Abstract
Hidradenitis suppurativa (HS) is a chronic inflammatory skin condition characterized by painful nodules, abscesses, and scarring, predominantly affecting intertriginous regions and it is often underdiagnosed. This study aimed to utilize single cell RNA and cell-surface protein sequencing (CITE-Seq) to delineate the immune composition of circulating cells in Hidradenitis suppurativa (HS) peripheral blood compared to healthy controls. CITE-Seq was used to analyze the gene and protein expression profiles of peripheral blood mononuclear cells (PBMCs) from 9 HS and 29 healthy controls. The study identified significant differences cell composition between HS patients and healthy controls, including increased proportions of CD14+ and CD16+ monocytes, cDC2, plasmablasts, and proliferating CD4+ T cells in HS patients. Differential expression analysis revealed upregulation of inflammatory markers such as TNF, IL1B, and NF-κB in monocytes, as well as chemokines and cell adhesion molecules involved in immune cell recruitment and tissue infiltration. Pathway enrichment analysis highlighted the involvement of IL-17, IL-26 and TNF signaling pathways in HS pathogenesis. Machine learning identified key markers for diagnostics and therapeutic development. The findings also support the potential for machine learning models to aid in the diagnosis of HS based on immune cell markers. These insights may inform future therapeutic strategies targeting specific immune pathways in HS.
Keywords: hidradenitis suppurativa, single cell, CITE-seq, machine learning, diagnostic test, pathway enrichment analyses, cell-cell interaction
1. Introduction
Hidradenitis suppurativa (HS) is a chronic inflammatory skin condition characterized by painful lesions, including nodules, abscesses, skin tunnels, and scarring1. These manifestations primarily affect intertriginous areas, notably the axillary, groin, perianal, perineal, and inframammary regions. The impact of the condition extends beyond physical discomfort; the associated pain, drainage, malodor, and scarring often lead to profound negative psychosocial effects. The estimated global prevalence of HS is 1–2%, but it is likely underreported due to underdiagnosis and inadequate treatment. HS is also associated with comorbidities including psoriasis, Crohn’s disease, metabolic syndrome, and spondyloarthritis2.
Single-cell technologies have generated large immunological datasets to delineate the biological mechanisms of immune-mediated disease pathways at high resolution.3 4. In HS, a study by Straalen et al. identified TNF-α, IL-1β, SFRP4+, and CXCL13+ as relevant therapeutic targets for HS patients through spatial sequencing and complement analysis5. Another study explored inflammatory responses in HS, revealing that the immune responses were centered on IFN-γ, IL-36, and TNF, with a lesser contribution from IL-17A6.
Treatment options for HS remain limited. Currently, two drugs, adalimumab and secukinumab, are the only FDA approved targeted therapies for HS. Briefly, adalimumab acts as a TNF inhibitor, achieving a clinical response in just 40 to 60% of patients. Secukinumab, an inhibitor targeting the IL-17A, plays a significant role in the pathophysiology of HS by preventing interaction with the IL-17 receptor 7.
In this study, we utilized single-cell cellular indexing of transcriptomes and protein epitopes (CITE-Seq) to define the immune composition of circulating cells in HS peripheral blood compared to healthy controls. Our results provide a high-resolution view of HS systemic immunity, identifying dysregulated cell types and novel cell-cell interactions that contribute to disease inflammation. We also identify HS pathways that may serve as potential targets for future therapeutic intervention. Finally, we developed several machine learning models for the accurate classification of HS and healthy individuals based on the expression of multiple cell markers, thereby advancing biomarker discovery.
2. Methodology
2.1. Subject Recruitment and Sampling
Peripheral blood mononuclear cells (PBMCs) were collected from nine HS subjects at Henry Ford Hospital, Detroit, Michigan, USA. The HS subjects comprised 6 females and 3 males with mean age 43.0 (SD 13.2). All subjects were either Hurley Stage II or III. All subjects provided written informed consent (Henry Ford Health IRB# 12826). The clinical diagnosis of HS was confirmed by a dermatologist. None of the subjects were receiving biologic or systemic therapy for HS during PBMC collection. Additionally, PBMCs from 29 healthy controls, age- and sex-matched, were collected at the University of California San Francisco under IRB approval 10–02830. Detailed subject information is in Supplementary Table 1. Peripheral blood was collected in Vacutainer ACD tubes, PBMCs were isolated using Ficoll, and stored in liquid nitrogen.
2.2. Sample and Library Preparation for CITE-Seq Sequencing
For each sample, 500 μL of thawed PBMCs were mixed with 10 mL of EasySep buffer (StemCell Technologies, Cat. 20144) and centrifuged at 300g for 5 minutes at room temperature. The resulting cell pellets were resuspended in 1 mL of a buffer solution containing 18 mL EasySep and 21 μL Benzonase Nuclease (MilliporeSigma, Cat. 70664) to break down extracellular nucleic acids. This mixture was incubated at room temperature for 15 minutes. The cell suspensions treated with nuclease were then passed through a 40 μm Flowmi Cell Strainer (Bel-Art, Cat. H13680–0040), centrifuged again at 300g for 5 minutes at room temperature, and resuspended in 100 μL EasySep buffer. Final cell counts were obtained by diluting the suspensions 1:100 and staining with 0.4% trypan blue, followed by counting with a Countess I FL Automated Cell Counter (Thermo Fisher Scientific).
cDNA libraries for gene expression were prepared following the manufacturer’s guidelines (Chromium Next GEM Single Cell 3’ v3.1), including 12 cycles of PCR amplification. Cellometer (Nexcelom - Lawrence, MA) was used to determine the cell viability and concentration to normalize to 1E6 cells/ml, 40,000 cells were targeted for each library. For antibody-derived tags (ADT) libraries from feature barcoding antibodies, size purification was repeated on the supernatant from the initial cDNA library size purification, using a 7:8 volumetric ratio of 2.0X SPRIselect reagent (Beckman Coulter, Cat. B23317) to the sample. Indexing amplification was carried out using Kapa HiFi HotStart ReadyMix (Kapa Biosystems, Cat. KK2601) and TruSeq Small RNA RPI primers (Illumina) under the following conditions: initial denaturation at 98°C for 2 minutes, followed by 15 cycles of 98°C for 20 seconds, 60°C for 30 seconds, and 72°C for 20 seconds, and a final extension at 72°C for 5 minutes. The amplified libraries underwent another round of size purification using a 5:6 volumetric ratio of 1.2X SPRIselect reagent to the sample. Libraries were quantified with a Bioanalyzer 2100 (Agilent) and sequenced on a NovaSeq 6000 (Illumina).
2.3. Antibody-Tag protein Sequencing
For the antibody-tagged protein 10X sequencing, staining of cell surface protein was performed using a modified version of the Totalseq-A protocol (details at https://www.biolegend.com/en-us/protocols/totalseq-a-dual-index-protocol). A pooled cell suspension, comprising 100,000 cells from up to 10 subjects, was centrifuged at 300g for 5 minutes at 4°C. The cell pellets were then resuspended in 100 μL Cell Staining Buffer (BioLegend, Cat. 420201) and incubated for 10 minutes at 4°C with 10 μL Human TruStain FcX™ Fc Blocking Solution (BioLegend, Cat. 422301). Following this, the cell suspensions were stained for 30 minutes at 4°C with 100 μL of a TotalSeq antibody cocktail. The stained cells were then divided into two aliquots of 105 μL each.
Each aliquot was subjected to three washes by resuspending the cells in 15 mL of Cell Staining Buffer and centrifuging at 300g for 5 minutes at 4°C. After washing, the cells were resuspended in 150 μL of 10% FBS in PBS, recombined, and filtered through a 40 μm Flowmi Cell Strainer. To assess cell viability, 10 μL of the filtered cells were mixed with 10 μL of 0.4% Trypan Blue and counted manually using a hemocytometer. The cell density was then adjusted to 2,500 cells/μL, and the cells were processed on the Chromium Controller (10X Genomics) using the Single Cell 3’ v3.1 Assay (10X Genomics), targeting 50,000 cells per reaction.
2.4. Genotyping of PBMCs
Genomic DNA was extracted from peripheral blood mononuclear cells (PBMCs) using the DNeasy Blood and Tissue Kit (Qiagen, Cat. 69504) according to the manufacturer’s instructions. The concentration and purity of extracted DNA quality were determined using a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific). Further, the extracted DNA samples were genotyped using the Affymetrix UK Biobank Axiom Array (ThermoFisher Scientific) on a GeneTitan Multi-Channel Instrument (Applied Biosystems) following the recommended protocols.
2.5. Genotype Calling and Quality Control
The genotype samples from the DNA array were processed using Thermo-Analysis Power Tools 2.10.2.2 (Affymetrix) to generate genotype calls. Standard quality control filters were applied, including sample call rate >98%, SNP call rate >98%, minor allele frequency >1%, and Hardy-Weinberg Equilibrium p-value > 1×10^6. The resulting genotype data files (VCFs) were further scanned using snpflip (https://github.com/biocore-ntnu/snpflip) against the GRCh37 human genome reference sequence from the University of California, Santa Cruz (http://hgdownload.cse.ucsc.edu/goldenPath/hg19/bigZips/hg19.fa.gz). Reversed and ambiguous-stranded SNPs were identified, flipped, and removed, respectively, using Plink 1.90 (http://pngu.mgh.harvard.edu/purcell/plink/). The remaining SNPs were sorted using Plink 2.00a3LM (www.cog-genomics.org/plink/2.0/). Additional untyped SNPs were imputed using the Michigan Imputation Server (https://imputationserver.sph.umich.edu) with the 1000G Phase 3 v5 GRCh37 reference panel, Eagle v2.4 phasing, and the EUR population. The SNP positions were then converted to GRCh38 coordinates using the ‘LiftoverVcf’ command of Picard 2.23.3 (http://broadinstitute.github.io/picard/). Finally, Vcftools 0.1.13 was used to exclude non-exonic SNPs and SNPs with minor allele frequency < 0.05. The resulting high-quality genotype data were used for demuxlet of sequencing data.
2.6. Cell Demultiplexing and Doublet Removal
We sequenced multiple samples into a single aliquot per Chromium run, resulting in multiple samples combined into one count matrix. To determine the subject of origin for all droplet barcodes within each RNA count matrix, we used ‘demuxlet’ as part of the ‘popscle’ suite (https://github.com/statgen/popscle). This R-package, applied to imputation-augmented exonic SNP genotypes as described earlier, enabled the exclusion of doublets detected between different individuals. The count matrices for each Chromium library were then imported into R for further analysis using the ‘Seurat’ 5.0.0 R package. The demuxlet identified heterogenic doublets, which are doublets formed from cells originating from different donors8. Additionally, the ‘Doubletfinder’ V2.0 into R package was utilized to remove doublets formed by both heterogenic and homogenic doublets, as it relies on transcriptional signatures rather than genetic variation.
2.7. Clustering and Annotation of Clusters
ADT expression was estimated for cells with measured RNA but not ADT according to the Seurat reference mapping protocol (https://satijalab.org/seurat/articles/multimodal_reference_mapping.html), and unless otherwise noted, all function names described here belong to the Seurat package. Briefly, the integrated dataset above was split into the subset of cells with ADT measurements (reference subset) and the subset of cells without ADT measurements (query subset). RNA expression normalization and scaling were performed using ‘SCTransform’ on both subsets, adjusting for the number of features and total counts in each cell. While ADT expression normalization for the reference subset was performed using the Centered Log-Ratio (CLR), followed by mean centering and scaling. For the reference subset, PCA was then run for both the SCTransformed RNA (SCT) expression and the ADT expression, and a weighted nearest-neighbor network for the reference subset was calculated from first 30 for and ADT, respectively, using the ‘FindMultiModalNeighbors’ function. Next, SCT from the reference subset was transformed again using supervised PCA (via the ‘RunPCA’ function) to identify the principal components that best capture the combined RNA and ADT expression variation represented by the weighted nearest-neighbor network.
2.8. Cluster Annotation through Azimuth
After QC, all samples were merged into one object and cluster annotation was performed through Azimuth using labels from the Human PBMC reference9.
2.9. Samples Batch-correction and Integration
Batch effects were corrected via integration of SCT expression data according to the Seurat integration protocol (https://satijalab.org/seurat/articles/seurat5_integration). Briefly, samples were split, and 3000 genes consistently variable among the individual SCT matrices were selected using SelectIntegrationFeatures. PrepSCTIntegration was then used to prepare reduced SCT expression matrices for these genes. PCA was calculated for each reduced SCT matrix using RunPCA, and the first 30 principal components were used to identify transcriptionally similar cells between each pair of reduced SCT matrices. The IntegrateLayers function and Harmony method were then used to integrate the SCT matrices, and UMAP was re-generated to visualize the batch effect between HS and Healthy samples.
2.10. Cell Cluster Proportion Comparison
We compared the cell type proportions of each cluster within each subject group relative to their total cell count. To determine significant differences in cell proportions among the subject cohorts, we employed the Kruskal-Walli’s test (kruskal.test in R). Each cell types with FDR-adjusted Kruskal-Wallis p-values less than 0.05 were considered as statistically different between the cohorts group.
2.11. Differential Feature Analysis of genes and cell-surface marker
Differentially expressed genes (DEGs) and proteins (DEPs) were identified using the FindMarkers function in Seurat. Separate analyses for DEGs and DEPs were performed, and each cell type included in the analysis had at least 100 cells. The non-parametric Wilcoxon rank sum test (test.use = “wilcox”) was used for identifying DEGs and DEPs. Genes were considered DEGs if they had both a Bonferroni-corrected p-value < 0.001 and an absolute log fold change > 1.5. Proteins were considered DEPs if they had a p-value < 0.005 and an absolute log fold change > 1.0.
2.12. Biological pathways and gene ontology analysis
Pathway and functional enrichment analysis was performed with the g:Profiler tool g:GOST using gprofiler2 in RStudio (v4.1.0). Functional enrichment analysis was performed separately for significantly up- and down-regulated genes (log2FC > 1.5 & p_value < 0.01) for each individual cell type with at least 100 cells. Input gene lists were ranked by log2FC and g:GOST was performed as an ordered query using KEGG, Reactome, and WikiPathway terms. Terms were then filtered for significance, retaining terms with query_size > 50, term_size > 10, and p_value < 0.05. Precision was defined as intersection_size / query_size and recall was defined as intersection_size / term_size, where query_size refers to the n number of top genes (determined by ranking) that are evaluated by g:GOST for a given term, intersection_size refers to the number of genes within query_size that match a given term, and term_size refers to the number of genes contained within a term.
2.13. Cell-Cell Interaction analysis
Cell-cell interactions were inferred using CellPhoneDB (v5.0.0) 10,11 and querying a manually curated repository of receptors, ligands, and their interactions (cellphonedb-data v5.0.0). CellPhoneDB was performed separately for HS and healthy cells, providing log-normalized counts and Azimuth cell type annotations. CellPhoneDB analysis was run using the statistical_analysis method with default settings. Briefly, CellPhoneDB performs 1000 random permutations of cell labels to estimate a null distribution of the mean of the average ligand and receptor expression (mean_exp) in the interacting clusters and estimate a p-value. A given ligand and receptor pair are only considered if both are expressed in at least 10% of the corresponding cell type. We filtered for significant HS enriched interactions by only considering cell-cell interactions in which mean_exp is at least 10% greater in HS as compared to healthy and with a p-value of less than 0.05 in HS. Here, we only considered cell-cell interactions in which both cell pairs have at least 10 cells in both HS and healthy.
2.14. Machine learning classification model
Machine learning (ML) models were used to classify HS vs healthy patients, including Random Forest Classifier (RF), Gradient Boosting Classifier (GB), Support Vector Machine for Classification (SVC), and Neural Network (NN). sci-kit learn (v1.4.2) was used for all classifier models, except the NN model which was from the keras (v3.3.3) library in python3.4. The data were split into 60:40, as a Training and Test set. Data inputted into the ML models included differentially expressed genes and proteins, with their means normalized and centered, and expression values scaled. These expression values were calculated for each patient across all present cell types.
Each model underwent training and testing with a set of RNA expression only data, and was then repeated for an ADT only dataset, and finally a combined RNA + ADT expression dataset. Only training data were used to build the ML models while the test data were used for independent evaluation of prediction models. A five-fold cross validation step with hyperparameter tuning was performed on each model. Hyperparameters tested for RF included n_estimators = (100, 200, 500, 1000), GB included learning_rate = (0.1, 0.2, 0.4, 0.8), SVC included kernel = (linear, poly, rbf, sigmoid), and NN included learning_rate = (0.001, 0.0001) and rate = (0.4, 0.8). The evaluation metric used to determine the best performing models was accuracy. The best performing models were then used to extract the top 20 markers for each dataset (RNA only, ADT only, and RNA + ADT). An AUC-ROC curve was plotted for only the top performing models to calculate classification rate.
3. Results
3.1. Single-cell transcriptomic analysis of 29 cell types in HS vs healthy blood identifies cell composition differences in monocytes, T cells, B cells, dendritic cells, NK cells, and innate lymphoid cells
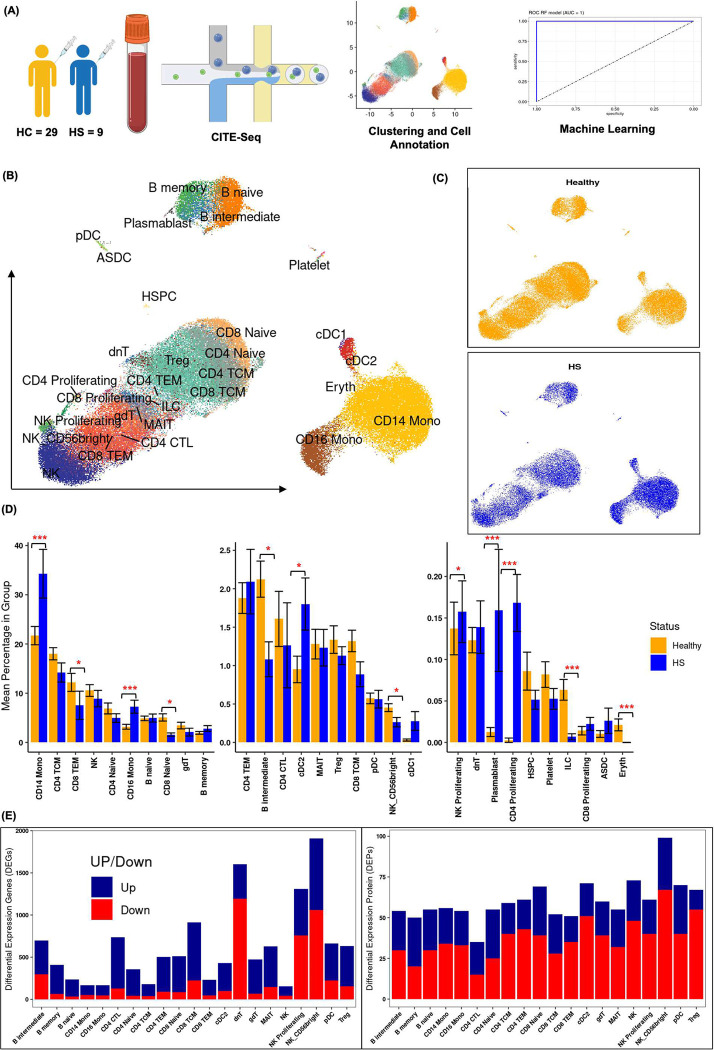
To delineate the different immunological features from 9 HS patients and 29 healthy controls, droplet-based single-cell RNA-sequencing (10X Genomics) was conducted to examine the gene expression patterns. The workflow is depicted in [Figure 1A]. After quality control (see Methods), 109,873 high-quality single cells with distinct transcriptome profiles were obtained, with 34,545 cells from 9 HS subjects and 75,303 cells from 29 healthy controls. Batch effected correction was done at the sample as well as subject wise [Supplementary Figure 1–2]. After quality control on the cell-surface antibody-tagged protein data, 258 high-quality protein markers were retained for both sample groups. As shown in [Figure 1B], 29 major PBMC cell types were delineated based on the expression of canonical gene markers in Azimuth. Both HS and healthy subjects harbored all 29 cell populations including monocytes (CD14+ and CD16+), CD4+ T cells (naive, central memory, effector memory, cytotoxic, and proliferating), CD8+ T cells (naive, effector memory, and central memory), regulatory T cells, gamma-delta T cells, double-negative T cells, mucosal-associated invariant T cells (MAIT), natural killer cells (NK, NK CD56bright, and proliferating NK), B cells (memory, naive, and intermediate), conventional dendritic cells (cDC1 and cDC2), plasmacytoid dendritic cells, plasmablasts, hematopoietic stem and progenitor cells, platelets, innate lymphoid cells, and erythrocytes. The details of cell cluster composition [Supplementary Figure 3] in HS and healthy and top three marker genes for each cell cluster are shown in dot expression plot [Supplementary Figure 4].
Figure 1.
(A) The study design is outlined, showing the process from patient recruitment to sample analysis. Peripheral blood mononuclear cells (PBMCs) were collected from 9 HS patients and healthy controls for comparison.
(B) Thirty different cell types were annotated using Azimuth and visualized through UMAP based on SCTransform-normalized RNA expression integrated with ADT expression, with distinct colors representing different cell subsets.
(C) UMAP plots depict the clustering of cell types in HS and healthy samples, highlighting differences between the two groups.
(D) The mean percentages of each cell type within the total PBMCs of each subject are shown, with error bars indicating the standard error of the mean. Significant differences between HS and healthy subjects were determined using Wilcoxon and FDR-adjusted Kruskal-Wallis tests, with * indicating p-values < 0.05.
(E) The number of differentially expressed genes (DEGs) and proteins (DEPs) in HS vs. healthy samples across all cell types are presented, with separate counts for upregulated (blue) and downregulated (red) genes and proteins.
We calculated the mean percentage composition in each of the 29 clusters in HS and compared the percentages to those of healthy control samples. The analysis revealed significant differences in the abundance of several immune cell populations between HS and healthy individuals [Figure 1C]. In comparison to healthy controls, HS subjects had a significantly higher proportion of CD14+ monocytes, CD16+ monocytes, cDC2, cDC1, plasmablasts, and proliferating CD4+ T cells. In contrast, HS subjects had a significantly lower proportion of CD8+ effector memory T cells (CD8 TEM), CD8+ naive T cells, B intermediate cells, CD56bright NK cells, and innate lymphoid cells (ILC). These findings indicate that various immune cell subsets, including monocytes, T cells, B cells, dendritic cells, NK cells, and innate lymphoid cells, exhibit differential abundance in the peripheral blood of HS patients compared to healthy individuals [Figure 1 D].
3.2. Differential expression of RNA and protein reveals altered immunity in HS
Differential expression analysis of genes and proteins was performed on all 29 cell types and the list of DEG and DEP are in (Supplementary Table 2 and 3). However, we focused our analysis on cell types with differential cell abundance and cell types with at least 100 cells per cluster (CD14+ monocytes, CD16+ monocytes, CD8+ TEM, CD8+ T Naïve, B Intermediate, cDC2, NK Proliferating, and NK 56 bright). The numbers of differentially expressed genes and protein of these cell types are shown in [Figure 1E].
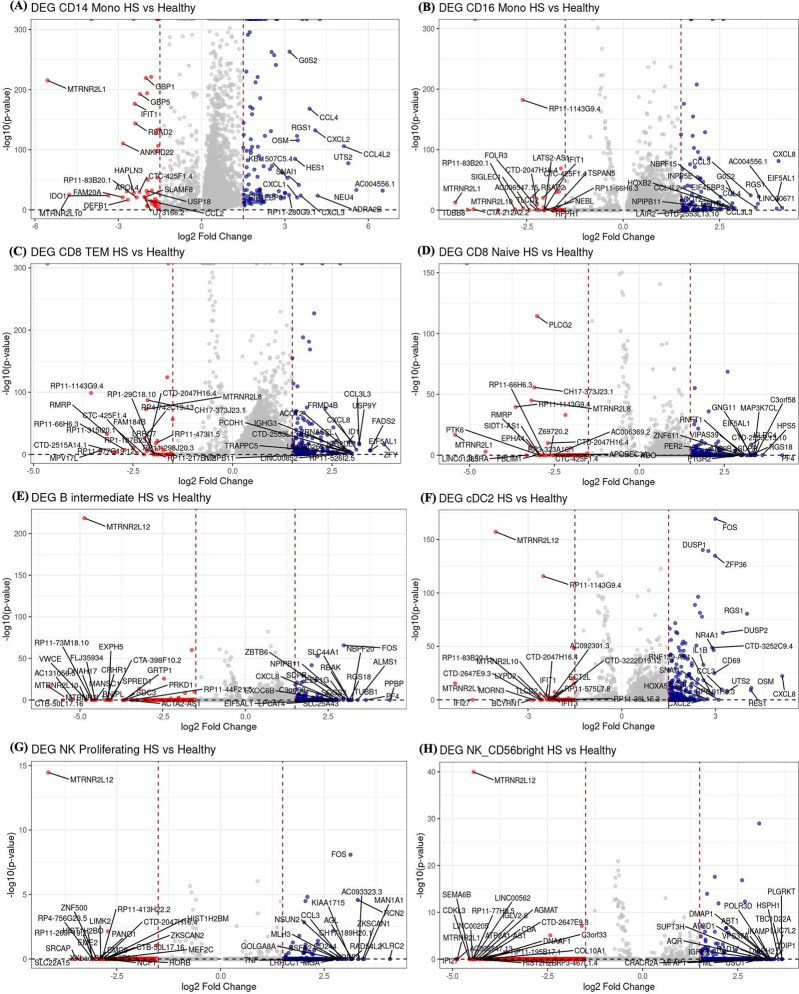
Within CD14+ monocytes, there was overexpression of the inflammatory markers TNF, IL1B, and NF-κB; chemokines CCL2, CXCL8, and CCL5; cell proliferation and migration markers MKI67, MMP9, and CDK1; and cell adhesion molecules ITGB2, ICAM1, and VCAM1. Similarly, CD16+ monocytes showed upregulation of TNF, ZFP36, and IL1B; DUSP1, a phosphatase regulating MAPKs; and the chemokines and cytokine CCL4, CXCL8, and IL6. The cell adhesion genes MKI67, VCAM1, ITGA4, and CD44 were upregulated, as were metabolic genes LDHA, GAPDH, and PKM. NOD2, relevant to HS pathology, was also upregulated [Figure 2 A–B].
Figure 2. Volcano plot of differentially expressed genes in various cell types.
The volcano plots of differentially expressed genes in HS versus healthy controls across several cell types: (A) CD14 monocytes, (B) CD16 monocytes, (C) CD8 TEM cells, (D) CD8 naïve cells, (E) B-intermediate cells, (F) cDC2 cells, (G) NK proliferating cells, and (H) NK56 bright cells. In eachplot, blue dots represent upregulated genes in HS, while red dots indicate downregulated genes. The top 20 genes for both upregulation and downregulation are labeled based on their p-values.
Within CD8+ Naive and CD8+ TEM cells, there was upregulation of FKBIA and CCL4L2, involved in cell recruitment, and CD69 [Figure 3C–D]. Proliferation and migration genes PMAIP1, PPP4C, and PHLDA1 were upregulated, as was RHOB. DEGs in B-intermediate cells included NF-κB, a key inflammatory pathway regulator; JUNB, influencing cytokine production; and FOS, part of the AP-1 complex. There was also upregulation of CD69, DUSP1, DUSP2, and AREG involved in cell recruitment and migration. S100A9, a calcium-binding protein that promotes T17 responses, and TNFSF12, a TNF ligand, were upregulated. [Figure 2 E–F]
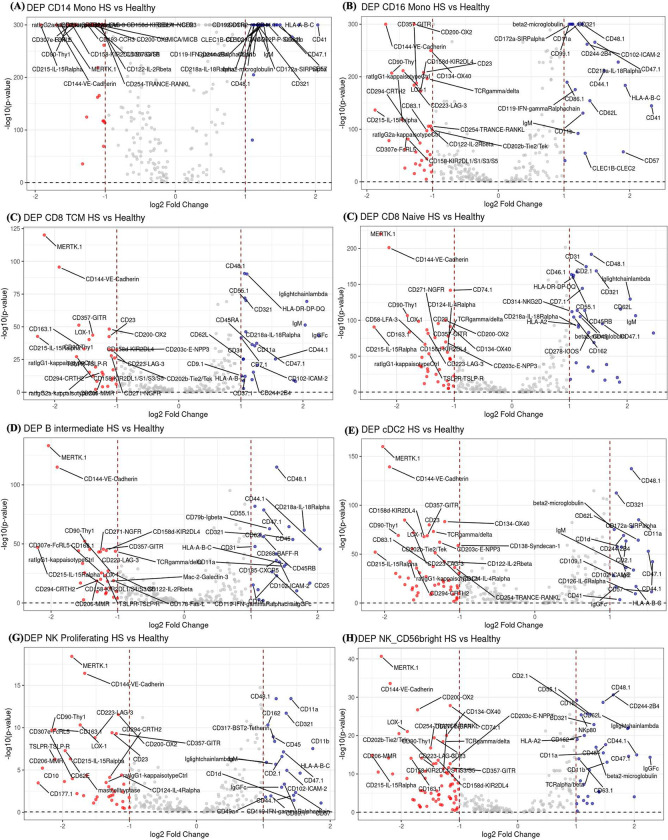
Figure 3. Volcano plot of differentially expressed proteins (DEPs) in various cell types.
The volcano plots of differentially expressed proteins (DEPs) in HS versus healthy controls across several cell types: (A) CD14 monocytes, (B) CD16 monocytes, (C) CD8 TEM cells, (D) CD8 naïve cells, (E) B-intermediate cells, (F) cDC2 cells, (G) NK proliferating cells, and (H) NK56 bright cells. In each plot, blue dots represent upregulated proteins in HS, while red dots indicate downregulated genes. The top 20 genes for both upregulation and downregulation are labeled based on their p-values.
Within cDC2 cells, there was upregulation of the inflammatory chemokines CXCL8, CCL3, CCL4 and cytokines IL1B, IL18RAP. NK cells had significant upregulation of the inflammatory mediators NF-κB, FOS, and IL6; cytotoxicity genes GZMB, PRF1, and CD69; and adhesion and migration genes ITGA4, ITGB2, and ICAM1 [Figure 2 F].
Differential expressed proteins (DEPs) in CD14+ and CD16+ monocytes included integrins such as CD41, CD11a, and CD11b. The adhesion molecules CD62L, CD44.1, and ICAM-2 were also elevated in many cell types, suggesting enhanced interactions with endothelial cells and tissue infiltration. Several cell surface markers were also found downregulated including CD83.1, CD137L-4–1BBLigand, CD177.1, and CD134-OX40 expressed in monocytes, CD8 Naïve and TEM, NK and Treg, which play critical roles in immune cell activation and regulation. Additional downregulated proteins were CD202b-Tie2/Tek, CD254-TRANCE-RANKL, and CD223-LAG-3 [Figure 3A–H].
3.3. Pathway enrichment analysis identifies pro-inflammatory signatures and points to a significant role of CD14 and CD16 monocytes
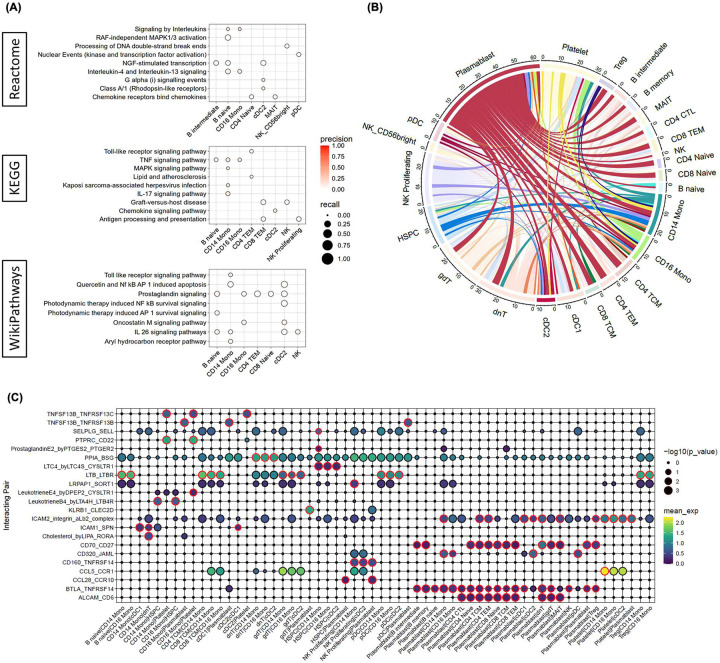
To further elucidate the cell type specific biological pathways underpinning disease pathogenesis in HS compared to healthy control, we performed functional enrichment analysis using g:Profiler (g:GOSt) 12. Briefly, for each cell type we performed separate g:GOSt analyses utilizing significantly up- or down-regulated genes (log2FC > 1.5 & pval < 0.01) as a ordered query. Genes were mapped to the gene sets sourced from KEGG, Reactome and WikiPathways databases. We observed positive enrichment of several interleukin signaling gene sets in the transcriptome of CD14/16 monocytes, B naïve cells, and NK cells. Specifically, we observed upregulation of the IL-17 signaling pathways in CD14/16 monocytes, the IL-4/13 and IL-26 signaling pathway in B naïve and CD14/16 monocytes, and the IL-26 signaling pathway in B naïve, CD14 monocytes, cDC2, and NK cells [Figure 4A]. Significantly, IL-17 has been shown to be elevated in the serum of patients with HS and positively correlated with disease severity13. Additionally, HS is associated with the Th1/Th17-driven inflammatory response that acts through downstream signaling pathways NF-κB, CCAAT/enhancer-binding protein (C/EBP) family, and mitogen-activated protein kinase (MAPK) 14,15. Supporting this, we also observed positive enrichment of the MAPK and TNF signaling pathway in CD14 monocytes (Figure 4A). Overall, our results suggest a potentially significant role of monocytes in the pathogenesis of HS acting through the IL-17 signaling pathway.
Figure 4. Pathway enrichment analysis for differentially expressed genes (DEGs) in various cell types in HS versus healthy controls.
The dot plot shows the precision and recall values for each pathway in (A) Wiki Pathway, REACTOME, andKEGG databases. Key pathways involved in inflammatory signaling and immune responses are highlighted, such as signaling by TNF, interleukins, MAPK1/3 activation, chemokine receptor binding, and IL-4/IL-13 signaling. Each dot represents a specific cell type, including B intermediate, B naïve, CD16 monocytes, CD4 naïve, CD4 memory, CD8 TEM, CD8 naïve, cDC2, MAIT, NK, NK_Proliferating, and NK_CD56bright cells.
(B) Circos plot depicting cell-cell interactions between various immune cell types in HS versus healthy controls. The plot visualizes interactions among different cell populations, including CD8 TEM, CD8 TCM, CD8 naïve, CD4 TCM, CD4 naïve, CD4 CTL, CD4 proliferating, CD14 monocytes, CD16 monocytes, cDC1, cDC2, plasmablasts, Treg, and various B cell subsets. The lines indicate interactions, with color-coded segments representing different cell types. The thickness of the lines corresponds to the interaction strength, highlighting the complexity of the immune network in HS. (C) Dot plot representing the average expression and p-value of a given receptor-ligand pair by cell pair. Of note, represented here are expression values and p-values in HS samples only. Dots with a red border represent a specific receptor-ligand interaction between a given cell-cell pair that is significantly enriched in HS compared to healthy, as defined in methods. For a given receptor_ligand pair detected in a cell pair, cellA_cellB, the receptor and ligand are inferred as expressed in cellA and cellB, respectively.
3.4. Cell-cell interaction analysis supports a pro-inflammatory role for monocytes and B-cells mediated through TNF signaling and T-cell activation
To characterize the intercellular interaction landscape in HS, we performed cell-cell interaction analysis using CellPhoneDB 10,11. Briefly, CellPhoneDB was run separately for HS and healthy using cell type annotated log-normalized expression values. We filtered for HS enriched cell-cell interactions if a given receptor-ligand pair had at least a 10% greater mean expression in HS compared to healthy and with p-value < 0.05 in HS. This resulted in a total of 188 HS-enriched significant cell-cell interactions, with the most common cell partner being plasmablasts (61) followed by double negative T-cells (31), platelets (31), NK proliferating (27), γδ T cells (26), and CD14 monocytes (23) (Figure 4B). To narrow our search, we focused on cell-cell interactions involving significantly expanded cell types in HS, namely CD14/16 monocytes, cDC2, and plasmablasts (Figure 4C). In concordance with our pathway enrichment analysis, we identified several cell-cell interactions primarily involving CD14/16 monocytes and plasmablasts that mediate signaling through the TNF signaling pathway (TNFSF13B-TNFRSF13C, TNFSF13B-TNFRSF13B) (Figure 4C). TNFSF13B (BAFF) interactions with TNFRSF13C (BAFF-R) and TNFRSF13B (TACI) are known to be critical for B-cell survival and activation through engagement with TRAF adaptors and the activation of NF-κB and MAPK 16–19. Notably, BAFF is primarily produced by macrophages, monocytes and dendritic cells, in agreement with our observations of BAFF interactions between CD16 monocytes/cDC1 cells and plasmablasts 20,21. Here, we also observe significant interactions between CD70, expressed by plasmablasts, and CD27, expressed by B-cells and T-cells (Figure 4C). Importantly, CD70 expression on activated antigen presenting cells (dendritic cells and B-cells) is known to bind CD27 on T-cells and promote T-cell activation/differentiation and stimulate production of pro-inflammatory cytokines through TRAF2/5 mediated activation of the NF-κB and JNK pathways 22–24. Similarly, our data further suggests T-cell activation in HS is mediated by interaction with plasmablasts through the ALCAM-CD6 interaction (Figure 4C). ALCAM has been shown to support T-cell migration and activation, promoting transendothelial migration and immune responses 25,26. Taken together, and in agreement with our functional enrichment analysis, these results support a potentially pathogenic role of monocytes and B-cells mediated by TNF signaling through BAFF and CD70-CD27 interactions.
3.5. Machine learning model for classification of HS and Healthy based on top identified markers
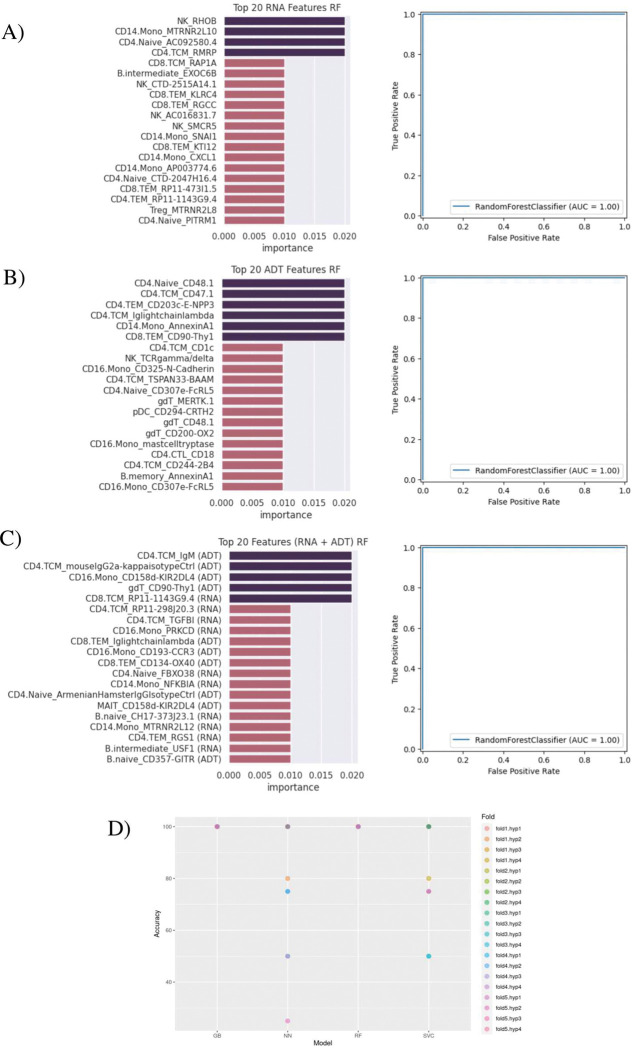
The top 20 markers for each dataset and each model showed varying results. For RF and GB models, the top 20 markers resulting from the combined gene + protein input data showed a relatively even split between RNA and ADT markers. Both models also had primarily ADT markers as the features with highest importance [Figure 5C, Supplementary Figure 6]. As for the SVC model, it showed only RNA markers populating its top 20 [Supplementary Figure 6].
Figure 5. Machine learning models to classify the HS and Healthy Patients based on the identified biomarkers.
(A) Top 20 ensemble features predicted using DEGs. (B) Top 20 ensemble features predicted using DEPs. (C) Combined predicted features from DEGs and DEPs. (A-C) Model performance was evaluated using an independent test and accuracy presented in AUC-ROC curves. (D) Accuracies for each cross-validation fold and hyperparameter tuning set are shown for 4 ML models: Gradient Boosting (GB), Neural Network (NN), Random Forest (RF), Support Vector Machine for Classification (SVC).
Using the best performing hyperparameters, all ML models were able to classify with a maximum accuracy of 100% as well as precision, recall, f1, and perfect AUC scores of 1.0. For RF and GB models in particular, accuracies did not go under 100% and AUC scores did not go under 1.0, even after being tested with different hyperparameters and training/validation folds [Figure 5D].
The top RNA discriminators included RHOB in NK cells, MTRNR2L10 in CD14 Monocytes, PITRM1 in CD4 Naïve cells, and RMRP in CD4 TCM cells. The top cell surface protein discriminators included MICA/MICB on CD8 TEM cells, CD314 (NKG2D) on NK cells, AnnexinA1 on CD14 Monocytes, and CD48.1 on CD4 Naïve cells [Figure 5A–B]. The MTRNR2L12 gene and the CD325 (N-Cadherin) surface protein were top discriminators in multiple different cell types [Supplementary Figure 6].
4. Discussion
4.1. Characterization of Dysregulated Immune Cell Populations in PBMCs Highlights Key Cell Types
Hidradenitis suppurativa is a highly debilitating and heterogeneous chronic inflammatory disease with diverse clinical presentations and responses to treatment, driven by distinct genetic and transcriptomic mechanisms. While several genetic and functional studies have identified various genetic risk factors and characterized the molecular mechanisms underpinning HS pathogenesis, little is known about the exact cell specific mechanisms that drive chronic and systemic inflammation observed in HS patients 14,27–30. To this end, and to the best of our knowledge, this study performs the first single-cell transcriptomic profiling of PBMCs isolated from patients with HS. Our findings underscore the complex interactions among different immune cell types and their regulatory mechanisms, shedding light on their potential roles in the pathogenesis of HS 5 31 32.
We identified several immune cells were significantly increased in HS, including CD14 monocytes and CD16 monocytes, cDC1/2, NK proliferating cells, plasmablasts, and CD4 proliferating cells. This suggests the contribution of these cell types to the inflammatory processes and immune dysregulation observed in HS5 33 34. In previous studies of HS in which single cell RNA-seq was performed on skin or mass cytometry by time of flight (CyTOF) was performed on blood, the cell types of interest included monocytes, B cells, plasma cells, dendritic cells (DCs), CD8 T cells, and CD4 T cells, which aligns with our PBMC CITEseq data35 6 36.
4.2. Overexpressed genes elucidate key cellular functions in HS
The upregulation of key inflammatory mediators such as TNF, ZFP36, IL1B, JUN, NFKBIA, and MAP2K1 in CD14 monocytes and CD16 monocytes and cDC1/cDC2 signify their pivotal role in initiating and sustaining inflammation through the TNF pathway 37. The upregulation of genes like MKI67 and MMP9 indicates active monocyte participation in tissue remodeling and repair, essential processes in chronic inflammatory conditions like HS38. In healthy human skin, a skin-tropic population of TCM cells expressing skin-homing receptors CCR7 and L-selectin has been observed, with overexpression in psoriasis and depletion by alemtuzumab therapy39. Consistent with these findings, our PBMC analysis also demonstrated increased expression of CCR7 in CD8 TEM and TCM cells.
Elevated chemokine genes such as CCL2/4 and CXCL1/23/8 highlight the migration of immune cells to sites of inflammation40 41. Moreover, the enhanced expression of adhesion molecules (ITGB2, ICAM1,VCAM1, NCAM1, ICAM1) highlights the crucial role of monocyte and CD8 T cell interactions in cell trafficking to inflamed tissues42. The notable upregulation of NOD2 and CARD9 in monocytes highlight their potential role in responding to microbial antigens, further implicating these cells in the pathogenesis of HS43 44. Lastly, the metabolic reprogramming of monocytes, macrophages, or other immune cells as evidenced by the increased expression of (LDHA, GAPDH, PKM and HK2), reflects the heightened energy demands associated with sustaining an inflammatory response44
4.3. Pathway and cell-cell interaction analysis supports a pathogenic role of IL-17 and TNF signaling and plasmablasts in HS
HS has been strongly correlated with the Th17 immune response, with various studies finding elevated Th1 and Th17 associated cytokines (IL-17, IFN-γ, IL-12, IL-23, IL-32, IL-1β, and TNF) in HS tissue or serum as well as increased expression of genes associated with the IL-17 signaling pathway (IFN family and JAK/STAT), suggesting a significant pathogenic role of IL-17 13,14,45–48. Indeed, anti-IL17 targeted therapy (secukinumab) has shown significant clinical activity in HS, gaining FDA approval in 2023 49. Here, utilizing pathway enrichment analysis to understand cell intrinsic mechanisms that contribute to HS we identified significant upregulation of the IL-17 signaling pathway in HS, in agreement with prior studies. Interestingly, our analysis identified this upregulation in CD14 and CD16 monocyte. Previous studies have also found increased frequencies of CD14 and CD16 monocytes in HS, with aberrant expression of genes associated with STAT1/IFN-signaling 50–52. Interestingly, monocyte migration in rheumatoid arthritis (RA) has been shown to be mediated through IL-17 signaling and p38 MAPK activation, with neutralization of IL-17 resulting in suppression of monocyte migration into the RA synovial fluid 53. IL-17 signaling has been shown to act through TNF receptor associated factors (TRAFs) as well as MAPK and NF-κB pathways 54. In keeping with these observations, we also observe upregulation of the TNF and MAPK signaling pathway in HS associated monocytes. Significantly, cell-cell interaction analysis also identified HS associated interactions between plasmablasts, expressing BTLA, and monocytes, expressing TNFRSF14 (HVEM). Cell enrichment analysis also identifies both cell types to be significantly expanded in HS. Interestingly, plasmablasts were the most frequent partner in cell-cell interactions when considering interactions enriched in HS. Plasmablasts have been found to be highly elevated in HS skin lesions and shown to play a significant role in the pathogenesis of HS by contributing to a dysregulated immune response through the increased production of auto-antibodies 55 56 6. Additionally, tertiary lymphoid structures have been found in HS lesions that function similar to germinal centers, facilitating B-cell maturation and differentiation55. Interestingly, previous work has found that neutrophil and B-cell/plasma cell frequency are correlated and that neutrophils support B-cell activation and function by expressing BAFF, with B-cells expressing its receptors BAFFR, TACI, and BCMA57. While this interaction between neutrophil and B-cells is not observed in our data, likely due to cells being sourced from peripheral blood as opposed to the lesional skin, we do observe an interaction between BAFF-BAFFR and BAFF-TACI occurring between monocytes and plasmablasts, suggesting a similar mechanism of B-cell activation may be mediated by both neutrophils and monocytes in the pathogenesis of HS.
Overall, our findings highlight a potentially significant role of IL-17 and TNF signaling in monocytes, as well as plasmablasts, in HS pathogenesis. Indeed, a previous study has shown that patients treated with anti-TNF therapy demonstrated a significant decrease in IL-17+ CD4 T-cells in lesional skin compared to treatment naïve patients 40. Taken together with our findings, this supports a potential synergistic role of anti-IL17 and anti-TNF therapy in HS. Finally, plasmablasts, found to be elevated in HS skin lesions and, from our analysis, in peripheral blood, drives disease pathogenesis through the production of autoantibodies and is supported through interactions with BAFF, expressed on neutrophils55 56 6 57. Although our data does not show a similar direct interaction between neutrophils and B-cells, here we report a similar mechanism of B-cell activation in peripheral blood mediated by monocytes, expressing BAFFR and TCI. Together, these observations warrant further investigation into the precise cell-intrinsic and cell-extrinsic contributions of monocytes and plasmablasts to HS pathogenesis.
4.4. ML Classification of HS and Healthy Subjects based on single-cell RNA and protein markers
We observed that HS-associated differences in cell-specific gene and surface protein expression could distinguish HS from healthy subjects with > 0.95 AUROC achieved by several machine learning algorithms [Figure 5], though the general performance of these models may be limited by sample size (particularly for HS subjects). We nevertheless note that cell surface protein expression of CD325 (N-Cadherin) was consistently identified as an important marker in protein feature sets used for model training, which, given its biological significance as discussed above, may warrant further investigation as a diagnostic and therapeutic target. Besides modest cohort size, other limitations of this study include the sampling of patients from a single center and the use of only molecular biomarkers for subject classification. Future studies can address these limitations by recruiting a larger number of patients including those with other inflammatory skin diseases as well as by incorporating clinical and demographic data into the classification model.
4.5. Conclusion
This comprehensive analysis of differentially expressed genes and proteins across various immune cell types in the PBMC of HS provides valuable insights into the molecular underpinnings of this chronic inflammatory condition. The identified pathways and dysregulated genes and proteins offer potential targets for therapeutic interventions aimed at modulating the immune response in HS. The involvement of Th2, Th17, Th23, and Th26, TNF pathways in particular emphasize the need to explore targeted therapies that can address these specific immune dysregulations. Cell-cell interaction analysis supports a pro-inflammatory role for monocytes and B-cells mediated through TNF signaling and T-cell activation. For instance, we observed elevated expression of CD134/OX40, which has been targeted in clinical trials for HS and atopic dermatitis 58 59. Additionally, through machine learning, we identified elevated expression of several other cell surface marker [Figure 5 A–C] could serve as a new potential therapeutic and diagnostic marker in CD14 monocytes. Further research is warranted to elucidate the precise mechanisms and develop effective treatments for HS.
Acknowledgments
We would like to thank the patients who contributed to this study.
Funding
This study was supported by NIH grant R01AR078688 and R21AR079089.
Footnotes
Conflict of Interest
WL has received research grant funding from Abbvie, Amgen, Janssen, Leo, Novartis, Pfizer, Regeneron, and TRex Bio. CP has received honoraria and/or travel support from MSD, BMS, Pierre Fabre, MERCK, Sanofi, Almirall, AbbVie, Pelpharma, Amgen, DSD, Takeda, Pfizer, Novartis, Leo, Janssen, Astra Zeneca, and Boehringer Ingelheim. AW received honoraria and travel support from AbbVie, Amgen, Biogen, Janssen, Leo, Novartis, UCB and Sanofi. HN has received grant support from AbbVie; consulting fees from 23andme, Abbvie, Aristea Therapeutics, Nimbus Therapeutics, Medscape, Sonoma Biotherapeutics, DAVA Oncology, Boehringer Ingelheim, Union Chimique Belge’s (UCB) and Novartis; investigator fees from Pfizer; and holds shares in Radera, Inc. She is also an Associate Editor for JAMA Dermatology and Vice President of the Hidradenitis Suppurativa Foundation. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Figure 1. Batch correction of HS and Healthy patient effects across all clusters using harmony batch correction.
Supplementary Figure 2. Evaluation of overlap and batch correction effects across all samples and clusters.
Supplementary Figure 3. Visualization of cell composition in Healthy and HS patients using a stacked plot.
Supplementary Figure 4. Identification of the top 3 marker expressions from each cell cluster type used for cluster annotation.
Supplementary Figure 5. Analysis of cell-cell interactions across all cell cluster types, where red indicates stronger interactions with other cell types and blue indicates weaker interactions.
Supplementary Figure 6. The top 20 markers were identified using machine learning classifiers, such as Gradient Boosting (GB) and Support Vector Machine with a linear kernel. The prediction classification rate for these markers was measured by the Area Under the Curve (AUC). These top markers were identified based on their expression in different cells and cell surface markers, both individually and combined.
Supplementary Table 1. Clinical characteristics of the study cohort.
Supplementary Table 2. List of differentially expressed genes across all 21 different cell types where the cell clusters have more than 100 cells in both HS and Healthy conditions.
Supplementary Table 3. List of differentially expressed proteins across all 21 different cell types where the cell clusters have more than 100 cells in both HS and Healthy conditions.
Data Availability Statement
The datasets generated for this study can be found in the GEO repository under the accession: GSE194315 for healthy and GSEXXX for HS samples
References:
- 1.Ballard K. & Shuman V. L. Hidradenitis Suppurativa. in StatPearls (StatPearls Publishing, Treasure Island (FL), 2024). [PubMed] [Google Scholar]
- 2.A Review of the Current Landscape of Hidradenitis Suppurativa Treatment Development. JDDonline - Journal of Drugs in Dermatology https://jddonline.com/articles/a-review-of-the-current-landscape-of-hidradenitis-suppurativa-treatment-development-S1545961623P1021X/. [DOI] [PubMed] [Google Scholar]
- 3.Liu J. et al. Combined Single Cell Transcriptome and Surface Epitope Profiling Identifies Potential Biomarkers of Psoriatic Arthritis and Facilitates Diagnosis via Machine Learning. Front. Immunol. 13, (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alber S. et al. Single Cell Transcriptome and Surface Epitope Analysis of Ankylosing Spondylitis Facilitates Disease Classification by Machine Learning. Front. Immunol. 13, (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Straalen K. R. van et al. Single-cell sequencing reveals Hippo signaling as a driver of fibrosis in hidradenitis suppurativa. J. Clin. Invest. 134, (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gudjonsson J. E. et al. Contribution of plasma cells and B cells to hidradenitis suppurativa pathogenesis. JCI Insight 5, e139930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stergianou D., Kanni T., Damoulari C. & Giamarellos-Bourboulis E. J. An evaluation of secukinumab for the treatment of moderate-to-severe hidradenitis suppurativa. Expert Opin. Biol. Ther. 24, 225–232 (2024). [DOI] [PubMed] [Google Scholar]
- 8.Kang H. M. et al. Multiplexed droplet single-cell RNA-sequencing using natural genetic variation. Nat. Biotechnol. 36, 89–94 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hao Y. et al. Integrated analysis of multimodal single-cell data. Cell 184, 3573–3587.e29 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Troulé K. et al. CellPhoneDB v5: inferring cell-cell communication from single-cell multiomics data. Preprint at 10.48550/arXiv.2311.04567 (2023). [DOI] [Google Scholar]
- 11.Efremova M., Vento-Tormo M., Teichmann S. A. & Vento-Tormo R. CellPhoneDB: inferring cell–cell communication from combined expression of multi-subunit ligand–receptor complexes. Nat. Protoc. 15, 1484–1506 (2020). [DOI] [PubMed] [Google Scholar]
- 12.Kolberg L. et al. g:Profiler-interoperable web service for functional enrichment analysis and gene identifier mapping (2023 update). Nucleic Acids Res. 51, W207–W212 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.C S, T H., N Y. & Re H. Expression of the IL-23/Th17 pathway in lesions of hidradenitis suppurativa. J. Am. Acad. Dermatol. 65, (2011). [DOI] [PubMed] [Google Scholar]
- 14.R T., S C., Sm S. J., C S. & Re H. Association of Hidradenitis Suppurativa With T Helper 1/T Helper 17 Phenotypes: A Semantic Map Analysis. JAMA Dermatol. 154, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu T. et al. The IL-23/IL-17 Pathway in Inflammatory Skin Diseases: From Bench to Bedside. Front. Immunol. 11, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schneider P. et al. Maturation of marginal zone and follicular B cells requires B cell activating factor of the tumor necrosis factor family and is independent of B cell maturation antigen. J. Exp. Med. 194, 1691–1697 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schiemann B. et al. An essential role for BAFF in the normal development of B cells through a BCMA-independent pathway. Science 293, 2111–2114 (2001). [DOI] [PubMed] [Google Scholar]
- 18.Batten M. et al. BAFF mediates survival of peripheral immature B lymphocytes. J. Exp. Med. 192, 1453–1466 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.The TNF-receptor-associated factor family: scaffold molecules for cytokine receptors, kinases and their regulators - PubMed. https://pubmed.ncbi.nlm.nih.gov/11384837/. [DOI] [PubMed]
- 20.Mackay F. & Browning J. L. BAFF: A fundamental survival factor for B cells. Nat. Rev. Immunol. 2, 465–475 (2002). [DOI] [PubMed] [Google Scholar]
- 21.Nardelli B. et al. Synthesis and release of B-lymphocyte stimulator from myeloid cells. Blood 97, 198–204 (2001). [DOI] [PubMed] [Google Scholar]
- 22.Han B. K., Olsen N. J. & Bottaro A. The CD27–CD70 pathway and pathogenesis of autoimmune disease. Semin. Arthritis Rheum. 45, 496–501 (2016). [DOI] [PubMed] [Google Scholar]
- 23.Hashimoto-Okada M. et al. The CD70–CD27 interaction during the stimulation with dendritic cells promotes naive CD4+ T cells to develop into T cells producing a broad array of immunostimulatory cytokines in humans. Int. Immunol. 21, 891–904 (2009). [DOI] [PubMed] [Google Scholar]
- 24.Arens R. et al. Constitutive CD27/CD70 Interaction Induces Expansion of Effector-Type T Cells and Results in IFNγ-Mediated B Cell Depletion. Immunity 15, 801–812 (2001). [DOI] [PubMed] [Google Scholar]
- 25.Cayrol R. et al. Activated leukocyte cell adhesion molecule promotes leukocyte trafficking into the central nervous system. Nat. Immunol. 9, 137–145 (2008). [DOI] [PubMed] [Google Scholar]
- 26.Chalmers S. A. et al. The CD6/ALCAM pathway promotes lupus nephritis via T cell–mediated responses. J. Clin. Invest. 132, e147334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Straalen K. R., Prens E. P., Willemsen G., Boomsma D. I. & van der Zee H. H. Contribution of Genetics to the Susceptibility to Hidradenitis Suppurativa in a Large, Cross-sectional Dutch Twin Cohort. JAMA Dermatol. 156, 1359–1362 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Genetic Variants Associated With Hidradenitis Suppurativa | Genetics and Genomics | JAMA Dermatology | JAMA Network. https://jamanetwork.com/journals/jamadermatology/fullarticle/2807701. [DOI] [PMC free article] [PubMed]
- 29.Rumberger B. E., Boarder E. L., Owens S. L. & Howell M. D. Transcriptomic analysis of hidradenitis suppurativa skin suggests roles for multiple inflammatory pathways in disease pathogenesis. Inflamm. Res. 69, 967–973 (2020). [DOI] [PubMed] [Google Scholar]
- 30.Witte-Händel E. et al. The IL-1 Pathway Is Hyperactive in Hidradenitis Suppurativa and Contributes to Skin Infiltration and Destruction. J. Invest. Dermatol. 139, 1294–1305 (2019). [DOI] [PubMed] [Google Scholar]
- 31.Mariottoni P. et al. Single-Cell RNA Sequencing Reveals Cellular and Transcriptional Changes Associated With M1 Macrophage Polarization in Hidradenitis Suppurativa. Front. Med. 8, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Freudenberg J. M. et al. A Hidradenitis Suppurativa molecular disease signature derived from patient samples by high-throughput RNA sequencing and re-analysis of previously reported transcriptomic data sets. PLOS ONE 18, e0284047 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mi Q.-S. et al. Dysregulated CD38 expression in blood and skin immune cells of patients with hidradenitis suppurativa. Res. Sq. rs.3.rs-2609421 (2023) doi: 10.21203/rs.3.rs-2609421/v1. [DOI] [Google Scholar]
- 34.Kanni T. et al. Compartmentalized Cytokine Responses in Hidradenitis Suppurativa. PLoS ONE 10, e0130522 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Frew J. W., Hawkes J. E. & Krueger J. G. A systematic review and critical evaluation of immunohistochemical associations in hidradenitis suppurativa. F1000Research 7, 1923 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Frew J. W., Hawkes J. E. & Krueger J. G. A systematic review and critical evaluation of immunohistochemical associations in hidradenitis suppurativa. F1000Research 7, 1923 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lowe M. M. et al. Immunopathogenesis of hidradenitis suppurativa and response to anti–TNF-α therapy. JCI Insight 5, (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Campos T. M. et al. Matrix Metalloproteinase 9 Production by Monocytes is Enhanced by TNF and Participates in the Pathology of Human Cutaneous Leishmaniasis. PLoS Negl. Trop. Dis. 8, e3282 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee B. H. et al. High-dimensional profiling of regulatory T cells in psoriasis reveals an impaired skin-trafficking property. eBioMedicine 100, 104985 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moran B. et al. Hidradenitis Suppurativa Is Characterized by Dysregulation of the Th17:Treg Cell Axis, Which Is Corrected by Anti-TNF Therapy. J. Invest. Dermatol. 137, 2389–2395 (2017). [DOI] [PubMed] [Google Scholar]
- 41.Verma S. S., Sharma K. & Chhabra S. Pathogenesis of Hidradenitis Suppurativa: An Immunological Perspective. Indian J. Dermatol. 68, 296–300 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Golden J. B. et al. Chronic Psoriatic Skin Inflammation Leads to Increased Monocyte Adhesion and Aggregation. J. Immunol. 195, 2006–2018 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Drummond R. A., Franco L. M. & Lionakis M. S. Human CARD9: A Critical Molecule of Fungal Immune Surveillance. Front. Immunol. 9, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cibrian D., Fuente H. de la & Sánchez-Madrid, F. Metabolic Pathways That Control Skin Homeostasis and Inflammation. Trends Mol. Med. 26, 975–986 (2020). [DOI] [PubMed] [Google Scholar]
- 45.Matusiak Ł., Szczęch J., Bieniek A., Nowicka-Suszko D. & Szepietowski J. C. Increased interleukin (IL)-17 serum levels in patients with hidradenitis suppurativa: Implications for treatment with anti-IL-17 agents. J. Am. Acad. Dermatol. 76, 670–675 (2017). [DOI] [PubMed] [Google Scholar]
- 46.Kelly G. et al. Dysregulated cytokine expression in lesional and nonlesional skin in hidradenitis suppurativa. Br. J. Dermatol. 173, 1431–1439 (2015). [DOI] [PubMed] [Google Scholar]
- 47.Hotz C. et al. Intrinsic Defect in Keratinocyte Function Leads to Inflammation in Hidradenitis Suppurativa. J. Invest. Dermatol. 136, 1768–1780 (2016). [DOI] [PubMed] [Google Scholar]
- 48.van der Zee H. H. et al. Elevated levels of tumour necrosis factor (TNF)-α, interleukin (IL)-1β and IL-10 in hidradenitis suppurativa skin: a rationale for targeting TNF-α and IL-1β. Br. J. Dermatol. 164, 1292–1298 (2011). [DOI] [PubMed] [Google Scholar]
- 49.Kimball A. B. et al. Secukinumab in moderate-to-severe hidradenitis suppurativa (SUNSHINE and SUNRISE): week 16 and week 52 results of two identical, multicentre, randomised, placebo-controlled, double-blind phase 3 trials. Lancet Lond. Engl. 401, 747–761 (2023). [DOI] [PubMed] [Google Scholar]
- 50.Mariottoni P. et al. Single-Cell RNA Sequencing Reveals Cellular and Transcriptional Changes Associated With M1 Macrophage Polarization in Hidradenitis Suppurativa. Front. Med. 8, 665873 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dimitrion P. M. et al. Disease associated monocytes contribute to the inflammatory milieu in hidradenitis suppurativa and have dysregulated expression of transposable elements. J. Immunol. 210, 72.10 (2023).36426999 [Google Scholar]
- 52.Dimitrion P. et al. Dysregulated CD38 expression in blood and skin immune cells of patients with hidradenitis suppurativa. bioRxiv 2023.01.27.525867 (2023) doi: 10.1101/2023.01.27.525867. [DOI] [Google Scholar]
- 53.Shahrara S., Pickens S. R., Dorfleutner A. & Pope R. M. IL-17 induces monocyte migration in rheumatoid arthritis. J. Immunol. Baltim. Md 1950 182, 3884–3891 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huangfu L., Li R., Huang Y. & Wang S. The IL-17 family in diseases: from bench to bedside. Signal Transduct. Target. Ther. 8, 1–22 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lowe M. M. et al. Tertiary lymphoid structures sustain cutaneous B cell activity in hidradenitis suppurativa. JCI Insight 9, e169870 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Byrd A. S. et al. Neutrophil extracellular traps, B cells, and type I interferons contribute to immune dysregulation in hidradenitis suppurativa. Sci. Transl. Med. 11, eaav5908 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sabat R. et al. Neutrophilic granulocyte-derived B-cell activating factor supports B cells in skin lesions in hidradenitis suppurativa. J. Allergy Clin. Immunol. 151, 1015–1026 (2023). [DOI] [PubMed] [Google Scholar]
- 58.Lé A. M. & Torres T. OX40-OX40L Inhibition for the Treatment of Atopic Dermatitis—Focus on Rocatinlimab and Amlitelimab. Pharmaceutics 14, 2753 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rewerska B. et al. Phase 2b randomized trial of OX40 inhibitor telazorlimab for moderateto-severe atopic dermatitis. J. Allergy Clin. Immunol. Glob. 3, 100195 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated for this study can be found in the GEO repository under the accession: GSE194315 for healthy and GSEXXX for HS samples