Abstract
The cycling characteristics of CD8+ T cells specific for two lytic-phase epitopes of murine gammaherpesvirus 68 (γHV68) have been analyzed for mice with high or low levels of virus persistence. The extent of cell division is generally reflective of the antigen load and suggests that γHV68 may be regularly reactivating from latency for some months after the resolution of the acute phase of the infectious process. Although γHV68 infection is also associated with massive proliferation of lymphocytes that are not obviously specific for the virus, the level of “bystander-induced” cycling in a population of influenza virus-specific CD8+ T cells was generally fourfold lower than the extent of cell division seen for the antigen-driven, γHV68-specific response. The overall conclusion is that turnover rates substantially in excess of 5 to 10% over 6 days for CD8+ “memory” T-cell populations are likely to be reflective of continued antigenic exposure.
Respiratory challenge with murine gammaherpesvirus 68 (γHV68) induces massive turnover in the CD8+ T-cell compartment (13, 24). This pattern of sustained lymphocyte proliferation persists long after both γHV68 replicative infection is controlled in epithelial sites and peak numbers of latently infected B lymphocytes are detected in the spleen (4). These cycling CD8+ T cells comprise at least two distinct populations. The first consists of multiple sets of γHV68-specific CD8+ T cells that are, in C57BL/6J (B6) mice, responding to at least six different viral peptides derived from proteins expressed during the lytic phase of the infectious process (18). The second is an unusual CD8+ T-cell population that expresses a Vβ4 T-cell receptor chain and expands greatly in numbers after the end of the initial, lytic phase of the infectious process (24). These CD8+ Vβ4+ T cells show some evidence of oligoclonality (14), although they do not seem to be recognizing viral peptides or major histocompatibility complex class I or II glycoproteins (6). Furthermore, the dramatic increase in the size of the CD8+ Vβ4+ set is totally dependent on concurrent, CD40 ligand-mediated CD4+ T-cell help (3, 9). This effect is not seen in major histocompatibility complex class II−/− (I-Ab−/−) mice.
In the present analysis, we used these CD4+ T-cell-deficient I-Ab−/− mice (12) and conventional B6 (I-Ab+/+) congenic mice to characterize the proliferation of virus-specific CD8+ T cells under conditions of differential, continuing γHV68 challenge. The I-Ab+/+ group effectively controls lytic γHV68 infection within 12 days of the initial intranasal (i.n.) exposure, while I-Ab−/− mice show persistent evidence of γHV68 replication in the respiratory tract and succumb to a late-onset wasting disease that develops after 80 to 100 days (2, 4). Latently infected B cells and macrophages can be demonstrated in both groups over the very long term by an infectious-center assay and by limiting-dilution analysis.
CD8+ T cells were obtained from the spleen and from the infected lungs (1) by bronchoalveolar lavage (BAL). The experiments focus on the two most prominent γHV68 epitopes, H2Dbp56 (Dbp56) and H2Kbp79 (Kbp79). The γHV68 p56 peptide (AGPHNDMEI) is derived from a single-stranded DNA binding protein, while the p79 peptide (TSINFKVI) is derived from the large ribonucleotide reductase (18). The possible contribution of “bystander” activation (8, 16, 22, 25, 26) to the total pool of cycling CD8+ T cells was also analyzed for a “memory” population specific for the prominent influenza virus DbNP366 epitope (10). Influenza-immune I-Ab+/+ mice were first infected intraperitoneally with H1N1 influenza A virus and then boosted at least 6 weeks prior to γHV68 challenge by respiratory infection with an H3N2 virus that shares the same nucleoprotein gene (10, 15).
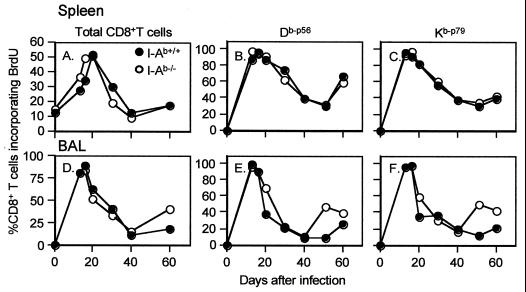
The bromodeoxyuridine (BrdU)-positive (BrdU+) CD8+ population showed maximal prevalence in both the spleen and BAL populations recovered from the I-Ab+/+ and I-Ab−/− mice at day 20 after the initial exposure to γHV68 (Fig. 1A to D). The percentages of BrdU+ T cells in the splenic CD8+ Dbp56+ and CD8+ Kbp79+ sets were also remarkably concordant for the I-Ab+/+ and I-Ab−/− mice until day 60 after the i.n. γHV68 challenge (Fig. 1B and C), with the values ranging from >80% on day 20 to about 30% on day 60. The same was true for the BAL populations until day 40, although there was evidence of more cycling in the I-Ab−/− mice by day 50 (Fig. 1E and F). This result presumably reflects the persistent, low-level production of lytic virus in the I-Ab−/− mouse respiratory tract (4).
FIG. 1.
Kinetic analysis of BrdU incorporation in the CD8+ Kbp79+ and CD8+ Dbp56+ sets during the first 60 days after γHV68 infection (13, 19, 23). I-Ab+/+ and I-Ab−/− mice were infected i.n. with 2 × 103 PFU of γHV68 and given 0.8 mg of BrdU/ml in their drinking water for 6 days prior to sampling. The results for pooled spleen (A, B, and C) and BAL (D, E, and F) populations from four or five mice at each time point show the percentages of BrdU+ cells for all gated CD8+ lymphocytes (A and D) and for the virus-specific Dbp56+(B and E) and Kbp79+ (C and F) sets. The lymphocytes were stained with anti-CD8–tricolor and phycoerythrin-labeled Kbp79 or Dbp56 tetramers, fixed, and stained with anti-BrdU–fluorescein isothiocyanate (13, 19, 23). The cells were then analyzed on a FACScan flow cytometer using CellQuest software (Becton Dickinson, Mountain View, Calif.). A minimum of 2,000 CD8+ tetramer-positive events were analyzed for each sample.
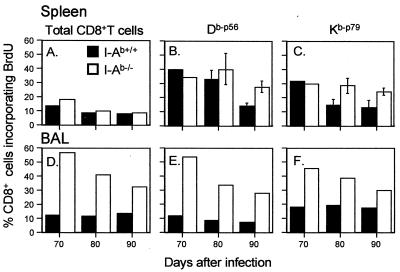
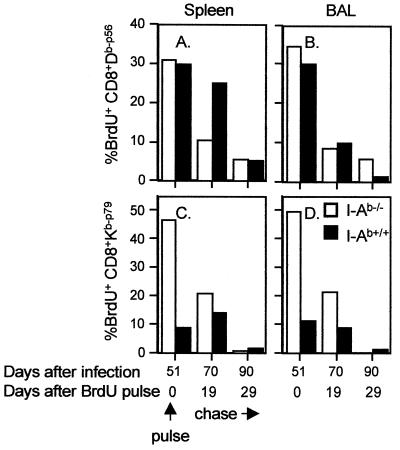
Analysis at later time points (days 70 to 90) showed a continuing profile of greater cycling for both the total and the virus-specific CD8+ T-cell populations in the BAL populations from the I-Ab−/− mice (Fig. 2D to F). This difference between the I-Ab+/+ and I-Ab−/− groups was also apparent for the virus-specific CD8+ T cells recovered on day 90 from the spleen (Fig. 2B and C), although the effect was not evident in the total CD8+ T-cell population (Fig. 2A). A further pulse-chase analysis (12) also indicated that both the CD8+ Dbp56+ (Fig. 3A and B) and the CD8+ Kbp79+ (Fig. 3C and D) sets were proliferating at a higher rate in the I-Ab−/− mice between day 50 and day 70 after infection.
FIG. 2.
Prevalence of BrdU+ CD8+ Kbp79+ and CD8+ Dbp56+ T cells in I-Ab+/+ and I-Ab−/− mice at 70 to 90 days after γHV68 infection. The spleen results (A, B, and C) are presented as means and standard deviations for five mice per group, while the BAL samples (D, E, and F) were pooled from the same mice. The values for the I-Ab+/+ and I-Ab−/− spleens were significantly different (P < 0.05) on day 90 for both epitopes (B and C). The other differences between the I-Ab+/+ and I-Ab−/− mice in panels A to C were not significant. The BrdU incorporation profiles are shown for total, gated CD8+ lymphocytes (A and D) and for the Dbp56+ (B and E) and Kbp79+ (C and F) populations. The mice were infected i.n. with 2 × 103 PFU of γHV68 and given BrdU in their drinking water for 6 days prior to sampling. The γHV68-specific CD8+ T-cell populations were analyzed as described in the legend to Fig. 1.
FIG. 3.
Pulse-chase analysis of γHV68-specific CD8+ T cells isolated after the peak of the protracted CD8+ Vβ4+ T-cell proliferation (24) in I-Ab+/+ mice. The mice were given drinking water containing BrdU for 6 days, commencing 45 days after i.n. infection with γHV68. They were assayed at the end of the pulse period (day 51) and again 19 and 29 days later (chase). Spleens (A and C) and BAL populations (B and D) were pooled from three to five animals for each time point, enriched for CD8+ T cells, stained with the Dbp56 and Kbp79 tetramers, and analyzed for BrdU incorporation as described in the legend to Fig. 1.
Some evidence of bystander activation for the influenza virus CD8+ DbNP366+ set was found in both the spleen and BAL populations recovered during the first 18 days after i.n. γHV68 challenge (Table 1). Although we refer to this effect throughout as bystander activation, a description that implies T-cell receptor-independent stimulation by cytokines (8, 19, 26), it is also formally possible that there is some unidentified cross-reactivity between a γHV68-encoded epitope and DbNP366 (17). The percentages of BrdU+ CD8+ DbNP366+ T cells in the spleen were significantly increased (P < 0.05) over background values (day 0) at 12 and 16 days after i.n. exposure to γHV68, although the prevalence of cycling CD8+ DbNP366+ T cells was three- to fourfold lower than that for the CD8+ Dbp56+ and CD8+ Kbp79+ sets (Table 1). The results are very comparable to those found previously during the acute phase of infection with influenza B virus (5) and suggest that γHV68 has no particular propensity to drive the proliferation of “irrelevant” CD8+ memory T cells.
TABLE 1.
Cycling characteristics of influenza virus-specific and γHV68-specific CD8+ T cells following γHV68 infection
| Site sampleda | Day | % CD8+ tetramer-positive BrdU+ cells specific fora:
|
||
|---|---|---|---|---|
| Influenza virus DbNP366a | γHV68
|
|||
| Dbp56 | Kbp79 | |||
| Spleen | 0 | 6.2 ± 1.5 | ||
| 12 | 18.9 ± 4.4 | 84.5 ± 3.8 | 92.5 ± 1.2 | |
| 16 | 19.2 ± 4.3 | 62.5 ± 8.9 | 79.9 ± 10.2 | |
| 18 | 11.7 ± 3.9 | 47.0 ± 5.1 | 70.6 ± 11.1 | |
| BAL | 0 | 1.5 | ||
| 12 | 8.7 | 46.7 | 52.4 | |
| 16 | 9.5 | 66.5 | 95.5 | |
| 18 | 4.5 | 18.6 | 53.5 | |
The spleen results are expressed as means and standard deviations for five mice, while the BAL samples were pooled.
I-Ab+/+ mice were infected intraperitoneally with 108.5 50% embryo-infective doses (EID50) of the PR8 (H1N1) influenza A virus, boosted i.n. 1 month later with 106.5 EID50 of the HKx31 (H3N2) influenza A virus (10, 11), and then challenged i.n. after another 6 weeks with γHV68. They were given drinking water containing BrdU for 6 days prior to sampling. The BrdU incorporation profiles were then analyzed for γHV68-specific (Dbp56+ and Kbp79+) and influenza virus nucleoprotein-specific (DbNP366) CD8+ T-cell populations.
In general, this analysis of cycling for the CD8+ Dbp56+ and CD8+ Kbp79+ T-cell populations in I-Ab+/+ and I-Ab−/− mice indicates that the extent of cell division above background levels is correlated with the antigen load. Although every CD8+ DbNP366+ T cell divides multiple times during the acute, antigen-driven phase of the host response to the readily eliminated influenza A viruses (11), the background turnover rates for influenza virus-specific CD8+ DbNP366+ memory T cells in the spleen average about 5% (5, 10) (Table 1, day 0). Particularly in the secondary H3N2→H1N1 response used to prime the mice analyzed for bystander activation in Table 1, the extent of BrdU incorporation drops to the level characteristic of long-term memory as soon as the virus is eliminated from the lungs (11).
Much higher levels of cycling (20 to 40%) (Fig. 1 and 2) were detected for the CD8+ Dbp56+ and CD8+ Kbp79+ T-cell populations recovered from γHV68-infected I-Ab+/+ mice for at least 2 months after evidence of virus replication (4) could no longer be found in the respiratory tract (day 12). This result suggests that, although infectious virus cannot be recovered directly from the spleen by a conventional plaque assay, there may be a continuing process of reactivation from latency in the in vivo situation. Virus reactivation could in turn lead to rapid CD8+ T-cell-mediated elimination of the now productively infected B cells and macrophages and, as a consequence, a progressive reduction in the extent of γHV68 latency. By 80 to 90 days after the initial γHV68 challenge, the numbers of cycling CD8+ Dbp56+ and CD8+ Kbp79+ T cells in I-Ab+/+ mice had fallen (Fig. 2) to levels more characteristic of those associated with “resting” memory for other viruses. The counts continued to be high in the I-Ab−/− congenic mice, where evidence of continuing lytic infection was found in the lung epithelium (4).
The results of these experiments thus support the idea that any evidence of CD8+ T-cell proliferation that is substantially above a “ homeostatic background” (7, 21, 23) of 5 to 10% over 6 days is reflective of continued exposure to antigen (20). Evidence of bystander activation can be found at the acute phase of the host response to an unrelated pathogen, but any bystander proliferation is at a much lower level than that associated with the antigen-driven effect.
Acknowledgments
We thank Gabriela Byers for technical assistance and Vicki Henderson for help with the manuscript.
This work was supported by U.S. Public Health Service grants AI29579, AI38359, and CA21765 and by the American Lebanese Syrian Associated Charities. G.T.B. is a C. J. Martin fellow of the Australian National Health and Medical Research Council.
REFERENCES
- 1.Allan W, Tabi Z, Cleary A, Doherty P C. Cellular events in the lymph node and lung of mice with influenza. Consequences of depleting CD4+ T cells. J Immunol. 1990;144:3980–3986. [PubMed] [Google Scholar]
- 2.Belz G T, Stevenson P G, Castrucci M R, Altman J D, Doherty P C. Postexposure vaccination massively increases the prevalence of gamma-herpesvirus-specific CD8+ T cells but confers minimal survival advantage on CD4-deficient mice. Proc Natl Acad Sci USA. 2000;97:2725–2730. doi: 10.1073/pnas.040575197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brooks J W, Hamilton-Easton A M, Christensen J P, Cardin R D, Hardy C L, Doherty P C. Requirement for CD40 ligand, CD4+ T cells, and B cells in an infectious mononucleosis-like syndrome. J Virol. 1999;73:9650–9654. doi: 10.1128/jvi.73.11.9650-9654.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cardin R D, Brooks J W, Sarawar S R, Doherty P C. Progressive loss of CD8+ T cell-mediated control of a gamma-herpesvirus in the absence of CD4+ T cells. J Exp Med. 1996;184:863–871. doi: 10.1084/jem.184.3.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Christensen J P, Doherty P C, Branum K C, Riberdy J M. Profound protection against respiratory challenge with a lethal H7N7 influenza A virus by increasing the magnitude of CD8(+) T-cell memory. J Virol. 2000;74:11690–11696. doi: 10.1128/jvi.74.24.11690-11696.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coppola A M, Flano E, Nguyen P, Hardy C L, Cardin R D, Shastri N, Woodland D L, Blackman M A. Apparent MHC-independent stimulation of CD8+ T cells in vivo during latent murine gammaherpesvirus infection. J Immunol. 1999;163:1481–1489. [PubMed] [Google Scholar]
- 7.Doherty P C, Topham D J, Tripp R A. Establishment and persistence of virus-specific CD4+ and CD8+ T cell memory. Immunol Rev. 1996;150:23–44. doi: 10.1111/j.1600-065x.1996.tb00694.x. [DOI] [PubMed] [Google Scholar]
- 8.Ehl S, Hombach J, Aichele P, Hengartner H, Zinkernagel R M. Bystander activation of cytotoxic T cells: studies on the mechanism and evaluation of in vivo significance in a transgenic mouse model. J Exp Med. 1997;185:1241–1251. doi: 10.1084/jem.185.7.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flano E, Woodland D L, Blackman M A. Requirement for CD4+ T cells in Vβ4+CD8+ T cell activation associated with latent murine gammaherpesvirus infection. J Immunol. 1999;163:3403–3408. [PubMed] [Google Scholar]
- 10.Flynn K J, Belz G T, Altman J D, Ahmed R, Woodland D L, Doherty P C. Virus-specific CD8+ T cells in primary and secondary influenza pneumonia. Immunity. 1998;8:683–691. doi: 10.1016/s1074-7613(00)80573-7. [DOI] [PubMed] [Google Scholar]
- 11.Flynn K J, Riberdy J M, Christensen J P, Altman J D, Doherty P C. In vivo proliferation of naive and memory influenza-specific CD8+ T cells. Proc Natl Acad Sci USA. 1999;96:8597–8602. doi: 10.1073/pnas.96.15.8597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grusby J M, Johnson R S, Papaioannou V E, Glimcher L H. Depletion of CD4+ T cells in major histocompatibility complex class II-deficient mice. Science. 1991;253:1417–1420. doi: 10.1126/science.1910207. [DOI] [PubMed] [Google Scholar]
- 13.Hamilton-Easton A M, Christensen J P, Doherty P C. Turnover of T cells in murine gammaherpesvirus 68-infected mice. J Virol. 1999;73:7866–7869. doi: 10.1128/jvi.73.9.7866-7869.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hardy C L, Silins S L, Woodlan D L, Blackman M A. Murine γherpesvirus infection causes Vβ4-specific CDR3-restricted clonal expansions within CD8+ peripheral blood T lymphocytes. Int Immunol. 2000;12:1193–1204. doi: 10.1093/intimm/12.8.1193. [DOI] [PubMed] [Google Scholar]
- 15.Kilbourne E D. Future influenza vaccines and the use of genetic recombinants. Bull W H O. 1969;41:643–645. [PMC free article] [PubMed] [Google Scholar]
- 16.Murali-Krishna K, Altman J D, Suresh M, Sourdive D J, Zajac A J, Miller J D, Slansky J, Ahmed R. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 1998;8:177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- 17.Selin L K, Nahill S R, Welsh R M. Cross-reactivities in memory cytotoxic T lymphocyte recognition of heterologous viruses. J Exp Med. 1994;179:1933–1943. doi: 10.1084/jem.179.6.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stevenson P G, Belz G T, Altman J D, Doherty P C. Changing patterns of dominance in the CD8+ T cell response during acute and persistent murine gamma-herpesvirus infection. Eur J Immunol. 1999;29:1059–1067. doi: 10.1002/(SICI)1521-4141(199904)29:04<1059::AID-IMMU1059>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 19.Stevenson P G, Belz G T, Castrucci M R, Altman J D, Doherty P C. A gamma-herpesvirus sneaks through a CD8+ T cell response primed to a lytic-phase epitope. Proc Natl Acad Sci USA. 1999;96:9281–9286. doi: 10.1073/pnas.96.16.9281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stewart J P, Usherwood E J, Ross A, Dyson H, Nash T. Lung epithelial cells are a major site of murine γherpesvirus persistence. J Exp Med. 1998;187:1941–1951. doi: 10.1084/jem.187.12.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tanchot C, Rocha B. The peripheral T cell repertoire: independent homeostatic regulation of virgin and activated CD8+ T cell pools. Eur J Immunol. 1995;25:2127–2136. doi: 10.1002/eji.1830250802. [DOI] [PubMed] [Google Scholar]
- 22.Tough D F, Borrow P, Sprent J. Induction of bystander T cell proliferation by viruses and type I interferon in vivo. Science. 1996;272:1947–1950. doi: 10.1126/science.272.5270.1947. [DOI] [PubMed] [Google Scholar]
- 23.Tough D F, Sprent J. Lifespan of γδ T cells. J Exp Med. 1998;187:357–365. doi: 10.1084/jem.187.3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tripp R A, Hamilton-Easton A M, Cardin R D, Nguyen P, Behm F G, Woodland D L, Doherty P C, Blackman M A. Pathogenesis of an infectious mononucleosis-like disease induced by a murine gamma-herpesvirus: role for a viral superantigen? J Exp Med. 1997;185:1641–1650. doi: 10.1084/jem.185.9.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tripp R A, Hou S, McMickle A, Houston J, Doherty P C. Recruitment and proliferation of CD8+ T cells in respiratory virus infections. J Immunol. 1995;154:6013–6021. [PubMed] [Google Scholar]
- 26.Zhang X, Sun S, Hwang I, Tough D F, Sprent J. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity. 1998;8:591–599. doi: 10.1016/s1074-7613(00)80564-6. [DOI] [PubMed] [Google Scholar]