Abstract
Background and Aims:
Tenecteplase is used as the standard of care treatment for thrombolysis in acute ischemic stroke (AIS) patients within 4.5 h of symptom onset. Documented reports were less certain to claim the benefits of it in an extended window period. EAST-AIS (CTRI/2022/03/040718) trial is designed to determine the success rate of thrombolysis in an extended window period for good clinical outcomes.
Study Design:
It is a randomized, placebo-controlled trial of tenecteplase administered within 4.5–24 h of stroke onset (with or without large vessel occlusion) based on evidence of salvageable tissue through baseline computed tomography perfusion (CTP) or magnetic resonance imaging (MRI) scan. Criteria of patient inclusion are as follows: patients of both genders (male and female), age >18 years, pre-stroke modified Ranking Scale (mRS) <2, baseline NIHSS >5, CTP showing penumbra–ischemic core ratio >1.8, absolute difference in volume >10 ml, and ischemic core volume <70 ml. The sample size for the study is 100 patients: 50 in the tenecteplase arm (0.25 mg/kg body weight; maximum- 25 mg) and 50 in the placebo arm (controls).
Study Outcomes:
The study’s primary objective is safety endpoints along with the efficacy of tenecteplase assessed using the mRS score at 90 days of stroke onset.
Conclusion:
The result obtained from EAST-AIS will determine the safety and efficacy of tenecteplase injection administered 4.5–24 h following the symptom onset for AIS patients within the territory of Internal Carotid Artery (ICA), Middle Cerebral Artery (MCA), or Anterior Cerebral Artery (ACA) occlusion.
Keywords: Acute ischemic stroke, eligible window period: 4.5–24 h, mRS, randomized controlled trial, study protocol, tenecteplase
INTRODUCTION
Stroke is the second major contributor of disability and the third leading cause of death in India. It is a neurologic disorder in which oxygen supply is decreased in the brain due to blockage of blood vessels. The crude prevalence of stroke in India ranges from 44.29 to 559/lakh persons and the incidence is 105–152/lakh persons over the last 20 years.[1] The rate of intravenous (IV) thrombolysis within the window period (4.5 h of onset) is very low (<5%) worldwide, including India, due to the narrow therapeutic window.[2] Generally, various situations arise in developing countries that conventionally delay the treatment process for acute ischemic stroke (AIS) patients. Some of the reasons for delay of treatment can be lack of awareness about brain stroke, failure of primary care physician to identify symptoms of stroke, unavailability of neuroimaging and thrombolysis facility, and patient affordability for treatment.[3] According to the guidelines for management of AIS, alteplase is the primary thrombolysis agent given to eligible patients of AIS (within 4.5 h of the onset of AIS symptoms) and is preferred before mechanical thrombectomy.[4] Previous studies show evidence to prove that good clinical outcome entirely depends upon the time length to reperfusion in AIS patients.[5,6] Furthermore, performing IV thrombolysis beyond the extended window period (>4.5 h) of onset could substantially improve patient outcomes.[4,7,8] The EXTEND trial showed benefit of thrombolysis with injection alteplase (Adjusted Risk Ratio [aRR]: 1.44; 95% confidence interval [CI]: 1.01–2.06, P = 0.04), in the window period of 4.5–9 h. However, the level of evidence is considered low due to premature closure of this trial.[9] Tenecteplase (tNK), a genetically modified form of recombinant tissue plasminogen activator, shows superior characteristics like prolonged half-life (~18 min), higher resistance to Plasminogen Activator Inhibitor -1 (PAI-1) (80-fold), higher fibrin specificity (14-fold), and rapid lysis rate, in comparison of alteplase.[10,11,12,13,14,15] A meta-analysis report observed that thrombolysis with tNK in AIS patients in 3–6 h had a promising primary neurologic recovery (Risk Ratio [RR] =1.56, 95% CI: 1–2.43, P = 0.05) in comparison of alteplase. However, no significant difference was found in excellent and good functional outcomes in intracerebral hemorrhage rate and mortality at 90 days between them.[16] Altogether, previous studies are in favor of a better outcome of tNK in terms of a lesser symptomatic hemorrhagic transformation rate compared to tPA when administered within 6 h of symptom onset.[10,11,12] A previous report has claimed tNK therapy to be safe as IV thrombolysis in AIS patients and has shown suitable imaging details of ischemic penumbra in 4.5–24 h of onset.[17] Still there is a limitation of thrombolysis with tNK after 4.5 h of onset in AIS patients because this study was conducted in small population of AIS patients and in a nonrandomized pattern. Recently, a multicentric, Phase III, double-blinded, randomized, placebo-controlled trial (TIMELESS trial) conducted in the USA reported no significant improvement in the primary efficacy endpoint (modified Rankin Scale [mRS]) at 90 days. Nonetheless, a higher complete recanalization rate was observed in the tNK group (77% vs. 64%, odds ratio [OR]: 1.85, P = 0.006) at 24 h without safety concerns, as the number of symptomatic ICH and fatal adverse events was balanced across groups.[18] Based on the evidence from previous data, this is the first study being conducted in the Indian population to evaluate the efficacy and safety of tNK injection in the extended time period (4.5–24 h) based on imaging-eligible window in stroke patients (with or without large vessel occlusion [LVO]). The main aim of the study was to determine the efficacy and safety of tNK administered IV beyond the approved window period (4.5–24 h) from the onset of symptoms in patients with or without LVO. Inclusion of patients in the study is based on evidence of salvageable tissue through imaging like computed tomography (CT) perfusion (CTP) or magnetic resonance perfusion imaging. The results of the study may change the overall paradigm of AIS patient management.
OBJECTIVES
Primary objective
Our primary objective is to determine the safety and efficacy of the injection of tNK along with standard treatment in AIS with or without LVO in patients presenting within the extended window period (4.5–24 h) of onset and with evidence of salvageable tissue on images of baseline CTP or magnetic resonance imaging scan, in comparison to standard treatment alone at 3 months.
Secondary objectives
To determine the rate of recanalization of vessels causing stroke
To determine the reduction of the need for decompressive hemicraniectomy in cases of large vessel obstruction
METHODOLOGY
Design of study and setting
This publicly funded, prospective, randomized controlled trial was initiated after obtaining approval from the Institute Ethics Committee (IEC-58/14.01.2022, RP-09/2022). The study is funded by the funding agency SERB-DST, from the Government of India, and the first case was enrolled on August 1, 2022. Patients in our study are being enrolled from All India Institute of Medical Sciences (AIIMS) (a tertiary care hospital), New Delhi, India and our trial is Clinical Trial Registry India (CTRI) registered with trial registration number (CTRI/2022/03/040718). A consort diagram of the study is shown in Figure 1.
Figure 1.
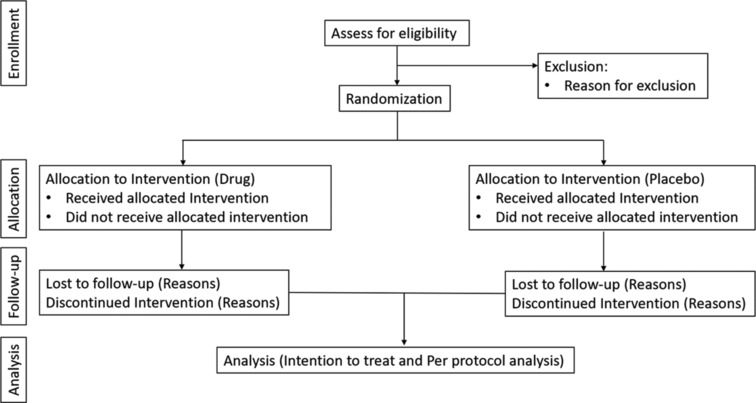
Consort diagram for the study
Consent
Consent (witnessed written information) is taken from all eligible patients or their next of kin willing to be enrolled in the study. Eligible patients or their next of kin give their written consent to the physician, with a nurse witnessing the consent. Patients or their relatives who are not educated have the contents of the consent form read out and explained by the treating physician and their thumb impression is taken for maintenance of record.
Study population
All AIS patients arrive at the emergency department of AIIMS, New Delhi. Potentially eligible patients undergo emergency head noncontrast CT for diagnosis. All patients of AIS from emergency are screened and data is maintained in a logbook, along with reasons of exclusion. Purpose of logbook register is to estimate the proportion of potentially eligible patients screened for the study and to assess the generalizability of study results. Study will be conducted on 100 eligible patients, randomized 1:1 (50 in the drug arm and 50 in the placebo arm) as per the eligibility criteria of our study. A descriptive explanation of the eligibility criteria of AIS patients in the trial is presented in Table 1.
Table 1.
Descriptive explanation of the eligibility criteria
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Age >18 years | Imaging shows >1/3 of the MCA territory infarct or more than one vascular territory |
| AIS symptom onset withing the past 4.5-24 h | Acute or previous intracranial hemorrhage |
| AIS consistent within Internal Carotid Artery (ICA), Middle Cerebral Artery (MCA) or Anterior Cerebral Artery (ACA) territory | Active internal organ bleeding |
| Baseline mRS 0-2 | Known hypersensitivity to Tenecteplase |
| NIHSS score of >5 at baseline and prior to randomization | Seizures at stroke onset |
| Imaging eligibility is defined as: Perfusion lesion-ischemic core mismatch should be greater than 1.8. Difference between the volume of hypoperfusion and volume of the ischemic core should be greater than 10ml, and an ischemic-core volume should be >70ml. | Severe, uncontrolled hypertension |
| If CT perfusion is technically inadequate and CT perfusion shows a penumbra and core volume of 0ml then patients will be screened by brain MRI and then randomized. If there is MRI DWI-FLAIR (Difussion-weighted imaging/Fluid attenuated inversion recovery) mismatch or DWI-ASL perfusion (Diffusion-weighted imaging/Arterial spin labelling) mismatch | Treatment with thrombolytic agent within the last three months |
| Gastrointestinal malignancy or gastrointestinal bleed within 21 days | |
| Occlusion in >1 vascular territory | |
| Patients on anti-coagulation therapy should not be randomized in the trial |
AIS=Acute Ischemic Stroke, mRS=modified Rankin Scale, NIHSS=National Institutes of Health Stroke Scale, CT=Computed Tomography, MRI=Magnetic resonance imaging
Randomization and allocation concealment
A list for randomization of stroke patients was prepared in the Department of Biostatistics, AIIMS (New Delhi). Distribution of patients was done in the ratio of 1:1 (intervention and placebo arms). After obtaining consent, patients are randomized and each patient is assigned a unique identification number. Patients and their next of kin, treatment allocating biostatistician, the treating resident, the principal investigator, the research staff, the outcome assessor, and the biostatistician analyzing the final outcome in the project will remain blinded to the allocation of patients throughout the study.
Intervention
tNK injection (0.25 mg/kg body weight; 25 mg maximum) is administered to patients randomized to the intervention arm, and a placebo (similar-looking injection of tNK placed in visibly matched packing) is given to patients. The injection Tenectase used in this trial is not a biosimilar for the indication, and manufacturers are innovators for this indication. Standard treatment is provided to patients in both arms as per the national or international guidelines for management of AIS patients.
Data monitoring body
An independent data safety and monitoring board (DSMB) oversees patient safety in the study. Three interim reviews are planned according to the enrollment of the patients, in which unblinded data will be reviewed by DSMB. Two safety reviews will be performed after the first 25 and the first 50 patients have completed the 72-h assessments postrandomization. Furthermore, one more efficacy and safety interim analysis will be performed after 50% of total patients have completed their assessment on Day 90. An interim efficacy is evaluated at 0.003 significance level (two sided).
Study endpoints and expected outcomes
Mainly, the present study is conducted to determine the safety and efficacy of injection tNK, with the primary endpoint being ordinal mRS at Day 90, as illustrated in Figure 2.
Figure 2.
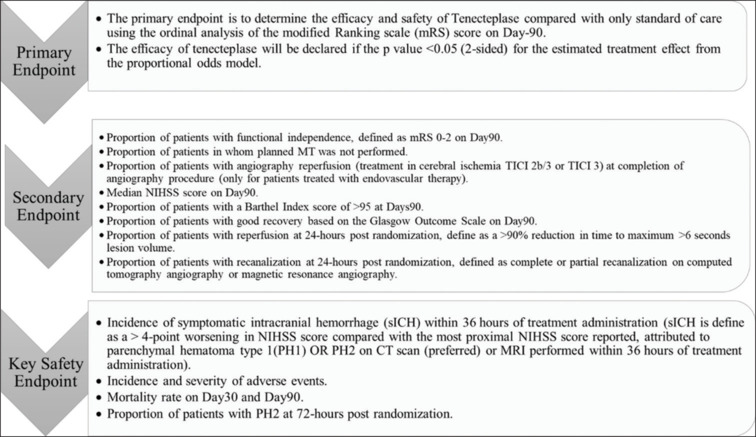
Study endpoints
Primary outcome
Safety and efficacy will be determined by measuring the physical function through mRS after a follow-up period of 3 months (90 days).
Secondary outcome
Proportion of participants with a poor outcome, which is defined as death or major disability. Major disability is defined as a score of 4–5 on mRS at 90 days after randomization.
All-cause mortality in the first 30 days postrandomization
Adverse events
The Cochrane review on this topic states, “The most common side effects of tenecteplase treatment are intracranial and or extracranial bleeding, allergic reactions but none of them had reached up to the statistically significant difference between the treatment and control groups.” All adverse effects are provided standard treatment.
It is the duty of the principal investigator (PI) to inform the sponsor (funding agency) and DSMB within 48 h of when the event comes to knowledge. PI shall also be responsible to prepare safety updates for informing serious adverse events to the institute ethics committee within 14 days. Flow diagram for severe adverse events happening in the clinical trial is shown in Figure 3.
Figure 3.
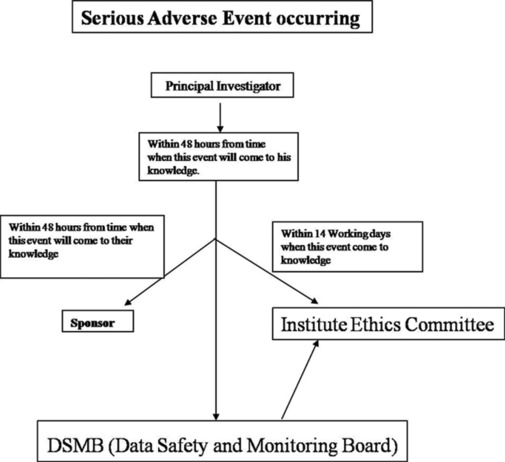
Flow diagram for adverse events
Follow-up and data collection
After discharge from the hospital, all subjects are followed up at 3 months by a trained research worker who is blinded to the allocation arm of the patient. Follow-up of the patient is either done telephonically or in person.
Statistical analysis
Sample size calculation
Considering the DAWN trial (5) wherein thrombectomy was performed between 6 and 24 h of the onset of stroke the power two means 3.4, 5.5, sd1 (3.1) sd2 (3.8) [Control, thrombectomy case]. Considering an alpha of 0.05, a power of 80%, and approximately about 10% lost to follow-up, 100 cases will be randomized (50 cases in each arm).
Analysis
The analysis of the data will be performed as per intention-to-treat and per-protocol-analysis principles. The primary outcome of the study is to evaluate the efficacy determined by mRS at 90 days in the injection tNK arm compared to the placebo arm. This analysis will be estimated as an OR with ordinal logistics regression. Any chance of imbalance in prognostics will be adjusted using multivariable regression analysis.
Analysis of secondary outcome variables will be performed using simple two by two tables with Mann–Whitney tests, t-tests, multivariable linear and logistic regression models as per the requirements. All statistical uncertainty will be expressed using 95% CI in all statistical analyses. A P-value < 0.05 for the estimated treatment effect will be considered significant.
DISCUSSION
A previous report states that tNK administration before thrombectomy is accompanied by an improved frequency of reperfusion and a good rate of functional outcome. Results showed that treatment of 22% of patients with tNK versus 10% of patients who received alteplase met the primary end point of reperfusion of greater than 50% in involved territory or absence of retrievable thrombus in initial angiography.[7] In the largest ASSENT-2 trial, patients with acute myocardial infarction randomized to tNK had decreased rates of complications like noncerebral bleeding (26.43% vs. 28.95%, P =0.0003) along with lesser rate of blood transfusion (4.25% vs. 5.49%, P =0.0002) in comparison of patients who had received alteplase.[19] Our EAST-AIS trial will provide an understanding of the safety and efficacy of injection tNK in AIS patients (with or without LVO) presenting in the late window period (4.5–24 h) and having imaging eligibility. It shall also help to determine the rate of recanalization of vessels causing stroke and reduction of need for decompressive hemicraniectomy in cases of LVOs. This study will also ascertain the clinical significance of tNK by evaluating mRS, National Institutes of Health Stroke Scale (NIHSS), and Thrombolysis in Cerebral Infarction (TICI) scores to assess patient’s health. This trial is also beneficial in cases of wake-up stroke, where the actual onset of stroke is unknown but sleeping time is known. It also signifies the affordability of therapy due to its lower price in comparison to alteplase and its more practical use: a single bolus and there is no requirement for an IV infusion pump.
Trial status
The study is ongoing and recruiting eligible patients. Patient enrolment began on 1st August, 2022 and expected date of completion of study is January 2026.
Financial support and sponsorship
The study is funded by Science and Engineering Research Board, Department of Science and Technology, (SERB-DST), Government of India.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Kamalakannan S, Gudlavalleti ASV, Gudlavalleti VSM, Goenka S, Kuper H. Incidence and prevalence of stroke in India: A systematic review. Indian J Med Res. 2017;146:175–85. doi: 10.4103/ijmr.IJMR_516_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen CH, Tang SC, Tsai LK, Hsieh MJ, Yeh SJ, Huang KY, et al. Stroke code improves intravenous thrombolysis administration in acute ischemic stroke. PLoS One. 2014;9:e104862. doi: 10.1371/journal.pone.0104862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Badachi S, Mathew T, Prabhu A, Nadig R, Sarma GR. Hurdles in stroke thrombolysis: Experience from 100 consecutive ischemic stroke patients. Ann Indian Acad Neurol. 2015;18:415–8. doi: 10.4103/0972-2327.165460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. 2018 guidelines for the early management of patients with acute ischemic stroke: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018;49:e46–110. doi: 10.1161/STR.0000000000000158. [DOI] [PubMed] [Google Scholar]
- 5.Saver JL, Goyal M, van der Lugt A, Menon BK, Majoie CB, Dippel DW, et al. Time to treatment with endovascular thrombectomy and outcomes from ischemic stroke: A meta-analysis. JAMA. 2016;316:1279–88. doi: 10.1001/jama.2016.13647. [DOI] [PubMed] [Google Scholar]
- 6.Emberson J, Lees KR, Lyden P, Blackwell L, Albers G, Bluhmki E, et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: A meta-analysis of individual patient data from randomised trials. Lancet. 2014;384:1929–35. doi: 10.1016/S0140-6736(14)60584-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campbell BCV, Mitchell PJ, Churilov L, Yassi N, Kleinig TJ, Dowling RJ, et al. Tenecteplase versus alteplase before thrombectomy for ischemic stroke. N Engl J Med. 2018;378:1573–82. doi: 10.1056/NEJMoa1716405. [DOI] [PubMed] [Google Scholar]
- 8.Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P, et al. Thrombectomy 6 to 24 Hours after Stroke with a Mismatch between Deficit and Infarct. N Engl J Med. 2018;378:11–21. doi: 10.1056/NEJMoa1706442. [DOI] [PubMed] [Google Scholar]
- 9.Ma H, Campbell BCV, Parsons MW, Churilov L, Levi CR, Hsu C, et al. Thrombolysis guided by perfusion imaging up to 9 hours after onset of stroke. N Engl J Med. 2019;380:1795–803. doi: 10.1056/NEJMoa1813046. [DOI] [PubMed] [Google Scholar]
- 10.Parsons MW, Miteff F, Bateman GA, Spratt N, Loiselle A, Attia J, et al. Acute ischemic stroke: Imaging-guided tenecteplase treatment in an extended time window. Neurology. 2009;72:915–21. doi: 10.1212/01.wnl.0000344168.05315.9d. [DOI] [PubMed] [Google Scholar]
- 11.Haley EC, Jr., Lyden PD, Johnston KC, Hemmen TM Investigators TNKiS. A pilot dose-escalation safety study of tenecteplase in acute ischemic stroke. Stroke. 2005;36:607–12. doi: 10.1161/01.STR.0000154872.73240.e9. [DOI] [PubMed] [Google Scholar]
- 12.Parsons M, Spratt N, Bivard A, Campbell B, Chung K, Miteff F, et al. A randomized trial of tenecteplase versus alteplase for acute ischemic stroke. N Engl J Med. 2012;366:1099–107. doi: 10.1056/NEJMoa1109842. [DOI] [PubMed] [Google Scholar]
- 13.Smalling RW. Molecular biology of plasminogen activators: What are the clinical implications of drug design? Am J Cardiol. 1996;78:2–7. doi: 10.1016/s0002-9149(96)00736-9. [DOI] [PubMed] [Google Scholar]
- 14.Collen D, Stassen JM, Yasuda T, Refino C, Paoni N, Keyt B, et al. Comparative thrombolytic properties of tissue-type plasminogen activator and of a plasminogen activator inhibitor-1-resistant glycosylation variant, in a combined arterial and venous thrombosis model in the dog. Thromb Haemost. 1994;72:98–104. [PubMed] [Google Scholar]
- 15.Keyt BA, Paoni NF, Refino CJ, Berleau L, Nguyen H, Chow A, et al. A faster-acting and more potent form of tissue plasminogen activator. Proc Natl Acad Sci U S A. 1994;91:3670–4. doi: 10.1073/pnas.91.9.3670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thelengana A, Radhakrishnan DM, Prasad M, Kumar A, Prasad K. Tenecteplase versus alteplase in acute ischemic stroke: Systematic review and meta-analysis. Acta Neurol Belg. 2019;119:359–67. doi: 10.1007/s13760-018-0933-9. [DOI] [PubMed] [Google Scholar]
- 17.Kate M, Wannamaker R, Kamble H, Riaz P, Gioia LC, Buck B, et al. Penumbral imaging-based thrombolysis with tenecteplase is feasible up to 24 hours after symptom onset. J Stroke. 2018;20:122–30. doi: 10.5853/jos.2017.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Albers GW, Campbell BC, Lansberg MG, Broderick J, Butcher K, Froehler MT, et al. A phase III, prospective, double-blind, randomized, placebo-controlled trial of thrombolysis in imaging-eligible, late-window patients to assess the efficacy and safety of tenecteplase (TIMELESS): Rationale and design. Int J Stroke. 2022;18:237–41. doi: 10.1177/17474930221088400. [DOI] [PubMed] [Google Scholar]
- 19.Assessment of the Safety and Efficacy of a New Thrombolytic (ASSENT-2) Investigators. Van De Werf F, Adgey J, Ardissino D, Armstrong PW, Aylward P, et al. Single-bolus tenecteplase compared with front-loaded alteplase in acute myocardial infarction: The ASSENT-2 double-blind randomised trial. Lancet. 1999;354:716–22. doi: 10.1016/s0140-6736(99)07403-6. [DOI] [PubMed] [Google Scholar]


