Abstract
Introduction:
Cerebral Venous Thrombosis (CVT) poses a rare but life-threatening challenge, warranting meticulous treatment approaches. Traditional therapy involves Vitamin K Antagonists (VKAs), but Newer Oral Anticoagulants (NOACs) offer potential advantages. This study addresses a crucial knowledge gap in the Indian context, analyzing real-world data to guide CVT management decisions.
Methods:
A single-center, ambispective cohort study included consecutive adult CVT patients. Data collection encompassed demographics, clinical data, imaging, and treatment details. Patients were categorized into VKA and NOAC groups. Outcomes measured recanalization status, functional outcomes, bleeding events, and adverse drug reactions.
Results:
Among 181 enrolled patients, NOAC-treated (Group B) individuals had significantly higher rates of complete recanalization (58.5% vs. 31.1%) with a similar incidence of adverse events and also displayed better functional outcomes at weeks 8 and 12 compared to VKA-treated (Group A) patients. Recurrent thromboembolic events were absent in both groups during follow-up.
Conclusion:
This study highlights NOACs’ potential advantages in CVT management, including improved functional outcomes, enhanced recanalization, and similar bleeding risk. Adverse events were milder with NOACs. While acknowledging limitations, these findings support NOACs as a promising alternative to VKAs, advancing CVT care and outcomes.
Keywords: CVT, NOACs in CVT, NOACs versus VKA
INTRODUCTION
Cerebral venous thrombosis (CVT), a relatively rare but potentially life-threatening condition, is characterized by the formation of thrombus within the dural sinuses or the cerebral veins. It often affects individuals across age groups, including young adults and women of childbearing age, and has a prevalence of up to 1.6 per 100,000 persons.[1] The diverse clinical spectrum and complex pathophysiology of CVT demand a meticulous approach to treatment decisions. Traditionally, the standard of care to prevent recurrence of CVT has been vitamin K antagonists (VKAs) such as warfarin. However, the advent of newer oral anticoagulants (NOACs) has introduced alternative therapeutic options that offer potential advantages such as predictable pharmacokinetics, fixed dosing, and limited dietary interactions.[2]
Emerging evidence suggests the noninferiority and efficacy of NOACs in the treatment of CVT, as indicated by the outcomes of several clinical trials and research studies.[3,4] The utilization of NOACs has demonstrated comparable clinical and radiographic results, along with a favorable safety profile, when compared to traditional warfarin therapy. Nevertheless, a significant knowledge gap persists concerning the applicability of these findings to the Indian population. The primary objectives of this study are to evaluate the impact of NOACs and VKAs on CVT patients in terms of functional outcomes, recanalization status, the incidence of bleeding events, and adverse drug reactions. By analyzing real-world data derived from a regionally representative cohort, the study aims to provide valuable insights that can guide evidence-based decision-making in the management of CVT within the Indian population.
METHODOLOGY
Study methods and population
This ambispective observational cohort study was carried out at a tertiary care center in Chennai after obtaining ethical clearance and approval from the university ethics committee (CSP/22/OCT/117 / 532). Consecutive adult patients with CVT, diagnosed by imaging over a period of 4 years (2019–2022), were included in the study. All patients included in the study were treated in the acute phase with injectable heparin. Patients with CVT were identified from inpatient and outpatient departments and medical records. The patient data was then obtained during outpatient visits from their medical and imaging records and by telephonic consults. Patient identification and clinical data collection were done by a separate team, and the follow-up data, including the safety and efficacy data, were collected by a separate team. These teams were blinded to each other and to the drug taken by the patient. Patients with CVT associated with infection, individuals with malignancy, pregnant women and peripartum patients, subjects exhibiting hypersensitivity to the study drugs, those with known bleeding disorders or autoimmune disorders including antiphospholipid antibody syndrome, patients who were not compliant with the drugs, and those in whom therapeutic international normalised ratio (INR) was maintained were excluded from the study.
The primary outcomes being assessed are as follows: (i) The recanalization status of CVT on venous imaging, obtained from a computed tomography or magnetic resonance venogram (complete, partial, or no recanalization). Complete recanalization was defined as full recanalization of the thrombosed vein or sinus without any residual thrombus. Partial recanalization was defined as improved opacification or flow in the affected cerebral sinus or vein, but with residual thrombus present on follow-up imaging. No recanalization was defined as no change or worsening of opacification or flow in the affected cerebral sinus or vein from baseline imaging. Recanalization status was assessed by a radiologist team which was blinded to the study participants. (ii) Analysis of the safety outcomes of the anticoagulant being used to prevent CVT. Bleeding events were categorized into intracranial and extracranial. Nonbleeding adverse events were also recorded. (iii) Recurrence of venous thrombosis (recurrent CVT, Deep vein thrombosis (DVT) of any limb, pulmonary embolism (PE), or splanchnic vein thrombosis) during follow-up. Recurrent CVT included an extension of the existing clot and a new thrombus formation. The secondary outcome analysis included the study of the patient’s disability at 3 months and any deaths recorded during follow-up.
Study variables and statistical analysis
Detailed demographic information, clinical data pertinent to CVT, risk factors, investigative data, imaging variables, and data on treatment were collected for each patient from the medical records and outpatient records. Analysis of the medical and outpatient records and telephonic data collection was done to collect follow-up data and adverse events related to the treatment. The participants were divided into two groups: Group A, consisting of individuals undergoing treatment with VKAs, and Group B, comprising patients on treatment with NOACs (dabigatran 110 mg twice daily [BD] or apixaban 5 mg BD).
Descriptive statistics, including frequency analysis, percentage analysis, mean, median, interquartile range, and standard deviation, was employed to characterize the data. For univariate analyses, we categorized patients into two distinct groups based on their oral anticoagulant treatment: those exclusively receiving VKAs and those exclusively using NOACs. When analyzing clinical outcomes, we considered the entire dataset, and endpoints that occurred during oral anticoagulation treatment were attributed to the respective drug in use at that time. The Mann–Whitney U test was utilized to discern significant differences between bivariate samples in independent groups. For multivariate analysis involving repeated measures, the Friedman test served as the primary tool, complemented by the Wilcoxon signed-rank test. Categorical data were assessed for significance using the Chi-square test. In all statistical analyses, a probability value of P < 0.05 was considered the threshold for statistical significance. The study procedure adhered diligently to the principles of the International Conference on Harmonization of Good Clinical Practice. The collected data was analyzed using the International Business Machine Statistical Package for the Social Sciences Statistics software, version 29.0.
RESULTS
A total of 181 patients were enrolled in this study after applying specific inclusion and exclusion criteria. Figure 1 shows the enrollment and categorization process employed in the study. The patient cohort was categorized into two groups according to the anticoagulation treatment they received. No crossovers occurred between the groups, and each patient did not receive both treatments. The choice of anticoagulation was made after discussion and in concurrence with patients and their attendants after consideration of many factors like requirement of monitoring (INR), specific diet, cost, reversibility, and dosing. Group A comprised 94 individuals receiving treatment with VKAs, and Group B consisted of 87 patients treated with NOACs, of which 49 patients used dabigatran (Group B1) and 38 patients used apixaban (Group B2). Table 1 shows the baseline characteristics of both groups. The mean age of the patients in this study was 53.5 years and there was a slightly highe r prevalence of CVT (54.2%) among female patients, although the observed difference was not statistically significant (P = 0.13). There were no statistically significant differences in the prevalence of comorbidities or in the usage of drugs across both groups.
Figure 1.
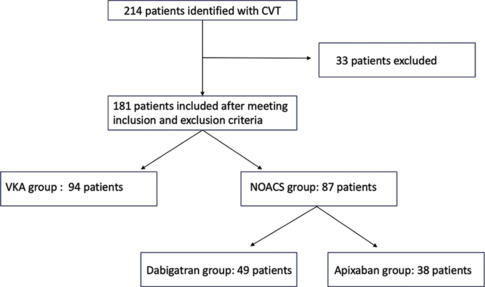
Study flow chart
Table 1.
Baseline characteristics of the study participants in the two groups
| Variables | Group A (n=94) | Group B (n=87) |
|---|---|---|
| Age in yearsa | 53 (10.7) | 54 (10.6) |
| Gender | ||
| Male | 45 (47.8) | 36 (41.4) |
| Female | 49 (52.2) | 51 (58.6) |
| Medical history | ||
| Alcohol consumption | 10 (10.6) | 4 (4.6) |
| Hypertension | 25 (26.6) | 26 (29.9) |
| Diabetes | 24 (25.5) | 25 (28.7) |
| Obese | 35 (37.3) | 32 (36.8) |
| Medication used | ||
| CCB | 35 (37.4) | 29 (33.3) |
| ARBs | 23 (24.3) | 22 (25.3) |
| Statins | 10 (10.6) | 7 (8.1) |
| Antidepressants | 8 (8.5) | 6 (6.9) |
| Antidiabetic | 6 (6.4) | 12 (13.8) |
| Occupation | ||
| Employed | 37 (39.4) | 27 (31.0) |
| Unemployed | 14 (14.9) | 11 (12.7) |
| Business | 23 (24.5) | 31 (35.6) |
| Daily wages | 7 (7.4) | 11 (12.7) |
| Pensioner | 13 (13.8) | 7 (8.0) |
| Socioeconomic class | ||
| Upper | 7 (7.5) | 4 (4.6) |
| Upper middle | 27 (28.7) | 15 (17.3) |
| Lower middle | 24 (25.5) | 29 (33.3) |
| Upper lower | 23 (24.5) | 33 (37.9) |
| Lower | 13 (13.8) | 6 (6.9) |
| Radiographic findings | ||
| MRI venography | 56 (59.6) | 46 (52.9) |
| CT venography | 38 (40.4) | 41 (47.1) |
| Sinuses involved | ||
| Superior sagittal sinus | 49 (52.1) | 43 (49.4) |
| Right lateral sinus | 11 (11.7) | 8 (9.2) |
| Left lateral sinus | 14 (14.9) | 19 (21.8) |
| Straight sinus | 21 (22.3) | 16 (18.4) |
| Cortical veins | 8 (8.5) | 13 (14.9) |
| Jugular veins | 9 (9.6) | 11 (12.5) |
| Parenchymal lesion on diagnostic neuroimaging | ||
| Nonhemorrhagic lesion | 28 (29.8) | 31 (35.7) |
| Hemorrhagic lesion | 32 (34.0) | 27 (31.0) |
| Clinical presentation | ||
| Seizure | 46 (48.9) | 41 (47.1) |
| Aphasia | 29 (30.9) | 26 (29.9) |
| Headache | 64 (68.0) | 59 (67.8) |
| Hemiparesis | 27 (28.7) | 18 (20.7) |
| Surgical decompression | 3 | 2 |
| mRS at admission (median) | 4 | 3.8 |
Except age, other values are presented as n (%). Group A: vitamin K antagonist; Group B: newer oral anticoagulants. aValues are given as mean (SD). ARBs=angiotensin II receptor blockers, CCB=calcium channel blocker, CT=computed tomography, MRI=magnetic resonance imaging, mRS=modified Rankin Scale
Analysis of recanalization status revealed that patients in Group B (NOACs) had a higher rate of complete recanalization (59.7%) compared to Group A (VKAs) patients (52.1%), with a corresponding decrease in partial recanalization (34 [39.0%] in Group B vs. 41 [43.7%] in Group A). In addition, Group B showed a lower rate of no recanalization (1.1%) compared to Group A (4.2%). The difference in recanalization rates was found to be statistically significant (P = 0.04), suggesting a distinct advantage for NOACs in promoting more complete recanalization. Subgroup analysis within the NOACs group showed that there was a marginal increase in the number of recanalized patients in the dabigatran group, but it was statistically insignificant (P = 0.11). None of the patients experienced recurrent thromboembolic events during the follow-up period.
Bleeding events, categorized as gastrointestinal, intracranial, and urogenital, were documented and compared between the groups. There were one gastrointestinal, one intracranial, and three urogenital bleeds observed in the VKA group, whereas three gastrointestinal bleeds and two intracranial bleeds were observed in the NOACs group. No statistically significant differences were identified in these categories (P > 0.05). On comparing the other adverse drug events between VKA and NOAC groups, it was found that Group A reported marginally more adverse drug events, which were as follows: urticaria 4.25%, thrombocytopenia 2.1%, abdominal pain 1.06%, and cough 1.06%. In Group B1, 3.7% reported urticaria, 1.8% had diarrhea, and 3.7% had cough. In Group B2, 2.4% reported urticaria, 2.4% had abdominal pain, and 4.8% had diarrhea. Causality, preventability, and severity scales indicated that the reaction was possible, probably preventable, and mild in both groups.
Analysis of functional outcomes was done using the modified Rankin Scale (mRS) scores at baseline and 4th, 8th, and 12th weeks of treatment. The results showed that when the functional outcome was compared between the groups, at the 8th and 12th weeks, Group B (NOACs) exhibited significantly better outcomes compared to Group A (VKAs) (P < 0.01). These findings underscore the potential superiority of NOACs in achieving favorable functional outcomes for CVT patients. Moreover, a comparison of functional status between the VKA group and the dabigatran group showed statistically significant functional improvement at the 8th and 12th weeks, and VKAs when compared to apixaban individually showed statistically significant improvement at the 12th week. Comparison of NOACs within Group B, namely dabigatran (Group B1) and apixaban (Group B2) subgroups, showed no significant differences in mRS scores between the two subgroups [Figure 2].
Figure 2.
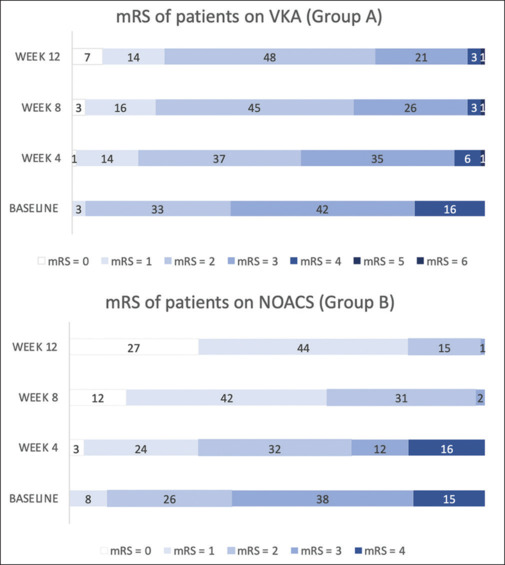
Comparison of mRS of patients in groups A and B over four different time points (baseline, week 4, week 8, and week 12)
mRS = modified Rankin Scale
DISCUSSION
This ambispective observational study involved 181 consecutive patients diagnosed with CVT, and we analyzed the safety profile and efficacy of anticoagulation treatments, specifically comparing VKAs and NOACs. There were no crossovers, ensuring a clear demarcation of their effects. Baseline characteristics of the patient cohort revealed a slightly higher prevalence of CVT among female patients, although this gender-based difference did not achieve statistical significance. In addition, there were no substantial differences in the prevalence of comorbidities or medication usage between the VKA and NOAC groups. The distribution of CVT across genders noted in our study correlates with the data from previous studies from India as well, with no significant gender bias, indicating a general shift in CVT prevalence from predominantly affecting women to showing an equal or a male predilection.[5,6] This is likely due to an increase in alcohol consumption, improved diagnostics, and better obstetric care.[5]
In general, patients diagnosed with CVT typically commence treatment with intravenous anticoagulation while simultaneously undergoing an evaluation for potential underlying thrombogenic causes. Subsequently, the intravenous anticoagulation regimen is transitioned to oral anticoagulants, and this is where the comparison of safety and efficacy profiles between the two categories becomes significant. NOACs offer advantages in terms of not requiring dietary restrictions or regular monitoring of blood parameters, potentially promoting greater patient adherence. However, they are relatively costly, and their efficacy and safety profiles have only recently demonstrated noninferiority to VKAs.
The RE-SPECT CVT study, a randomized clinical trial, represents one of the pioneering investigations to contrast NOACs with VKAs. This study assessed the efficacy and safety of dabigatran etexilate in comparison to warfarin in a cohort of 120 CVT patients, which revealed similar bleeding and recurrence rates in both treatment groups.[3] Another study conducted by Wasay et al.[7] included 111 patients and compared NOACs (specifically rivaroxaban and dabigatran) with VKAs, which yielded analogous results. It is worth noting that there is currently limited data regarding the use of NOACs in CVT within an Indian patient population.
The patients included in this study were prescribed either VKAs or NOACS, based on their preference, considering their financial burden and their willingness to follow a strict diet and undergo regular blood tests. Their functional status, imaging status, and side effect profile were followed up and analyzed.
The concept of recanalization as a therapeutic target for treating CVT has been investigated in previous studies. Some of this research has demonstrated a positive association between recanalization and improved functional outcomes, while the absence of recanalization has been linked to poorer functional outcomes.[8,9] It is worth noting that the timing of recanalization can extend up to 4 months and, in some cases, even up to 11 months.[10] The findings of this study shed light on this aspect, revealing that patients who received non-VKA oral anticoagulants (NOACs) in Group B exhibited notably higher rates of complete recanalization compared to their counterparts in Group A who received VKAs. These elevated rates of recanalization also corresponded with superior functional outcomes in the NOAC group. Functional outcome scores assessed using mRS at various treatment intervals showed that both at the 8th and 12th weeks, patients in the NOAC group (Group B) displayed statistically significantly improved functional outcomes compared to those in the VKA group (Group A). Furthermore, a subgroup analysis conducted within the NOAC group did not reveal any significant differences in functional outcomes between users of dabigatran and apixaban. Although there was a marginal increase in recanalization rates among dabigatran users in the subgroup analysis, the lack of statistical significance suggests that the overall efficacy of NOACs remained consistent regardless of the specific agent employed. In addition, during a 6-month follow-up period, there were no instances of recurrent thromboembolic events, which aligns with similar findings reported in other studies.[3,4]
On the safety front, the adverse events were categorized into bleeding events and others, and the bleeding events were characterized by the site of bleeding. This suggests that both anticoagulation strategies are comparable in terms of bleeding risk. However, when exploring adverse drug events, VKA users reported a marginally higher incidence of adverse events, including urticaria, thrombocytopenia, abdominal pain, and cough. These findings resonate with previous research in other patient populations, where NOACs exhibited favorable safety profiles compared to VKAs, especially in terms of intracranial hemorrhage risk. The results of this study add significant weight to the growing body of evidence supporting the use of NOACs as a viable alternative to VKAs in the management of CVT.
However, it is crucial to acknowledge the study’s limitations, including its ambispective nature, the potential for selection bias, and the small sample size. Moreover, follow-up for functional outcomes was not done until 6 months. Future research is needed to confirm and extend these findings.
In conclusion, the findings from this study strongly suggest that NOACs, with their comparatively better recanalization rates, similar recurrence rates and safety profiles, and favorable functional outcomes, offer a promising alternative to VKAs in the management of CVT. As the options in anticoagulation therapy continue to grow, these results hold the potential to improve the care and outcomes of individuals with CVT and given the multifactorial nature of CVT, personalized treatment strategies remain essential in its management.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Payne AB, Adamski A, Abe K, Reyes NL, Richardson LC, Hooper WC, et al. Epidemiology of cerebral venous sinus thrombosis and cerebral venous sinus thrombosis with thrombocytopenia in the United States, 2018 and 2019. Res Pract Thromb Haemost. 2022;6:e12682. doi: 10.1002/rth2.12682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mekaj YH, Mekaj AY, Duci SB, Miftari EI. New oral anticoagulants: Their advantages and disadvantages compared with vitamin K antagonists in the prevention and treatment of patients with thromboembolic events. Ther Clin Risk Manag. 2015;11:967–77. doi: 10.2147/TCRM.S84210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferro JM, Coutinho JM, Dentali F, Kobayashi A, Alasheev A, Canhão P, et al. Safety and efficacy of dabigatran etexilate vs dose-adjusted warfarin in patients with cerebral venous thrombosis: A randomized clinical trial. JAMA Neurol. 2019;76:1457–65. doi: 10.1001/jamaneurol.2019.2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yaghi S, Shu L, Bakradze E, Salehi Omran S, Giles JA, Amar JY, et al. Direct Oral anticoagulants versus warfarin in the treatment of cerebral venous thrombosis (ACTION-CVT): A multicenter international study. Stroke. 2022;53:728–38. doi: 10.1161/STROKEAHA.121.037541. [DOI] [PubMed] [Google Scholar]
- 5.Narayan D, Kaul S, Ravishankar K, Suryaprabha T, Bandaru VC, Mridula KR, et al. Risk factors, clinical profile, and long-term outcome of 428 patients of cerebral sinus venous thrombosis: Insights from Nizam′s Institute Venous Stroke Registry, Hyderabad (India) Neurol India. 2012;60:154–9. doi: 10.4103/0028-3886.96388. [DOI] [PubMed] [Google Scholar]
- 6.Parikh PM, Sukthankar RU, Parikh A, Pipalia DH, Sidhva SJ, Ramakanten R, et al. Cerebral venous thrombosis. J Assoc Physicians India. 1987;35:349–51. [PubMed] [Google Scholar]
- 7.Wasay M, Khan M, Rajput HM, Farooq S, Memon MI, AlRukn SA, et al. New oral anticoagulants versus warfarin for cerebral venous thrombosis: A multi-center, observational study. J Stroke. 2019;21:220–3. doi: 10.5853/jos.2019.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aguiar de Sousa D, Lucas Neto L, Canhão P, Ferro JM. Recanalization in cerebral venous thrombosis. Stroke. 2018;49:1828–35. doi: 10.1161/STROKEAHA.118.022129. [DOI] [PubMed] [Google Scholar]
- 9.Kim DJ, Honig A, Alimohammadi A, Sepehry AA, Zhou LW, Field TS. Recanalization and outcomes after cerebral venous thrombosis: A systematic review and meta-analysis. Res Pract Thromb Haemost. 2023;7:100143. doi: 10.1016/j.rpth.2023.100143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arauz A, Vargas-González JC, Arguelles-Morales N, Barboza MA, Calleja J, Martínez-Jurado E, et al. Time to recanalisation in patients with cerebral venous thrombosis under anticoagulation therapy. J Neurol Neurosurg Psychiatry. 2016;87:247–51. doi: 10.1136/jnnp-2014-310068. [DOI] [PubMed] [Google Scholar]


