Abstract
In the feline immunodeficiency virus system, immunization with a fixed-infected-cell vaccine conferred protection against virulent homologous challenge but the immune effectors involved remained elusive. In particular, few or no neutralizing antibodies were detected in sera from vaccinated cats. Here we show that, when preadsorbed with selected feline cells, the same sera revealed clearly evident virus-neutralizing activity. Because high titers of neutralizing antibody in cell-adsorbed sera from 23 cats immunized with fixed-infected-cell or whole-inactivated-virus vaccines correlated with protection, it is likely that they were more important for protection than formerly realized. In vitro, the fixed-cell vaccine efficiently removed neutralizing antibody from immune sera while the whole-inactivated-virus vaccine was much less effective.
Studies with animal models have shown that certain experimental vaccines can prevent lentiviral infections or retard progression to disease, but the immune effectors responsible for these protective effects have remained elusive (reviewed in references 9, 10, 15, 17, and 25). In the feline immunodeficiency virus (FIV) system (26, 33), substantial levels of protection have been achieved with several immunogens, including fixed-infected-cell (FC) and whole-inactivated-virus (WIV) vaccines (3, 6, 11, 12, 18, 19, 21, 34, 35), two types of immunogens that have provided some satisfactory results also against simian immunodeficiency virus (5, 14). Thus, FIV is a practical model for investigating correlates of vaccine-induced immunity to lentiviruses.
In previous studies, it was found that an FC vaccine, consisting of feline lymphoid cells acutely infected with the clade B primary isolate FIV-M2, fixed with paraformaldehyde (1.25%, 37°C for 24 h) at the peak of viral antigen surface expression, effectively protected cats against systemic challenge with fully virulent, ex vivo-derived cell-free and cell-associated homologous virus (18, 19). However, thorough investigation of the elicited immune response failed to identify correlates that might explain the protection. Due to their importance in prophylactic immunization in general (27), virus-neutralizing antibodies (NA) were a special focus of attention but were detected in only a few sera from vaccinated animals, without correlation to protected or unprotected status (22). Here, we show that failure to detect NA in such sera was due to the presence of vaccine-induced antibodies directed to cellular antigens and removable by adsorption with selected feline cells. In light of this finding, we have reinvestigated the levels of NA in cell-adsorbed sera of cats immunized with the above-mentioned FC vaccine (hereafter referred to as FC vaccine sera) and with a nonprotective WIV vaccine.
FC vaccine sera contain anticell antibodies that prevent NA detection in vitro.
Because the anti-FIV FC vaccine was known to elicit moderate levels of antibodies to substrate cell antigens (19), before definitely excluding NA as possible contributors to its protective action, we checked whether failure of vaccinated-cat sera to inhibit FIV infectivity in vitro might be due to the presence of cell-reactive factors that interfered with the outcome of in vitro neutralization assays. To this end, we adsorbed with selected cell types the sera of vaccinated specific-pathogen-free (SPF) cats that had repeatedly been found to be NA negative in previous assays (22) and retested their ability to inhibit FIV infectivity in vitro. The cells used for adsorption were MBM cells (i.e., the same feline lymphoid cells as used for vaccine preparation), freshly harvested feline peripheral blood mononuclear cells (PBMC), primary lymphoblasts obtained from PBMC stimulated with concanavalin A for 3 (PLB-d3) or 12 (PLB-d12) days, Crandell feline kidney (CrFK) cells, and human oral epidermoid carcinoma KB cells. For adsorption, 0.8 ml of a 1:8 dilution of heat-inactivated sera was incubated with 106 viable packed cells at 4°C for 1 h with occasional shaking, spun down, incubated with the same number of fresh cells at 37°C for 1 h, and then centrifuge clarified. Adsorbed and untreated sera, diluted 1:16, 1:64, 1:256, and 1:1,024 (dilutions before the addition of virus and cells), were tested in parallel for NA against 10 50% tissue culture infectious doses of a stock of low-passage FIV-M2 prepared in MBM cells. The NA assay was routinely carried out using indicator MBM cells. The only deviation from the previously described procedure (4) was that the virus-serum mixtures were removed from the indicator cultures and replaced with fresh complete medium 3 h after inoculation. This modification was suggested by findings showing that, by this time, FIV-M2-exposed MBM cells already contain substantial copy numbers of proviral DNA (results not shown).
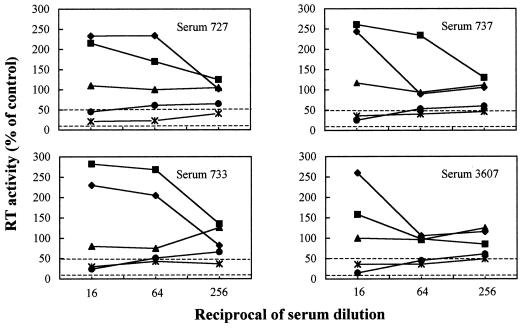
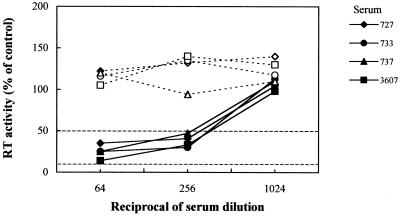
Table 1 shows the NA titers exhibited by cell-adsorbed and untreated sera of FC-vaccinated cats. Similar to their untreated counterparts, FC vaccine sera preadsorbed with PBMC or PLB-d3 or KB cells had minimal or no neutralization activity. In contrast, following adsorption with MBM, PLB-d12, or CrFK cells, the same sera effectively inhibited FIV replication. It is also important to note that, at low dilutions, the untreated FC vaccine sera caused a moderate but clearly evident enhancement of FIV replication and that this effect was lost after adsorption with MBM, PLB-d3, or PLB-d12 cells but not with freshly harvested PBMC (Fig. 1). When probed by flow cytometry with vaccine sera strongly reactive with MBM cells PLB-d3, PLB-d12, and CrFK cells were found to share increasing amounts of surface antigen(s) with MBM cells, while PBMC tested totally negative (data not shown). On the other hand, adsorption with MBM cells had no effect on NA-positive and NA-negative control sera obtained from infected and naive cats (Table 1). We also examined whether the FIV-inhibitory effect of cell-adsorbed FC vaccine sera was affected by immunoglobulin G (IgG) depletion. The sera, adsorbed with MBM cells and diluted 1:64, were incubated at room temperature for 2 h in microwells that had been coated overnight with 10 μg of goat anti-cat IgG (whole molecule) serum (Sigma, St. Louis, Mo.) and postcoated with skim milk and, as a control, in microwells coated with skim milk alone. Effective IgG capture was demonstrated by probing the wells with biotinylated mouse anti-cat IgG serum (Sigma) followed by an antibiotin-peroxidated conjugate and reading the optical density at 450 nm. As shown by Fig. 2, the FIV-inhibitory effect of adsorbed FC vaccine sera was abolished by IgG depletion, thus demonstrating that it was the result of true virus antibody-mediated neutralization and not of unidentified virus-blocking factors released by the cells used for adsorbing the sera. Further experiments demonstrated that, following MBM cell adsorption, FC vaccine sera acquired the ability to neutralize FIV infectivity also for mitogen-stimulated PBMC. Furthermore, the adsorbed sera failed to neutralize two heterologous clade B viruses grown in MBM cells exactly as the homologous virus (results not shown), thus showing the virus isolate specificity typical of FIV-infected cat sera (1, 4).
TABLE 1.
Effects of preadsorbing with selected cell types on the FIV-neutralizing activity of FC vaccine sera and control infected and naive cat sera
| Serum no. | Titer of NAa with indicated cell type
|
||||||
|---|---|---|---|---|---|---|---|
| None | MBM | PBMC | PLB-d3 | PLB-d12 | CrFK | KB | |
| Vaccinated | |||||||
| 727 | <16 | 512 | <16 | <16 | 64 | 512 | 16 |
| 733 | <16 | 512 | <16 | <16 | 128 | 64 | <16 |
| 737 | <16 | 512 | <16 | <16 | 64 | 32 | <16 |
| 3607 | <16 | 512 | <16 | <16 | 128 | 512 | 16 |
| Infectedb | |||||||
| 753 | 256 | 512 | NDc | ND | ND | ND | ND |
| 792 | 512 | 512 | ND | ND | ND | ND | ND |
| 3573 | 256 | 512 | ND | ND | ND | ND | ND |
| 3588 | 512 | 512 | ND | ND | ND | ND | ND |
| 3603 | 512 | 512 | ND | ND | ND | ND | ND |
| Uninfected, unvaccinated (6 sera) | <16 | <16 | ND | ND | ND | ND | ND |
The titer is expressed as the reciprocal of the highest serum dilution that gave 50% inhibition of reverse transcriptase production by 10 50% tissue culture infectious doses of FIV-M2 mixed with the corresponding dilution of a pool of 10 normal cat sera and calculated by the Reed and Müench method (28). The experiment was repeated twice, with comparable results.
These cats were infected with plasma from FIV-M2-infected cats; sera were obtained 1 year later.
ND, not done.
FIG. 1.
Effects of adsorption with selected cell types on the FIV neutralization curves produced by FC vaccine sera. The sera were adsorbed with MBM cells (×|), PBMC (⧫), or PLB-d3 (▴) or PLB-d12 (●) cells or manipulated in the same manner except for omission of cells (■). RT, reverse transcriptase.
FIG. 2.
Effects of IgG depletion on the FIV-neutralizing activity of MBM cell-adsorbed FC vaccine sera. Prior to neutralization, the indicated sera were depleted of IgG by capture onto anti-cat IgG (whole molecule) serum-coated microwells (broken lines) or manipulated in the same manner except for omission of anti-IgG serum (continuous lines). RT, reverse transcriptase.
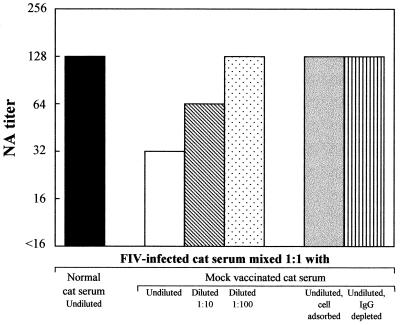
We also examined whether the addition of sera obtained from cats immunized with uninfected paraformaldehyde-fixed MBM cells affected the NA titer of otherwise neutralizing sera. Pooled sera from 3 cats infected with FIV-M2 1 year earlier were mixed 1:1 with undiluted or diluted pooled sera from 2 cats immunized with a mock MBM cell-based FC vaccine (hereafter referred to as mock-vaccine sera) in incomplete Freund's adjuvant (cats 743 and 3583 in reference 19) and, as a control, with undiluted pooled sera from 10 normal cats. The mixtures were then titrated for NA. As shown by Fig. 3, the mock-vaccine serum reduced the NA titers of infected cat sera in a dose-dependent fashion whereas naive cat serum did not. Importantly, mock-vaccine sera failed to neutralize, regardless of whether they were MBM cell adsorbed. Sera from two cats immunized with fixed CrFK cells exerted a similar though slightly less pronounced effect, while sera from cats immunized with autologous PLB-d3 cells did not (data not shown). Furthermore, the effect of mock-vaccine serum was abolished by MBM cell adsorption and IgG depletion (Fig. 3).
FIG. 3.
Effects on the FIV-neutralizing activities of serum from FIV-infected cats of mixing with sera from cats immunized with a mock vaccine consisting of fixed uninfected MBM cells. NA titers are expressed as in Table 1. The experiment was repeated three times, with comparable results.
Collectively, the above findings demonstrated that the FC vaccine had elicited virus-specific antibodies capable of neutralizing the FIV strain used for vaccine preparation and that previous failure to demonstrate such NA in in vitro neutralization tests was due to the presence in the sera of antibodies directed to the surfaces of feline cells and elicited by the cell substrate used for vaccine preparation. It is well known that, during budding from the plasma membrane, lentiviruses incorporate into their envelopes relatively vast amounts of a wide array of functional host cell materials (reviewed in reference 31). Thus, it is possible that anticell antibodies compete physically with NA on the virion surface or counteract their action by some other mechanism. The presence of certain host cell-derived molecules in the viral envelope has been seen to increase the resistance of human immunodeficiency virus type 1 (HIV-1) to antibody-mediated neutralization (31), and it is plausible that antibodies reactive with such molecules might magnify this effect. Alternatively, anticell antibodies might cross-link virions and cells and hence augment FIV infectivity for neutralization indicator cells. Previous studies have shown that in the case of HIV-1, some ligands, such as oligomers of the RANTES CC-chemokine, can facilitate virus infection also indirectly, namely by binding to cells and increasing their permissiveness (32). However, preincubation of MBM cells with the mock-vaccine sera that in the experiment described above had effectively reduced the neutralizing activity of FIV-infected cat sera had no effect on FIV replication (results not shown), thus excluding the latter type of mechanism. Future studies are warranted to investigate in depth how anticell antibodies can counteract NA in vitro as well as the precise nature of the cellular antigen(s) to which they are directed. The spectrum of cells which proved effective at removing the relevant antibodies suggests that such an antigen(s) is especially abundant or present solely on actively cycling cells.
Levels of NA in cell-adsorbed day-of-challenge sera from protected and unprotected vaccinated cats.
We systematically adsorbed with MBM cells and compared for NA content three groups of day-of-challenge FC-vaccinated SPF cat sera which, as discussed in a previous report (22), were considered particularly informative due to differences in timing and outcome of challenge, which was performed with homologous ex vivo cell-free or cell-associated FIV. The results with untreated sera were in line with our previous findings (22) in that only one group 1 serum neutralized FIV (titer, 256). Following cell adsorption, all group 1 and 2 sera exhibited NA at a titer of 512 (10 cats) or 256 (2 cats), while only half of group 3 sera neutralized FIV and, with one exception, their titers were uniformly lower (Table 2). Thus, these results demonstrated that NA were at higher titers in protected than in unprotected vaccinees, supporting the concept that NA had played a role in protection, possibly in concert with other immune effectors (13, 22). It is noteworthy that a 50% end point neutralization titer of 500 has been proposed as a desirable target for HIV-1 vaccines because it is considered adequate for conferring solid protection (23).
TABLE 2.
Titers of FIV-neutralizing antibodies in MBM cell-adsorbed day-of-challenge sera from vaccinated cats that had proven protected or unprotected against homologous challenge
| Group no. | Vaccine (immunization and challenge history)a | Outcome of challengeb | Serum no. | Titer of NAc
|
|
|---|---|---|---|---|---|
| No adsorption | Cell adsorbed | ||||
| 1 | FC (5 vaccine doses; challenge 4 mo after last dose) | Protected from intravenous cell-free virus | 733 737 806 3532 3535 3585 | <16 <16 256 <16 <16 <16 | 512 512 512 512 512 512 |
| 2 | FC (5 vaccine doses; challenge 12 mo after last dose) | Protected from intravenous cell-associated but not from cell-free virus | 727 759 824 3558 3587 3607 | <16 <16 <16 <16 <16 <16 | 512 256 512 512 256 512 |
| 3 | FC (5 vaccine doses + booster after 26 mo; challenge 2 mo after booster) | Not protected from intravenous cell-free virus | 733 737 806 3532 3535 3585 | <16 <16 <16 <16 <16 <16 | <16 <16 16 256 <16 128 |
| 4 | WIV (5 vaccine doses; challenge 4 mo after last dose) | Not protected from intravenous cell-free virus or from cell-associated mucosal challenge | 90 101 234 1166 1169 | <16 <16 <16 <16 <16 | 32 16 16 <16 <16 |
The vaccine doses, each containing 3 × 107 FC (groups 1, 2, and 3) or 250 μg of WIV (group 4), were administered subcutaneously in Freund's incomplete adjuvant.
Cell-free challenge consisted of plasma from infected cats, while cell-associated challenge consisted of PBMC collected directly from infected cats (group 2) or PBMC activated and infected at a multiplicity of infection of 0.0015 in vitro (group 4).
Expressed as in Table 1. The experiment was repeated twice, with comparable results.
Since under certain immunization conditions host-derived proteins bound to lentiviral virions may trigger the formation of cell-reactive antibodies (2, 16, 30), it was also of interest to determine whether elicitation of anticell antibodies capable of counteracting the in vitro activity of NA extended to a cell-free WIV vaccine. We therefore adsorbed with MBM cells and tested for NA day-of-challenge sera (group 4 in Table 2) from SPF cats that had been immunized with paraformaldehyde-inactivated (0.5%, at 37°C for 24 h), gradient-purified FIV-M2 produced in MBM cells. These cats had not resisted a mild systemic challenge with homologous ex vivo virus (20). When tested untreated, the sera failed to neutralize as in previously performed tests (20), but following cell adsorption, three of five sera proved clearly neutralizing, albeit at lower titers than those observed with group 1 and 2 FC vaccine sera. These findings hence showed that WIV vaccines can also elicit anticell antibodies capable of affecting the results of in vitro NA assays. They further indicated that the WIV vaccine had elicited a poorer NA response than the FC vaccine, thus correlating with failure to protect.
Depletion of NA in vitro by different immunogens.
In an attempt to understand why the WIV vaccine had triggered less NA formation than the FC vaccine, we evaluated the two immunogens for the ability to deplete the FIV-neutralizing activity of immune sera in vitro. For comparison, a preparation of viable FIV-M2 produced and gradient purified as for the WIV vaccine preparation (19) but not paraformaldehyde inactivated and FIV-M2 glycoproteins purified with Galanthus nivalis lectin as described previously (8) were also examined in this regard. FIV-immune sera, diluted 1:64, were incubated in microwells that contained 2 × 105 FC vaccine cells or had been coated overnight with 1 μg of the other antigens being tested. Incubation was carried out first at 4°C for 1 h and then, on fresh microwells, at 37°C for 1 h. The sera thus treated were high-speed centrifuged, heated at 56°C for 1 h to eliminate any acquired viral infectivity, and finally tested for NA. As shown in Table 3, preincubation of sera with the FC vaccine led to substantial reductions of the NA titers of test immune sera. Preincubation with viable FIV-M2 also removed NA, albeit slightly less efficiently. In contrast, preincubation with the WIV vaccine had only marginal effects on the NA titers of immune sera, indicating that paraformaldehyde inactivation had impaired the functionality of neutralization-relevant epitopes present on virions, possibly as a consequence of conformational changes (7, 24, 29). As expected, purified FIV glycoproteins also failed to adsorb NA.
TABLE 3.
Abilities of different antigens to consume the FIV-neutralizing activities of immune sera
Conclusions.
This study has shown that the sera of cats immunized with anti-FIV FC and WIV vaccines can contain antibodies directed to the substrate cells used for vaccine preparation and capable of preventing the detection of virus-specific neutralizing activity in in vitro assays. Interestingly, following removal of such masking antibodies, day-of-challenge sera of FC-vaccinated cats that had been found to be protected against fully virulent homologous FIV exhibited higher NA titers than the sera of unprotected FC- or WIV-vaccinated animals. Clearly, this raises the possibility that NA were more important effectors of FC vaccine-induced protection than formerly realized (22). A role for NA in vaccine-induced anti-FIV immunity has always been deemed likely, although attempts to unequivocally correlate them with protection have generated inconsistent findings (13, 22). Also of interest is that in vitro, the WIV vaccine removed far fewer NA from immune sera than the FC vaccine or viable cell-free virus, indicating that paraformaldehyde treatment is more harmful for the neutralization epitopes present on cell-free virions than for the ones expressed on virus-infected cells. Since this meager immune reactivity in vitro corresponded to a poor NA-inducing capacity of the WIV vaccine in vivo, the ability to adsorb NA in vitro should be further evaluated as a possible parameter for screening candidate anti-FIV immunogens prior to their use in animals. It is hoped that this and other evidence raised in the FIV model will be of value also in the design and evaluation of other antilentiviral vaccines.
Acknowledgments
This work was supported by grants from the Ministero della Sanità—Istituto Superiore di Sanità, “Programma per l'AIDS,” and from the Ministero della Università e Ricerca Tecnologica.
REFERENCES
- 1.Baldinotti F, Matteucci D, Mazzetti P, Giannelli C, Bandecchi P, Tozzini F, Bendinelli M. Serum neutralization of feline immunodeficiency virus is markedly dependent on passage history of the virus and host system. J Virol. 1994;68:4572–4579. doi: 10.1128/jvi.68.7.4572-4579.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bergmeier L A, Walker J, Tao L, Cranage M, Lehner T. Antibodies to human and non-human primate cellular and culture medium components in macaques vaccinated with the simian immunodeficiency virus. Immunology. 1994;83:213–220. [PMC free article] [PubMed] [Google Scholar]
- 3.Bishop S A, Stokes C R, Gruffydd-Jones T J, Whiting C V, Humphries J E, Osborne R, Papanastasopoulou M, Harbour D A. Vaccination with fixed feline immunodeficiency virus (FIV) infected cells: protection, breakthrough and specificity of response. Vaccine. 1996;14:1243–1250. doi: 10.1016/s0264-410x(96)00023-0. [DOI] [PubMed] [Google Scholar]
- 4.Del Mauro D, Matteucci D, Giannecchini S, Maggi F, Pistello M, Bendinelli M. Autologous and heterologous neutralization analyses of primary feline immunodeficiency virus isolates. J Virol. 1998;72:2199–2207. doi: 10.1128/jvi.72.3.2199-2207.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Thé G. Roundtable: can experience with veterinary retroviral vaccines be applied to the human situation? AIDS Res Hum Retrovir. 1996;12:365–373. doi: 10.1089/aid.1996.12.365. [DOI] [PubMed] [Google Scholar]
- 6.Elyar J S, Tellier M C, Soos J M, Yamamoto J K. Perspectives on FIV vaccine development. Vaccine. 1997;15:1437–1444. doi: 10.1016/s0264-410x(97)00056-x. [DOI] [PubMed] [Google Scholar]
- 7.Giannecchini S, Matteucci D, Bendinelli M. Effect of enzymatic deglycosylation on feline immunodeficiency virus sensitivity to antibody-mediated neutralization. AIDS Res Hum Retrovir. 1998;14:199–204. doi: 10.1089/aid.1998.14.199. [DOI] [PubMed] [Google Scholar]
- 8.Gilljam G. Envelope glycoproteins of HIV-1, HIV-2, and SIV purified with Galanthus nivalis agglutinin induce strong immune responses. AIDS Res Hum Retrovir. 1993;9:431–438. doi: 10.1089/aid.1993.9.431. [DOI] [PubMed] [Google Scholar]
- 9.Haynes B F, Pantaleo G, Fauci A S. Toward an understanding of the correlates of protective immunity to HIV infection. Science. 1996;271:324–328. doi: 10.1126/science.271.5247.324. [DOI] [PubMed] [Google Scholar]
- 10.Heeney J L, Beverley P, McMichael A, Shearer G, Strominger J, Wahren B, Weber J, Gotch F. Immune correlates of protection from HIV and AIDS—more answers but yet more questions. Immunol Today. 1999;20:247–251. doi: 10.1016/s0167-5699(98)01437-6. [DOI] [PubMed] [Google Scholar]
- 11.Hohdatsu T, Okada S, Motokawa K, Aizawa C, Yamamoto J K, Koyama H. Effect of dual-subtype vaccine against feline immunodeficiency virus infection. Vet Microbiol. 1997;58:155–165. doi: 10.1016/S0378-1135(97)00164-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hosie M J, Dunsford T, Klein D, Willett B J, Cannon C, Osborne R, MacDonald J, Spibey N, Mackay N, Jarrett O, Neil J C. Vaccination with inactivated virus but not viral DNA reduces virus load following challenge with a heterologous and virulent isolate of feline immunodeficiency virus. J Virol. 2000;74:9403–9411. doi: 10.1128/jvi.74.20.9403-9411.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hosie M J, Flynn J N. Feline immunodeficiency virus vaccination: characterization of the immune correlates of protection. J Virol. 1996;70:7561–7568. doi: 10.1128/jvi.70.11.7561-7568.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Israel Z R, Edmonson P F, Maul D H, O'Neil S P, Mossman S P, Thiriart C, Fabry L, Van Opstal O, Bruck C, Bex F, Burny A, Fultz P N, Mullins J I, Hoover E A. Incomplete protection, but suppression of virus burden, elicited by subunit simian immunodeficiency virus vaccines. J Virol. 1994;68:1843–1853. doi: 10.1128/jvi.68.3.1843-1853.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnston M I. HIV/AIDS vaccine development: challenges, progress and future directions. Rev Med Virol. 1996;6:123–140. doi: 10.1002/(SICI)1099-1654(199609)6:3<123::AID-RMV170>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 16.Langlois A J, Weinhold K J, Matthews T J, Greenberg M L, Bolognesi D P. The ability of certain SIV vaccines to provoke reactions against normal cells. Science. 1992;255:292–293. doi: 10.1126/science.1549775. [DOI] [PubMed] [Google Scholar]
- 17.Levy J A. HIV and the pathogenesis of AIDS. 2nd ed. Washington, D.C.: ASM Press; 1998. [Google Scholar]
- 18.Matteucci D, Pistello M, Mazzetti P, Giannecchini S, Del Mauro D, Lonetti I, Zaccaro L, Pollera C, Specter S, Bendinelli M. Studies of AIDS vaccination using an ex vivo feline immunodeficiency virus model: protection conferred by a fixed cell vaccine against cell-free and cell-associated challenge differs in duration and is not easily boosted. J Virol. 1997;71:8368–8376. doi: 10.1128/jvi.71.11.8368-8376.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matteucci D, Pistello M, Mazzetti P, Giannecchini S, Del Mauro D, Zaccaro L, Bandecchi P, Tozzini F, Bendinelli M. Vaccination protects against in vivo-grown feline immunodeficiency virus even in the absence of detectable neutralizing antibodies. J Virol. 1996;70:617–622. doi: 10.1128/jvi.70.1.617-622.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matteucci D, Pistello M, Mazzetti P, Giannecchini S, Isola P, Merico A, Zaccaro L, Rizzuti A, Bendinelli M. AIDS vaccination studies using feline immunodeficiency virus as a model: immunisation with inactivated whole virus suppresses viraemia levels following intravaginal challenge with infected cells but not following intravenous challenge with cell-free virus. Vaccine. 1999;18:119–130. doi: 10.1016/s0264-410x(99)00189-9. [DOI] [PubMed] [Google Scholar]
- 21.Matteucci D, Poli A, Mazzetti P, Sozzi S, Bonci F, Isola P, Zaccaro L, Giannecchini S, Calandrella M, Pistello M, Specter S, Bendinelli M. Immunogenicity of an anti-clade B feline immunodeficiency fixed-cell virus vaccine in field cats. J Virol. 2000;74:10911–10919. doi: 10.1128/jvi.74.23.10911-10919.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mazzetti P, Giannecchini S, Del Mauro D, Matteucci D, Portincasa P, Merico A, Chezzi C, Bendinelli M. AIDS vaccination studies using an ex vivo feline immunodeficiency virus model: detailed analysis of the humoral immune response to a protective vaccine. J Virol. 1999;73:1–10. doi: 10.1128/jvi.73.1.1-10.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moore J P, Burton D R. HIV-1 neutralizing antibodies: how full is the bottle? Nat Med. 1999;5:142–144. doi: 10.1038/5502. [DOI] [PubMed] [Google Scholar]
- 24.Moore J P, Ho D D. Antibodies to discontinuous or conformationally sensitive epitopes on the gp120 glycoprotein of human immunodeficiency virus type 1 are highly prevalent in sera of infected humans. J Virol. 1993;67:863–875. doi: 10.1128/jvi.67.2.863-875.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nathanson N, Mathieson B J. Biological considerations in the development of a human immunodeficiency virus vaccine. J Infect Dis. 2000;182:579–589. doi: 10.1086/315707. [DOI] [PubMed] [Google Scholar]
- 26.Pedersen N C, Ho E W, Brown M L, Yamamoto J K. Isolation of a T-lymphotropic virus from domestic cats with an immunodeficiency-like syndrome. Science. 1987;235:790–793. doi: 10.1126/science.3643650. [DOI] [PubMed] [Google Scholar]
- 27.Quinnan G V. Immunization against viral diseases. In: Galasso G, Whitley R, Merigan T C, editors. Antiviral agents and human viral diseases. New York, N.Y: Raven Press; 1997. pp. 791–834. [Google Scholar]
- 28.Reed L J, Müench H A. A simple method for estimating fifty percent end points. Am J Hyg. 1938;27:493–497. [Google Scholar]
- 29.Richardson J, Fossati I, Moraillon A, Castelot S, Sonigo P, Pancino G. Neutralization sensitivity and accessibility of continuous B cell epitopes of the feline immunodeficiency virus envelope. J Gen Virol. 1996;77:759–777. doi: 10.1099/0022-1317-77-4-759. [DOI] [PubMed] [Google Scholar]
- 30.Stott E J. Anti-cell antibody in macaques. Nature (London) 1991;353:393. doi: 10.1038/353393a0. [DOI] [PubMed] [Google Scholar]
- 31.Tremblay M J, Fortin J-F, Cantin R. The acquisition of host-encoded proteins by nascent HIV-1. Immunol Today. 1998;19:346–351. doi: 10.1016/s0167-5699(98)01286-9. [DOI] [PubMed] [Google Scholar]
- 32.Trkola A, Gordon C, Matthews J, Maxwell E, Ketas T, Czaplewski L, Proudfoot A E I, Moore J P. The CC-chemokine RANTES increases the attachment of human immunodeficiency virus type 1 to target cells via glycosaminoglycans and also activates a signal transduction pathway that enhances viral infectivity. J Virol. 1999;73:6370–6379. doi: 10.1128/jvi.73.8.6370-6379.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Willett B J, Flynn J N, Hosie M J. FIV infection of the domestic cat: an animal model for AIDS. Immunol Today. 1997;18:182–189. doi: 10.1016/s0167-5699(97)84665-8. [DOI] [PubMed] [Google Scholar]
- 34.Yamamoto J K, Hohdatsu T, Olmsted R A, Pu R, Louie H, Zochlinski H A, Acevedo V, Johnson H M, Soulds G A, Gardner M B. Experimental vaccine protection against homologous and heterologous strains of feline immunodeficiency virus. J Virol. 1993;67:601–605. doi: 10.1128/jvi.67.1.601-605.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamamoto J K, Okuda T, Ackley C D, Louie H, Pembroke E, Zochlinski H, Munn R J, Gardner M B. Experimental vaccine protection against feline immunodeficiency virus. AIDS Res Hum Retrovir. 1991;7:911–922. doi: 10.1089/aid.1991.7.911. [DOI] [PubMed] [Google Scholar]