Abstract
We demonstrate that dendritic cells loaded in vitro with human immunodeficiency virus type 1 (HIV-1) protein-liposome complexes activate HLA class I-restricted anti-HIV-1 cytotoxic T-lymphocyte and gamma interferon (IFN-γ) responses in autologous CD8+ T cells from late-stage HIV-1-infected patients on prolonged combination drug therapy. Interleukin-12 enhanced this effect through an interleukin-2- and IFN-γ-mediated pathway. This suggests that dendritic cells from HIV-1-infected persons can be engineered to evoke stronger anti-HIV-1 CD8+ T-cell reactivity as a strategy to augment antiretroviral therapy.
Maintenance of high levels of anti-human immunodeficiency virus type 1 (HIV-1) CD8+ memory cytotoxic T-lymphocyte (CTL) and gamma interferon (IFN-γ) responses may be important in preventing HIV-1 disease (3). We (17, 18) and others (12, 14) have shown that anti-HIV-1 CTL and IFN-γ responses decline with HIV-1 disease progression. Therapies that could reverse this decline in HIV-1-specific T-cell function may be important in delaying or preventing disease progression. Recent evidence indicates that suppression of HIV-1 RNA by combination antiretroviral therapy can augment anti-HIV-1 CD8+ T-cell responses, but this effect is only temporary (11, 16, 18). New strategies are thus needed to enhance HIV-1-specific CD8+ T-cell responses in patients receiving combination antiretroviral therapy.
Dendritic cells (DC) are highly specialized antigen-presenting cells (APC) for both primary and secondary T-cell responses (1). This function of DC is related to their expression of high levels of major histocompatibility complex (MHC) class I and II antigens, costimulatory molecules CD40, CD80, and CD86, and production of cytokines such as interleukin 12 (IL-12). DC can process antigens in various forms by MHC class I and II pathways to induce antigen-specific CD4+ and CD8+ T-cell reactivity (19). We hypothesize that DC harvested from HIV-1-infected subjects can be engineered to evoke stronger anti-HIV-1 CD8+ T-cell reactivity and thus potentially augment the antiviral activity of currently available antiretroviral therapies. This hypothesis is based on our previous observation that, in the absence of effective antiretroviral therapy, DC maintain their capacity to activate anti-HIV-1 CTL in vitro for many years of HIV-1 infection, even though CD8+ T cells eventually lose their ability to respond to the DC (5).
We have developed an approach for delivery of HIV-1 Gag, Pol, and Env proteins to autologous DC by cationic liposome to activate anti-HIV-1 CD8+ T cells (21). In the present study, we assessed the ability of autologous DC loaded with HIV-1 protein-liposome complexes to augment HIV-1-specific CD8+ T-cell responses in cells obtained from five HIV-1-infected patients with advanced immunodeficiency who were receiving combination antiretroviral therapy (zidovudine, lamivudine, and indinavir) in the Merck 035 trial (patients A1160, A1179, B1178, C1156, and C1158) (18) and one subject (E18) in the Multicenter AIDS Cohort Study (MACS). Additional peripheral blood mononuclear cells (PBMC) were obtained from two subjects in the MACS who were being treated with indinavir, lamivudine, stavudine, and DMP-266 (subject N1) or indinavir, lamivudine, stavudine, and nelfinavir (subject E16). At the time of this immunologic study, HIV-1 RNA load was very low or undetectable (<50 to 300 copies per ml of serum) by the Roche ultrasensitive PCR assay (18), and the number of CD4+ T cells was a median of 517 (range, 294 to 1,008) per mm3 of blood. Normal, HIV-1-negative volunteers served as controls.
We first characterized the phenotype of DC cultured from PBMC of these patients on long-term antiretroviral therapy. To obtain enough DC for phenotypic analysis, cryopreserved PBMC were in some cases combined from different time points late in the course of therapy, when the patients' viral loads were consistently below detectable limits. Plastic-adherent PBMC were cultured for 7 to 21 days in AIM V medium containing recombinant human IL-4 (1,000 U/ml) (Schering-Plough, Kenilworth, N.J.) and recombinant human granulocyte-monocyte colony-stimulating factor (GM-CSF) (1,000 U/ml) (Schering-Plough) at 37°C in humidified air containing 5% CO2 (5, 21). Fresh IL-4 and GM-CSF were added at 2- to 3-day intervals. In certain experiments, DC were loaded with liposome-complexed HIV-1 proteins on days 5 to 7, incubated at 37°C for 16 h, and then treated with CD40 ligand (CD40L; 2.5 μg/ml; Immunex, Seattle, Wash.) for 1 to 2 days to augment phenotypic and functional maturation.
The DC cultures were analyzed for background staining using isotypic controls and then stained with PE5 (phycoerythrin-cyanin 5.1)-labeled anti-HLA DR monoclonal antibody (MAb) (Immunotech, Marsille, France) and phycoerythrin (PE)-labeled MAb to lineage markers CD3, CD14, CD16, CD19, and CD56 (Becton Dickinson, Mountain View, Calif.). The cells were then assessed by flow cytometry (Elite; Coulter) by gating on large cells with high side scatter (DC gate) and analyzing on a two-color histogram. Only cells that were positive for HLA DR and negative for the lineage markers were defined as DC. These HLA DR+ DC were further gated and analyzed for DC markers with fluorescein isothiocyanate (FITC)-conjugated MAbs specific for CD80 (Immunotech), CD86 (Ancell, Bayport, Minn.), and CD40 (Ancell). Results were expressed as a percentage of the HLA DR+ DC. Cells within the DC gate were also analyzed with anti-CD83-PE (Immunotech).
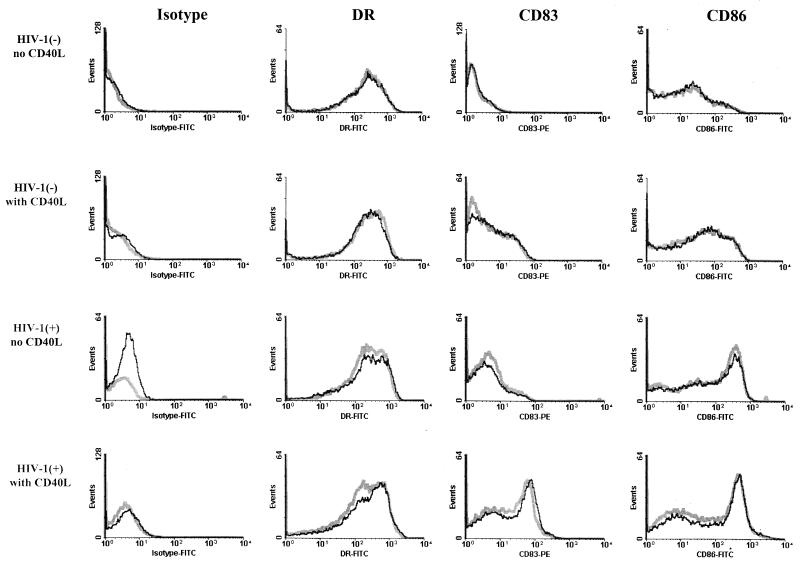
The results show that these cells had the phenotypic characteristics of immature DC (1) after 7 days of culture in that they expressed relatively high levels of CD40 and HLA DR, moderate levels of CD80 and CD86, and very small amounts of CD83 (representative data are shown in Fig. 1). This was comparable to the expression of these markers on DC derived from healthy, HIV-1-negative persons. After culture for an additional 7 to 14 days, the levels of these markers did not increase (data not shown). In contrast, treatment of the 7-day cultures of DC for 2 days with CD40L consistently induced a phenotypic maturation of the DC similar to that noted in DC from HIV-1-negative persons, shown by an increase in the percentage and mean fluorescence intensity of DC expressing CD86 and CD83 (Fig. 1). The surface expression of these markers was not affected by loading the DC with the liposome–HIV-1 protein preparations prior to stimulation of the T cells. Thus, immature DC can be derived by culture of blood monocytes in IL-4 and GM-CSF in late-stage patients whose viral load was suppressed by antiretroviral therapy and can be matured by treatment with CD40L.
FIG. 1.
Immature DC loaded with cationic liposome complexed with HIV-1 p55 Gag were treated with CD40L and showed phenotypic characteristics of mature DC. The DC were derived from PBMC obtained from a representative HIV-1-negative subject and an HIV-1-positive subject (E18; 224 weeks of combination drug therapy). DC were cultured for 5 days in IL-4 and GM-CSF, at which time a portion of the DC were loaded with liposome and p55 Gag. On day 6, a portion of the antigen-loaded DC and nonloaded DC were treated with CD40L. Phenotyping with the four MAbs was done after 7 days of culture. Gray lines and black lines represent fluorescence intensity of DC cultured without p55 antigen and with p55 antigen, respectively.
PBMC were obtained from the patients on long-term combination therapy when CD8+ CTL activity specific for Gag, Pol, and Env was very low (3% ± 0.8% [mean ± standard error (SE)] HIV-1-specific lysis, n = 36, at all effector-to-target cell [E:T] ratios) by our standard antigen stimulation method that uses autologous B lymphoblastoid cell lines (B-LCL) infected with vaccinia virus–HIV-1 vectors as APC (18). Autologous, immature DC were loaded with HIV-1 Gag, Pol, or Env or mock bacterial recombinant proteins (Protein Sciences, Meriden, Conn.) that were complexed with cationic liposome (Lipofectin; Gibco, Grand Island, N.Y.) (106 DC with 15 μg of Lipofectin and 20 μg of protein in 1 ml of AIM-V medium) (21). We have shown that cationic liposome augments the entry of protein into both immature DC and mature DC (X.-L. Huang et al., unpublished data). The DC were then incubated for 4 h at 37°C in a 5% CO2 atmosphere and either treated with psoralen (10 μg/ml; Sigma, St. Louis, Mo.) and long-wave UV irradiation for 5 min or left untreated. The psoralen-UV treatment of the DC was done to enhance immunogenicity and inactivate any residual HIV-1 (7, 10).
The PBMC were stimulated repetitively with the psoralen-UV-treated or untreated DC at a 10-to-1 ratio in RPMI 1640 with 15% fetal calf serum and recombinant IL-2 (Chiron, Emeryville, Calif.; 100 U/ml) for an initial 2-week interval, followed by restimulation at 1-week intervals for up to 6 weeks. Fresh complete medium and IL-2 were added every 5 days. In some experiments, gamma-irradiated PBMC from HIV-1-uninfected allogeneic donors were added as feeder cells. We have found that such feeder cells increase the outgrowth of T cells by approximately 50% in these cultures compared to cultures without feeder cells (i.e., the number of cells after 2 weeks of culture with feeders was 0.11 [±0.09] × 106, compared to 0.08 [±0.07] × 106 cells without feeders; n = 4, P = 0.02, paired t test, with >90% being T cells). However, comparable levels of anti-HIV-1 CD8+ T-cell activity were induced by the HIV-1 antigen-expressing DC with or without the addition of feeder cells (data not shown). The stimulated cells were assessed for anti-HIV-1 bulk CTL activity by a standard 51Cr release assay at a 20:1 E:T ratio using autologous B-LCL infected with vaccinia virus–HIV-1 vectors or loaded with synthetic peptides (Peptide Synthesis Facility, University of Pittsburgh Cancer Institute) representing known HLA A∗02 epitopes for HIV-1 as targets (18). Data were expressed as percent HIV-1-specific lysis, i.e., percent lysis of the HIV-1 antigen-expressing targets minus the percent lysis of the non-antigen-expressing targets. For assessment of IFN-γ production, a quantitative single-cell enzyme immunoassay was done on the stimulated PBMC or enriched CD8+ T cells (9, 18). The latter data were expressed as the number of spots produced in response to HIV-1 antigen minus the number of spots produced in the control cultures per 106 total cells.
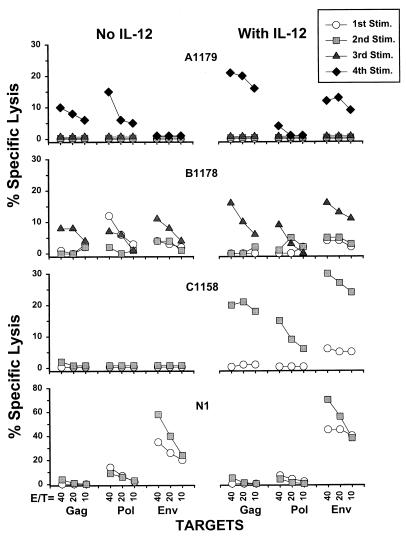
A single 2-week stimulation of PBMC with autologous, immature DC that had been loaded with liposome-complexed HIV-1 proteins and treated with psoralen and UV induced HIV-1-specific CTL responses in only two of the four patients (Pol for patient B1178 and Env for patient N1; Fig. 2). Two to four repetitive stimulations with these DC loaded with the HIV-1 protein-liposome complexes resulted in CTL activity against at least 1 HIV-1 protein in three of the four patients. This enhancing effect was also noted with DC and T cells obtained from the patients at later times after initiation of therapy (data not shown). Sequential, multiple rounds of stimulation with the immature DC were required to activate the CD8+ T cells, as one or two rounds of stimulation with antigen-loaded DC followed by sequential stimulations with antigen-loaded PBMC induced several-fold-lower levels of anti-HIV-1 CTL (data not shown). MHC blocking and immunomagnetic-bead negative selection studies (9) indicated that the CTL response induced by the antigen-loaded DC and using the vaccinia virus vector-infected targets was HLA class I restricted and mediated by CD8+ T cells (data not shown).
FIG. 2.
Augmented CTL responses to HIV-1 by up to four repetitive stimulations (Stim.) of PBMC obtained from patients on combination antiretroviral therapy (duration of 128 weeks for patient A1179, 88 weeks for patients B1178 and C1158, and 60 weeks for patient N1) with autologous, immature, and psoralen-UV-treated DC loaded with liposome complexed with one of the three major HIV-1 structural proteins (Gag, Pol, and Env) and added with or without IL-12. Percent virus-specific lysis is defined in the text. The mean ±SE background lysis of the vaccinia virus-mock-infected targets in our laboratory was 22% ± 1.4%, n = 154, 17% ± 1.0%, n = 218, and 11% ± 0.8%, n = 217 at E:T ratios of 40:1, 20:1, and 10:1, respectively.
Anti-HIV-1 CTL responses were augmented by recombinant IL-12 (R & D Systems, Minneapolis, Minn.) (Fig. 2) added at a concentration of 100 pg/ml that we (Rinaldo et al., unpublished results) and others (2) have previously found to enhance antiviral CD8+ T-cell activity. Higher CTL responses were induced in the presence of IL-12 against Gag and Env in patients A1179 and B1178; against Gag, Pol, and Env in patient C1158; and against Env in patient N1 (P < 10−5 for specific lysis induced by DC in the presence of IL-12 compared to no IL-12 for the four patients, paired t test).
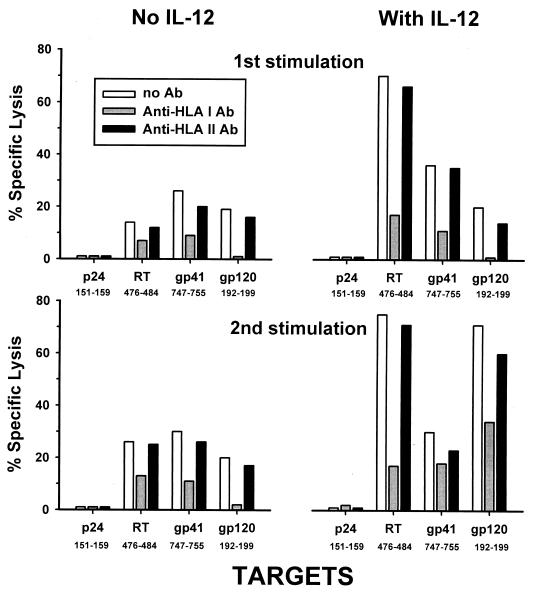
We next examined whether the protein-liposome-loaded immature DC could stimulate CTL activity to multiple known epitopes of the virus. Figure 3 shows that PBMC which were stimulated with immature DC loaded with Gag, Pol, and Env had CTL activity specific for three HLA A∗02 epitopes of HIV-1 (RT476–484, gp41747–755, and gp120192–199), with no CTL response noted to a fourth HIV-1 epitope (p24151–159). The CTL responses to RT476–484 and gp120192–199 but not to gp41742–755 were also enhanced by addition of IL-12 to the cultures. This response was predominantly HLA class I restricted, as shown by blockage of the CTL responses (approximately 70%) by anti-HLA class I MAb but not by HLA class II MAb.
FIG. 3.
CTL responses specific for HIV-1 peptides of known HLA A∗02 epitopes induced by one or two stimulations in vitro with immature, psoralen-UV-treated DC loaded with HIV-1 p24 Gag, Pol, or Env complexed to liposome, with or without IL-12, in patient N1 (week 60 of treatment). The CTL responses were inhibited by anti-HLA class I MAb but not anti-HLA class II MAb. Data are percent virus-specific lysis at an E:T ratio of 20:1.
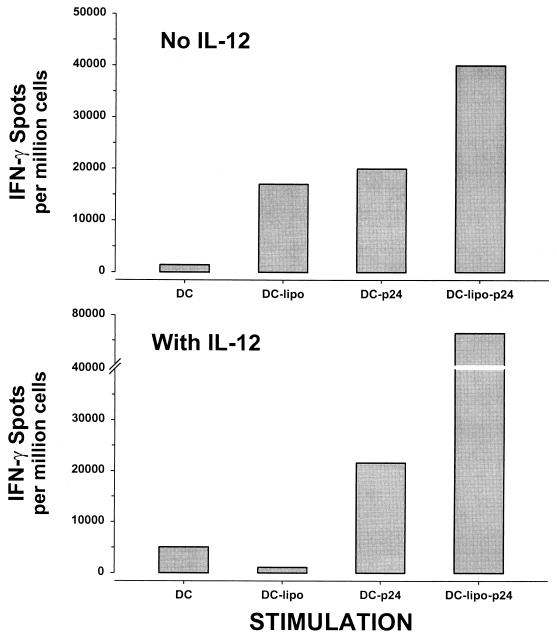
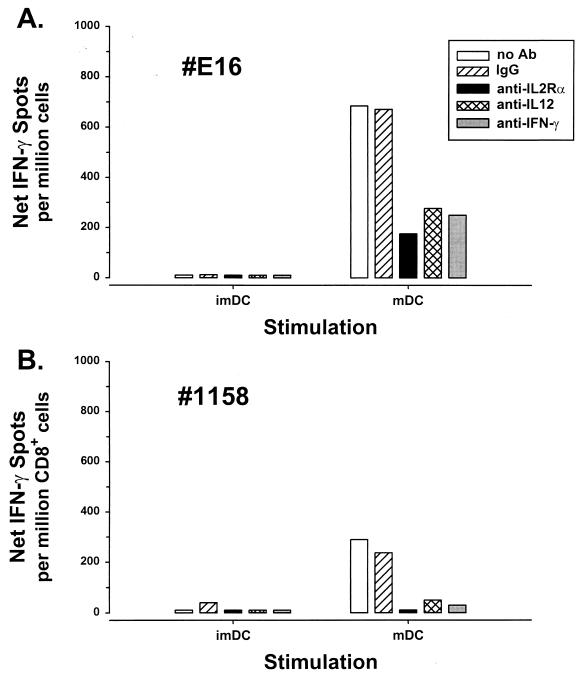
Next, we investigated another important antiviral activity, IFN-γ production, in CD8+ T cells from these patients. We first used a procedure similar to that for repetitive stimulation of CTL activity in PBMC with immature DC loaded with liposome-complexed HIV-1 protein. As with CTL responses, HIV-1-specific IFN-γ production was induced in PBMC or enriched CD8+ T cells from HIV-1-infected patients on long-term combination drug therapy that were repetitively stimulated with autologous, immature DC loaded with HIV-1 p24 Gag and liposome (Fig. 4). This T-cell response was enhanced by addition of IL-12. Because maturation of DC is known to enhance activation of antiviral T-cell responses (8), we directly compared immature DC with CD40L-matured DC for activation of HIV-1-specific IFN-γ production. CD40L-matured DC were superior to immature DC in that they consistently activated HIV-1-specific IFN-γ production after either one or two repetitive stimulations of CD8+ T cells from patients who had no detectable IFN-γ production by conventional stimulation with HIV-1 antigen-expressing B-LCL (Fig. 5). Moreover, mature DC that were loaded with p24 and liposome and treated with psoralen and UV stimulated T-cell activity similar to that by antigen-loaded, untreated DC (215 spots per 106 PBMC stimulated by antigen-loaded, psoralen-UV-treated DC compared to 345 spots per 106 PBMC for antigen-loaded, untreated DC).
FIG. 4.
Anti-HIV-1 production of IFN-γ by CD8+ T cells after two in vitro stimulations of CD8+ T cells from patient A1179 (week 124 of treatment) with immature, psoralen-UV-treated DC loaded with HIV-1 Gag p24-liposome, with or without addition of IL-12. DC, no liposome or antigen added; DC-lipo, liposome alone added; DC-p24, p24 Gag added; DC-lipo-p24, liposome-complexed p24.
FIG. 5.
Anti-HIV-1 IFN-γ production by (A) PBMC from subject E16 at 146 weeks of treatment or (B) CD8+ T cells from patient C1158 at 114 weeks of treatment, stimulated twice for 1 week each with immature DC (imDC) or CD40L-matured DC (mDC), non-psoralen-UV-treated DC loaded with liposome-complexed p24 Gag and IL-12. The responses were inhibited by anti-IL-2Rα, anti-IL-12, and anti-IFN-γ MAbs that were added to the wells containing responder cells for 3 days before the T-cell assay.
Activation of anti-HIV-1 CD8+ T-cell reactivity by the mature DC was inhibited by anti-IL-2 receptor α (IL-2Rα) and anti-IFN-γ MAbs as well as by anti-IL-12 MAb (1 μg/ml; R & D Systems) (Fig. 5). We found in further studies that the psoralen-UV-treated and untreated DC did not differ in the amount of IL-12 p70 produced in response to CD40L (85 ± 48 pg/ml for treated DC versus 107 ± 61 pg/ml for untreated DC; n = 8, P = 0.38, Student t test; IL-12 ELISA, R & D Systems). This supports the role of IL-12 in activation of CD8+ T-cell immunity by CD40L-matured DC (4, 13) and suggests that the IL-12 effect was mediated through these IL-2 and IFN-γ pathways.
To confirm the specificity of CD8+ T-cell responses activated by DC and IL-12, immature DC were loaded with an HLA A∗02-associated, synthetic HIV-1 Gag peptide (p1777–85) (100 μg/ml) without liposome for 2 h at 37°C in a 5% CO2 atmosphere. In a representative experiment, two repetitive stimulations of PBMC from patient A1160 (at 84 weeks of combination therapy) with the peptide-loaded DC without IL-12 stimulated 25 IFN-γ spots per 106 PBMC, whereas addition of IL-12 during the stimulation resulted in 7,150 IFN-γ spot-forming cells per 106 PBMC. Addition of anti-IL-2Rα or anti-IL-12 MAb to the untreated cultures blocked all detectable IFN-γ-producing cells, and addition of these MAb to the IL-12-treated cultures reduced the number of IFN-γ-producing cells to 225 and 0 spots per 106 PBMC, respectively.
Thus, DC from late-stage HIV-1-infected patients on combination therapy retained their APC function for activation of anti-HIV-1 CD8+ T-cell responses in that repetitive stimulation by immature DC induced such T-cell activity. Mature DC, defined by their increased capacity to express antigen and cytokines as well as surface costimulatory and activation markers, are considered optimal for stimulation of T cells (1, 19). In this regard, we found that maturation of the DC by treatment with CD40L increased their ability to stimulate anti-HIV-1 CD8+ T-cell reactivity. These data support the use of CD40L as a consistent, controlled approach for induction of DC maturation. Of additional note is that the antigen-presenting properties of immature and mature DC were similar for psoralen-UV-treated and untreated DC. Thus, both forms of in vitro-manipulated DC may have application to clinical immunotherapy trials of HIV-1 infection using infusion of ex vivo-engineered DC.
The results show that, in addition to having monocytic precursors that are capable of becoming immunocompetent DC, HIV-1-specific CD8+ T cells can still be activated in patients with late-stage HIV-1 infection after suppression of their viral load with combination therapy. This concurs with our finding that there is a residual population of memory T cells specific for binding of HLA class I–HIV-1 peptide tetramers in patients on long-term combination drug therapy (18). Furthermore, others have reported that interruption of therapy with combination antiretroviral drugs results in the temporary return of anti-HIV-1 CD8+ T-cell reactivity in late-stage patients (15). Thus, immunocompetent, HIV-1-specific CD8+ T cells remain in the circulation of persons with advanced HIV-1 infection who are responding to antiretroviral therapy. This supports the concept that approaches using either ex vivo loading and reinfusion of DC or direct in vivo targeting of DC with liposome-complexed HIV-1 antigen could augment host anti-HIV-1 T-cell immunity in such patients. Our protein-based antigen method also has the potential advantage over live HIV-1 vectors in safety and ease of use and over HIV-1 peptides in the lack of restriction to specific HLA genotypes.
Addition of IL-12 in vitro enhanced the anti-HIV-1 T-cell responses induced by the antigen-loaded DC in most patients. Indeed, we have observed that liposome-complexed HIV-1 protein induces IL-12 in both immature and mature DC (C. R. Rinaldo, Jr., unpublished results). The IL-12 enhancement of anti-HIV-1 CTL activity and IFN-γ production by the protein-liposome-loaded DC was shown to be an IL-2- and IFN-γ-related effect, similar to that reported for other IL-12-augmented CD8+ T-cell responses (20). This does not exclude other possible mechanisms of IL-12 activation of T-cell reactivity, such as inhibition of CD8+ T-cell apoptosis. These data support the use of recombinant IL-12 protein or potentially IL-12 DNA (6) as part of an adjunct therapy for augmenting DC-mediated antiviral T-cell responses.
We have recently shown that a portion of protein complexed with cationic liposome is processed through the HLA class I pathway in DC (21; Huang et al., unpublished results). Repetitive stimulation of PBMC with these DC that are loaded with either HIV-1 proteins or ovalbumin can prime HIV-1-and ovalbumin-specific CD8+ T cells from HIV-1-negative persons. These studies suggest that anti-HIV-1 CD8+ T-cell responses induced in the present investigation were mediated by either residual memory T cells or naive T cells. This anti-HIV-1 CD8+ T-cell induction system could therefore apply to development of both therapeutic and prophylactic HIV-1 vaccines.
Acknowledgments
This project was supported by NIH grants R01-AI41870, U01-AI37984, and U01-AI35041 and a grant from Merck Research Laboratories.
We thank E. Thomas and K. Picha of Immunex for the gift of CD40L; S. Narula of Schering-Plough for the gift of IL-4 and GM-CSF; M. Lotze for the gift of a portion of the IL-12; A. Zeevi and W. Hildebrand for HLA typing; J. Liebmann, A. Bazmi, C. Kalinyak, E. Molina, S. McQuiston, B. Colleton, H. Li, M. Tseng, and E. Ramirez for technical assistance; and W. Buchanan, B. Calhoun, T. Silvestre, L. Johnson, N. Mantz, J. Stewart, A. Sparks, and R. Kudray for clinical assistance.
REFERENCES
- 1.Bell D, Young J W, Banchereau J. Dendritic cells. Adv Immunol. 1999;72:255–324. doi: 10.1016/s0065-2776(08)60023-1. [DOI] [PubMed] [Google Scholar]
- 2.Bhardwaj N, Seder R A, Reddy A, Feldman M V. IL-12 in conjunction with dendritic cells enhances antiviral CD8+ CTL responses in vitro. J Clin Investig. 1996;98:715–722. doi: 10.1172/JCI118843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brander C, Walker B D. T lymphocyte responses in HIV-1 infection: implications for vaccine development. Curr Opin Immunol. 1999;11:451–459. doi: 10.1016/S0952-7915(99)80076-4. [DOI] [PubMed] [Google Scholar]
- 4.Clarke S R. The critical role of CD40/CD40L in the CD4-dependent generation of CD8+ T cell immunity. J Leukocyte Biol. 2000;67:607–614. doi: 10.1002/jlb.67.5.607. [DOI] [PubMed] [Google Scholar]
- 5.Fan Z, Huang X-L, Zheng L, Wilson C, Borowski L, Liebmann J, Gupta P, Margolick J, Rinaldo C. Cultured blood dendritic cells retain HIV-1 antigen-presenting capacity for memory CTL during progressive HIV-1 infection. J Immunol. 1997;159:4973–4982. [PubMed] [Google Scholar]
- 6.Gurunathan S, Prussin C, Sacks D L, Seder R A. Vaccine requirements for sustained cellular immunity to an intracellular parasitic infection. Nat Med. 1998;4:1409–1415. doi: 10.1038/4000. [DOI] [PubMed] [Google Scholar]
- 7.Hanson C V. Photochemical inactivation of viruses with psoralens: an overview. Blood Cells. 1992;18:7–25. [PubMed] [Google Scholar]
- 8.Herr W, Ranieri E, Olson W, Zarour H, Gesualdo L, Storkus W J. Mature dendritic cells pulsed with freeze-thaw cell lysates define an effective in vitro vaccine designed to elicit EBV-specific CD4(+) and CD8(+) T lymphocyte responses. Blood. 2000;96:1857–1864. [PubMed] [Google Scholar]
- 9.Huang X L, Fan Z, Kalinyak C, Mellors J W, Rinaldo C R., Jr CD8+ T-cell gamma interferon production specific for human immunodeficiency virus type 1 (HIV-1) in HIV-1-infected subjects. Clin Diagn Lab Immunol. 2000;7:279–287. doi: 10.1128/cdli.7.2.279-287.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson R P, Glickman R L, Yang J Q, Kaur A, Dion J T, Mulligan M J, Desrosiers R C. Induction of vigorous cytotoxic T-lymphocyte responses by live attenuated simian immunodeficiency virus. J Virol. 1997;71:7711–7718. doi: 10.1128/jvi.71.10.7711-7718.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kalams S A, Goulder P J, Shea A K, Jones N G, Trocha A K, Ogg G S, Walker B D. Levels of human immunodeficiency virus type 1-specific cytotoxic T-lymphocyte effector and memory responses decline after suppression of viremia with highly active antiretroviral therapy. J Virol. 1999;73:6721–6728. doi: 10.1128/jvi.73.8.6721-6728.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klein M R, van Baalen C A, Holwerda A M, Kerkhof Garde S R, Bende R J, Keet I P, Eeftinck-Schattenkerk J K, Osterhaus A D, Schuitemaker H, Miedema F. Kinetics of Gag-specific cytotoxic T lymphocyte responses during the clinical course of HIV-1 infection: a longitudinal analysis of rapid progressors and long-term asymptomatics. J Exp Med. 1995;181:1365–1372. doi: 10.1084/jem.181.4.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mosca P J, Hobeika A C, Clay T M, Nair S K, Thomas E K, Morse M A, Lyerly H K. A subset of human monocyte-derived dendritic cells expresses high levels of interleukin-12 in response to combined CD40 ligand and interferon-gamma treatment. Blood. 2000;96:3499–3504. [PubMed] [Google Scholar]
- 14.Ogg G S, Kostense S, Klein M R, Jurriaans S, Hamann D, McMichael A J, Miedema F. Longitudinal phenotypic analysis of human immunodeficiency virus type 1-specific cytotoxic T lymphocytes: correlation with disease progression. J Virol. 1999;73:9153–9160. doi: 10.1128/jvi.73.11.9153-9160.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ortiz G M, Nixon D F, Trkola A, Binley J, Jin X, Bonhoeffer S, Kuebler P J, Donahoe S M, Demoitie M A, Kakimoto W M, Ketas T, Clas B, Heymann J J, Zhang L, Cao Y, Hurley A, Moore J P, Ho D D, Markowitz M. HIV-1-specific immune responses in subjects who temporarily contain virus replication after discontinuation of highly active antiretroviral therapy. J Clin Investig. 1999;104:R13–R18. doi: 10.1172/JCI7371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pontesilli O, Kerkhof-Garde S, Notermans D W, Foudraine N A, Roos M T, Klein M R, Danner S A, Lange J M, Miedema F. Functional T cell reconstitution and human immunodeficiency virus-1-specific cell-mediated immunity during highly active antiretroviral therapy. J Infect Dis. 1999;180:76–86. doi: 10.1086/314837. [DOI] [PubMed] [Google Scholar]
- 17.Rinaldo C R, Jr, Gupta P, Huang X, Fan Z, Mullins J I, Gange S, Farzadegan H, Shankarappa R, Muñoz A, Margolick J B. Anti-HIV-1 memory cytotoxic T lymphocyte responses associated with changes in CD4+ T cell numbers in progression of HIV-1 infection. AIDS Res Hum Retrovir. 1998;14:1423–1433. doi: 10.1089/aid.1998.14.1423. [DOI] [PubMed] [Google Scholar]
- 18.Rinaldo C R, Jr, Huang X L, Fan Z, Margolick J B, Borowski L, Hoji A, Kalinyak C, McMahon D K, Riddler S A, Hildebrand W H, Day R B, Mellors J W. Anti-human immunodeficiency virus type 1 (HIV-1) CD8+ T-lymphocyte reactivity during combination antiretroviral therapy in HIV-1-infected patients with advanced immunodeficiency. J Virol. 2000;74:4127–4138. doi: 10.1128/jvi.74.9.4127-4138.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steinman R M, Inaba K, Turley S, Pierre P, Mellman I. Antigen capture, processing, and presentation by dendritic cells: recent cell biological studies. Hum Immunol. 1999;60:562–567. doi: 10.1016/s0198-8859(99)00030-0. [DOI] [PubMed] [Google Scholar]
- 20.Trinchieri G. Proinflammatory and immunoregulatory functions of interleukin-12. Int Rev Immunol. 1998;16:365–396. doi: 10.3109/08830189809043002. [DOI] [PubMed] [Google Scholar]
- 21.Zheng L, Huang X-L, Fan Z, Borowski L, Wilson C C, Rinaldo C R., Jr Delivery of liposome-encapsulated HIV type 1 proteins to human dendritic cells for stimulation of HIV type 1-specific memory cytotoxic T lymphocyte responses. AIDS Res Hum Retrovir. 1999;15:1011–1020. doi: 10.1089/088922299310520. [DOI] [PubMed] [Google Scholar]