Abstract
Rationale:
Quantifying functional small airways disease (fSAD) requires additional expiratory computed tomography (CT) scan, limiting clinical applicability. Artificial intelligence (AI) could enable fSAD quantification from chest CT scan at total lung capacity (TLC) alone (fSADTLC).
Objectives:
To evaluate an AI model for estimating fSADTLC and study its clinical associations in chronic obstructive pulmonary disease (COPD).
Methods:
We analyzed 2513 participants from the SubPopulations and InteRmediate Outcome Measures in COPD Study (SPIROMICS). Using a subset (n = 1055), we developed a generative model to produce virtual expiratory CTs for estimating fSADTLC in the remaining 1458 SPIROMICS participants. We compared fSADTLC with dual volume, parametric response mapping fSADPRM. We investigated univariate and multivariable associations of fSADTLC with FEV1, FEV1/FVC, six-minute walk distance (6MWD), St. George’s Respiratory Questionnaire (SGRQ), and FEV1 decline. The results were validated in a subset (n = 458) from COPDGene study. Multivariable models were adjusted for age, race, sex, BMI, baseline FEV1, smoking pack years, smoking status, and percent emphysema.
Measurements and Main Results:
Inspiratory fSADTLC was highly correlated with fSADPRM in SPIROMICS (Pearson’s R = 0.895) and COPDGene (R = 0.897) cohorts. In SPIROMICS, fSADTLC was associated with FEV1 (L) (adj.β = −0.034, P < 0.001), FEV1/FVC (adj.β = −0.008, P < 0.001), SGRQ (adj.β = 0.243, P < 0.001), and FEV1 decline (mL / year) (adj.β = −1.156, P < 0.001). fSADTLC was also associated with FEV1 (L) (adj.β = −0.032, P < 0.001), FEV1/FVC (adj.β = −0.007, P < 0.001), SGRQ (adj.β = 0.190, P = 0.02), and FEV1 decline (mL / year) (adj.β = −0.866, P = 0.001) in COPDGene. We found fSADTLC to be more repeatable than fSADPRM with intraclass correlation of 0.99 (95% CI: 0.98, 0.99) vs. 0.83 (95% CI: 0.76, 0.88).
Conclusions:
Inspiratory fSADTLC captures small airways disease as reliably as fSADPRM and is associated with FEV1 decline.
INTRODUCTION
Small conducting airways of the lungs are the primary sites of airflow limitation in early obstructive lung disease (1, 2), and their loss is extensive by the time that airflow limitation is detectable by spirometry (3). Identifying early small airways disease (SAD) in susceptible individuals who have inhalational exposures linked to COPD is essential to the development of disease-modifying therapies for this leading cause of worldwide death and disability.
Currently, the only in vivo method to quantify SAD relies on image registration for demonstrating non-emphysematous air-trapping on chest computed tomography (CT) scans obtained at inspiration and expiration (4). These co-registered scans are analyzed through parametric response mapping (PRM) to quantify such air-trapping, termed functional small airways disease (fSADPRM) (4). MicroCT analysis of resected lungs demonstrated that fSADPRM accurately reflects SAD in advanced COPD (5). However, the need for additional expiratory CT incurs increased cost and ionizing radiation exposure. Additionally, expiratory CT scan acquisition in clinical settings requires specialized technician training and there is no harmonized protocol to coach and acquire images at different lung volumes (6). These drawbacks preclude application of PRM analysis to clinical settings where expiratory CT scans are unavailable. By contrast, inspiratory chest CT scans are common in many clinical settings, including lung cancer screening. Hence, a method for estimating fSAD from inspiratory chest CT scans alone would be valuable.
Deep generative modeling can reliably generate multimodal medical images by converting a medical image from one modality to the other (7). Recently, we developed a method for estimating an expiratory chest CT scan solely from a given inspiratory CT image (7). The synthesized virtual expiratory image could be used in combination with the available inspiratory CT scan for estimating SAD. We hypothesize that this single inspiratory volume fSAD estimation from chest CT at total lung capacity (TLC) can be used to identify regions of SAD and that these regions will be associated with poor outcomes in COPD. To test our hypothesis, we analyzed data from the SubPopulations and InteRmediate Outcome Measures in COPD Study (SPIROMICS) cohort (8). The results were validated in a cohort from the Genetic Epidemiology of COPD (COPDGene) study (9). We also investigated the repeatability of inspiratory CT fSAD and compared its repeatability with the conventional fSADPRM extracted from two volumes.
METHODS
Study Populations
We analyzed data from the SubPopulations and InteRmediate Outcome Measures in COPD Study (SPIROMICS) which is a multicenter prospective cohort study being conducted at 14 clinical centers across the United States (US) (8). SPIROMICS enrolled 2981 participants between 40 and 80 years of age across four strata: with smoking history ≤ 1 pack-year (stratum 1) or a smoking history ≥ 20 pack-years (strata 2 – 4). The participants underwent high-resolution chest CT scans on full inspiration and expiration, i.e., at both total lung capacity (TLC) and residual volume (RV), respectively at their enrollment visit. We divided SPIROMICS participants as described below into two non-overlapping subsets, which were respectively used to train our generative model (to transform inspiratory CT to synthetic expiratory CT scans), and for the association of the resulting fSAD measurements from the single inspiratory and synthetic expiratory scans.
We externally validated our model on a subset from the Genetic Epidemiology of COPD (COPDGene) study (9). COPDGene is a multicenter observational cohort study that recruited nearly 10,300 participants across 21 clinical centers in the US. COPDGene used a different image acquisition protocol from SPIROMICS. COPDGene acquired chest CT scans at TLC and functional residual capacity (FRC). However, in a sub-study at a single clinical site (Iowa), some participants were also scanned at RV (n = 458), and this group was used in this analysis.
Both studies were conducted according to the principles of the Helsinki accord. Written informed consent for both studies was provided by all participants, and the protocols were approved by the Institutional Review Boards (IRBs) of each participating study center.
Estimating fSAD from Inspiratory Chest CT
To synthesize virtual expiratory CT scans from a participant’s inspiratory chest CT scan alone, we used a recently developed generative adversarial network (GAN), called LungViT (7). Before training the model, we registered the expiratory chest CT scan (treated as moving image) to the inspiratory chest CT scan (treated as the registration reference). We trained the generative model using registered scan pairs, obtained at TLC and RV, from SPIROMICS. The training set (n = 1055) comprised 55 individuals who never smoked, and 200 participants randomly sampled from each COPD Global Initiative for Chronic Lung Disease (GOLD) stage (0 – 4).
GANs use image pairs to learn a transformation from one image to the other, in our case, to transform TLC scans to RV scans. Generative models rely on two coupled neural networks, where an additional model (called the discriminator) is used to enhance and assess the image quality generated by the generative model (the generator) (7). Iterative feedback from the discriminator helps the generator to produce perceptually realistic images. Once trained, the generator can be used to synthesize a virtual chest CT scan at RV from a given TLC CT scan alone. For more details on model architecture and training please refer to the work in (7) and the online supplement.
Next, we used the trained generator to synthesize virtual expiratory chest CT scans for the remaining SPIROMICS (n = 1458) and COPDGene participants (n = 458). These virtual expiratory CT scans were employed, in combination with the TLC scans, to compute a variable called fSADTLC. We calculated fSADTLC as the voxels between −950 HU to −810 HU on inspiratory chest CT scan and between −1000 HU to −857 HU on the virtual or synthetic expiratory CT scan (4, 10).
We compared the proposed fSADTLC with the conventional dual volume, image registration-based fSAD, obtained using the PRM (fSADPRM). The fSADPRM was computed by registering the expiratory CT scans at RV to the inspiratory CT scans at TLC. The fSADPRM was defined as voxels between −950 HU and −810 HU on inspiratory chest CT and between −1000 HU and −857 HU on the expiratory CT (4, 10). The only difference between the fSADPRM and fSADTLC calculation was that the latter used a virtual expiratory CT scan at RV instead of the actual RV scan acquired by SPIROMICS. Both fSADPRM and fSADTLC were expressed as a percentage of total lung volume.
Predictors
Predictors in this study included age, sex, race, body mass index (BMI), smoking status (current or former defined as ≥ 6-month cessation), smoking pack years, post-bronchodilator forced expiratory volume in 1 second (FEV1), percent emphysema defined as percentage of low-attenuation areas (LAA%) below −950 Hounsfield units (HU), and fSADTLC or fSADPRM.
Outcomes
We studied the association of fSADTLC with various functional and clinical outcomes of respiratory morbidity in both SPIROMICS and COPDGene cohorts. Our study outcomes included lung function measures such as the post-bronchodilator forced expiratory volume in 1 second (FEV1) in liters (L) and the ratio of postbronchodilator FEV1/FVC. We also analyzed the respiratory quality of life quantified by the total St. George’s Respiratory Questionnaire (SGRQ) score (11). SGRQ score ranges between 0 and 100, where 0 indicates no symptom burden with a good quality of life, while 100 indicates a poor quality of patient life with high symptom burden (11). We also studied the association of fSADTLC with six-minute walk distance (6MWD) and mMRC (modified Medical Research Council) dyspnea scale that ranges from 0 to 4 in increasing order of shortness of breath (12).
In both cohorts, we further investigated the relationship between fSADTLC and change in FEV1 between baseline and follow-up visit after five years. The change in FEV1 was calculated as a difference between baseline FEV1 and the follow-up FEV1 around five years, which was then divided by the time between visits to calculate the change in mL / year.
Statistical Analysis
To initially investigate the relationship between percent fSADTLC and fSADPRM, we used Pearson’s correlation. Scatter plots were also generated to assess if fSADTLC was able to reliably capture the distribution of fSADPRM. We also conducted a Bland-Altman analysis between the means of fSADTLC and fSADPRM to assess any systematic bias or variability in the differences across the range of measurements. Univariate and multivariable regression analysis was also conducted for investigating the association of fSADTLC with spirometry and clinical outcomes in COPD. We tested associations between baseline fSADTLC and post-bronchodilator FEV1 (L) and FEV1/FVC, with age, sex, race, body-mass index (BMI), smoking status, smoking pack years, and percent emphysema or LAA% as covariates. For assessing the association of baseline fSADTLC with SGRQ, 6MWD (ft), and mMRC dyspnea scale, we added baseline post-bronchodilator FEV1 (L) as an additional covariate for adjustment.
We studied the association of with change in FEV1 (mL / year) after adjusting for the same set of variables mentioned above. We repeated the multivariable analysis for change in FEV1 (mL / year) among GOLD 0 and GOLD 1 – 4 participants. To assess the relationship between fSADTLC and all-cause mortality, we categorized participants in four quartiles of fSADTLC and conducted Kaplan-Meier curve analysis for each quartile (13). Survival curves were plotted for each quartile, and the log-rank test was used to compare survival distributions across quartiles. All association studies and Kaplan-Meier curve analysis was repeated for the fSADPRM. A two-sided P value < 0.05 was considered to be significant.
Repeatability Analysis
We conducted a repeatability study to assess the overall reproducibility of fSADTLC. We used data from the SPIROMICS repeatability study that enrolled 100 participants at the primary study sites between 2012 and 2015 (14). The study participants were scanned at TLC and RV during their enrollment visit. Repeat scans at both TLC and RV were acquired 2 – 6 weeks after the enrollment visit (14). For both visits, we generated synthetic RV scans for computing fSADTLC. To test the repeatability of fSADTLC, we computed the intraclass correlation coefficient (ICC) using the single measurement, same raters’ case (15). This was followed by a Bland-Altman analysis to test any systematic bias across the range of fSADTLC. ICC and Bland-Altman plots were also generated for fSADPRM to compare its repeatability with fSADTLC.
RESULTS
Study Design
SPIROMICS enrolled 2981 participants during its first phase from Nov 12, 2010, to July 31, 2015. Of 2981 participants, eight withdrew consent, and 1055 were reserved for training the TLC to RV generative model, as shown in Figure E1. Of 1918 scans, we removed 177 participants with change in TLC and RV volume less than 1L. After further eliminating the missing clinical information, we were able to obtain complete case data for 1458 participants at baseline in SPIROMICS (see Figure E1). At five-year follow-up, spirometry data was available only for 650 individuals (see Figure E1).
Of 10,305 individuals from the first phase of COPDGene, 473 had RV scans available for analysis at enrollment (see Figure E1). After eliminating the cases with missing clinical information, we analyzed baseline data from 458 participants to validate our results. For studying change in FEV1, eight subjects were lost to follow-up at five years, as shown in Figure E1.
Participant Characteristics
The mean age at baseline was 62.9 (9.1) years in SPIROMICS and 63.3 (8.5) in COPDGene (see Table 1). Both cohorts were fairly balanced by biological sex with 788 (54%) and 215 (47%) males in SPIROMICS and COPDGene cohorts, respectively. In SPIROMICS, 588 (40%) individuals were current smokers, and the fraction was smaller for COPDGene with 130 (28%) individuals who smoked currently (see Table 1). As shown in Table 1, the mean baseline fSADTLC (%) in SPIROMICS was 12.98 (13.25) compared to a mean baseline fSADPRM (%) of 13.35 (12.21). Similarly, in COPDGene, the mean fSADTLC (%) was 9.55 (10.99) and the mean fSADPRM (%) was 10.13. (9.46) (see Table 1). Participant characteristics at five-year follow-up are reported in Table E1 of the online supplement.
Table 1:
Participant characteristics from the SPIROMICS and COPDGene cohorts at baseline.
| SPIROMICS | COPDGene | P Value | |
|---|---|---|---|
|
| |||
| (n = 1458) | (n = 458) | ||
|
| |||
| Age, years | 62.86 (9.13) | 63.30 (8.52) | 0.6 |
| Race | < 0.001 | ||
| White | 1,138 (78%) | 454 (99%) | |
| Non-White | 320 (22%) | 4 (0.9%) | |
| Sex | 0.008 | ||
| Male | 788 (54%) | 215 (47%) | |
| Female | 670 (46%) | 243 (53%) | |
| BMI, kg / m2 | 28.08 (5.18) | 29.93 (6.16) | < 0.001 |
| Smoking status | < 0.001 | ||
| Former or never smokers | 870 (60%) | 328 (72%) | |
| Current smokers | 588 (40%) | 130 (28%) | |
| Smoking pack years | 46.62 (28.11) | 39.42 (23.77) | < 0.001 |
| Postbronchodilator FEV1, L | 2.29 (0.87) | 2.50 (0.75) | < 0.001 |
| FEV1 / FVC ratio | 0.64 (0.15) | 0.71 (0.12) | < 0.001 |
| GOLD stage | < 0.001 | ||
| 0 | 579 (40%) | 240 (52%) | |
| 1 | 165 (11%) | 44 (9.6%) | |
| 2 | 468 (32%) | 78 (17%) | |
| 3 | 164 (11%) | 25 (5.5%) | |
| 4 | 33 (2.3%) | 1 (0.2%) | |
| Never smokers | 49 (3.4%) | 18 (3.9%) | |
| PRISm | NA | 52 (11%) | |
| Total SGRQ score | 30.18 (20.48) | 12.48 (15.26) | < 0.001 |
| 6MWD, ft | 1,342.01 (361.23) | 1,599.13 (336.83) | < 0.001 |
| mMRC dyspnea scale | 0.95 (0.95) | 0.64 (1.05) | < 0.001 |
| fSADPRM, % | 13.35 (12.21) | 10.13 (9.46) | < 0.001 |
| fSADTLC, % | 12.98 (13.25) | 9.55 (10.99) | 0.002 |
| CT emphysema (LAA < −950 HU), % | 6.59 (9.07) | 3.84 (5.88) | < 0.001 |
Data reported as mean (SD) or n (%). SPIROMICS = SubPopulations and InteRmediate Outcome Measure In COPD; COPDGene = Genetic Epidemiology of CoPD; BMI = body-mass index (kg / m2); FEV1 = forced expiratory volume in 1 second; FVC = forced vital capacity; GOLD = Global Initiative for Obstructive Lung Disease; PRISm = preserved ratio impaired spirometry; SGRQ = St. George’s Respiratory Questionnaire; 6MWD = six minute walk distance (ft); mMRC = modified Medical Research Council; fSAD = functional small airways disease; TLC = total lung capacity; PRM = parametric response mapping; CT = computed tomography; LAA = low-attenuation areas (%). P values were generated using Wilcoxon’s rank sum test or Pearson’s Chi-squared test.
Relationship between fSADTLC and fSADPRM
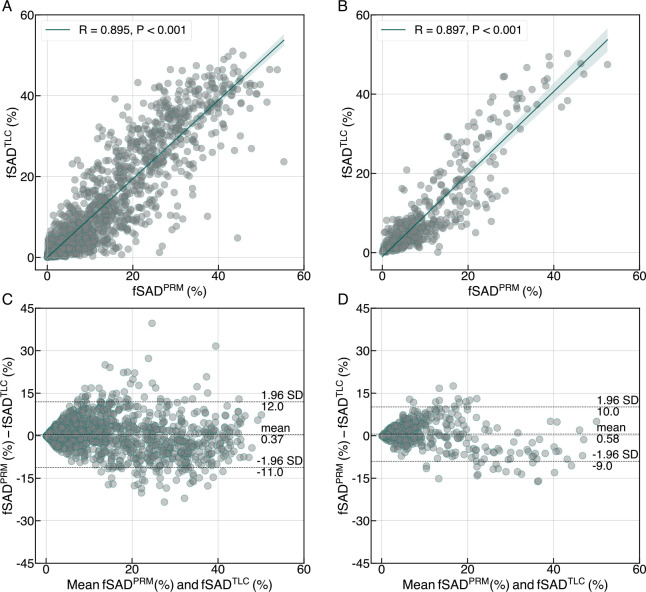
We show a qualitative comparison between the spatial distributions of fSADPRM and fSADTLC (see columns 4 and 5 in Figure 1) across three different SPIROMICS subjects with varying degrees of fSAD. We also show mid-coronal slices from the virtual RV images generated by our AI model (see column 3 in Figure 1). Perceptually, there were negligible differences between the real and virtual RV image slices, shown respectively in columns 2 and 3 of Figure 1). We observed a high Pearson’s correlation between fSADTLC and fSADPRM in SPIROMICS (R = 0.895, P < 0.001) and COPDGene (R = 0.897, P < 0.001), suggesting a strong linear relationship between two variables (see Figure 2A and 2B). Scatter plots showed a high agreement between the overall distributions of fSADTLC and fSADPRM in both cohorts (see Figure 2A and 2B). Bland-Altman analysis between the means of fSADTLC and fSADPRM showed minimal bias of 0.37 and 0.58 in SPIROMICS (see Figure 2C) and COPDGene (see Figure 2D), respectively.
Figure 1:
Spatial distribution of fSADPRM and fSADTLC, shown on mid-coronal slices from three different individuals with varying degrees of small airways disease. The first and the second columns indicate chest CT scans at total lung capacity (TLC) and residual volume (RV), respectively. The TLC and RV scans were used to compute fSADPRM. The third column shows the virtual or synthetic RV scans generated from the TLC chest CT scan alone. fSADTLC was computed using the TLC scan and the virtual RV scan, which allowed for a single-volume estimation of fSAD from TLC alone.
Figure 2:
Relationship between fSADTLC with fSADPRM in both (A and C) SPIROMICS (n = 1458) and (B and D) COPDGene (n = 458) cohorts through scatter plots and Bland-Altman analysis. Pearson’s correlation, R is also reported for both cohorts.
Lung Function and Respiratory Morbidity Predicted by fSADTLC
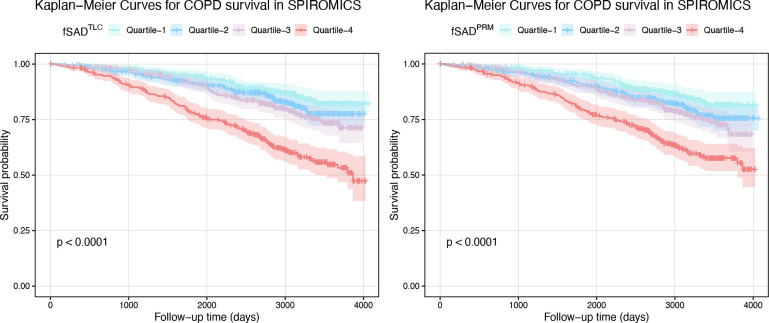
Univariate regression analysis suggested significant association of fSADTLC with lung function and respiratory morbidity in SPIROMICS and COPDGene cohorts (see Tables E2 and E3). On multivariable analysis, fSADTLC was significantly associated with lung function measures in SPIROMICS: postbronchodilator FEV1 (L) (adjusted β = −0.034, 95% CI: −0.037, −0.031; P < 0.001) and postbronchodilator FEV1/FVC (adjusted β = −0.008, 95% CI: −0.008, −0.007; P < 0.001) (see Table 2), independent of age, sex, race, BMI, smoking status, pack years, BMI, and percent emphysema. Similarly, fSADTLC was associated with FEV1 (L) (adjusted β = −0.032, 95% CI: −0.038, −0.027; P < 0.001) and FEV1 / FVC (adjusted β = −0.007, 95% CI: −0.008, −0.007; P < 0.001) in COPDGene (see Table 3). fSADTLC was also associated with SGRQ in SPIROMICS (adjusted β = 0.240, 95% CI: 0.127, 0.353, P < 0.001) and COPDGene (adjusted β = 0.190, 95% CI: 0.030, 0.350, P = 0.02). To compare fSADTLC with fSADPRM, we repeated the association studies for fSADPRM as well (see Tables 2 and 3). A Kaplan-Meier curve analysis revealed significantly increased rates of mortality (log rank P < 0.001) in individuals with increased fSADTLC (see Figure 3).
Table 2:
Multivariable linear regression analysis for assessing associations of baseline fSADTLC with lung function and respiratory morbidity in SPIROMICS (Estimate, 95% CI, P Value).
| fSADTLC | P Value | fSADPRM | P Value | |
|---|---|---|---|---|
|
| ||||
| Postbronchodilator FEV1, L | −0.034 (−0.037, −0.031) | P < 0.001 | −0.034 (−0.037, −0.030) | P < 0.001 |
| Postbronchodilator FEV1 / FVC | −0.008 (−0.008, −0.007) | P < 0.001 | −0.007 (−0.008, −0.007) | P < 0.001 |
| SGRQ | 0.240 (0.127, 0.353) | P < 0.001 | 0.177 (0.064, 0.290) | 0.002 |
| 6MWD, ft | −1.364 (−3.548, 0.819) | 0.22 | −1.887 (−4.057, 0.284) | 0.09 |
| mMRC Dyspnea Scale | 0.011 (−0.002, 0.024) | 0.10 | 0.01 (−0.003, 0.023) | 0.12 |
CI = confidence interval; fSAD = functional small airways disease; TLC = total lung capacity; FEV1 = forced expiratory volume in 1 second; FVC = forced vital capacity; SGRQ = St. George’s Respiratory Questionnaire; 6MWD = six-minute walk distance; mMRC = modified Medical Research Council. Multivariable models were adjusted for age, sex, race, smoking status, smoking pack years, body-mass index (BMI), baseline postbronchodilator FEV1, and percent emphysema define as percent LAA < −950 Hounsfield units (HU).
Table 3:
Multivariable associations of baseline fSADTLC with lung function, symptom burden, and exercise capacity in the COPDGene cohort (Estimate, 95% CI, P Value).
| fSADTLC | P Value | fSADPRM | P Value | |
|---|---|---|---|---|
|
| ||||
| Postbronchodilator FEV1, L | −0.032 (−0.038, −0.027) | P < 0.001 | −0.032 (−0.038, −0.026) | P < 0.001 |
| Postbronchodilator FEV1 / FVC | −0.007 (−0.008, −0.007) | P < 0.001 | −0.007 (−0.008, −0.006) | P < 0.001 |
| SGRQ | 0.190 (0.030, 0.350) | 0.02 | 0.131 (−0.043, 0.304) | 0.14 |
| 6MWD, ft | −2.211 (−5.755, 1.332) | 0.22 | −2.505 (−6.332, 1.321) | 0.20 |
| mMRC Dyspnea Scale | 0.004 (−0.023, 0.03) | 0.76 | −0.008 (−0.037, 0.021) | 0.60 |
CI = confidence interval; fSAD = functional small airways disease; TLC = total lung capacity; FEV1 = forced expiratory volume in 1 second; FVC = forced vital capacity; SGRQ = St. George’s Respiratory Questionnaire; 6MWD = six-minute walk distance; mMRC = modified Medical Research Council. Multivariable models were adjusted for age, sex, race, smoking status, smoking pack years, body-mass index (BMI), baseline postbronchodilator FEV1, and percent emphysema define as percent LAA < −950 Hounsfield units (HU).
Figure 3:
Kaplan-Meier curve analysis for studying overall survival in different quartiles of fSADTLC in both (A) SPIROMICS and (B) COPDGene cohorts.
Change in FEV1 and fSADTLC
In both SPIROMICS (β = −1.156, 95% CI: −1.699, −0.613; P < 0.001) and COPDGene (β = −0.866, 95% CI: −1.386, −0.345; P < 0.001), fSADTLC was significantly associated with change in FEV1 (mL / year) for all subjects (see Table 4). We conducted stratified linear regression analysis for GOLD 0 and GOLD 1 – 4 to investigate the impact of fSADTLC on FEV1 decline based on baseline airflow limitation (see Table 4). In both cohorts, fSADTLC was associated with FEV1 decline for GOLD 1 – 4 participants (see Table 4). In SPIROMICS, for every additional 1% increase in fSADTLC, a decline in FEV1 of 1.050 mL / year (P = 0.001) was observed for GOLD 1 – 4 participants. Similarly, in COPDGene, for a 1% increase fSADTLC, FEV1 declined by 1.175 mL / year (P = 0.003) (see Table 4).
Table 4:
Association between fSADTLC and change in FEV1 (mL / year) stratified by baseline COPD GOLD stage (Estimate, 95% CI, P Value).
| fSADTLC | P Value | fSADPRM | P Value | ||
|---|---|---|---|---|---|
|
| |||||
| SPIROMICS | Overall | −1.156 (−1.699, −0.613) | P < 0.001 | −0.742 (−1.280, −0.205) | 0.007 |
|
| |||||
| GOLD 0 | −2.994 (−5.900, −0.088) | 0.04 | −0.084 (−1.244, 1.077) | 0.89 | |
| GOLD 1 – 4 | −1.050 (−1.691, −0.408) | 0.001 | −0.899 (−1.598, −0.201) | 0.01 | |
|
| |||||
| COPDGene | Overall | −0.866 (−1.386, −0.345) | 0.001 | −0.871 (−1.440, −0.303) | 0.003 |
|
| |||||
| GOLD 0 | −0.230 (−1.760, 1.300) | 0.77 | −0.514 (−1.845, 0.817) | 0.45 | |
| GOLD 1 – 4 | −1.175 (−1.943, −0.406) | 0.003 | −1.067 (−1.934, −0.200) | 0.02 | |
CI = confidence interval; fSAD = functional small airways disease; TLC = total lung capacity; GOLD = Global Initiative for Obstructive Lung Disease; FEV1 = forced expiratory volume in 1 second. Multivariable models were adjusted for age, sex, race, smoking status, smoking pack years, body-mass index (BMI), baseline postbronchodilator FEV1, and percent emphysema define as percent LAA < −950 Hounsfield units (HU).
Repeatability of fSADTLC
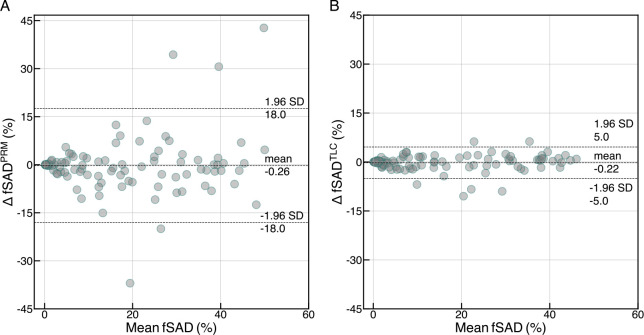
Over a short follow-up of 2 – 6 weeks, we observed fSADTLC to be highly repeatable with ICC of 0.99 (95% CI: 0.98, 0.99). This was significantly higher than the ICC of fSADPRM (0.83 (95% CI: 0.76, 0.88)). A Bland-Altman analysis between the fSADTLC and fSADPRM computed at visit 1 and visit 2 chest CT scans from the SPIROMICS Repeatability study revealed minimal bias for both fSADPRM (0.26) and fSADTLC (0.22) (see Figure 4A and 4B). The limits of agreement were significantly larger for fSADPRM (18%) as compared to fSADTLC (5%), suggesting greater overall variability in fSADPRM between 2 – 6 weeks follow-up.
Figure 4:
Repeatability study of (A) fSADPRM and (B) fSADTLC between baseline and 2 to- 6-week follow-up (n = 98) through Bland-Altman analysis.
Discussion
We aimed to determine whether generative AI can be used to derive fSAD from a single CT scan at TLC, and whether this single volume fSAD estimation correlates with clinical and functional outcomes in COPD. Traditionally, fSAD estimation has relied on an additional expiratory CT scan, a requirement that limits its clinical applicability (4, 10). We believe this is the first study to utilize a generative AI model for estimating fSAD from a single inspiratory CT scan. We demonstrated that, in two large cohorts, fSAD measured by TLC CT alone is associated with poor lung function, respiratory quality of life, and FEV1 decline.
A comparison of fSADTLC and fSADPRM suggested a strong linear relationship between the two measurements, supporting our hypothesis that SAD can be accurately measured from TLC CT using generative AI. During training, our model learned to transform TLC scans to RV scans from a large number of TLC and RV pairs. Exposing our model to a large training dataset improved virtual RV image generation, allowing us to capture SAD from TLC as accurately as fSADPRM. An equally strong relationship between fSADTLC and fSADPRM was observed in the COPDGene cohort suggested model generalizability to different cohort. For both cohorts, Bland-Altman analysis between the means of percent fSADTLC and fSADPRM showed minimal bias across the range of two measurements, and the limits of agreement, while not too narrow, were reasonable, suggesting that the two methods are generally consistent and could potentially be used interchangeably within an acceptable range.
We demonstrated that fSADTLC was significantly associated with FEV1 decline in both SPIROMICS and COPDGene cohorts. Our findings were consistent with those of Bhatt et al., who found similar associations of fSADPRM with FEV1 decline in COPDGene (16). Further, in individuals with established airflow limitation and mild-to-severe COPD (GOLD 1 – 4), fSADTLC was strongly associated with FEV1 decline independent of baseline percent emphysema. This suggested that there was an involvement of a small airways disease component at baseline contributing towards FEV1 decline. A consistency of these findings with fSADPRM indicated the potential of single chest CT volume fSADTLC as an alternative for characterizing disease progression in COPD. For both univariate and multivariable models, the direction of regression coefficients were consistent, and magnitudes were comparable between fSADTLC and fSADPRM. This suggested a consistent influence of unit change in fSADTLC and fSADPRM on the outcome variables further signifying an agreement between them.
An important part of this study was the repeatability analysis conducted using data short-term follow-up data from the SPIROMICS Repeatability study (14). The repeat scans over short follow-up prevented any long-term variabilities that might occur due to disease. fSADTLC was significantly more repeatable than fSADPRM, however, this was expected due to the potential for variations in patient effort between the repeat RV scans. Less common acquisition of RV scans in clinical practice results in inadequate coaching to expiratory volumes (RV and FRC), making them hard to reproduce. Since fSADPRM relies on RV scans, its repeatability is more dependent on adequate patient effort each time they are being scanned. Since expiration scans are thus harder to reproduce (14), this complicates the repeatability of fSADPRM. On the contrary, the proposed fSADTLC relies only on inspiratory chest CT scans, which are more easily reproducible and less prone to inadequate patient effort (17). These findings were also consistent with the results from the Repeatability Study where the dual volume CT biomarkers showed significantly lower ICC values compared to single volume metrics (14).
A few limitations of this study need to be recognized. Our spatial estimation of fSADTLC consistent with fSADPRM with a few differences in some isolated regions. Still, the major fSAD clusters were picked up by our method. We developed our model using scans from the SPIROMICS study that were acquired using a quality-controlled imaging protocol. This may not be true for the TLC scans acquired in clinical settings. It would be important to test our method in TLC scans with lower dosage and different acquisition protocols. We had to remove subjects with unreliable TLC or RV (volume difference < 1L) and acknowledge this as a quality control step in our analysis; fortunately, only 10% of the scans were considered unusable. For assessing change in FEV1, we were limited to only two lung function measurements separated by a follow-up of almost five years. Another limitation of our method was that all the scans were acquired without any contrast agent, and whether these results are reproducible in CT scans with contrast remains to be evaluated.
As our findings suggest, the current study has a number of strengths. Inspiratory CT scans are common in clinical settings, and our method allows for the detection of functional small airway disease by using them to create virtual expiratory scans. Large-scale characterization and phenotyping of individuals with small airways disease has been limited due to the fact that fSADPRM requires RV scans which are not routinely acquired in most settings. Also, retrospective evaluation of individuals with a TLC scan but no expiratory scan is not possible. The proposed method addresses these concerns by allowing an assessment of fSAD in patient cohorts with only TLC scans, an example being the National Lung Screening Trial (NLST) (19). Our method can also be used for the retrospective evaluation of large patient cohorts where expiratory chest CT scans were not acquired, an instance being the Multi-Ethnic Study of Atherosclerosis (MESA) (18). Also, this method has the potential to reduce radiation exposure to patients in future studies of small airway disease.
Supplementary Material
Acknowledgements
The authors thank the SPIROMICS participants and participating physicians, investigators, and staff for making this research possible. More information about the study and how to access SPIROMICS data is available at www.spiromics.org. The authors would like to acknowledge the University of North Carolina at Chapel Hill BioSpecimen Processing Facility for sample processing, storage, and sample disbursements (http://bsp.web.unc.edu/).
We would like to acknowledge the following current and former investigators of the SPIROMICS sites and reading centers: Neil E Alexis, MD; Wayne H Anderson, PhD; Mehrdad Arjomandi, MD; Igor Barjaktarevic, MD, PhD; R Graham Barr, MD, DrPH; Patricia Basta, PhD; Lori A Bateman, MSc; Surya P Bhatt, MD; Eugene R Bleecker, MD; Richard C Boucher, MD; Russell P Bowler, MD, PhD; Stephanie A Christenson, MD; Alejandro P Comellas, MD; Christopher B Cooper, MD, PhD; David J Couper, PhD; Gerard J Criner, MD; Ronald G Crystal, MD; Jeffrey L Curtis, MD; Claire M Doerschuk, MD; Mark T Dransfield, MD; Brad Drummond, MD; Christine M Freeman, PhD; Craig Galban, PhD; MeiLan K Han, MD, MS; Nadia N Hansel, MD, MPH; Annette T Hastie, PhD; Eric A Hoffman, PhD; Yvonne Huang, MD; Robert J Kaner, MD; Richard E Kanner, MD; Eric C Kleerup, MD; Jerry A Krishnan, MD, PhD; Lisa M LaVange, PhD; Stephen C Lazarus, MD; Fernando J Martinez, MD, MS; Deborah A Meyers, PhD; Wendy C Moore, MD; John D Newell Jr, MD; Robert Paine, III, MD; Laura Paulin, MD, MHS; Stephen P Peters, MD, PhD; Cheryl Pirozzi, MD; Nirupama Putcha, MD, MHS; Elizabeth C Oelsner, MD, MPH; Wanda K O’Neal, PhD; Victor E Ortega, MD, PhD; Sanjeev Raman, MBBS, MD; Stephen I. Rennard, MD; Donald P Tashkin, MD; J Michael Wells, MD; Robert A Wise, MD; and Prescott G Woodruff, MD, MPH. The project officers from the Lung Division of the National Heart, Lung, and Blood Institute were Lisa Postow, PhD, and Lisa Viviano, BSN; SPIROMICS was supported by contracts from the NIH/NHLBI (HHSN268200900013C, HHSN268200900014C, HHSN268200900015C, HHSN268200900016C, HHSN268200900017C, HHSN268200900018C, HHSN26820-0900019C, HHSN268200900020C), grants from the NIH/NHLBI (U01 HL137880 and U24 HL141762), and supplemented by contributions made through the Foundation for the NIH and the COPD Foundation from AstraZeneca/MedImmune; Bayer; Bellerophon Therapeutics; Boehringer-Ingelheim Pharmaceuticals, Inc.; Chiesi Farmaceutici S.p.A.; Forest Research Institute, Inc.; GlaxoSmithKline; Grifols Therapeutics, Inc.; Ikaria, Inc.; Novartis Pharmaceuticals Corporation; Nycomed GmbH; ProterixBio; Regeneron Pharmaceuticals, Inc.; Sanofi; Sunovion; Takeda Pharmaceutical Company; and Theravance Biopharma and Mylan.
This work was supported by NHLBI grants U01 HL089897 and U01 HL089856 and by NIH contract 75N92023D00011. The COPDGene study (NCT00608764) has also been supported by the COPD Foundation through contributions made to an Industry Advisory Committee that has included AstraZeneca, Bayer Pharmaceuticals, Boehringer-Ingelheim, Genentech, GlaxoSmithKline, Novartis, Pfizer, and Sunovion.
Funding Source:
This work was supported by NHLBI grants R01 HL142625, U01 HL089897 and U01 HL089856, by NIH contract 75N92023D00011, and by a grant from The Roy J. Carver Charitable Trust (19-5154). The COPDGene study (NCT00608764) has also been supported by the COPD Foundation through contributions made to an Industry Advisory Committee that has included AstraZeneca, Bayer Pharmaceuticals, Boehringer-Ingelheim, Genentech, GlaxoSmithKline, Novartis, Pfizer, and Sunovion.
References
- 1.Hogg JC, Macklem PT, Thurlbeck WM. Site and Nature of Airway Obstruction in Chronic Obstructive Lung Disease. N Engl J Med 1968;278:1355–1360. [DOI] [PubMed] [Google Scholar]
- 2.Yanai M, Sekizawa K, Ohrui T, Sasaki H, Takishima T. Site of airway obstruction in pulmonary disease: direct measurement of intrabronchial pressure. J Appl Physiol 1992;72:1016–1023. [DOI] [PubMed] [Google Scholar]
- 3.Koo H-K, Vasilescu DM, Booth S, Hsieh A, Katsamenis OL, Fishbane N, et al. Small airways disease in mild and moderate chronic obstructive pulmonary disease: a cross-sectional study. Lancet Respir Med 2018;6:591–602. [DOI] [PubMed] [Google Scholar]
- 4.Galbán CJ, Han MK, Boes JL, Chughtai KA, Meyer CR, Johnson TD, et al. Computed tomography–based biomarker provides unique signature for diagnosis of COPD phenotypes and disease progression. Nat Med 2012;18:1711–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vasilescu DM, Martinez FJ, Marchetti N, Galbán CJ, Hatt C, Meldrum CA, et al. Noninvasive Imaging Biomarker Identifies Small Airway Damage in Severe Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med 2019;200:575–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Comellas AP, Newell JD, Kirby M, Sieren JP, Peterson S, Hatt C, et al. Residual Volume versus FRC Computed Tomography Assessment of Functional Small Airway Disease in Smokers with and without Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med 2023;207:1536–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaudhary MFA, Gerard SE, Christensen GE, Cooper CB, Schroeder JD, Hoffman EA, et al. LungViT: Ensembling Cascade of Texture Sensitive Hierarchical Vision Transformers for Cross-Volume Chest CT Image-to-Image Translation. IEEE Trans Med Imaging 2024;43:2448–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Couper D, LaVange LM, Han ML, Barr RG, Bleecker E, Hoffman EA, et al. Design of the subpopulations and intermediate outcomes in copd study (SPIROMICS). Thorax 2014;doi: 10.1136/thoraxjnl-2013-203897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Regan EA, Hokanson JE, Murphy JR, Make B, Lynch DA, Beaty TH, et al. Genetic epidemiology of COPD (COPDGene) study design. COPD J Chronic Obstr Pulm Dis 2010;doi: 10.3109/15412550903499522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pompe E, Galbán CJ, Ross BD, Koenderman L, Ten Hacken NH, Postma DS, et al. Parametric response mapping on chest computed tomography associates with clinical and functional parameters in chronic obstructive pulmonary disease. Respir Med 2017;123:48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones PW. St. George’s Respiratory Questionnaire: MCID. COPD J Chronic Obstr Pulm Dis 2005;2:75–79. [DOI] [PubMed] [Google Scholar]
- 12.Mahler DA, Wells CK. Evaluation of Clinical Methods for Rating Dyspnea. Chest 1988;93:580–586. [DOI] [PubMed] [Google Scholar]
- 13.Ranstam J, Cook JA, editors. Kaplan–Meier curve. Br J Surg 2017;104:442–442. [DOI] [PubMed] [Google Scholar]
- 14.Motahari A, Barr RG, Han MK, Anderson WH, Barjaktarevic I, Bleecker ER, et al. Repeatability of Pulmonary Quantitative Computed Tomography Measurements in Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med 2023;208:657–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weir JP. Quantifying Test-Retest Reliability Using the Intraclass Correlation Coefficient and the SEM. J Strength Cond Res 2005;19:231. [DOI] [PubMed] [Google Scholar]
- 16.Bhatt SP, Soler X, Wang X, Murray S, Anzueto AR, Beaty TH, et al. Association between Functional Small Airway Disease and FEV 1 Decline in Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med 2016;194:178–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brown MS, Kim HJ, Abtin F, Da Costa I, Pais R, Ahmad S, et al. Reproducibility of Lung and Lobar Volume Measurements Using Computed Tomography. Acad Radiol 2010;17:316–322. [DOI] [PubMed] [Google Scholar]
- 18.Bild DE. Multi-Ethnic Study of Atherosclerosis: Objectives and Design. Am J Epidemiol 2002;156:871–881. [DOI] [PubMed] [Google Scholar]
- 19.National Lung Screening Trial Research Team. The National Lung Screening Trial: Overview and Study Design. Radiology 2011;258:243–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.