Abstract
Trace amine-associated receptor 1 (TAAR1) is known to negatively regulate dopamine (DA) release. The partial TAAR1 agonist RO5263397 promotes wakefulness and suppresses NREM and REM sleep in mice, rats, and non-human primates. We tested the hypothesis that the TAAR1-mediated effects on sleep/wake were due, at least in part, to DA release. Male C57BL6/J mice (n=8) were intraperitoneally administered the D1R antagonist SCH23390, the D2R antagonist eticlopride, a combination of D1R+D2R antagonists or saline at ZT5.5, followed 30 min later by RO5263397 or vehicle (10% DMSO in DI water) at ZT6 per os. EEG, EMG, subcutaneous temperature, and activity were recorded in each mouse across the 8 treatment conditions and sleep architecture was analyzed for 6 hours post-dosing. Consistent with our previous reports, RO5263397 increased wakefulness as well as the latency to NREM and REM sleep. D1, D2, and D1+D2 pretreatment reduced RO5263397-induced wakefulness during the first 1–2 hours after dosing, but only the D1+D2 combination attenuated the wake-promoting effect of RO5263397 from ZT6–8, mostly by increasing NREM sleep. Although D1+D2 antagonism blocked the wake-promoting effect of RO5263397, only the D1 antagonist significantly reduced the TAAR1-mediated increase in NREM latency. Neither the D1 nor the D2 antagonist affected TAAR1-mediated suppression of REM sleep. These results suggest that, whereas TAAR1 effects on wakefulness are mediated in part through the D2R, D1R activation plays a role in reversing the TAAR1-mediated increase in NREM sleep latency. By contrast, TAAR1-mediated suppression of REM sleep appears not to involve D1R or D2R mechanisms.
Keywords: Trace amine-associated receptor 1(TAAR1), agonists, dopaminergic antagonists, sleep
1. Introduction
Trace amines (TAs) such as β-phenylethylamine, m- and p-tyramine, and tryptamine are endogenous amines that are related to the biogenic amine neurotransmitters. Compared to other neurotransmitters, TAs in mammalian brains have much lower concentrations (~1000-fold less) and rapid turnover (<30s) which makes it difficult to measure them directly [1]. TAs have physiological roles in the mammalian CNS by binding to Trace Amine-Associated Receptors (TAARs), a family of G protein-coupled receptors (GPCRs) which were discovered in 2001 [2, 3]. There are six different types of TAARs in humans and nine in rats and mice [4, 5]. Unlike other TAARs which are expressed mostly in the olfactory epithelia in mammals [6], TAAR1 is expressed in the rodent and human brain and has been well-studied compared to other TAARs [7]. TAAR1 is expressed in monoaminergic and limbic system nuclei and is known to modulate dopaminergic (DA) neurons in ventral tegmental area (VTA), serotonergic neurons in dorsal raphe nucleus (DRN), and glutamatergic signaling in the prefrontal cortex [7–9].
TAAR1 has been extensively studied in relation to psychostimulants over the past 20 years. Multiple studies have shown that the hyperlocomotion caused by elevated DA activity is increased in TAAR1 knockout mice when exposed to psychostimulants such as amphetamine [10–12], methamphetamine [10], methylenedioxymethamphetamine (MDMA) [13], and nicotine [14, 15]. Conversely, mice that overexpress TAAR1 decrease locomotion after amphetamine administration [16], supporting the concept that TAAR1 is a negative regulator of DA release. TAAR1 full and partial agonists have anti-psychostimulant effects in rodents and non-human primates [17, 18]. Ulotaront, a TAAR1 agonist with 5-HT1A agonist activity, has been in Phase 3 clinical trials for the treatment of schizophrenia [19, 20], raising the prospect of TAAR1 agonism as a clinical intervention for psychiatric disorders involving hyperdopaminergic signaling.
Like other monoamines, DA is known to be involved in sleep/wake regulation since DA neurons in VTA have been found to be active during wakefulness and rapid eye movement (REM) sleep [21–24]. We have previously shown that TAAR1 partial agonists promote wakefulness and suppress non-REM (NREM) and REM sleep in mice [25, 26], rats [17, 18] and non-human primates [27]. Given the role of TAAR1 in regulation of DA release [7, 16], we hypothesized that the wakefulness-promoting effects of TAAR1 partial agonism may be mediated by DA receptor activation. To test this hypothesis, we administered DA receptor antagonists as pretreatments prior to administration of the TAAR1 partial agonist RO5263397 in mice and monitored sleep/wake, activity, and subcutaneous body temperature. We confirmed that TAAR1 partial agonism increased Wake Time and reduced both NREM and REM sleep and found that only a combination of D1 and D2 receptor antagonism blocked the TAAR1-mediated increase in Wakefulness but that the TAAR1-mediated suppression of REM sleep was independent of DA signaling.
2. Results
As illustrated in Figure 1A, all mice received experimental treatments at both ZT5.5 and 30 min later at ZT6; the experimenter was blinded to the dosing condition. The D1 receptor antagonist SCH23390 (hereafter D1; 0.25 mg/kg), the D2 antagonist eticlopride (hereafter D2; 1 mg/kg), a combination of D1+D2, or saline were administered intraperitoneally (i.p.) at ZT5.5. Since the goal of this study was to determine whether the wakefulness-promoting and sleep-suppressing effects of TAAR1 partial agonism were mediated through DA release, the primary focus of the analyses was on D1 and D2 antagonism of TAAR1 effects. The experimental design also resulted in the data collection on D1 and D2 antagonist effects on sleep/wake but, since this aspect of sleep/wake control has been addressed elsewhere in the literature [28–33], the analyses discussed below focus on TAAR1-DA interactions. All statistical analyses cited below are presented in Table S1.
Figure 1.
Schematic illustrating the experimental design and Wake Time for 6 hours after the second dosing. A. Male C57BL/6 mice received a dopaminergic antagonist i.p. at ZT5.5 followed 30 min later by either p.o. RO3397 or saline at ZT6. B-G. For ease of visualization, data are split into three subgroups in which the results from the negative (Sal+Veh) and positive (Sal+RO3397) control treatments are repeated in each graph. Hourly Wake Time (mean+SEM) for the first 6 hours after the second dosing with (B) D1 antagonist +Veh or +RO3397, (C) D2 antagonist +Veh or +RO3397, and (D) D1+D2 antagonist +Veh or +RO3397. Colored symbols indicate statistical significance for that hour compared to Sal+Veh(*) or Sal+RO3397(#) based on RM-ANOVA. Panels E-G present Wake Time (mean+SEM) summed in 3-h bins (ZT6–8 and ZT9–11) for each treatment. *, # p < 0.05; **, p < 0.01; ***, p < 0.005; ****, p < 0.001.
Wakefulness.
RM-ANOVA revealed significant variation in hourly Wake Time due to drug treatment (Figs. 1B–1D; F(7, 48) = 3.919, p = 0.0019) and a treatment × time interaction (F(35, 240) = 2.693, p = 5.2 × 10−6). Tukey’s post hoc test indicated that Saline+RO3397 had wake-promoting effects for the first two hours (ZT6–7) post-dosing compared to Saline+Vehicle (p < 0.0001). When Wake Time was analyzed in 3-h bins (Figs. 1E–1G), Saline+RO3397 increased wakefulness from ZT6–8 (p < 0.0001) due primarily to more Wake bouts (p < 0.01; Table S2) with no significant difference from ZT9–11. RM-ANOVA revealed significant variation for Cumulative Wakefulness over the 6-h recording period (F(35, 240) = 2.882, p = 1.1 × 10−6; Figure S1); post hoc testing indicated that Saline+RO3397 also increased Cumulative Wakefulness across the entire 6-h compared to Saline+Vehicle (p < 0.0001). The results from the Saline+RO3397 combination replicate those from our previous studies in mice, rats and non-human primates in which no Saline pretreatment occurred [17, 18, 25–27].
Pretreatment with the D1 antagonist SCH23390 followed by RO3397 did not affect Wake Time compared to the Saline+RO3397 combination in either the hourly (Figure 1B) or 3-h ZT6–8 bin (Figure 1E; p = 0.13) comparisons. In contrast, pretreatment with the D2 antagonist eticlopride attenuated the increased Wake Time induced by Saline+RO3397 for the first 2-h after dosing (Figure 1C, p = 0.0060), although this decrease did not reach significance for the ZT6–8 bin (Figure 1F; p = 0.16) even though the mean Wake Bout Duration was reduced by >50% (p < 0.0001; Table S2). However, pretreatment with the D1+D2 antagonist combination reduced the Saline+RO3397-induced Wake Time increase in both the hourly analysis (Figure 1D; p = 0.0075) and the ZT6–8 binned results (Figure 1G; p = 0.0086) due to shorter Wake bouts (p < 0.0001; Table S2). When analyzed over the entire 6-h period, the Cumulative Wakefulness induced by the Saline+RO3397 combination was reduced by pretreatment with the D1 antagonist (Figure S1A; p = 0.0464), the D2 antagonist (Figure S1B; p = 0.0033), and the D1+D2 combination (Figure S1C; p = 0.00163).
NREM Sleep.
ANOVA indicated significant variation (F(7, 32.4) = 15.99, p < 0.0001) in the latency to NREM sleep produced by different treatments (Figs. 2A–2C). Post hoc comparisons revealed that Saline+RO3397 significantly increased NREM latency compared to Saline+Vehicle (p = 0.0005; Figs. 2A–2C), the D1 antagonist+Vehicle (p = 0.0003; Figure 2A) and the D2 antagonist+Vehicle (p = 0.0003; Figure 2B). The Saline+RO3397 increase in NREM latency was blocked by pretreatment with the D1 antagonist+RO3397 (p = 0.0004; Figure 2A) but not by the pretreatment with the D2 antagonist+RO3397 (p = 0.1264; Figure 2B). Surprisingly, the D1+D2 antagonist combination had no effect on the RO3397-induced increase in NREM latency (p > 0.5, Figure 2C).
Figure 2.
Latency to NREM sleep and NREM time for the first 6 hours after the second dosing. For ease of visualization, data are split into three subgroups in which the results from the negative (Sal+Veh) and positive (Sal+RO3397) control treatments are repeated in each graph. A-C. Latency to NREM sleep (mean+SEM). D-F. Hourly NREM time (mean+SEM) for the first 6 hours after the second dosing with (D) D1 antagonist +Veh or +RO3397, (E) D2 antagonist +Veh or +RO3397, and (F) D1+D2 antagonist +Veh or +RO3397. Colored symbols indicate statistical significance for that hour compared to Sal+Veh(*) or Sal+RO3397(#) based on RM-ANOVA. G-I. NREM Time (mean+SEM) summed in 3-h bins (ZT6–8, ZT9–11) for each treatment. *, p < 0.05; **, p < 0.01; ***, p < 0.005; ****, p < 0.001.
RM-ANOVA indicated significant effects on hourly NREM time across treatments (Figs. 2D–2F; F(7, 48) = 2.391, p = 0.035) and a treatment x time interaction (F(35, 240) = 2.864, p < 0.0001). Inverse to the Wake Time results, post hoc tests revealed that Saline+RO3397 significantly reduced NREM sleep for the first 2-h after treatment compared to Saline+Vehicle (Figure 2D–F; p = 0.0003). Whereas D1+RO3397 did not significantly affect NREM time (Figure 2D; p = 0.29), post hoc tests revealed that D2+RO3397 (Figure 2E; p = 0.0023) and D1+D2+RO3397 (Figure 2F; p = 0.0256) significantly increased NREM time compared to the Saline+RO3397 group. Although 2-way ANOVA indicated significant variation in NREM sleep in the 3-h bin analysis from ZT6–8 (F(7,48) = 4.179, p = 0.0012) and Tukey’s post hoc test confirmed that Saline+RO3397 significantly reduced NREM sleep compared to Saline+Vehicle (Figs. 2G–I; p = 0.0004), only the D1+D2+RO3397 combination blocked the Saline+RO3397-induced decrease in NREM sleep (p = 0.0246; Figure 2I), due to almost twice as many NREM bouts (p < 0.01; Table S2).
RM-ANOVA revealed a significant treatment effect on Cumulative NREM Time (Figure S2; F(7, 48) = 5.474, p = 0.0001) as well as treatment by time (F(35, 240) = 2.108 p = 0.0006). Pretreatment with D1 (Figure S2A; p = 0.04), D2 (Figure S2B; p = 0.0007), and D1+D2 (Figure S2C; p = 0.005) all attenuated the Saline+RO3397 reduction of NREM sleep. Most of the effect occurred the initial 3-h (ZT6–8), but there was also some contribution during ZT9–11.
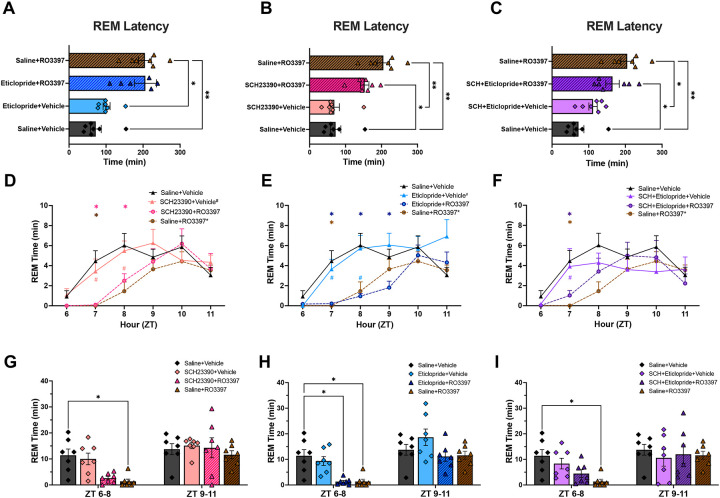
REM Sleep.
ANOVA revealed significant variation in REM sleep latency (Figure 3A–C; F(7, 28.2) = 10.55, p < 0.0001), with Tukey’s post hoc test indicating that Saline+RO3397 increased REM latency relative to Saline+Vehicle (p = 0.0019). In contrast to NREM sleep, neither pretreatment with the D1 (p = 0.43), D2 (p > 0.99), nor D1+D2 antagonist combination (p = 0.91) significantly affected the Saline+RO3397 increase in REM sleep latency.
Figure 3.
Latency to REM sleep and REM time for the first 6 hours after the second dosing. For ease of visualization, data are split into three subgroups in which the results from the negative (Sal+Veh) and positive (Sal+RO3397) control treatments are repeated in each graph. A-C. Latency to REM sleep (mean+SEM). D-F. Hourly REM time (mean+SEM) for the first 6 hours after the second dosing with (D) D1 antagonist +Veh or +RO3397, (E) D2 antagonist +Veh or +RO3397, and (F) D1+D2 antagonist +Veh or +RO3397. Colored symbols indicate statistical significance for that hour compared to Sal+Veh(*) or Sal+RO3397(#) based on RM-ANOVA. G-I. REM time (mean+SEM) summed in 3-h bins (ZT6–8, ZT9–11) for each treatment. *, # p < 0.05; **, p < 0.01; ***, p < 0.005; ****, p < 0.001.
RM-ANOVA revealed significant effects of treatment (F(7, 48) = 2.458, p = 0.031) and treatment × time (F(35, 240) = 1.755, p = 0.0079) on hourly REM Sleep Time (Figure 3D–F). Post hoc tests indicated a significant reduction of hourly REM Time by Saline+RO3397 but neither the D1, D2 nor the D1+D2 combination affected the RO3397-induced decrease in REM Time. Although Figure 3E shows even less REM Time in the D2+RO3397 group than in the Saline+RO3397 treatment at ZT8, this effect did not reach significance overall. This inhibition of REM sleep was sustained in the 3-h analysis from ZT6–8 (Figure 3G–I; p = 0.047) without an effect of the DA antagonist treatments. Lastly, although RM-ANOVA revealed significant effects of treatment (F(7, 48) = 4.091, p = 0.0014) and treatment x time (F(35, 240) = 2.188, p = 0.0003) on Cumulative REM time, there were no effects of any pre-treatment on RO3397-reduced REM time (Figure S3).
EEG Spectral Analysis.
Figure S4A–C presents the 6-h averaged power spectra for Wake, NREM, and REM sleep, normalized to Saline+Vehicle. Figure 4D–I shows the spectral composition of the conventional bands from ZT6–8 and ZT9–11. As we have observed previously [26], the only significant difference across the various EEG bandwidths is the elevated gamma power in the low (30.2–60.0 Hz) ranges after the Saline+RO3397 treatment.
Figure 4.
Subcutaneous body temperature and activity for the first 6 hours after the second dosing of male C57BL/6 mice that received a dopaminergic antagonist i.p. at ZT5.5 followed 30 min later by either p.o. RO3397 or saline at ZT6. Colored symbols indicate statistical significance (p < 0.05) for that hour compared to Sal+Veh(*) or Sal+RO3397(#) based on RM-ANOVA.
Subcutaneous Temperature (Tsc).
Figure 4A–C shows Tsc for the first 6-h after the second dosing. RM-ANOVA indicated significant effects of treatment (F(7, 48) = 2.49; p = 0.03) and treatment by time (F(35, 240) = 6.18; p < 1.10 × 10−8) when compared to Saline+Vehicle. Post hoc tests revealed significant treatment effects for D2+RO3397 (Figure 4B, p = 0.017), D1+D2+Vehicle (Figure 4C; p = 0.013), and D1+D2+RO3397 (Figure 4C; p = 0.032) but not for Saline+RO3397 (Figs. 4A–C; p = 0.29) or D1+RO3397 (Figure 4A; p = 0.15). The D2+3397 effect was profound across the 6-h recording. As with the D2+3397 treatment, the D1+D2+Vehicle and D1+D2+RO3397 effects were prolonged in duration but not as profound. Post hoc tests for treatment by time indicated that Saline+RO3397 reduced Tsc for the first 2-h post-treatment. After the first hour, the D2+RO3397 (Figure 4B) and D1+D2+RO3397 (Figure 4C) treatments appeared to exacerbate the RO3397-induced decline in Tsc.
Activity.
RM-ANOVA also indicated highly significant effects of both treatment (F(7, 48) = 9.66; p = 1.95 × 10−7) and treatment by time (F(35, 240) = 2.68; p = 5.61 × 10−6) for LMA. When compared to Saline+Vehicle, treatment effects were evident for Saline+RO3397 (Figs. 4D, E, F; p = 0.047), D2+RO3397 (Figure 4E; p = 0.0004) and D1+D2+RO3397 (Figure 4F; p = 0.0001) but not for D1+RO3397 (Figure 4D; p = 0.19). Post hoc tests for treatment by time revealed that all treatments reduced activity relative to Saline+Vehicle for the first post-dosing hour. Whereas LMA recovered after the first hour for the D1 treatment, this inhibition of activity persisted for most of the 6-h period for the 4 treatments that involved use of the D2 antagonist (Figs. 4E, F).
3. Discussion
In the present study conducted in mice, the TAAR1 agonist RO5263397 increased wakefulness as well as the latency to NREM and REM sleep as we have shown previously in three species of mammals [17, 18, 25–27]. Pretreatment with both the D1 and D2 antagonists reduced RO5263397-induced wakefulness during the first 1–2 hours after dosing, but only the D1+D2 antagonist combination attenuated the wake-promoting effect of RO5263397 from ZT6–8, mostly by increasing NREM sleep. Although D1+D2 antagonism blocked the wake-promoting effect of RO5263397, only the D1 antagonist significantly reduced the TAAR1-mediated increase in NREM latency. Surprisingly, neither the D1 nor the D2 antagonist affected TAAR1-mediated suppression of REM sleep. These results suggest that, whereas TAAR1 effects on wakefulness are mediated in part through the D2R, D1R activation plays a role in reversing the TAAR1-mediated increase in NREM sleep latency. In contrast, the TAAR1-mediated suppression of REM sleep either requires a higher level of receptor occupancy than that achieved in the current study or it may not involve D1R or D2R mechanisms.
3.1. Dopaminergic effects on sleep/wake in rodents
The experimental design utilized here involved administration of DA antagonists as pretreatments prior to use of the TAAR1 partial agonist RO5263397. Consequently, it is important to consider the known effects of DA antagonists on sleep/wake. D1 antagonists such as SCH23390 have been shown to increase both NREM and REM sleep while decreasing wakefulness [28–31] when administered systemically. In contrast, the effects of D2 receptor antagonists or agonists on sleep depend on the dosage, as D2 receptors are located both pre- and post-synaptically. Eticlopride, a D2 receptor antagonist, activates autoreceptors at low doses, resulting in increased NREM and REM sleep, increased EEG delta power, and reduced locomotor activity. However, high doses of eticlopride activate postsynaptic D2 receptors, leading to arousal and decreased NREM and REM sleep [32, 33]. We chose doses of the D1 antagonist SCH23390 (0.25 mg/kg) and the D2 antagonist eticlopride (1mg/kg) for use in the present study because these doses did not affect sleep-wake amounts in a previous sleep study in mice [34]. Consistent with that study, Table 1 shows that, at those doses, neither SCH23390+Vehicle, eticlopride+Vehicle, nor the D1+D2+Vehicle combination had any effect on Wake (Figure 1E–1G), NREM (Figure 2G–2I) or REM (Figure 3G–3I) sleep time nor on the latency to NREM (Figure 2A–2C) or REM (Figure 3A–3C) sleep relative to Saline+Vehicle. Thus, despite being “subthreshold” doses for effects on sleep and wake, these doses attenuated the RO5263397-induced wakefulness during the first 1–2 hours after dosing and the D1+D2 combination had a more sustained effect, supporting our conclusion that the wake-promoting effects of TAAR1 agonists are mediated, at least in part, through D1 and D2 receptor activation.
Table 1.
D1 and D2 antagonist effects on physiological parameters when followed by vehicle or by the TAAR1 partial agonist RO5263397
| Change Relative to Saline + Vehicle | Change Relative to Saline + R05263397 | ||||||
|---|---|---|---|---|---|---|---|
| Parameter | D1 antagonist + Vehicle | D2 antagonist + Vehicle | D1+D2 antagonists + Vehicle | Saline + R05263397 | D1 antagonist + R05263397 | D2 antagonist + R05263397 | D1+D2 antagonists + R05263397 |
| Wake Time (ZT6–7) | No effect | No effect | No effect | Increased | No effect | Attenuated increase | Reduced increase |
| Wake Time (ZT6–8) | No effect | No effect | No effect | Increased | No effect | Not significant | Reduced increase |
| Cumulative Wake Time (ZT6–11) | Increased | No effect | No effect | Increased | Reduced increase | Reduced increase | Reduced increase |
| Latency to NREM Sleep | No effect | No effect | No effect | Increased | Blocked increase | No effect | No effect |
| NREM Sleep Time (ZT6–7) | No effect | No effect | No effect | Decreased | No effect | Increased | Increased |
| NREM Sleep Time (ZT6–8) | No effect | No effect | No effect | Decreased | No effect | Not significant | Blocked decrease |
| Cumulative NREM Time (ZT6–11) | No effect | No effect | No effect | Decreased | Attenuated decrease | Attenuated decrease | Blocked decrease |
| Latency to REM Sleep | No effect | No effect | No effect | Increased | No effect | No effect | No effect |
| REM Sleep Time (ZT6–8) | No effect | No effect | No effect | Decreased | No effect | No effect | No effect |
| Cumulative REM Time (ZT6–11) | No effect | No effect | No effect | Decreased | No effect | No effect | No effect |
| Subcutaneous Body Temperature | Mild ↓: ZT7 | Delayed ↓: ZT10–11 | Prolonged ↓: ZT7–11 | ZT6–7 only | Mild ↓: ZT6–7 | Profound ↓↓:ZT7–9 | Prolonged ↓: ZT7–11 |
| Activity | Reduced @ ZT6 only | Reduced throughout | Reduced throughout | Reduced @ ZT6 only | Reduced @ ZT6 only | Reduced throughout | Reduced throughout |
Nonetheless, D2+Vehicle did affect sleep architecture by increasing the number of both Wake (p < 0.01) and NREM (p < 0.05) bouts (Table S2). The D1+D2+Vehicle combination had similar effects, increasing the number of both Wake (p < 0.001) and NREM sleep (p < 0.05) bouts (Table S2).
3.2. Dopaminergic antagonism reduces TAAR1-mediated wakefulness
The wake-promoting effects of the partial TAAR1 agonist RO5263397 shown here are consistent with those from our previous studies in mice, rats and non-human primates [17, 18, 25–27]. TAAR1 is thought to be a negative regulator of DA release [7] and elevated DA levels are normally associated with wakefulness and locomotor activity. Thus, the wake-promoting effects of a TAAR1 partial agonist may seem unexpected but have been interpreted to indicate that RO5263397 antagonizes endogenous TAAR1 tone, increases DA release and thereby activates DA receptors [17, 18]. In contrast, the TAAR1 full agonist RO5256390 which, presumably, lacks TAAR1 antagonist activity, has no effect on sleep/wake [18].
At the doses used here, the D1 and D2 antagonists both produced slight, non-significant reductions in the TAAR1-mediated Wake Time increase from ZT6–8. However, the D1+D2 antagonist combination was very effective in reducing this TAAR1-mediated increase in Wake Time (Figure 1G; Table 1). Moreover, when assessed across the entire 6-h recording period, the D1 and D2 antagonists as well as the D1+D2 combination significantly reduced the TAAR1-mediated increase in Cumulative Wake Time (Table 1; Figure S1). Together, these results indicate that the wake-promoting effects of RO3397 are mediated, at least in part, through dopaminergic neurotransmission.
3.3. Distinct dopaminergic effects on TAAR1-mediated NREM sleep latency vs. NREM sleep time
Consistent with the wake-promoting effects of partial TAAR1 agonism shown here and previously, Saline+RO3397 increased the latency to NREM sleep (Figs. 2A–C; Table 1). Pretreatment with the D1 antagonist completely blocked this increase in NREM latency (Figure 2A), which is also reflected in NREM Time during ZT6, the first post-dosing hour (Figure 2D). However, neither D2+RO3397 nor the D1+D2+RO3397 combination significantly affected the TAAR1-mediated NREM sleep latency increase. D1 blockade of the TAAR1-mediated increase in NREM latency is consistent with the concept that TAAR1 partial agonists antagonize endogenous TAAR1 tone which results in DA release, as the D1 antagonist should block post-synaptic D1 receptors and counteract the TAAR1-mediated increase in NREM latency. Only the D1+D2+RO3397 combination blocked the TAAR1-mediated reduction in NREM time from ZT6–8 (Figure 2I) but all three treatments mitigated the TAAR1-mediated decrease in Cumulative NREM Sleep Time (Figure S2; Table 1).
3.4. Absence of dopaminergic involvement in TAAR1 effects on REM sleep or EEG spectra
In contrast to wakefulness or NREM sleep, DA seems to have no role in the TAAR1-mediated increase in REM latency (Figure 3A–C), the suppression of REM sleep time (Figure 3G–I), or Cumulative REM sleep time (Figure S3) – at least at the doses tested. As described previously, RO3397 increased spectral power in the gamma range but there was no statistical difference after any of the DA treatments, likely due to the large interindividual variation evident in Figure S4.
3.5. Tsc and Activity
All of the pharmacological treatments affected Tsc, although to different magnitudes and on different timecourses. As we have discussed elsewhere [35], while Tsc can provide a relative measure of Tcore, Tsc can dissociate from Tcore and instead reflect activation of heat loss mechanisms through vasodilatation. The Saline+RO3397 treatment transiently reduced Tsc for the first 2-h after treatment. Curiously, although neither the D2+RO3397 nor the D1+D2+RO3397 treatments affected Tsc during the first hour after treatment, both D2-related treatments exacerbated the decline in Tsc evident after Saline+RO3397 and prolonged the effect for several hours. In contrast, the D1 antagonist produced a mild, transient decline in Tsc.
LMA as measured by the DSI transmitter was suppressed by all treatments during the first treatment hour. As with Tsc, the D1-related effect was transient whereas the D2-related effects were prolonged. These prolonged effects on Tsc and LMA are surprising since there are no differences in sleep/wake states evident from ZT9–11.
3.6. Differential effects of D1 vs. D2 antagonism on TAAR1-mediated effects
Previous studies have shown functional interactions between D2R and TAAR1 at both pre- and post-synaptic levels [36–40], but no interactions with D1R or modulation of DAT have been found to date [4, 8, 17, 41]. Figs. 1B, 1C and Figs. 2D, 2E respectively show distinct differences between the effects of the D1R antagonist SCH23390 and the D2R antagonist eticlopride on TAAR1 agonist-mediated Wake and NREM Sleep. Hourly Wake (Figure 1B) and NREM Time (Figure 2D) indicate that D1+Vehicle and D1+RO3397 have similar patterns during ZT6–7 that are significantly different from Saline+RO3397, suggesting that D1 antagonism can mitigate TAAR1 effects. By contrast, D2+Vehicle and D2+RO3397 have different patterns from each other; the effect of D2+RO3397 is more similar to that of Saline+RO3397 from ZT6–8 (Figs. 1C, 2E), suggesting limited D2 efficacy when paired with RO3397. These results are consistent with those from a study on nucleus accumbens slices in which administration of the D2 antagonist L-741626 blocked the modulation of cocaine-induced DA uptake by the TAAR1 agonist RO5256390 [36]. Consistent with the efficacy of D1+D2+RO3397 treatment to block the TAAR1-mediated increase in Wake (Figure 1G) and decrease in NREM (Figure 2I) from ZT6–8, the Wake and NREM patterns produced by this combination are also distinct. Perhaps due to the half-life of these compounds, the significant hourly results from these three conditions are only partially reflected in the 3-h bins as only the D1+D2+RO3397 combination blocks the TAAR1-mediated increase in Wake and reduction in NREM sleep. Consequently, Table 1 summarizes the results for ZT6–7 as well as for the 3-h ZT6–8 bin.
The D1 and D2 antagonists also differed in their effects on the TAAR1-mediated increase in NREM latency: whereas the D1 antagonist blocked this increase, the D2 antagonist had no effect. Whether this difference reflects a differential involvement of D1 vs. D2 receptors in facilitating sleep onset or simply different pharmacokinetic/pharmacodynamic properties of the compounds tested is unclear at this time, but this difference is related to the differential amounts of wakefulness in the first hour after dosing (Figure 1B vs. 1C). In contrast to NREM sleep, the TAAR1-mediated increase in REM sleep latency was unaffected by antagonism of either the D1R or D2R.
Overall, blockade of dopaminergic neurotransmission using D1 and D2 antagonists affected wakefulness and NREM sleep but had no effect on REM sleep as summarized in Table 1. The differential effects of D1+RO3397 and D2+RO3397 on Wake and NREM time is particularly evident in the initial post-dosing hours (ZT6–7), where D2+RO3397 appears to alter the RO3397-induced increase in Wake and decrease in NREM. Tsc and LMA show similar patterns to wake and NREM in that D1+RO3397 produces an hourly pattern similar to Sal+RO3397 (Figure 4B, 4E) whereas D2+RO3397 exacerbated the Tsc decline produced by Sal+RO3397 (Figure 4B). Due to the different half-lives of the D1, D2 antagonists and RO3397, direct comparison between treatments is limited. Nonetheless, these results indicate complex interactions between DA neurotransmission and TAAR1 partial agonism on physiology and behavioral states.
3.7. Limitations of the present study
The experimental design utilized here involved administration of a single dose of DA antagonists as pretreatments prior to use of the TAAR1 partial agonist RO5263397. As summarized above, the DA system has profound effects on sleep/wake in both rodents and humans. However, the doses of the D1 and D2 antagonists we chose had previously been shown to be without effect on sleep/wake in a study from a well-regarded laboratory [34] and, as summarized above, these doses when combined with vehicle were without effect in our study as well. Nonetheless, many of the components of the DA system are known to be rhythmic at either the mRNA or protein level and it is conceivable that testing at another time of day might yield different results.
Secondly, we used pharmacological pre-treatment with D1 and D2 antagonists rather than evaluating the effects of the TAAR1 agonists in D1 and/or D2 receptor knockout (KO) mice. Further studies might employ such animal models, although such approaches would require sleep/wake phenotyping of the receptor KO strain.
Thirdly, our approach involved systemic drug administration to establish the principle that the DA system is involved in TAAR1 agonist-mediated effects on wakefulness. Future studies may be directed towards elucidating the underlying circuits using approaches such as local drug injection or more sophisticated tools such as cell specific knockouts, fiber photometry or GRAB sensors [42, 43] to measure activity of the DA system.
4. Conclusions
TAAR1 has been shown to play an important role in modulating monoaminergic neurotransmission, particularly in relation to psychostimulants and addiction, and TAAR1 agonists are increasingly recognized as a novel mechanism for treatment of psychiatric disorders [20, 44–46]. Similar to RO5263397, Ulotaront, a TAAR1 agonist with 5HT1A agonist activity, has been shown to have wake-promoting and REM sleep-suppressing activity in both preclinical [47] and clinical [48] studies. Ulotaront has also recently been shown to reduce REM sleep without atonia in human subjects [19]. Since Ulotaront (previously, SEP-363856) inhibits firing of VTA neurons [47], it likely also affects DA release as part of its mechanism of action. However, the mechanisms underlying TAAR1 partial agonist effects to promote wakefulness and suppress REM sleep have not been extensively studied. The results of the current study indicate that D2 antagonists can attenuate or block TAAR1-mediated wakefulness without affecting NREM sleep latency, whereas D1 antagonists can counteract the delayed NREM latency produced by TAAR1 agonism. Whether these differential effects reflect differential D1 vs D2 involvement in sleep onset vs. wake maintenance or a differential time course of action of the particular compounds studied is unclear at this time. Nonetheless, it is noteworthy that neither D1 nor D2 antagonists affected the TAAR1 suppression of REM sleep, particularly since many psychoactive compounds such as antidepressants suppress REM sleep.
5. Materials and Methods
5.1. Animals
Adult (>9 week) male C57BL6/J mice (n=8) were used; all mice were >23 g at the time of surgery. Mice were maintained on a 12:12 light/dark cycle with food and water ad libitum. Room temperature (23 ± 2°C), humidity (50 ± 20% relative humidity), and lighting conditions were monitored continuously (Rees Scientific, Trenton, NJ). All studies were conducted following the Guide for Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee (IACUC) at SRI International.
5.2. Surgical Procedures
Mice were implanted with sterilized F20-EET wireless transmitters (DSI, Inc., St. Paul, MN) subcutaneously under their left dorsum to record electroencephalogram (EEG), electromyogram (EMG), locomotor activity (LMA) and subcutaneous body temperature (Tsc). During surgery, mice were anesthetized with 1.5%−2% isoflurane and EMG and EEG leads were routed subcutaneously. EEG leads were inserted through the intracranial burr holes, with one lead placed over the hippocampal area (−2 mm A/P from bregma, +2 mm M/L) and the ground lead over the cerebellum (−1 mm A/P from lambda, +2 mm M/L). After placement of the EEG leads, dental cement was applied to the skull to affix the wires. EMG leads were sutured to the right nuchal muscle. An analgesia cocktail of meloxicam (5 mg/kg, s.c.) and buprenorphine (0.05 mg/kg, s.c.) was administered for two days post-surgery. Meloxicam was administered for an additional day.
5.3. EEG, EMG, LMA and Tsc Recording
Three weeks after surgery, mice were acclimated to handling and oral gavage dosing at least seven days before data collection. During each recording session, the wireless transmitters were activated at ZT23 (Day 0) and turned off at ZT24 the following day (Day 1). Physiological signals collected from transmitters were thus continuously recorded for 25 h using Ponemah (DSI, Inc., St. Paul, MN) and subsequently analyzed. Due to signal attenuation in the F20-EET transmitters, the cutoff for EEG spectral analysis was 60 Hz.
5.4. Experimental Protocols
As illustrated in Figure 1A, all mice received experimental treatments at both ZT5.5 and 30 min later at ZT6; the experimenter was blinded to the dosing condition. The D1 receptor antagonist SCH23390 (hereafter D1; 0.25 mg/kg), the D2 antagonist eticlopride (hereafter D2; 1 mg/kg), a combination of D1+D2, or saline were administered intraperitoneally (i.p.) at ZT5.5. The doses of the D1 antagonist SCH23390 and the D2 antagonist eticlopride were selected as these doses did not affect sleep-wake amounts in a previous sleep study conducted in mice [34]. After 30 min, the TAAR1 agonist RO5263397 (“3397”; 1 mg/kg in the vehicle) or vehicle (10% DMSO) was administered at ZT6 per os (p.o.). This dose of 3397 and time of day were chosen because our previous study had shown a robust wake-promoting effect of 3397 when administered at ZT6 [26]. Although the T½ of 3397 was determined to be 6.0 h when mice were dosed at 4 mg/kg, p.o. [18], both the wake-promoting and REM sleep-suppressing effects of 3397 dissipated within 3 h when mice were dosed at 1 mg/kg, p.o. [26]. A repeated measures design was utilized in which all mice received all treatment combinations and drug treatments were administered in a balanced order. To ensure adequate wash-out between each treatment, at least 72 h elapsed between dosings.
5.5. Drugs
SCH23390, eticlopride, and RO5263397 (Sigma Aldrich, St. Louis, MO) were prepared as fresh solutions daily, with sonication for 15 min and then diluted serially using deionized water (DI H2O) or 10% DMSO (Sigma Aldrich, St. Louis, MO) in DI H2O as the vehicle. Drugs were administered at a volume of 20 ml/kg.
5.6. Data and Statistical Analysis
EEG and EMG recordings, collected for 6 hours after the second dosing (ZT6-ZT12), were scored in 10-sec epochs as wakefulness, NREM or REM sleep using Neuroscore (DSI, Inc., MN) by expert scorers blinded to drug treatments as described previously [25, 26, 49]. The resultant data were analyzed using custom MATLAB (Mathworks, MA) scripts and Prism 9 (Graphpad, CA). Dependent variables measured included hourly percent time in Wake, NREM, and REM sleep, cumulative time in Wake, NREM and REM, measures of sleep/wake consolidation (mean hourly bout duration and the number of bouts of Wake, NREM and REM), spectral analysis of the EEG within each state, and effects on Tsc and LMA. Due to poor signal quality on one of the dosing days, all recordings for that day were excluded from the analysis; consequently, 7 of the 8 possible treatment combinations were analyzed for each mouse. Two-way repeated measures ANOVA (RM-ANOVA), with drug treatment and time as factors followed by Dunnett’s post hoc test, or one-way ANOVA followed by Tukey’s multiple comparison test were used to assess statistical significance. The statistical tests used for each figure panel are described in Table S1.
Supplementary Material
Funding:
Research supported by NIH R01NS103529.
Footnotes
Supplementary material: Supplementary material is available at IJMS online.
Institutional Review Board Statement: All studies were conducted following the Guide for Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee (IACUC) at SRI International.
Conflicts of Interest: Drs. Park, Ms. Heu and Dr. Kilduff reported no biomedical financial interests or potential conflicts of interest. Dr. Hoener is an employee of F. Hoffmann-La Roche Ltd.
References
- 1.Berry M. D., Mammalian central nervous system trace amines. Pharmacologic amphetamines, physiologic neuromodulators. J Neurochem 2004, 90, 257–271. [DOI] [PubMed] [Google Scholar]
- 2.Borowsky B.; Adham N.; Jones K. A.; Raddatz R.; Artymyshyn R.; Ogozalek K. L.; Durkin M. M.; Lakhlani P. P.; Bonini J. A.; Pathirana S.; Boyle N.; Pu X.; Kouranova E.; Lichtblau H.; Ochoa F. Y.; Branchek T. A.; Gerald C., Trace amines: Identification of a family of mammalian G protein-coupled receptors. Proc Natl Acad Sci USA 2001, 98, 8966–8971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bunzow J. R.; Sonders M. S.; Arttamangkul S.; Harrison L. M.; Zhang G.; Quigley D. I.; Darland T.; Suchland K. L.; Pasumamula S.; Kennedy J. L.; Olson S. B.; Magenis R. E.; Amara S. G.; Grandy D. K., Amphetamine, 3,4-methylenedioxymethamphetamine, lysergic acid diethylamide, and metabolites of the catecholamine neurotransmitters are agonists of a rat trace amine receptor. Mol Pharmacol 2001, 60, 1181–1188. [DOI] [PubMed] [Google Scholar]
- 4.Gainetdinov R. R.; Hoener M. C.; Berry M. D., Trace Amines and Their Receptors. Pharmacol Rev 2018, 70, 549–620. [DOI] [PubMed] [Google Scholar]
- 5.Lindemann L.; Ebeling M.; Kratochwil N. A.; Bunzow J. R.; Grandy D. K.; Hoener M. C., Trace amine-associated receptors form structurally and functionally distinct subfamilies of novel G protein-coupled receptors. Genomics 2005, 85, 372–385. [DOI] [PubMed] [Google Scholar]
- 6.Liberles S. D.; Buck L. B., A second class of chemosensory receptors in the olfactory epithelium. Nature 2006, 442, 645–650. [DOI] [PubMed] [Google Scholar]
- 7.Lindemann L.; Meyer C. A.; Jeanneau K.; Bradaia A.; Ozmen L.; Bluethmann H.; Bettler B.; Wettstein J. G.; Borroni E.; Moreau J. L.; Hoener M. C., Trace amine-associated receptor 1 modulates dopaminergic activity. J Pharmacol Exp Ther 2008, 324, 948–956. [DOI] [PubMed] [Google Scholar]
- 8.Revel F. G.; Moreau J. L.; Gainetdinov R. R.; Bradaia A.; Sotnikova T. D.; Mory R.; Durkin S.; Zbinden K. G.; Norcross R.; Meyer C. A.; Metzler V.; Chaboz S.; Ozmen L.; Trube G.; Pouzet B.; Bettler B.; Caron M. G.; Wettstein J. G.; Hoener M. C., TAAR1 activation modulates monoaminergic neurotransmission, preventing hyperdopaminergic and hypoglutamatergic activity. Proc Natl Acad Sci USA 2011, 108, 8485–8490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Espinoza S.; Lignani G.; Caffino L.; Maggi S.; Sukhanov I.; Leo D.; Mus L.; Emanuele M.; Ronzitti G.; Harmeier A.; Medrihan L.; Sotnikova T. D.; Chieregatti E.; Hoener M. C.; Benfenati F.; Tucci V.; Fumagalli F.; Gainetdinov R. R., TAAR1 Modulates Cortical Glutamate NMDA Receptor Function. Neuropsychopharmacology 2015, 40, (9), 2217–2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Achat-Mendes C.; Lynch L. J.; Sullivan K. A.; Vallender E. J.; Miller G. M., Augmentation of methamphetamine-induced behaviors in transgenic mice lacking the trace amine-associated receptor 1. Pharmacol Biochem Behav 2012, 101, 201–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sotnikova T. D.; Zorina O. I.; Ghisi V.; Caron M. G.; Gainetdinov R. R., Trace amine associated receptor 1 and movement control. Parkinsonism Relat Disord 2008, 14 Suppl 2, S99–102. [DOI] [PubMed] [Google Scholar]
- 12.Wolinsky T. D.; Swanson C. J.; Smith K. E.; Zhong H.; Borowsky B.; Seeman P.; Branchek T.; Gerald C. P., The Trace Amine 1 receptor knockout mouse: An animal model with relevance to schizophrenia. Genes Brain Behav 2007, 6, 628–639. [DOI] [PubMed] [Google Scholar]
- 13.di Cara B.; Maggio R.; Aloisi G.; Rivet J. M.; Lundius E. G.; Yoshitake T.; Svenningsson P.; Brocco M.; Gobert A.; de Groote L.; Cistarelli L.; Veiga S.; de Montrion C. D.; Rodriguez M.; Galizzi J. P.; Lockhart B. P.; Cogé F.; Boutin J. A.; Vayer P.; Verdouw P. M.; Groenink L.; Millan M. J., Genetic Deletion of Trace Amine 1 Receptors Reveals Their Role in Auto-Inhibiting the Actions of Ecstasy (MDMA). J Neurosci 2011, 31, 16928–16940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sukhanov I.; Dorofeikova M.; Dolgorukova A.; Dorotenko A.; Gainetdinov R. R., Trace Amine-Associated Receptor 1 Modulates the Locomotor and Sensitization Effects of Nicotine. Front Pharmacol 2018, 9, 329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sukhanov I.; Dorotenko A.; Dolgorukova A.; Dorofeikova M.; Gainetdinov R. R., The trace amine-associated receptor 1 modulates nicotine behavioural effects. Eur Neuropsychopharmacology 2017, 27, S673–S674. [Google Scholar]
- 16.Revel F. G.; Meyer C. A.; Bradaia A.; Jeanneau K.; Calcagno E.; André C. B.; Haenggi M.; Miss M.-T.; Galley G.; Norcross R. D.; Invernizzi R. W.; Wettstein J. G.; Moreau J.-L.; Hoener M. C., Brain-Specific Overexpression of Trace Amine-Associated Receptor 1 Alters Monoaminergic Neurotransmission and Decreases Sensitivity to Amphetamine. Neuropsychopharmacology 2012, 37, 2580–2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Revel F. G.; Moreau J.-L.; Gainetdinov R. R.; Ferragud A.; Velázquez-Sánchez C.; Sotnikova T. D.; Morairty S. R.; Harmeier A.; Groebke Zbinden K.; Norcross R. D.; Bradaia A.; Kilduff T. S.; Biemans B.; Pouzet B.; Caron M. G.; Canales J. J.; Wallace T. L.; Wettstein J. G.; Hoener M. C., Trace amine-associated receptor 1 partial agonism reveals novel paradigm for neuropsychiatric therapeutics. Biol Psychiatry 2012, 72, 934–42. [DOI] [PubMed] [Google Scholar]
- 18.Revel F. G.; Moreau J. L.; Pouzet B.; Mory R.; Bradaia A.; Buchy D.; Metzler V.; Chaboz S.; Groebke Zbinden K.; Galley G.; Norcross R. D.; Tuerck D.; Bruns A.; Morairty S. R.; Kilduff T. S.; Wallace T. L.; Risterucci C.; Wettstein J. G.; Hoener M. C., A new perspective for schizophrenia: TAAR1 agonists reveal antipsychotic- and antidepressant-like activity, improve cognition and control body weight. Mol Psychiatry 2013, 18, (5), 543–56. [DOI] [PubMed] [Google Scholar]
- 19.Feemster J. C.; Westerland S. M.; Gossard T. R.; Steele T. A.; Timm P. C.; Jagielski J. T.; Strainis E.; McCarter S. J.; Hopkins S. C.; Koblan K. S.; St. Louis E. K., Treatment with the novel TAAR1 agonist ulotaront is associated with reductions in quantitative polysomnographic REM sleep without atonia in healthy human subjects: Results of a post-hoc analysis. Sleep Med 2023, 101, 578–586. [DOI] [PubMed] [Google Scholar]
- 20.Kane J. M., A New Treatment Paradigm: Targeting Trace Amine-Associated Receptor 1 (TAAR1) in Schizophrenia. J Clin Psychopharmacol 2022, 42, (5 Suppl 1), S1–S13. [DOI] [PubMed] [Google Scholar]
- 21.Dahan L.; Astier B.; Vautrelle N.; Urbain N.; Kocsis B.; Chouvet G., Prominent Burst Firing of Dopaminergic Neurons in the Ventral Tegmental Area during Paradoxical Sleep. Neuropsychopharmacology 2007, 32, (6), 1232–1241. [DOI] [PubMed] [Google Scholar]
- 22.Eban-Rothschild A.; Rothschild G.; Giardino W. J.; Jones J. R.; De Lecea L., VTA dopaminergic neurons regulate ethologically relevant sleep-wake behaviors. Nature Neurosci 2016, 19, 1356–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hasegawa E.; Miyasaka A.; Sakurai K.; Cherasse Y.; Li Y.; Sakurai T., Rapid eye movement sleep is initiated by basolateral amygdala dopamine signaling in mice. Science 2022, 375, 994–1000. [DOI] [PubMed] [Google Scholar]
- 24.Miller J. D.; Farber J.; Gatz P.; Roffwarg H.; German D. C., Activity of mesencephalic dopamine and non-dopamine neurons across stages of sleep and waking in the rat. Brain Res 1983, 273, 133–141. [DOI] [PubMed] [Google Scholar]
- 25.Black S. W.; Schwartz M. D.; Chen T. M.; Hoener M. C.; Kilduff T. S., Trace Amine-Associated Receptor 1 Agonists as Narcolepsy Therapeutics. Biol Psychiatry 2017, 82, 623–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schwartz M. D.; Black S. W.; Fisher S. P.; Palmerston J. B.; Morairty S. R.; Hoener M. C.; Kilduff T. S., Trace Amine-Associated Receptor 1 Regulates Wakefulness and EEG Spectral Composition. Neuropsychopharmacology 2017, 42, (6), 1305–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goonawardena A. V.; Morairty S. R.; Dell R.; Orellana G. A.; Hoener M. C.; Wallace T. L.; Kilduff T. S., Trace amine-associated receptor 1 agonism promotes wakefulness without impairment of cognition in Cynomolgus macaques. Neuropsychopharmacology 2019, 44, (8), 1485–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trampus M.; Ongini E., The D1 dopamine receptor antagonist SCH 23390 enhances REM sleep in the rat. Neuropharmacology 1990, 29, 889–893. [DOI] [PubMed] [Google Scholar]
- 29.Bo P.; Ongini E.; Giorgetti A.; Savoldi F., Synchronization of the EEG and sedation induced by neuroleptics depend upon blockade of both D-1 and D-2 dopamine receptors. Neuropharmacology 1988, 27, 799–805. [DOI] [PubMed] [Google Scholar]
- 30.Eder D. N.; Zdravkovic M.; Wildschiødtz G., Selective alterations of the first NREM sleep cycle in humans by a dopamine D1 receptor antagonist (NNC-687). J Psychiatric Res 2003, 37, 305–312. [DOI] [PubMed] [Google Scholar]
- 31.Ongini E.; Bonizzoni E.; Ferri N.; Milani S.; Trampus M., Differential effects of dopamine D-1 and D-2 receptor antagonist antipsychotics on sleep-wake patterns in the rat. J Pharmacol Exp Ther 1993, 266, (2), 726–31. [PubMed] [Google Scholar]
- 32.Bagetta G.; Corasaniti M. T.; Strongoli M. C.; Sakurada S.; Nisticò G., Behavioural and ECoG spectrum power effects after intraventricular injection of drugs altering dopaminergic transmission in rats. Neuropharmacology 1987, 26, 1047–1052. [DOI] [PubMed] [Google Scholar]
- 33.Ongini E.; Bo P.; Dionisotti S.; Trampus M.; Savoldi F., Effects of remoxipride, a dopamine D-2 antagonist antipsychotic, on sleep-waking patterns and EEG activity in rats and rabbits. Psychopharmacology 1992, 107, 236–242. [DOI] [PubMed] [Google Scholar]
- 34.Burgess C. R.; Tse G.; Gillis L.; Peever J. H., Dopaminergic regulation of sleep and cataplexy in a murine model of narcolepsy. Sleep 2010, 33, (10), 1295–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun Y.; Tisdale R. K.; Yamashita A.; Kilduff T. S., Peripheral vs. Core Body Temperature as Hypocretin/Orexin Neurons Degenerate: Exercise Mitigates Increased Heat Loss. Peptides 2023, 164, 171002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Asif-Malik A.; Hoener M. C.; Canales J. J., Interaction Between the Trace Amine-Associated Receptor 1 and the Dopamine D(2) Receptor Controls Cocaine’s Neurochemical Actions. Sci Rep 2017, 7, (1), 13901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bradaia A.; Trube G.; Stalder H.; Norcross R. D.; Ozmen L.; Wettstein J. G.; Pinard A.; Buchy D.; Gassmann M.; Hoener M. C.; Bettler B., The selective antagonist EPPTB reveals TAAR1-mediated regulatory mechanisms in dopaminergic neurons of the mesolimbic system. Proc Natl Acad Sci USA 2009, 106, 20081–20086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Espinoza S.; Ghisi V.; Emanuele M.; Leo D.; Sukhanov I.; Sotnikova T. D.; Chieregatti E.; Gainetdinov R. R., Postsynaptic D2 dopamine receptor supersensitivity in the striatum of mice lacking TAAR1. Neuropharmacology 2015, 93, 308–313. [DOI] [PubMed] [Google Scholar]
- 39.Espinoza S.; Salahpour A.; Masri B.; Sotnikova T. D.; Messa M.; Barak L. S.; Caron M. G.; Gainetdinov R. R., Functional interaction between trace amine-associated receptor 1 and dopamine D2 receptor. Mol Pharmacol 2011, 80, 416–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leo D.; Mus L.; Espinoza S.; Hoener M. C.; Sotnikova T. D.; Gainetdinov R. R., Taar1-mediated modulation of presynaptic dopaminergic neurotransmission: Role of D2 dopamine autoreceptors. Neuropharmacology 2014, 81, 283–291. [DOI] [PubMed] [Google Scholar]
- 41.Leo D.; Sukhanov I.; Zoratto F.; Illiano P.; Caffino L.; Sanna F.; Messa G.; Emanuele M.; Esposito A.; Dorofeikova M.; Budygin E. A.; Mus L.; Efimova E.; Niello M.; Espinoza S.; Sotnikova T. D.; Hoener M. C.; Laviola G.; Fumagalli F.; Adriani W.; Gainetdinov R. R., Pronounced Hyperactivity, Cognitive Dysfunctions, and BDNF Dysregulation in Dopamine Transporter Knock-out Rats. J Neurosci 2018, 38, 1959–1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun F.; Zeng J.; Jing M.; Zhou J.; Feng J.; Owen S. F.; Luo Y.; Li F.; Wang H.; Yamaguchi T.; Yong Z.; Gao Y.; Peng W.; Wang L.; Zhang S.; Du J.; Lin D.; Xu M.; Kreitzer A. C.; Cui G.; Li Y., A Genetically Encoded Fluorescent Sensor Enables Rapid and Specific Detection of Dopamine in Flies, Fish, and Mice. Cell 2018, 174, (2), 481–496 e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patriarchi T.; Cho J. R.; Merten K.; Howe M. W.; Marley A.; Xiong W. H.; Folk R. W.; Broussard G. J.; Liang R.; Jang M. J.; Zhong H.; Dombeck D.; von Zastrow M.; Nimmerjahn A.; Gradinaru V.; Williams J. T.; Tian L., Ultrafast neuronal imaging of dopamine dynamics with designed genetically encoded sensors. Science 2018, 360, (6396). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dedic N.; Dworak H.; Zeni C.; Rutigliano G.; Howes O. D., Therapeutic Potential of TAAR1 Agonists in Schizophrenia: Evidence from Preclinical Models and Clinical Studies. Intl J Mol Sci 2021, 22, 13185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Halff E. F.; Rutigliano G.; Garcia-Hidalgo A.; Howes O. D., Trace amine-associated receptor 1 (TAAR1) agonism as a new treatment strategy for schizophrenia and related disorders. Trends Neurosci 2023, 46, (1), 60–74. [DOI] [PubMed] [Google Scholar]
- 46.Alnefeesi Y.; Tamura J. K.; Lui L. M. W.; Jawad M. Y.; Ceban F.; Ling S.; Nasri F.; Rosenblat J. D.; McIntyre R. S., Trace amine-associated receptor 1 (TAAR1): Potential application in mood disorders: A systematic review. Neurosci Biobehav Rev 2021, 131, 192–210. [DOI] [PubMed] [Google Scholar]
- 47.Dedic N.; Jones P. G.; Hopkins S. C.; Lew R.; Shao L.; Campbell J. E.; Spear K. L.; Large T. H.; Campbell U. C.; Hanania T.; Leahy E.; Koblan K. S., SEP-363856, a novel psychotropic agent with a unique, non-D2 receptor mechanism of action. Journal of Pharmacology and Experimental Therapeutics 2019, 371, 1–14. [DOI] [PubMed] [Google Scholar]
- 48.Hopkins S. C.; Dedic N.; Koblan K. S., Effect of TAAR1/5-HT(1A) agonist SEP-363856 on REM sleep in humans. Transl Psychiatry 2021, 11, (1), 228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morairty S. R.; Dittrich L.; Pasumarthi R. K.; Valladao D.; Heiss J. E.; Gerashchenko D.; Kilduff T. S., A role for cortical nNOS/NK1 neurons in coupling homeostatic sleep drive to EEG slow wave activity. Proc Natl Acad Sci USA 2013, 110, 20272–20277. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.