%) | 135 (78.9) | 36 (29.4) | |
| eGFR (ml/min/1.73 m2) | |||
| G2, n (%) | 45 (65.2) | 24 (34.8) | 0.229 |
| G1, n (%) | 99 (73.3) | 36 (26.7) | |
| NIHSS, median (IQR) | 9 (7.2-10.5) | 18 (13.2-19.1) | 0.001 |
| AKI, n=89, n (%) | 49 (34.02) | 40 (66.7) | 0.001 |
| Time frames, median (IQR) | |||
| Onset to hospitalization (hours) | 6 (5.6-9.0) | 11.5 (10.5-15.4) | 0.001 |
| Hospital stay (days) | 6 (4.4-10.6) | 12 (11.3-17.3) | 0.001 |
| Duration to death (days) | 6 (3.5-9.6) | 12.5 (10.9-16.8) | 0.045 |
| Duration to AKI (days) | 1.5 (1.1-3.2) | 2 (1.3-3.5) | 0.918 |
| Discharge disposition, n (%) | |||
| Favorable (n=56) | 53 (36.8) | 3 (5) | 0.001 |
| Unfavorable (n=148) | 91 (63.2) | 57 (95) | |
| Baseline investigations, mean±SD | |||
| Hemoglobin (g/dl) | 13.24±2.36 | 12.59±2.25 | 0.363 |
| TLC (/microliter) | 9522.43±3191.65 | 12484.67±4641.46 | 0.001 |
| Platelet count (lakhs/mm3) | 2.05±0.79 | 1.71±0.68 | 0.957 |
| Creatinine (mg/dl) | 0.79±0.17 | 0.82±0.19 | 0.186 |
| Urea (mg/dl) | 30.18±13.63 | 45.03±22.67 | 0.001 |
| HbA1c | 6.68±2.13 | 5.85±1.19 | 0.055 |
| Sodium (mEq/l) | 137.62±3.94 | 140.45±6.08 | 0.098 |
| Potassium (mEq/l) | 4.18±0.55 | 4.00±0.67 | 0.328 |
| Serum calcium (mg/dl) | 8.82±0.74 | 8.57±0.73 | 0.029 |
| Serum protein (g/dl) | 6.85±0.83 | 6.82±1.03 | 0.085 |
| SGOT (U/l) | 38.47±28.67 | 72.53±185.90 | 0.053 |
| SGPT (U/l) | 33.75±33.14 | 49.43±157.09 | 0.584 |
| Total bilirubin | 0.87±0.42 | 0.89±0.53 | 0.908 |
| Total cholesterol (mg/dl) | 167.26±44.39 | 167.15±48.67 | 0.987 |
| LDL (mg/dl) | 111.21±35.69 | 106.25±41.27 | 0.38 |
AKI=Acute kidney injury, BP=Blood pressure, DBP=Diastolic blood pressure, eGFR=Estimated glomerular filtration rate, GCS=Glasgow Coma Scale, GFR=Glomerular filtration rate, G1=GFR 90 ml/min/1.73 m2 and above, G2=GFR 60-89 ml/min per 1.73 m2, HbA1c=Glycated hemoglobin, LDL=Low-density lipoprotein, NIHSS=National Institutes of Health Stroke Scale, SBP=systolic blood pressure, SD=Standard deviation, SGOT=Serum glutamic oxaloacetic transaminase, SGPT=Serum glutamic pyruvic transaminase, TLC=Total leukocyte count
Supplementary Table 1.
Baseline demographic characteristics of the overall stroke cohort
| Category | Characteristic | Value |
|---|---|---|
| Demographic characteristics | Age in years, mean (SD) | 55.52 (14.15) |
| Sex: male/female, n (%) | 148 (72.6)/56 (27.4) | |
| Time delay in presentation, median (IQR) | 7 (13) | |
| Median NIHSS (IQR) | 10 (13) | |
| Duration of stay in days, median (IQR) | 6 (7) | |
| Type of stroke: ischemic/hemorrhagic, n (%) | 144 (70.6)/60 (29.4) | |
| Vitals | SBP, mean (SD) | 160 (67) |
| DBP, mean (SD) | 100 (18) | |
| GCS, median (IQR) | 15 (5) | |
| Laboratory parameters | eGFR, mean (SD) | 96.79 (16.75) |
| Urea, mean (SD) | 34.55 (18.06) | |
| Creatinine, mean (SD) | 0.80 (0.18) | |
| Hemoglobin, mean (SD) | 13.05 (2.34) | |
| TLC, mean (SD) | 10,393.68 (3907.41) | |
| Platelet count, mean (SD) | 1.95 (0.78) | |
| Sodium, mean (SD) | 138.45 (4.83) | |
| Potassium, mean (SD) | 4.12 (0.59) | |
| HbA1c, mean (SD) | 6.42 (1.93) | |
| TSH, mean (SD) | 2.72 (4.46) | |
| SGOT, mean (SD) | 48.49 (104.23) | |
| SGPT, mean (SD) | 38.36 (89.42) | |
| Total cholesterol, mean (SD) | 167.22 (45.56) | |
| LDL, mean (SD) | 109.75 (37.38) | |
| HDL, mean (SD) | 39.29 (10.78) | |
| VLDL, mean (SD) | 18.60 (8.11) | |
| In-hospital management | Intervention (yes), n (%) | 83 (40.7) |
| Intravenous thrombolysis, n (%) | 26 (12.7) | |
| Intravenous thrombolysis and mechanical thrombectomy, n (%) | 6 (2.9) | |
| EVD, n (%) | 15 (7.4) | |
| Decompressive hemicraniectomy, n (%) | 11 (5.39) | |
| Hematoma evacuation, n (%) | 11 (5.39) | |
| Decompressive hemicraniectomy and hematoma evacuation, n (%) | 3 (1.47) | |
| EVD and decompressive hemicraniectomy, n (%) | 1 (0.49) | |
| Mechanical thrombectomy, n (%) | 7 (3.4) | |
| Mechanical thrombectomy and decompressive hemicraniectomy, n (%) | 1 (0.49) | |
| Carotid artery stenting, n (%) | 2 (0.98) |
DBP=Diastolic blood pressure, eGFR=estimated glomerular filtration rate, EVD=External ventricular drain, GCS=Glasgow Coma Scale, IQR=Interquartile range, LDL=Low-density lipoprotein, NIHSS=National Institutes of Health Stroke Scale, SBP=Systolic blood pressure, SD=Standard deviation, SGOT=Serum glutamic oxaloacetic transaminase, SGPT=Serum glutamic pyruvic transaminase, TLC=Total leukocyte count, TSH=Thyroid stimulating hormone, VLDL=Very low-density lipoprotein, HbA1c = Glycated hemoglobin, HDL = High density lipoprotein
On comparing between the AKI and non-AKI cohorts, median GCS was found to be lower in the AKI cohort (P = 0.001) [see Table 2]. In the AKI group, the number of patients requiring tracheostomy and ventilatory support was higher (P = 0.001 each) and contrast use was more common (P = 0.004). Median hospital stay was 9 days in the AKI group and 6 days in the non-AKI group (P = 0.001). Upon comparing the baseline investigations, the AKI group was found to have raised TLC and decreased platelets (P = 0.002 and 0.004, respectively). This can be attributed to sepsis, which may lead to AKI, and both AKI and sepsis can cause thrombocytopenia. Six out of 89 patients with AKI (6.74%) required dialysis, and all of them died. Thirty-four patients died, of whom six (17.67%) were related to AKI (those requiring dialysis). Moreover, 44% of patients who died were in AKIN stage 3. A comparison of characteristics between ischemic and hemorrhagic stroke patients after stratification into AKI and non-AKI subgroups is shown in Table 3. A comparison of characteristics between AKI and non-AKI subgroups of patients according to the subtyping of stroke is shown in Supplementary Table 2. Thirty-four patients died (mortality rate 16.7%), and the difference in the mortality rate of ischemic stroke (8.3%) and hemorrhagic stroke (36.7%) patients was statistically significant. Mortality was more common in hemorrhagic (47.5%) stroke patients with AKI compared to ischemic stroke patients with AKI (22.4%). Twelve patients in the ischemic group and 22 in the hemorrhagic group died (P = 0.001), and greater severity of AKI was associated with more significant crude mortality (P = 0.001) for both cohorts.
Table 2.
Comparison of baseline characteristics between AKI and non-AKI subgroups of patients
| Baseline characteristics | AKI (n=89) | No AKI (n=115) | P |
|---|---|---|---|
| Age, mean (in years) ± SD | 55.08±14.07 | 56.09±14.30 | 0.616 |
| Males, n (%) | 67 (75.3) | 81 (70.4) | 0.442 |
| BP (mmHg), median (IQR) | |||
| SBP | 158 (148.4-168.3) | 150 (148.6-160.5) | 0.094 |
| DBP | 92 (83.5-92.4) | 90 (83.5-92.4) | 0.848 |
| GCS at presentation, median (IQR) | 11 (9.2-12.1) | 15 (11.2-15) | 0.001 |
| GCS <8 n (%) | 25 (75.8) | 8 (24.2) | 0.001 |
| GCS 9-15 n (%) | 64 (37.4) | 107 (62.6) | |
| NIHSS, median (IQR) | 13 (9.5-13.8) | 9 (8.4 - 12.3) | 1.029 |
| Risk factors prevalence, n(%) | |||
| Hypertension | 51 (57.3) | 66 (57.4) | 0.990 |
| Diabetes mellitus | 23 (25.8) | 31 (27) | 0.858 |
| Smoking | 22 (24.7) | 33 (28.7) | 0.526 |
| Alcohol intake | 10 (11.4) | 22 (19.1) | 0.174 |
| Dyslipidemia | 7 (7.9) | 7 (6.1) | 0.618 |
| Heart disease | 13 (14.6) | 30 (26.1) | 0.046 |
| Previous stroke | 6 (6.7) | 8 (7) | 0.952 |
| Family history | 3 (3.4) | 2 (1.7) | 0.381 |
| Use of antiplatelets | 4 (4.5) | 18 (15.7) | 0.012 |
| Factors in hospital, n(%) | |||
| Contrast used | 89 (77.4) | 52 (58.4) | 0.004 |
| Ventilatory requirement | 48 (53.9) | 15 (13) | 0.001 |
| Tracheostomized | 24 (34) | 10 (8.7) | 0.001 |
| eGFR G2 | 35 (39.33) | 38 (33.04) | 0.245 |
| eGFR G1 | 54 (60.67) | 77 (66.96) | |
| eGFR (ml/min/1.73 m2), mean±SD | 94.52±16.79 | 98.55±16.58 | 0.08 |
| Time frames, median (IQR) | |||
| Onset to hospitalization (hours) | 8 (6.5-12.6) | 7 (5.5-13.6) | 0.676 |
| Hospital stay (days) | 9 (6.5-12.3) | 6 (4.5-8.0) | 0.001 |
| Duration to death (days) | 10 (8.1-11.3) | 9 (7.8-11.4) | 0.699 |
| Duration to AKI (days) | 2 (1.3-3.5) | - | - |
| Comparison of baseline investigations between AKI and non-AKI subgroups of patients (Mean±SD) | |||
| Hemoglobin (g/dl) | 12.89±2.37 | 13.18±2.33 | 0.39 |
| TLC (/microliter) | 11.35±4.14 | 9.65±3.29 | 0.0019 |
| Platelet count (in lakhs/microliter) | 1.77±0.6 | 2.08±0.87 | 0.0044 |
| S. creatinine (mg/dl) | 0.83±0.19 | 0.77±0.16 | 0.02 |
| Blood urea (mg/dl) | 29.06±12.10 | 41.64±21.73 | 0.001 |
| HbA1c | 6.35+2.02 | 6.48+1.88 | 0.62 |
| Sodium (mEq/l) | 137.51±4.28 | 139.67±5.23 | 0.123 |
| Potassium (mEq/l) | 4.14±0.55 | 4.11±0.63 | 0.72 |
| Serum calcium (mg/dl) | 8.92±0.65 | 8.52±0.80 | 0.343 |
| Serum protein (g/dl) | 7.00±0.81 | 6.63±0.93 | 0.08 |
| SGOT (U/l) | 37.73±25.02 | 62.39±154.61 | 0.09 |
| SGPT (U/l) | 31.4±29.41 | 47.35±131.08 | 0.207 |
| Total bilirubin | 0.88±0.39 | 0.87±0.53 | 0.904 |
| Total cholesterol (mg/dl) | 165.51±43.38 | 169.43±48.39 | 0.54 |
| HDL (mg/dl) | 38.63±10.71 | 40.14±10.86 | 0.32 |
| LDL (mg/dl) | 109.20±35.12 | 110.44±40.30 | 0.81 |
AKI=Acute kidney injury, BP=Blood pressure, DBP=Diastolic blood pressure, eGFR=Estimated glomerular filtration rate, GCS=Glasgow Coma Scale, GFR=Glomerular filtration rate, G1=GFR 90 ml/min/1.73 m2 and above, G2=GFR 60-89 ml/min/1.73 m2, HbA1c=Glycated hemoglobin, HDL=High-density lipoprotein, LDL=Low-density lipoprotein, NIHSS=National Institutes of Health Stroke Scale, SBP=Systolic blood pressure, SD=Standard deviation, SGOT=Serum glutamic oxaloacetic transaminase, SGPT=Serum glutamic pyruvic transaminase, TLC=Total leukocyte count
Table 3.
Comparison of characteristics between ischemic and hemorrhagic stroke subgroups (stratified by AKI)
| Characteristic | Ischemic (n=144) |
Hemorrhagic (n=60) |
||
|---|---|---|---|---|
| AKI | No AKI | AKI | No AKI | |
| Age in years, mean± SD | 56.09±14.3 | 55.09±14.07 | 57.55±12.75 | 57.10±12.98 |
| Males, n (%) | 41 (83.7) | 63 (66.3) | 26 (65) | 18 (90) |
| eGFR (ml/min/1.73 m2), mean±SD | 94.52±16.79 | 98.55±16.58 | 91.01±16.02 | 97.93±16.80 |
| BP (mmHg), median (IQR) | ||||
| SBP | 158 (152.6-166.3) | 150 (145.6-156.5) | 186 (168.5-189) | 170 (149.6-178.1) |
| DBP | 92 (90.2-98.1) | 90 (84.6-90.3) | 99 (98.4-108.4) | 100 (88.8-103.9) |
| Risk factors prevalence, n (%) | ||||
| Hypertension | 22 (44.9) | 54 (56.8) | 29 (72.5) | 12 (60) |
| Diabetes mellitus | 15 (30.6) | 28 (29.5) | 8 (20) | 3 (15) |
| Smoking | 14 (28.6) | 27 (28.4) | 8 (20) | 6 (30) |
| Alcohol intake | 5 (10.2) | 18 (18.9) | 5 (12.5) | 4 (20) |
| Dyslipidemia | 4 (8.2) | 7 (7.4) | 3 (7.5) | 0 |
| Heart disease | 13 (26.5) | 28 (29.5) | 0 | 2 (10) |
| Previous stroke | 5 (10.2) | 6 (6.3) | 1 (2.5) | 2 (10) |
| Use of antiplatelets | 2 (4.1) | 15 (15.8) | 2 (5) | 3 (15) |
| Family history | 1 (2) | 1 (1.1) | 2 (5) | 1 (5) |
| Contrast used | 47 (95.9) | 89 (93.7) | 5 (12.5) | 0 |
| Ventilatory requirement | 17 (34.7) | 5 (5.3) | 31 (77.5) | 10 (50) |
| Tracheostomized | 6 (12.2) | 4 (4.2) | 18 (45) | 6 (30) |
| GCS at presentation, median (IQR) | 11 (10.4-12.0) | 15 (12.8-15.0) | 8.5 (7.0-10.5) | 11 (9.5-13.2) |
| GCS <8, n (%) | 5 (10.2) | 4 (4.2) | 20 (50) | 4 (20) |
| GCS 9-15, n (%) | 44 (89.8) | 91 (95.8) | 20 (50) | 16 (80) |
| eGFR G2/G1 | G2: 33 (67.3) | G1: 16 (32.7) | G2: 18 (45) | G1: 22 (55) |
| NIHSS, median (IQR) | 13 (9.0-14.3) | 9 (8.1-11.6) | 22 (13.9-22) | 16 (9.2-18.7) |
| Time frames, median (IQR) | ||||
| Onset to hospitalization (hours) | 8 (6.2-14.5) | 7 (5.6-14.5) | 11.5 (12.0-21.1) | 9.5 (7.4-19.6) |
| Hospital stay (days) | 9 (6.6- 14.3) | 6 (4.6-9.9) | 12 (11.2- 18.9) | 12 (9.6-17.2) |
| Duration to death (days) | 10 (6.5-16.5) | 8.5 (5.6-13.4) | 13 (10.5-15.6) | 12 (9.6-13.4) |
| Duration to AKI (days) | 2 (1.3- 3.5) | - | 2 (1.3-3.5) | - |
AKI=Acute kidney injury, BP=Blood pressure, DBP=Diastolic blood pressure, eGFR=Estimated glomerular filtration rate, GCS=Glasgow Coma Scale, GFR=Glomerular filtration rate, G1=GFR 90 ml/min/1.73 m2 and above, G2=GFR 60-89 ml/min/1.73 m2, LDL=Low-density lipoprotein, NIHSS=National Institutes of Health Stroke Scale, SBP=Systolic blood pressure, SD=Standard deviation, SGOT=Serum glutamic oxaloacetic transaminase, SGPT=Serum glutamic pyruvic transaminase, TLC=Total leucocyte count
Supplementary Table 2.
Comparison of characteristics between AKI and non-AKI subgroup of patients according to subtyping of strokes
| Characteristics | AKI (n=89) | No AKI (n=115) | P |
|---|---|---|---|
| ICH score (n=60) | 0.334 | ||
| 0 | 3 (7.5%) | 5 (25%) | |
| 1 | 6 (15%) | 4 (20%) | |
| 2 | 12 (30%) | 6 (30%) | |
| 3 | 13 (32.5%) | 3 (15%) | |
| 4 | 6 (15%) | 2 (10%) | |
| 5 | 0 | 0 | |
| Site of bleed (n=60) | 0.242 | ||
| Putamen | 21 (52.5%) | 15 (75%) | |
| Thalamus | 10 (25%) | 4 (20%) | |
| Pons | 1 (2.5%) | 0 | |
| Cerebellum | 3 (7.5%) | 0 | |
| Lobar | 5 (12.5%) | 1 (5%) | |
| Stroke subtypes - TOAST (n=144) | n=49 | n=95 | 0.343 |
| Large vessel | 31 (63.27%) | 46 (48.42%) | |
| Cardioembolic | 8 (16.33%) | 17 (17.89%) | |
| Small vessel | 7 (14.29%) | 16 (16.84%) | |
| Other classified | 2 (4.08%) | 8 (8.42%) | |
| Undetermined | 1 (2.04%) | 8 (8.42%) | |
| Vascular territory (n=144) | n=49 | n=95 | |
| MCA | 34 (69.39%) | 63 (66.32%) | 0.415 |
| ACA | 0 | 4 (4.21%) | |
| PCA | 3 (6.12%) | 2 (2.11%) | |
| Vertebrobasilar | 7 (14.29%) | 13 (13.68%) | |
| ICA | 5 (10.2%) | 13 (13.68%) |
ACA=Anterior cerebral artery, AKI=Acute kidney injury, ICA=Internal carotid artery, ICH=Intracerebral hemorrhage, MCA=Middle cerebral artery, PCA=Posterior cerebral artery, TOAST=This classification denotes five subtypes of ischemic stroke: 1) Large-artery atherosclerosis, 2) Cardioembolism, 3) Small-vessel occlusion, 4) Stroke of other determined etiology, and 5) Stroke of undetermined etiology
After stratifying into stroke type, three separate models were created to predict mortality of AKI and test the association between AKI and all-cause hospital mortality. Separate models were created to predict AKI in ischemic and hemorrhagic stroke. Logistic regression was carried out to predict AKI, considering all covariates. In this multivariate analysis, hypertension was the strongest predictor of AKI in hemorrhagic stroke, followed by diabetes mellitus. In ischemic stroke, ventilatory requirement was the strongest predictor of AKI (P = 0.001) [Tables 4 and 5]. Because of the baseline severity of stroke, the patient might have needed ventilatory support, and both ventilator-associated pneumonia and sepsis can cause AKI. A robust association was found between AKI and mortality after ischemic stroke in all models. In the fully adjusted model, AKI complicating an ischemic stroke hospitalization was associated with 30.4 greater odds of in-hospital mortality (95% confidence interval [CI] 3.58–257.59). In the models for hemorrhagic stroke, the association between AKI and increased mortality was also significant in magnitude (OR 5.21, CI 1.26-21.69) and became further significant once the NIHSS score and smoking were added (odds ratio [OR] 15.32, 95% CI 0.59–393.02). Correlation with creatinine rise was studied along with other parameters, including blood sugar levels, temperature, use of nephrotoxic antibiotics, diuretics (mannitol and frusemide), and antiepileptic drugs (levetiracetam) using logistic regression [see Figure 1a–d]. Estimates of mean SCr by group at various time points were calculated using repeated-measures analysis of variance. SCr was the only parameter showing statistical significance (taken at baseline, 3 days, 7 days, and 14 days). Coefficients were calculated using different parameters, including fluid balance and nephrotoxic drugs (antibiotics, diuretics, antiepileptic drugs, and other nephrotoxic drugs like telmisartan). Statistically significant values were obtained using antibiotics, antiepileptic drugs, and TLC. Adjusted multivariate analysis revealed statistically significant results only for TLC (P = 0.001). Overall, AKI was common and developed in 89 patients (43.6% of the overall cohort, with significantly higher rates among intracranial hemorrhage [ICH] cases compared to ischemic stroke [66.7% vs. 34%] cases) (P < 0.05). Results showed that 66% of all AKIs were stage 1, and 48 patients (53.93%) had normalized renal parameters during their stay. Forty-nine patients with ischemic stroke developed AKI, of which 37, seven, and five were in stages 1, 2, and 3, respectively. Forty patients with hemorrhagic stroke developed AKI, of which 22, six, and 12 were in stages 1, 2, and 3, respectively. The median duration of stay in the overall cohort was 6 days. The median duration to AKI was 2 days in the overall cohort. Patients detected to have AKI received standard-of-care treatment, potential nephrotoxic drugs were stopped, adequate hydration was ensured, regular monitoring of all renal parameters was done, and dialysis was initiated if required.
Table 4.
Model for prediction of AKI in ischemic and hemorrhagic stroke
| Ischemic stroke | |||
|---|---|---|---|
| Mortality | Odds ratio | P | 95% CI |
| Age | 0.98 | 0.630 | 0.91-1.05 |
| Sex | 1.82 | 0.640 | 0.288-11.55 |
| Ventilatory requirement | 22.38 | 0.004 | 2.75-182.004 |
| Tracheostomized | 0.213 | 0.118 | 0.03-1.48 |
| Previous HTN | 5.132 | 0.057 | 0.95-27.57 |
| NIHSS at admission | 1.38 | 0.001 | 1.14-1.68 |
| eGFR | 0.91 | 0.004 | 0.85-0.97 |
|
Hemorrhagic stroke | |||
| AKI | Odds ratio | P | 95% CI |
|
| |||
| Age | 0.80 | 0.129 | 0.61-1.06 |
| Sex | 0.15 | 0.333 | 0.03-6.92 |
| Ventilatory requirement | 1.27 | 0.908 | 0.02-76.03 |
| Tracheostomized | 3.07 | 0.560 | 0.07-134.93 |
| DM | 7.40 | 0.478 | 0.02-1876.83 |
| Previous HTN | 8.02 | 0.237 | 0.25-252.84 |
| Smoker | 0.12 | 0.123 | 0.04-3.29 |
| Alcohol | 0.85 | 0.949 | 0.06-105.51 |
| NIHSS at admission | 1.83 | 0.136 | 0.82-4.08 |
| eGFR | 0.86 | 0.128 | 0.72-1.04 |
AKI=Acute kidney injury, CI=Confidence interval, eGFR=Estimated glomerular filtration rate, DM=Diabetes mellitus, HTN=Hypertension, NIHSS=National Institute of Health Stroke Scale
Table 5.
Model for predicting AKI causing in-hospital mortality
| Model | Hemorrhagic stroke (n=60) | Ischemic stroke (n=144) | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Odds ratio | P | 95% CI | Odds ratio | P | 95% CI | |
| Model 1 | 5.12 | 0.02 | 1.29-20.28 | 27.21 | 0.002 | 3.39-218.13 |
| Model 2 | 5.21 | 0.023 | 1.25-21.68 | 27.06 | 0.002 | 3.31-221.09 |
| Model 3 | 15.32 | 0.099 | 0.59-393.02 | 30.40 | 0.002 | 3.58-257.59 |
Model 1: AKI only. Model 2: AKI plus age plus sex. Model 3: AKI plus age plus sex plus smoker plus hypertension plus NIHSS. AKI=Acute kidney injury, CI=confidence interval, NIHSS=National Institutes of Health Stroke Scale
Figure 1.
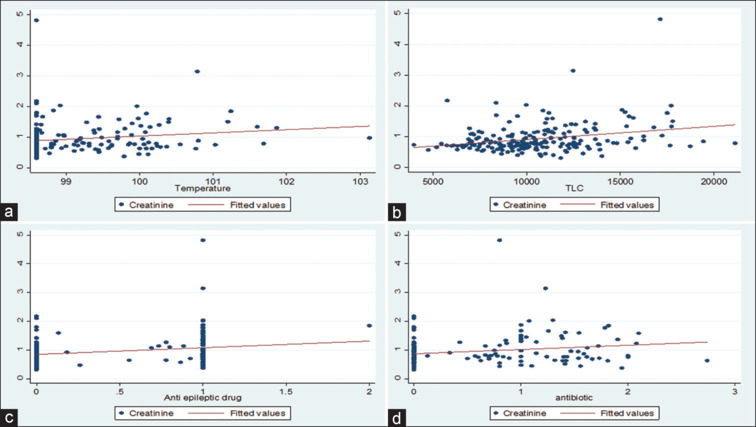
Scatter plot showing correlation of rise in creatinine with Temperature (a), Rise in Total Leucocyte Count (TLC) (b), Number of antiepileptic drugs (c) and Number of Antibiotics (d)
DISCUSSION
Acute stroke constitutes a significant number of patients admitted to neurology services, and AKI comprises a significant proportion of this cohort. Ours is the first study to assess AKI in acute stroke patients in the Indian population. In this analysis, we found that AKI was a common complication of stroke (43.6%), which is relatively high compared to other studies. This may be because of various reasons. Most of the previous studies have been retrospective analyses from hospital records and have considered only creatinine and urine output as measures for assessing AKI.[30] We performed the adjusted analysis of all these parameters to look for the crucial triggers for AKI. Our study was the first to focus on the transient and persistent renal dysfunction in the setting of acute stroke. Ours is a tertiary referral center; the admission bias reflects more admissions of sick patients who are more prone to AKI because of sepsis, medications, and fluid/nutrition balance. Another reason for the high incidence would be the high-sensitivity definition of AKI. Lastly, because of the nonavailability of preadmission creatinine, the actual baseline level of kidney function is unknown. However, at discharge, only 20.1% of patients had persistent AKI. Covi et al.[31] reported a slightly lower incidence (14.5% of patients developed AKI out of 932 patients). A meta-analysis reported the pooled prevalence rate of AKI after all stroke types was 11.6% (95% CI 10.6–12.7).[21] Ischemic stroke patients with AKI had 30 times the odds of in-hospital death compared to those without. Similar to our study, Khatri et al. showed that AKI was linked to increased hospital mortality due to ischemic stroke (OR 3.08), but not ICH (OR 0.82, 95% CI 0.50–1.35).[8] Recent studies have shown that AKI independently predicts short-term mortality after acute stroke. Another study found that SCr independently predicted mortality among stroke survivors even after adjustment for confounders.[32] The mean length of hospital stay in ICH patients with AKI was 13 days compared to 8 days in patients without AKI.[4] AKI requiring dialysis constitutes 2.9% of the overall stroke cohort and 6.7% of patients developing AKI. The incidence of Acute Kidney injury requiring Dialysis (AKI-D) was higher (8.3%) in hemorrhagic stroke than in ischemic stroke (0.69%) (P = 0.001). Nadkarni et al. ascertained the incidence of AKI-D at 0.15% in hospitalized patients with ischemic stroke and 0.35% in ICH, with a significantly higher chance of mortality and adverse outcomes at discharge.[33] Of 89 patients who developed AKI, 30 died; mortality was 33.7% in the AKI group (vs. 3.4% in the non-AKI group). Khatri et al. reported a 33% mortality in ischemic stroke versus a 40% mortality rate in hemorrhagic stroke patients who developed AKI.[8] In our study, previous hypertension and ventilatory requirement were predictors of AKI.
The higher prevalence of AKI in ICH (compared to AIS) may be related to the high prevalence of preexisting arterial hypertension in patients with ICH, and may be due to the inherent difference in managing hemorrhagic and ischemic stroke concerning the use of mannitol, nephrotoxic drugs, and various antibiotics. The proportion of patients on single, double, and triple nephrotoxic antibiotics was 21.5%, 12.3%, and 1%, respectively. Cefoperazone sulbactam was the most common antibiotic prescribed (14.2%), followed by piperacillin tazobactam in 12.7% of patients and meropenem and amikacin each prescribed to 11.7% patients. Vancomycin was prescribed to nine patients (4.4%), and netilmicin was received by only two patients (1%). Moreover, 24% of patients received a single diuretic and 22.5% of patients were on two diuretics during the course of their stay. Mannitol and frusemide were the most common diuretics used. A study reported that age, renal function at admission expressed by estimated GFR, presence of comorbidities, and type of stroke were independent predictors for AKI in the logistic regression analysis.[11] AKI was a predictor of mortality in both types of stroke, with more odds of death in ischemic stroke with AKI than in hemorrhagic stroke with AKI.[4,14] The use of nephrotoxic antibiotics, antiepileptic drugs, fluid balance, and presence of sepsis as measured by fever and TLC had statistically significant coefficients, and adjusted multivariate analysis revealed only TLC as an essential predictor of AKI in this population. However, a primary focus of this analysis was on changes in kidney function after hospital admission, where interventions could be attempted to prevent or treat AKI. In our study population, the proportion of AKI patients with ICH was high, which is Per se associated with significantly higher mortality than that of patients with ischemic stroke; in the case of ICH, we believe that the severity of the stroke itself negated any smaller effect that AKI may have. This finding may nullify the effect of AKI on mortality in hemorrhagic stroke. However, subgroup analysis still revealed a significant association between AKI and ischemic stroke mortality. Another limitation could be the lack of use of any biomarker for defining AKI.
CONCLUSION
We found that AKI is a common problem during stroke hospitalizations and is associated with in-hospital mortality from both types of stroke (ischemic more than hemorrhagic). The vast majority of subjects with AKI were stage 1, highlighting the importance of even minor elevation in creatinine during hospitalization that gives a time window to determine the cause at the earliest and manage it. The severity of impaired kidney function in patients hospitalized with acute stroke is associated with increased all-cause mortality independent of age, sex, and significant comorbidities. Our findings highlight that in patients with stroke at risk of developing AKI, parameters like hemorrhagic stroke, higher age, high baseline creatinine, and diabetes mellitus are independent predictors of outcome. Additional studies are required to determine if the development of AKI after stroke is a causal relationship or if it is simply a marker of end organ damage from long-standing arterial stiffness of small and large arteries due to atherosclerosis and its associated vascular risk factors. Further studies should be planned to determine whether interventions designed to prevent the development of AKI or treat early manifestations of AKI aggressively would reduce stroke mortality.
Author contributions
Conceptualization: MVPS and VVY; methodology: MVPS, VVY, and SA; software: SA; validation: MVPS, RB, and SA; formal analysis: MVPS, VVY, and SA; investigation: all authors; resources: all authors; data curation: MVPS, VVY, and SA; writing – original draft preparation: MVPS and SA; writing – review and editing: SA, AA, VVY, and MVPS; visualization: SA; supervision: VVY and MVPS; project administration: all authors. All authors have read and agreed to the published version of the manuscript.
Data availability statement
Qualified researchers may request study protocol, statistical analysis, and patient-level data access. Patient data will be anonymized to protect the privacy of the participants.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We acknowledge the help of all our colleagues, nursing staff, and supporting team.
REFERENCES
- 1.Feigin VL, Forouzanfar MH, Krishnamurthi R, Mensah GA, Connor M, Bennett DA, et al. Global and regional burden of stroke during 1990–2010: Findings from the Global Burden of Disease Study 2010. Lancet. 2014;383:245–55. doi: 10.1016/s0140-6736(13)61953-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arnold J, Ng KP, Sims D, Gill P, Cockwell P, Ferro C. Incidence and impact on outcomes of acute kidney injury after a stroke: A systematic review and meta-analysis. BMC Nephrol. 2018;19:283. doi: 10.1186/s12882-018-1085-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Katan M, Luft A. Global burden of stroke. Semin Neurol. 2018;38:208–11. doi: 10.1055/s-0038-1649503. [DOI] [PubMed] [Google Scholar]
- 4.Writing Group Members; Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart disease and stroke statistics—2016 update: A report From the American Heart Association. Circulation. 2016;133:e38–360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 5.Kamalakannan S, Gudlavalleti ASV, Gudlavalleti VSM, Goenka S, Kuper H. Incidence & prevalence of stroke in India: A systematic review. Indian J Med Res. 2017;146:175. doi: 10.4103/ijmr.IJMR_516_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koza Y. Acute kidney injury: Current concepts and new insights. J Inj Violence Res. 2016;8:58–62. doi: 10.5249/jivr.v8i1.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang D, Guo Y, Zhang Y, Li Z, Li A, Luo Y. Epidemiology of acute kidney injury in patients with stroke: A retrospective analysis from the neurology ICU. Intern Emerg Med. 2018;13:17–25. doi: 10.1007/s11739-017-1703-z. [DOI] [PubMed] [Google Scholar]
- 8.Khatri M, Himmelfarb J, Adams D, Becker K, Longstreth WT, Tirschwell DL. Acute kidney injury is associated with increased hospital mortality after stroke. J Stroke Cerebrovasc. Dis. 2014;23:25–30. doi: 10.1016/j.jstrokecerebrovasdis.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Snarska K, Kapica-Topczewska K, Bachórzewska-Gajewska H, Małyszko J. Renal function predicts outcomes in patients with ischaemic stroke and haemorrhagic stroke. Kidney Blood Press Res. 2016;41:424–33. doi: 10.1159/000443444. [DOI] [PubMed] [Google Scholar]
- 10.Qureshi AI, Aslam H, Zafar W, Huang W, Lobanova I, Naqvi SH, et al. Acute kidney injury in acute ischemic stroke patients in clinical trials. Crit Care Med. 2020;48:1334–9. doi: 10.1097/CCM.0000000000004464. [DOI] [PubMed] [Google Scholar]
- 11.Yaqub S, Aziz A. Acute kidney injury in acute stroke. J Pak Med Assoc 2022. 2022;72:1889. doi: 10.47391/JPMA.5549. [DOI] [PubMed] [Google Scholar]
- 12.Ramírez-Guerrero G, Baghetti-Hernández R, Ronco C. Acute kidney injury at the neurocritical care unit. Neurocrit Care. 2022;36:640–9. doi: 10.1007/s12028-021-01345-7. [DOI] [PubMed] [Google Scholar]
- 13.Lee M, Saver JL, Chang KH, Liao HW, Chang SC, Ovbiagele B. Low glomerular filtration rate and risk of stroke: Meta-analysis. BMJ, 2010;341:c4249. doi: 10.1136/bmj.c4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kumai Y, Kamouchi M, Hata J, Ago T, Kitayama J, Nakane H, et al. Proteinuria and clinical outcomes after ischemic stroke. Neurology. 2012;78:1909–15. doi: 10.1212/WNL.0b013e318259e110. [DOI] [PubMed] [Google Scholar]
- 15.McCullough PA. Cardiorenal syndromes. World J Cardiol. 2011;3:1. doi: 10.4330/wjc.v3.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nongnuch A, Panorchan K, Davenport A. Brain–kidney crosstalk. Crit Care. 2014;18:225. doi: 10.1186/cc13907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Husain-Syed F, Takeuchi T, Neyra JA, Ramírez-Guerrero G, Rosner MH, Ronco C, Tolwani AJ. Acute kidney injury in neurocritical care. Crit Care. 2023;27:341. doi: 10.1186/s13054-023-04632-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barnum SR. Complement in central nervous system inflammation. Immunol Res. 2002;26:7–14. doi: 10.1385/IR:26:1-3:007. [DOI] [PubMed] [Google Scholar]
- 19.Woiciechowsky C, Asadullah K, Nestler D, Eberhardt B, Platzer C, Schöning B, et al. Sympathetic activation triggers systemic interleukin-10 release in immunodepression induced by brain injury. Nat Med. 1998;4:808–13. doi: 10.1038/nm0798-808. [DOI] [PubMed] [Google Scholar]
- 20.Ott L, McClain CJ, Gillespie M, Young B. Cytokines and metabolic dysfunction after severe head injury. J Neurotrauma. 1994;11:447–2. doi: 10.1089/neu.1994.11.447. [DOI] [PubMed] [Google Scholar]
- 21.Zorrilla-Vaca A, Ziai W, Connolly ES, Jr, Geocadin R, Thompson R, Rivera-Lara L. Acute kidney injury following acute ischemic stroke and intracerebral hemorrhage: A meta-analysis of prevalence rate and mortality risk. Cerebrovasc Dis. 2018;45:1–9. doi: 10.1159/000479338. [DOI] [PubMed] [Google Scholar]
- 22.Saeed F, Adil MM, Khursheed F, Daimee UA, Branch LA, Jr, Vidal GA, et al. Acute renal failure is associated with higher death and disability in patients with acute ischemic stroke: Analysis of nationwide inpatient sample. Stroke. 2014;45:1478–80. doi: 10.1161/STROKEAHA.114.004672. [DOI] [PubMed] [Google Scholar]
- 23.Hao Z, Wu B, Lin S, Kong FY, Tao WD, Wang DR, Liu M. Association between renal function and clinical outcome in patients with acute stroke. Eur Neurol. 2010;63:237–42. doi: 10.1159/000285165. [DOI] [PubMed] [Google Scholar]
- 24.Praught ML, Shlipak MG. Are small changes in serum creatinine an important risk factor? Curr Opin Nephrol Hypertens. 2005;14:265–70. doi: 10.1097/01.mnh.0000165894.90748.72. [DOI] [PubMed] [Google Scholar]
- 25.Huang Y, Wan C, Wu G. Acute kidney injury after a stroke: A PRISMA-compliant meta-analysis. Brain Behav. 2020;10:e01722. doi: 10.1002/brb3.1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsagalis G, Akrivos T, Alevizaki M, Manios E, Theodorakis M, Laggouranis A, et al. Long-term prognosis of acute kidney injury after first acute stroke. Clin J Am Soc Nephrol. 2009;4:616–22. doi: 10.2215/CJN.04110808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spratt N, Wang Y, Levi C, Ng K, Evans M, Fisher J. A prospective study of predictors of prolonged hospital stay and disability after stroke. J Clin Neurosci. 2003;10:665–9. doi: 10.1016/j.jocn.2002.12.001. [DOI] [PubMed] [Google Scholar]
- 28.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, et al. Acute kidney injury network: Report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Notice. Kidney Int Suppl. 2012;2:1. doi: 10.1038/kisup.2012.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shrestha P, Thapa S, Shrestha S, Lohani S, Bk S, MacCormac O, et al. Renal impairment in stroke patients: A comparison between the haemorrhagic and ischemic variants. F1000Res. 2017;6:1531. doi: 10.12688/f1000research.12117.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Covic A, Schiller A, Mardare NG, Petrica L, Petrica M, Mihaescu A, et al. The impact of acute kidney injury on short-term survival in an Eastern European population with stroke. Nephrol Dial Transplant. 2008;23:2228–34. doi: 10.1093/ndt/gfm591. [DOI] [PubMed] [Google Scholar]
- 32.MacWalter RS, Wong SY, Wong KY, Stewart G, Fraser CG, Fraser HW, et al. Does renal dysfunction predict mortality after acute stroke? A 7-year follow-up study. Stroke. 2002;33:1630–5. doi: 10.1161/01.str.0000016344.49819.f7. [DOI] [PubMed] [Google Scholar]
- 33.Nadkarni GN, Patel AA, Konstantinidis I, et al. Dialysis Requiring Acute Kidney Injury in Acute Cerebrovascular Accident Hospitalizations. Stroke. 2015;46:3226–31. doi: 10.1161/STROKEAHA.115.010985. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Qualified researchers may request study protocol, statistical analysis, and patient-level data access. Patient data will be anonymized to protect the privacy of the participants.


