Abstract
Background:
Thrombocytopenia is common in pre-term neonates. Platelet transfusions are sometimes given to thrombocytopenic neonates with the hope of reducing the bleeding risk, however, there is little clinical data to support this practice and platelet transfusions may increase the bleeding risk or lead to adverse complications. Our group previously reported that fetal platelets expressed lower levels of immune related mRNA compared to adult platelets. In this study we focused on the effects of adult versus neonatal platelets on monocyte immune functions that may have an impact on neonatal immune function and transfusion complications.
Methods:
Using RNA-seq of post-natal day 7 (P7) and adult platelets, we determined age-dependent platelet gene expression. Platelets and naïve bone marrow isolated monocytes were co-cultured and monocyte phenotypes determined by RNA-seq and flow cytometry. An in vivo model of platelet transfusion in neonatal thrombocytopenic mice was used in which platelet deficient thrombopoietin receptor mutant mice (TPOR−/−) were transfused with adult or P7 platelets and monocyte phenotypes and trafficking were determined.
Results:
Adult and neonatal platelets had differential immune molecule expression, including Selp (P-selectin). Monocytes incubated with adult or neonatal mouse platelets had similar inflammatory (Ly6Chi), but different trafficking phenotypes, as defined by CCR2 and CCR5 mRNA and surface expression. Blocking P-selectin interactions with its its PSGL-1 receptor on monocytes limited the adult platelet induced monocyte trafficking phenotype, as well as adult platelet induced monocyte migration in vitro. Similar results were seen in vivo, when thrombocytopenic neonatal mice were transfused with adult or P7 platelets; adult platelets increased monocyte CCR2 and CCR5 as well as monocyte chemokine migration, while P7 platelets did not.
Conclusion:
These data provide comparative insights into adult and neonatal platelet transfusion regulated monocyte functions. The transfusion of adult platelets to neonatal mice was associated with an acute inflammatory and trafficking monocyte phenotype that was platelet P-selectin dependent and may have an impact on complications associated with neonatal platelet transfusions.
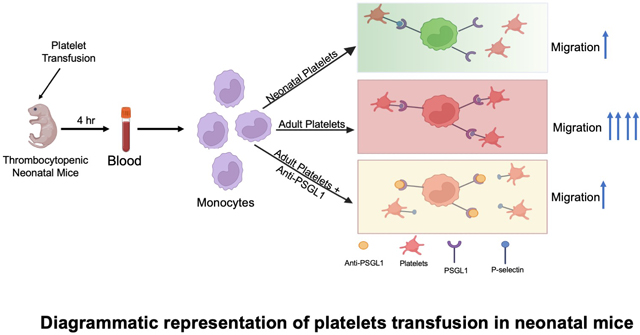
Graphical Abstract
INTRODUCTION
Thrombocytopenia is typically defined as a platelet count less than 150,000 platelets/μl of blood in humans and is common in preterm and low-birth-weight neonates, affecting 18–35% of neonatal intensive care unit (NICU) patients1. There are conflicting data on whether the degree of thrombocytopenia is associated with major bleeding risk in neonates, and on whether neonatal platelet transfusions increase or decrease the risk of a major bleeding episodes2–4. A recent study of 1221 preterm infants noted a lower platelet count was significantly associated with increased risks of hemorrhage and mortality2, but whether platelet transfusions are beneficial in neonates may be dependent on many factors. What is more clear is that multiple platelet transfusions to neonates is a risk factor for death5. The PlaNeT-2/MATISSE study brought more attention to neonatal platelet transfusions as it tracked what they defined as severe adverse events in addition to bleeding events and represents the largest randomized control trial of neonatal platelet transfusion thresholds to date6. As a primary outcome, PlaNeT-2/MATISSE found decreased survival without major bleeding in the high threshold transfusion group and noted that neonates who received platelet transfusions at the higher platelet threshold had increased rate of bronchopulmonary dysplasia (BPD), a potentially severe and long-term complication of platelet transfusion to neonates. Based on our past mouse model studies we speculated that neonatal platelet transfusion may be complicated by immune roles for platelets, since neonates are transfused with adult platelets.7, 8
Platelets are the cellular mediators of thrombosis and adhere to sites of vascular injury to prevent or stop bleeding. However, platelets also actively regulate innate and acquired immunity by delivering their immune messages through either direct/contact based or indirect/secreted molecule-based interactions with leukocytes9, 10. Platelets activate inflammatory pathways to provide early immune responses that are key in wound repair and preventing pathogen invasion at the sites of vascular injury9. In addition, persistent platelet and immune cell interactions initiate and exacerbate the development of chronic vascular diseases, such as atherosclerosis, that can lead to myocardial infarction11–13. Platelets also directly influence monocyte phenotypes, by secreting immune mediators that then effect the differentiation of monocytes to either an inflammatory or reparative phenotype7, 14. Platelets therefore serve both as cellular mediators of thrombosis and as immune cells, potentially complicating platelet transfusions, depending on the underlying cause of the thrombocytopenia and the immune/inflammatory context at the time of transfusion.
Our group has found numerous developmental differences between adult and neonatal platelets that may contribute to the complications associated with the transfusion of adult platelets into neonates8. Adult platelets express transcripts for many immune molecules at much higher levels compared to neonatal platelets, including Selp (P-selectin)8. These findings suggest that the transfusion of adult, ‘immune differentiated’, platelets to neonates may adversely alter the neonatal immune environment, thereby contributing to platelet transfusion complications.
We now demonstrate that adult and neonatal platelets differ in gene expression, particularly in the expression of immune-associated molecules that regulate monocyte phenotypes and functions. Using mouse platelet transfusion models, we found that the transfusion of adult versus neonatal platelets into thrombocytopenic neonatal mice resulted in more platelet-monocyte aggregates (PMAs) and increased monocyte trafficking. We propose that adult platelets promote a maladaptive trafficking of monocytes in neonates that may contribute to complications associated with the transfusion of adult platelets into neonates.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request. The authors have provided RNA-seq data as a publicly available GEO submissions (GSE225403 and GSE225401).
Animals
All mice used were on C57BL/6J background, except for the platelet RNA-seq in which ICR mice were used due to their larger size and greater blood volume. Both males and females were used in all studies. Platelet isolations and transfusions used littermates at a neonatal stage that is difficult to anatomically discern the sex. Therefore, all data and platelets represent pooled data from each sex to minimize data ambiguity. Platelets were isolated from P7 and 8–12 weeks old mice in this study. P14 TPOR−/− mice were used as platelet transfusion recipients. To isolate neonatal platelets, P7 pups were euthanized and decapitated to collect blood into heparinized Tyrodes buffer in a small petri dish as described8. For adult platelets, 8–12 weeks old mice were bled retro-orbitally and blood was collected in heparinized Tyrodes and platelets isolated as prior published7. For transfusions we infused 1X108 platelets into P14 old TPOR−/− mice via a retro-orbital route. Mice were randomly assigned to treatment groups. All animal studies were approved by the University of Rochester Institutional Animal Care and Use Committee. Animal diets are from Lab Diet (PicoLab® Rodent Diet 20, 5053). The major component in the diet is Crude Protein (21%). Males and females were used in approximately equal numbers with platelet donors and recipients randomly selected without sex consideration. All data are representative of both male and female mice.
Post-transfusion blood was collected into EDTA coated mini-tubes (Greiner Bio One) 4 hrs after platelet transfusion. Blood was centrifuged at 850 rcf for 10 mins and plasma was collected and stored at −80°C for ELISA. The buffy coat layer was isolated to assess monocytes by flow cytometry.
Enzyme-Linked Immunosorbent Assay
ELISAs were performed using either plasma samples or cell culture supernatants and ELISA kits included CCL2 DuoSet ELISA (Thermo Fisher Scientific, DY47905), mouse CXCL1 DuoSet ELISA (Thermo Fisher Scientific, DY45305) and b2M ELISA (LSBio, LS-F14141). FLUOstar OPTIMA software (BMG LABTECH) was used to determine the optical density and MyAssay for ELISA data analysis.
Cell culture
Primary monocytes were isolated from the bone marrow of mouse femurs and tibias. Bone marrow was flushed with buffer (1mM EDTA and 2% FBS in PBS; Thermo fisher scientific) using a 20 gauge needle. RBCs were lysed with ACK lysis buffer and cells passed through a 100 μm mesh strainer. EasySep™ mouse monocyte isolation kit (STEMCELL Technologies, 19861) was used to isolate primary monocytes. Monocytes were cultured in DMEM Glutamax media supplemented with 10% FBS, 1 mM sodium pyruvate, non-essential amino acid and 100 U/ml penicillin/streptomycin. Monocytes were treated with platelet releasates or platelets at a ratio of 20 platelets for every 1 monocyte. For isolation of platelets, Tyrode containing blood was centrifuged at 100 rcf for 10 mins and the upper layer (without RBCs) was transferred into a different tube. 1 μM PGI2 was added into the platelets containing tubes and centrifuged at 600 rcf for 5 minutes. Platelet pellets were resuspended in cell culture media. To make platelet releasate, platelets were activated with thrombin (1 U/ml) for 20 minutes and thrombin neutralized with an equal concentration of hirudin (1 U/ml). Platelet releasate was collected after centrifugation and used for the treatment of primary mouse monocytes.
qPCR
Monocytes were washed with PBS and lysed in RLT lysis buffer. RNA isolation was performed by RNeasy mini kit (Qiagen). NanoDrop™ 2000 (Thermo Fisher Scientific) was used to measure the concentration of RNA and cDNA conversion was performed with a high-capacity RNA to cDNA kit (Applied Biosystem). Real time PCR was executed using TaqMan Gene Expression Master Mix Protocol and a CFX Connect Real-Time PCR Detection System (Bio-rad Thermocycler). TaqMan gene expression primer (Thermo Fisher Scientific, Ccl2 (Mm00441242_m1), Ccr2 (Mm99999051_gH), Ccr5 (Mm01963251_s1) and Gapdh (Mm99999915_g1)) was used for the cDNA amplification in this study. The qPCR steps consist of 3 steps: Initiation (95 °C for 10 minutes), denaturation (95 °C for 15 sec) and annealing/extension (60 °C for 1 minute). Amplification consists of denaturation and annealing steps and in this study, target cDNA was amplifying for 40 cycles. Data analysis was done using calculation for fold change 2(−ΔΔCT) with the housekeeping gene Gapdh. Samples were normalized with control and fold change value reported.
Cell Migration study
In vitro monocyte migration assays were performed in trans-well chambers that were 5.0 μm diameter pore size polycarbonate membranes. Monocytes (1X105) were treated with neonatal and adult platelets as well as IgG or PSGL-1 blocking Ab and seeded into upper chamber. CCL2 (200 nM) or RANTES (200 nM) were used as chemoattractant in lower chamber. After 24 hr, non-migrating cells were wiped out of the top chamber with cotton top applicator and polycarbonate membranes (containing migrated cells) were washed with PBS and fixed with 4% formaldehyde for 10 minutes. Migrated cell staining was performed with 1% Crystal violet solution for 20 minutes. Images were taken under 200X magnification using BX51 upright microscope (Olympus). Image J software were used for the quantification of migratory cell number.
For in vivo migration P14 TPOR−/− mice were used. Mice were given adult or neonatal platelet transfusions retro-orbitally and CCL2 used as a chemoattractant by intraperitoneal injection following platelet transfusion. Peritoneal lavage was collected 24 hrs later. Cells were collected, centrifuges and resuspended for flow cytometry. Data were analyzed using Flow Jo software. Anti-mouse PSGL-1 (Cat number-BE0186, 20mg/kg delivered i.v.) and IgG (BP0091) were purchased from Bio X cell. Anti-mouse CD18 (BE0009, Clone-M18/2) was also purchased from Bio X Cell.
RNA-seq
P7 and adult platelet mRNA was isolated using our established methods8 that included the depletion of non-platelet cells using Ter119, CD9, CD19, Gr-1 antibodies and magnetic cell separation (Milteny Biotec) before RNA preparation. Bone marrow monocytes isolated by negative selection kit were treated with neonatal or adult mouse platelets for 4 hr. Total mRNA isolation was performed using RNeasy mini kits (Qiagen kit). To digest genomic DNA, mRNA were treated with DNase and processed for RNA-seq with TruSeq Stranded mRNA library preparation. RNA-seq analysis was performed by University of Rochester Genomic Research Core and R-4.0.2 was used for data normalization and differential expression analysis. The heatmap was generated using pheatmap v1.0.12, using the significantly differentially expressed genes within the specific comparison. PCA was performed using the r-log normalized counts values with pcaExplorer v2.20.0. EnrichR GSE was performed on the significantly up or down expressed genes for a given comparison using EnrichR v3.0. Inclusion or exclusion criteria are described within the Enrichr results section. We mapped against KEGG_2019, GO_Biological_Process_2018, WikiPathways_2019, and ChEA_2016. More information regarding the statistical test Enrichr uses can be found at: https://maayanlab.cloud/Enrichr/help#background.
Flow cytometry
For in vitro study, mouse primary monocytes were treated with platelets releasates or intact platelets for 4hr. Monocytes were washed and incubated with anti-mouse CD11b, LY6C, CCR2, CCR5 and CD41 antibodies for 30 minutes. Cells were washed and fixed with 1% formalin and data was acquired on LSR II flow cytometry machine.
For in vivo platelets transfusion study, whole blood was collected into EDTA coated tube, plasma was isolated and the buffy coat isolated and prepared for the flow cytometry following RBCs lysing in ACK lysis buffer. Samples were then incubated with anti-mouse CD45, CD11B, LY6G, LY6C, CCR2, CCR5 and CD41 antibodies. Cells were stained for 30 minutes and washed with PBS twice. Cells were fixed in 1% formalin and monocytes were gated as CD45+, CD11B+, LY6G− and LY6C+ cells. BD FACS LSR-II (4 laser 18 color fluorochrome, BD Biosciences) with FACS Diva was used for data acquisition. All the data were analyzed using FlowJo software (version 10.7.1).
Human Plasma Samples
Cord blood plasma and healthy control adult plasma were collected using established protocols15 approved by the University of Rochester Research Subject Review Board (STUDY00000729 and STUDY00000404). Cord blood was from 7 males and 6 females, 9 were greater than 38 weeks of gestation and 4 greater than 25 weeks of gestation. 8 were white, 2 black/African American and the remaining other racial background. Healthy adult donors were de-identified from within our medical center population with no known disease history and no use of anti-platelet or anti-inflammatory drugs.
Data analysis
Flow cytometry data analysis was performed using Flowjo version 10.7.1 software and GraphPad Prism used for further statistical analysis. RT-PCR data were also analyzed using GraphPad Prism. BMG Fluostar OPTIMA was used for acquiring ELISA optical density acquisition and MyAssay used for analysis.
GraphPad Prism (GraphPad Prism software, La Jolla, CA, USA) version 8.0 was used for all the statistical analysis. A Shapiro Wilk test was performed to check the normality of data when n>=6. For data that passed the normality test, and had more than two groups, a one-way ANOVA was used with a Bonferroni’s multiple comparison correction test. If the data sets did not pass the normality test, Kruskal-Wallis test with Dunn’s multiple comparison correction was performed. For two groups comparison, student t-test was used. P values equal or less than 0.05 was considered as statistically significant. All the data represent biological replicates.
Results
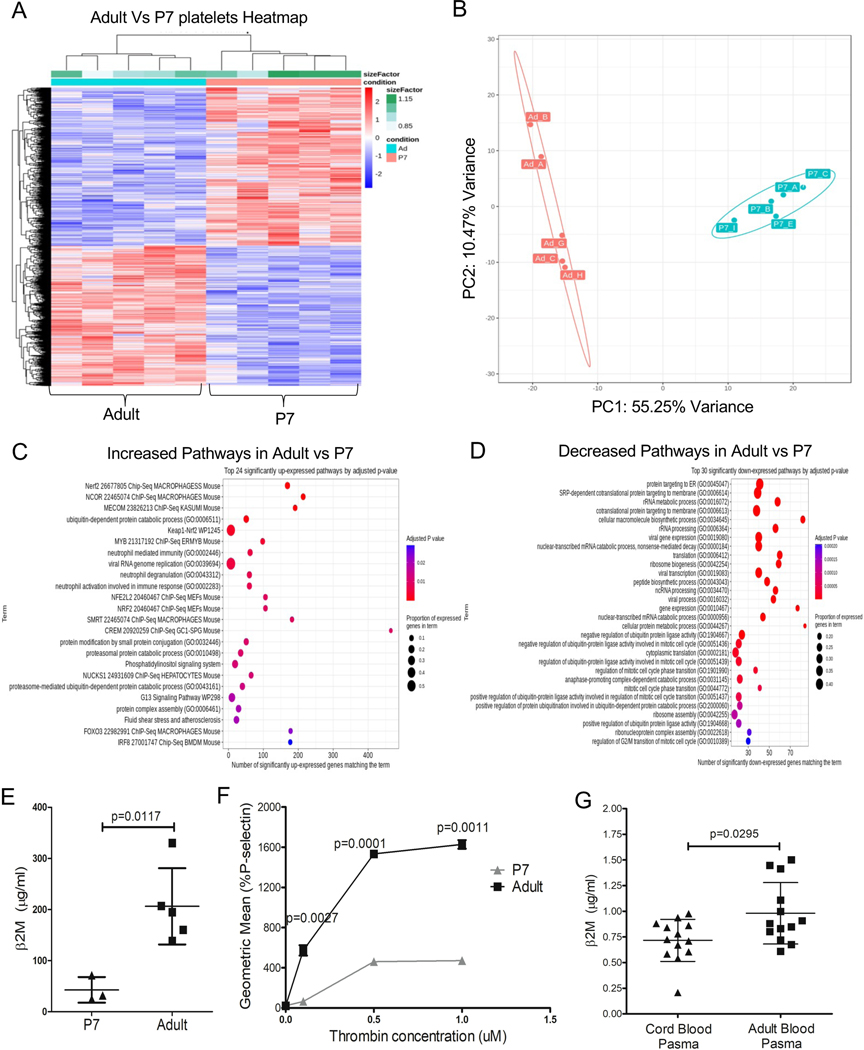
Past work by our group has characterized adult and fetal platelet transcripts in mice and found that adult platelets were enriched with immune transcripts compared to fetal platelets8. To determine whether neonatal and adult platelets also have differential mRNA expression, we isolated mRNA from adult and post-natal day 7 (P7) platelets and profiled their global gene expression by RNA-seq. Neonatal and adult platelets had differential mRNA expression (Figure 1A–B), including enrichment of adult platelets for ubiquitin/proteasome and immune related pathways (Figure 1C–D). Specific immune related genes with greater expression in adult platelets included Selp, B2m, Ppbp, Il1a, Il7, Cxcl3, and Cxcl5 (Table S1). To validate some of the mRNA findings, we measured neonatal and adult plasma β2-microglobulin (β2M) and platelet P-selectin (P-sel). Adult mouse plasma had more β2M compared to P7 plasma as determined by ELISA (Figure 1E), consistent with our past studies demonstrating that platelets are the major source of plasma β2M7, 14. We found significantly more P-selectin expression on thrombin-activated adult platelets compared to neonatal platelets (Figure 1F). This phenocopies stimulated human neonatal platelets that, like mouse neonatal platelets, expressed less stimulation induced P-selectin compared to adult platelets16. We also quantified β2M in human cord blood plasma (represents the fetal blood supply) and adult peripheral blood plasma using ELISA, and found that adult plasma contained significantly more β2M compared to cord blood plasma (Figure 1G). These data indicate that neonatal and adult platelets have many differentially expressed immune molecules.
Figure 1.
Adult and neonatal mouse platelets have different immune molecule expression. A-D) P7 and adult platelet mRNA were isolated for RNA-seq. A) Heat Map and B) PCI plot of platelet mRNA expression demonstrated differential mRNA expression. C-D). Pathway analysis. Adult platelets were enriched for immune related transcripts compared to P7 platelets. E-G) Validation of RNA-seq. E) P7 and adult mouse plasma β2M were measured by ELISA. Adult plasma had more β2M compared to P7 plasma. F) Adult and P7 platelets were stimulated with thrombin and surface P-selectin determined by flow cytometry. Adult platelets had more P-selectin compared to P7 platelets. G) Human cord blood plasma had less β2M compared to adult blood plasma as determined by ELISA (mean ± SEM, Student t-test).
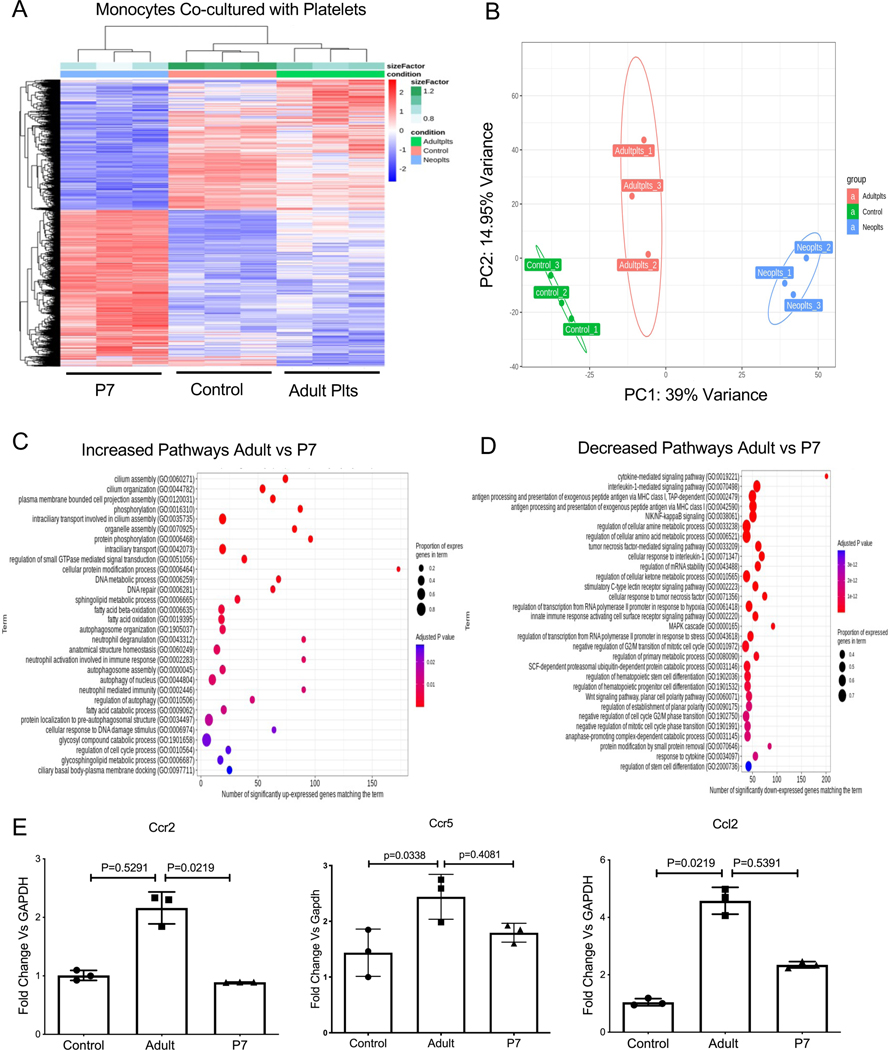
Platelet interactions with monocytes influences monocyte phenotypes7, 14. Given the immune molecule expression differences, we determined the effect that adult and P7 platelets have on monocytes. Naïve bone marrow monocytes (BMM) were isolated by negative selection and incubated with control buffer, adult platelets, or P7 platelets for 4 hrs to model immediate post-platelet transfusion conditions in vitro. Monocytes were then washed to remove platelets and monocyte RNA-seq performed. P7 and adult platelets induced different monocyte gene expression patterns (Figure 2A–B, Table S2). Adult platelets upregulated gene expression pathways related to monocyte inflammatory responses and cilium assembly, a major regulator of cell cycle and cell migration17 (Figure 2C). Down-regulated signaling pathways in adult vs neonatal platelet treated monocytes included interleukin-1 (IL-1)-mediated signaling, antigen processing and presentation via MHC class I, NIK-NF-kb signaling pathways, and the regulation of cellular amino acid metabolic processes (Figure 2D). To validate the RNA-seq data and explore the physiologic effects of adult and P7 platelets on monocyte trafficking responses, we incubated monocytes with control buffer, adult platelets, or P7 platelets for 4 hr, and determined the expression of Ccr2, Ccr5, and Ccl2 by qRT-PCR. Adult platelets preferentially induced the expression of Ccr2, Ccr5 and Ccl2 (Figure 2E), however adult and P7 platelets induced similar CCL2 protein production over 48 hrs in culture (Figure S1). These data indicate that adult and neonatal platelets may differentially regulate trafficking associated receptors in monocytes.
Figure 2.
Adult and P7 Platelets Induce Different Monocyte Phenotypes. A-D) Mouse BMM were co-incubated with adult or P7 mouse platelets for 4 hrs and mRNA expression was assessed by RNA-seq. A) Heat Map, and B) PCI of monocyte mRNA indicated that platelets induced monocyte gene expression in an age dependent manner. C-D) Top 30 monocyte gene expression pathways C) upregulated and D) downregulated by adult vs P7 platelets. E) Confirmation of RNA-seq. Monocytes were co-incubated with adult or P7 platelets and 4 hrs later gene expression was determined by qRT-PCR. Adult platelets increased Ccr2, Ccr5, and Ccl2 more than P7 platelets (mean ± SEM, 1-way ANOVA Kruskal-Wallis test with Dunn’s multiple comparison ).
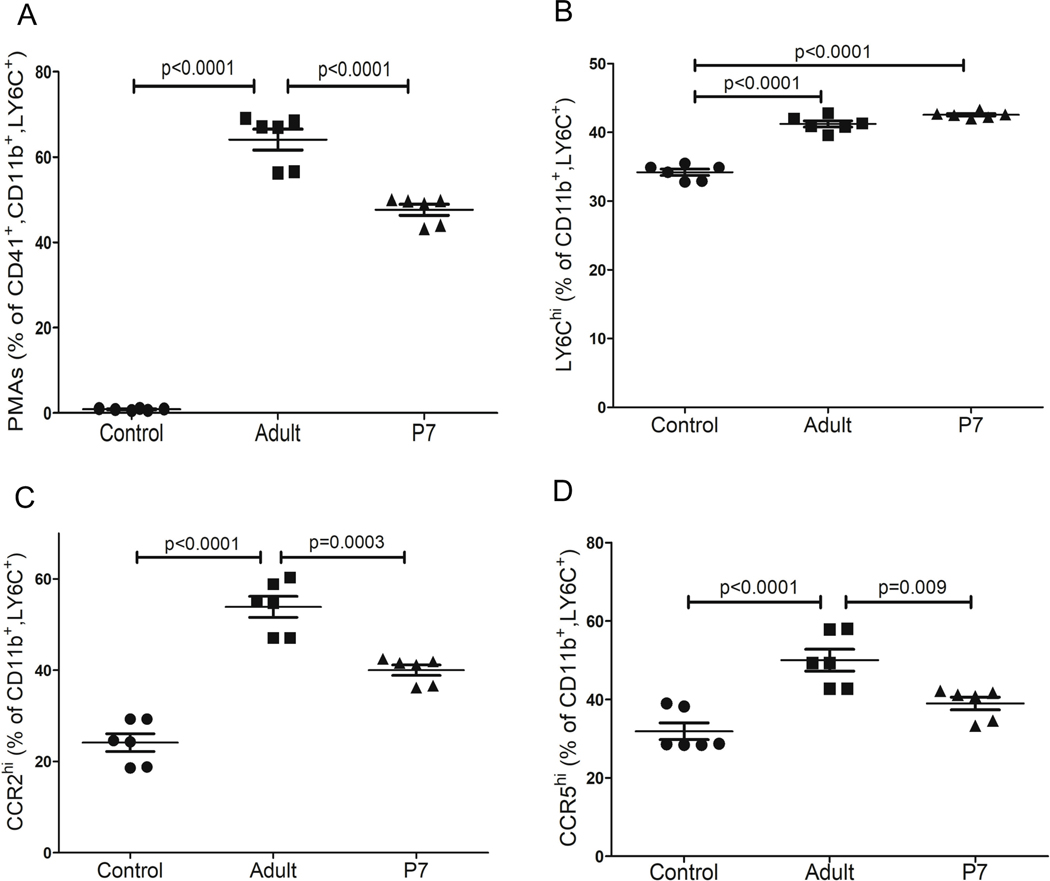
Platelet transfusions in general are associated with an increased risk of adverse immune/inflammatory complications18, 19. Providing neonates with adult platelets that are ‘immune mature’ may contribute to maladaptive monocyte responses at an early stage of post-natal immune and tissue development. To explore this hypothesis, we incubated naïve adult bone marrow monocytes with adult or P7 platelets and after 4 hrs we assessed platelet-monocyte aggregates (PMAs) and monocyte immune phenotypes. PMAs, identified as CD41-positive monocytes, were significantly greater in adult platelet-monocyte co-cultures compared to P7 platelet co-cultures (Figure 3A). Monocyte inflammatory differentiation was assessed by Ly6C expression. Adult and P7 platelets similarly increased Ly6Chi monocytes (Figure 3B). Since our RNA-seq data indicated that adult platelets upregulated gene pathways associated with cell migration, we assessed the trafficking monocyte population by CCR2 and CCR5 surface expression. Monocytes treated with adult platelets had increased surface expression of CCR2 (Figure 3C) and CCR5 (Figure 3D) compared to P7 platelet co-cultures. These data demonstrate that adult platelets, compared to neonatal platelets, increased the trafficking phenotype of monocytes.
Figure 3:
Adult platelets induce a trafficking monocyte phenotype in vitro. Primary mouse BMM were co-incubated with adult or P7 platelets and 4 hrs later monocyte CD41, Ly6C, CCR2 and CCR5 expression were determined. A) Adult platelets formed significantly more PMAs compared to P7 platelets, but B) induced similar inflammatory monocyte phenotypes. C-D). Adult, but not P7, platelets induced a trafficking monocyte phenotype. Adult platelets increased C) CCR2 and D) CCR5 expression compared to P7 platelets (mean ± SEM, 1-way ANOVA with Bonferroni correction).
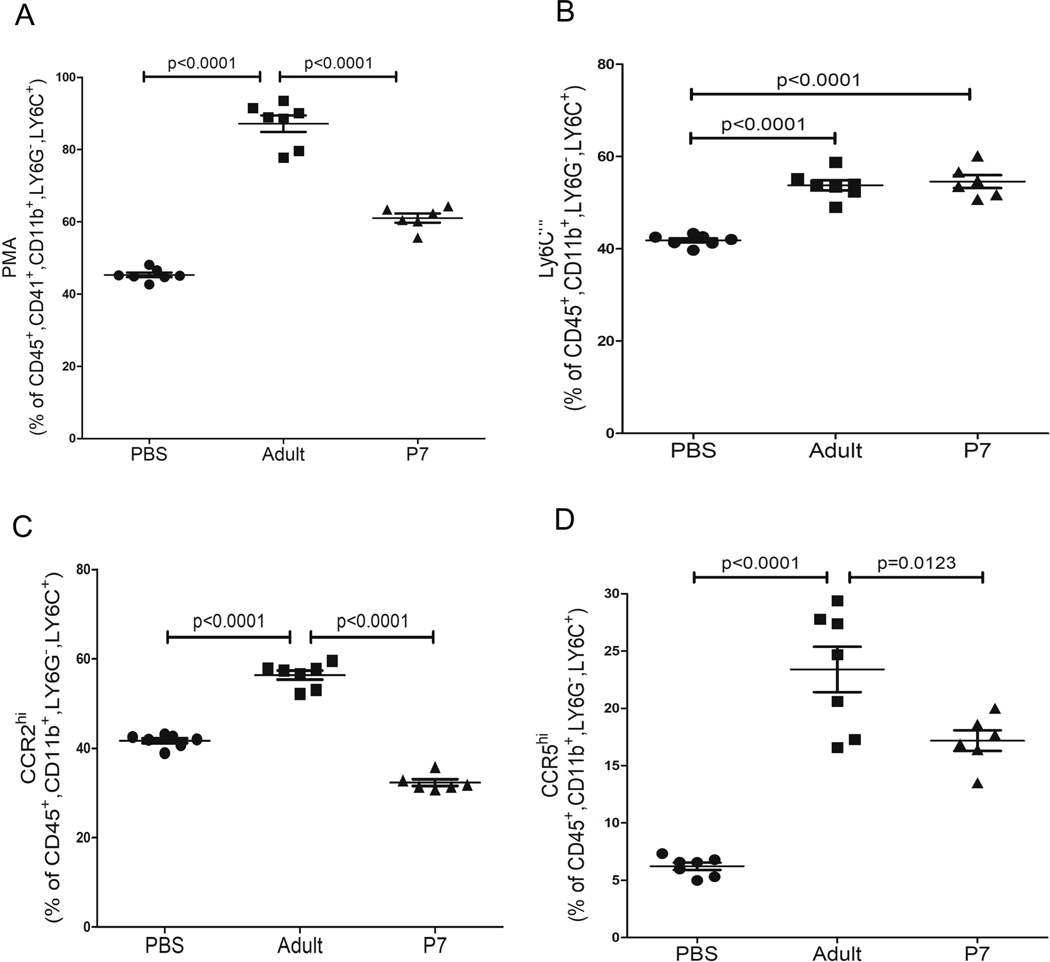
To determine the effect of adult and neonatal platelet transfusions on monocytes in vivo, we transfused platelets from adult (8–12 weeks old) mice or P7 mice into P14 platelet deficient TPOR−/− mice to better recapitulate platelet transfusions into thrombocytopenic neonatal recipients. 2 and 24 hrs after platelet transfusions, blood was collected from control or platelet transfused TPOR−/− mice. Complete blood counts (CBC) indicated that both adult and P7 platelet transfusions similarly increased platelet counts, and adult and P7 platelets remained in the circulation (Figure S2). The effects of adult versus P7 platelet transfusions on monocytes were determined 4hr after platelet transfusion. Transfusion of adult platelets significantly increased PMAs compared to neonatal platelet transfusions (Figure 4A). Ly6Chi monocytes were increased significantly both by adult and neonatal platelet transfusions (Figure 4B). However, trafficking monocytes typified by increased CCR2 (Figure 4C) and CCR5 (Figure 4D) were significantly increased in mice transfused with adult compared to neonatal platelets. Taken together, these data indicate that the transfusion of adult platelets to neonatal mice induced a trafficking monocyte phenotype that may lead to immune dysfunction.
Figure 4.
Adult, but not P7 platelet transfusions, induced a trafficking monocyte response in vivo. P14 TPOR−/− mice were transfused with PBS, adult platelets, or P7 platelets and 4 hrs later blood was collected. A) Circulating PMAs were quantified by flow cytometry and adult platelet transfusions formed more PMAs in vivo. B) Adult and P7 platelets both induced an inflammatory monocyte phenotype (Ly6Chi). C-D) Adult, but not P7, platelet transfusions induced a trafficking monocyte phenotype including increased C) CCR2 and D) CCR5 expression (mean ± SEM, 1-way ANOVA with Bonferroni correction).
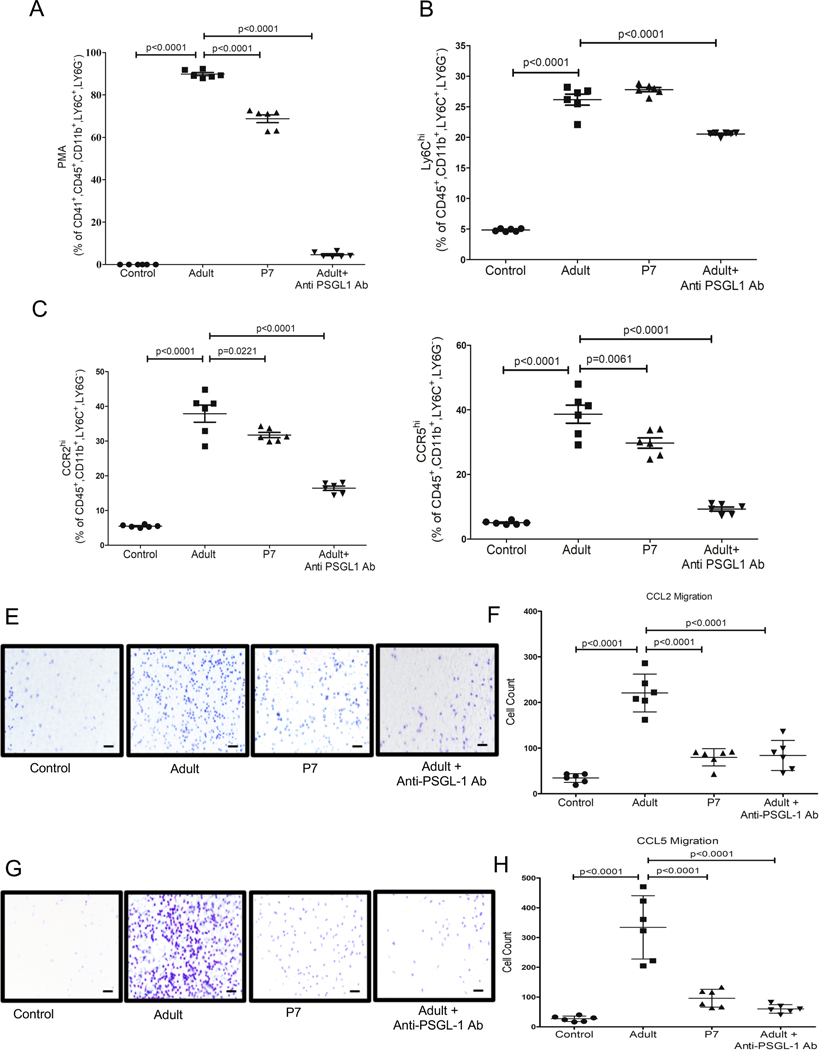
Major mediators of platelet - monocyte adhesion include interactions between PSGL-1 and Mac-1 on monocytes with P-selectin (P-sel/CD62P) and GPIbα or αIIbβ3 respectively on platelets9. We asked whether the altered monocyte trafficking phenotype mediated by adult platelets is contact dependent by incubating monocytes with adult or P7 platelet releasates. Consistent with our past studies, adult platelet releasates increased monocyte Ly6C expression7, as did P7 platelet releasates (Figure S3A). Neither adult, nor P7, platelet releasates increased monocyte CCR2, but adult platelets increased CCR5 slightly (Figure S3B–C). These data suggest that the adult platelet-mediated monocyte trafficking phenotype is in part contact dependent. To test this, we added blocking antibodies targeting P-sel, PSGL-1 or Mac-1 to platelet-monocyte co-cultures. Monocytes treated with adult platelets in the presence of anti-PSGL-1 antibody had a significant reduction in PMAs compared to adult platelet only co-cultures (Figure 5A). PSGL-1 blocking antibody did not change Ly6C expression, indicating that the platelet-mediated inflammatory phenotype is not dependent on PSGL-1 (Figure 5B). However, PSGL-1 blocking greatly limited adult platelet-induced CCR2 and CCR5 (Figure 5C and D). In contrast, Mac-1 blocking antibody had little effect on PMAs or adult platelet-mediated CCR2 expression, but did limit CCR5 expression somewhat (Figure S4). After 4 hrs, platelets that were not adherent to monocytes had some evidence of low-level activation in the culture conditions (Figure S5). There were however increased numbers of P-selectin positive monocytes in co-culture with adult platelets, but similar PF4 in the media, further indicating platelet P-selectin dependent monocyte adhesion rather than differences in platelet activation in the culture conditions that may drive the monocyte phenotype (Figure S5). These data indicate that P-selectin and PSGL-1 interactions are major drivers of the adult platelet mediated trafficking phenotype of monocytes that are not induced by neonatal platelets due to their low level of P-selectin expression.
Figure 5:
P-selectin-PSGL1 interactions mediate adult platelet induced trafficking monocyte phenotype. Adult or P7 platelets were co-incubated with monocytes with IgG or anti-PSGL1 Ab. 4 hrs later PMAs, Ly6C, CCR2 and CCR5 expression were determined. A) Adult platelet associated PMAs were reduced and B) Ly6C was unchanged by anti-PSGL1 Ab. C-D) Adult platelets increased CCR2 and CCR5 compared to P7 platelets, which was decreased by anti-PSGL1 antibody. E-H) Monocyte trafficking in vitro. Adult or P7 platelets and monocytes were co-cultured in the top of a transwell chamber with control IgG or anti-PSGL1. CCL2 or CCL5/RANTES were placed in the bottom chamber and monocyte migration determined. E-F) Adult platelets induced monocyte trafficking to CCL2 which was decreased by anti-PSGL1 Ab. E) Representative Image and F) Quantification. G-H) Adult platelets induced monocyte trafficking to CCL5, which was reduced by anti-PSGL1 Ab. G) Representative Image and H) Quantification (mean ± SEM, 1-way ANOVA with Bonferroni correction). Scale bar =200 μm.
We next assessed whether adult platelet-induced expression of CCR2 and CCR5 on monocytes led to differential cell trafficking towards their chemokine ligands in vitro. Monocytes were incubated either alone, with adult platelets, or with P7 platelets in the upper transwell chamber, and either CCL2 (CCR2 ligand, Figure 5E–F) or CCL5/RANTES (CCR5 ligand, Figure 5G–H) were placed in the lower chamber as a chemoattractant. Monocyte migration towards each chemokine was increased by co-incubation with adult platelets compared to P7 platelets. In addition, anti-PSGL-1 antibody decreased adult platelet-induced migration of monocytes towards CCL2 (Figure 5E–H). From these data we conclude that adult platelet interactions with monocytes induced monocyte trafficking at least in part through a P-selectin and PSGL-1 dependent manner.
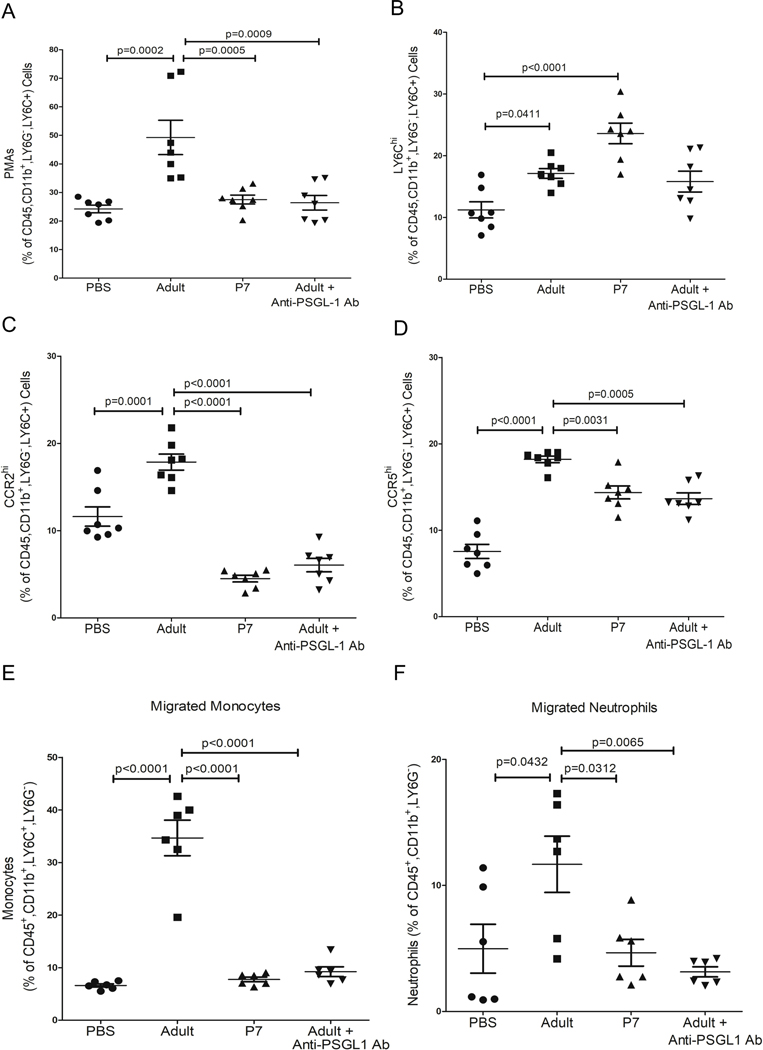
We next assessed transfusion-mediated monocyte trafficking in vivo using our platelet transfusion model. Transfusion-recipient mice were pre-treated with either control IgG or anti-PSGL-1 antibody, then transfused with either PBS, adult platelets or P7 platelets, and blood was collected 4hr later. Anti-PSGL-1 antibody treatment significantly reduced PMAs in mice transfused with adult platelets (Figure 6A) and reduced the expression of Ly6C on circulating monocytes (Figure 6B). Notably, monocyte CCR2 and CCR5 were also reduced with PSGL-1 blocking antibody (Figure 6C–D). We next asked whether transfusion of adult platelets led not only to an increase in the trafficking phenotype of monocytes, but also to an increase in their in vivo trafficking. Mice were given PBS or platelet transfusions and anti-PSGL-1 antibody. 4 hr later mice were treated with intraperitoneal CCL2 as a chemo-attractant. 24 hrs after CCL2, peritoneal lavage was performed to quantify monocyte lineage cells in the peritoneum. Transfusion with adult, but not P7, platelets increased the number of monocytes in the peritoneal-lavage in response to CCL2, as well as the number of neutrophils (Figure 6E–F). We also determined the plasma concentrations of CXCL1 and CCL2 in platelet transfused mice as systemic inflammatory markers. P7 platelet transfusions increased induced plasma CXCL1 and CCL2 compared to adult platelet transfusions (Figure S6), likely due to non-monocyte contact-dependent effects in the neonate, or a lack of negative feedback from CCR2 signaling. These data further emphasize that platelet transfusion related monocyte trafficking is dependent on a receptor rather than ligand-dependent effect on monocytes.
Figure 6:
P-selectin-PSGL1 mediated monocyte trafficking in vivo. P14 TPOR−/− mice were transfused with adult or P7 platelets in the presence IgG or anti-PSGL-1 Ab. 4hrs later A) CD41, B) Ly6C, C) CCR2 and D) CCR5 expression were determined. Adult platelet induced changes were inhibited by anti-PSGL-1 Ab. E-F) Mice were also transfused and treated with the CCR2 ligand CCL2 via an IP injection 4 hrs after platelet transfusions. 24 hrs later the number of migrated E) monocytes and F) neutrophils was determined by peritoneal lavage and flow cytometry. Adult platelet transfusions greatly increased monocyte and neutrophil peritoneal migration, which was blunted by anti-(mean ± SEM, 1-way ANOVA with Bonferroni correction).
Discussion
The transfusion of adult platelets to thrombocytopenic neonates may do little to prevent bleeding and may worsen outcomes. Because platelets have roles in both thrombosis and immune responses, the clinical outcome may be dependent on the tissue and inflammatory environment at the time of platelet transfusion. Neonates are not given age-matched platelet transfusions, so we asked whether adult and neonatal platelets in the murine system differ in gene expression and whether those differences may contribute to the platelet transfusion immune responses in a neonatal mouse model. We found that adult platelets preferentially expressed multiple immune-related genes, including Selp, compared to neonatal platelets. P-selectin mediates the physical interactions of platelets with leukocytes expressing PSGL-1, and the physical act of collecting and transfusing platelets can result in some platelet activation and P-selectin externalization. The transfusion of neonatal mice with adult platelets resulted in more PMAs compared to P7 platelet transfusions, consistent with enhanced platelet – monocyte interactions that may lead to immune dysfunction. The in vitro incubation of adult versus neonatal platelets with monocytes for only 4 hrs led to enhanced expression of CCR2 and CCR5 on monocytes, suggesting upregulation of a cell trafficking phenotype. It should be noted that a limitation of these in vitro studies is that our culture systems used adult monocytes. Although our in vitro data are largely recapitulated in vivo, future systems to isolate sufficient neonatal monocytes for our studies would add mechanistic value.
Our in vivo studies in a mouse model of neonatal thrombocytopenia also indicated that the transfusion of adult, but not P7 platelets to neonates induced a trafficking phenotype in circulating monocytes. Importantly, this occurred in a platelet P-selectin-dependent manner. These findings suggest that adult platelet-mediated monocyte trafficking may contribute to a more ‘immune primed’ environment in neonates and has the potential to contribute to adverse outcomes in the face of tissue lesions or infections at the time of transfusion. Our data also revealed that adult platelet mediated effects are contact dependent and that P7 platelet transfusions induced more cytokine responses in vitro, suggesting that platelet augmented monocyte trafficking is dependent on monocyte receptor expression rather than ligand production. Therefore, therapies to limit direct interactions between platelets and monocytes in transfused neonates may help to mitigate some platelet transfusion complications, particularly in the face of neonatal tissue injury and remodeling.
Platelet interactions with, and regulation of, immune cells is complex and can simultaneously involve a number of direct and indirect interactions9. Our results focused on P-selectin dependent interactions and represents just one of many pathways that may result in platelet transfusion-mediated immune dysfunction in neonates. For example, our RNA-seq data indicated that the expression of mRNA for secreted immune molecules such as Ppbb and B2m was greater in adult compared to neonatal platelets. It is possible that in a more chronic context, or with vascular injury and platelet activation, that other secreted platelet-derived mediators may contribute to post-transfusion immune dysfunction and long-term developmental complications. Our data also suggest that direct interactions between adult platelets and monocytes in a neonate with established infection or ongoing tissue remodeling may lead to adverse monocyte tissue trafficking and complications. More work needs to be done in disease specific contexts to define the roles of other platelet-derived mediators that differ between adults and neonates in immune regulation and platelet transfusion complications.
Our findings that fetal and neonatal murine platelets share several differences with adult platelets raises the possibility that platelet-like particles derived from human induced pluripotent stem cells (iPSCs) may ultimately be beneficial for the treatment of thrombocytopenia in both adults and neonates if they are more neonatal-like in immune profile. Inflammatory complications are also associated with platelet transfusions to adults, so iPSC-derived platelets may be more immune ‘immature’ and present fewer transfusion complications compared to traditional platelet transfusions. These data may also present the opportunity to genetically modify iPSC-derived platelets to a less immune phenotype, similar to neonatal platelets, to limit platelet transfusion-related immune complications in all age groups.
These studies may have physiologic implications beyond transfusions, as post-natal age-dependent platelet immune programming may have developmental benefits. These may include ensuring proper establishment of a post-natal microbiome and proper post-natal tissue development while limiting immune cell trafficking. If platelets are properly considered part of the immune cell family, this makes sense, because the early post-natal period is a time when immune development and immune tolerance must be properly balanced. Neonatal platelets have decreased aggregation in response to most agonists, but neonates have normal primary hemostasis20, further indicating that the developmental program of neonatal platelets is skewed towards being less inflammatory, but with normal functional hemostasis. Further study and model development are needed to understand the physiology of delayed platelet immune development and the implications of its disruption.
Supplementary Material
Highlights.
Neonatal and adult platelets have different immune molecule contents.
Neonatal and adult platelet acute interactions with monocytes leads to different monocyte responses, including adult platelets increasing monocyte trafficking molecule expression more than neonatal platelets.
In an in vivo mouse model of neonatal platelet transfusions, adult platelet transfusions led to more monocyte peritoneal trafficking.
The transfusion of adult platelets to neonates may lead to monocyte trafficking and adverse neonatal platelet transfusion responses.
Sources of Funding:
R21HL153409 to JP and CNM, R01HL160610 and R01 HL141106 to CNM.
Non-standard abbreviations
- PMA
platelet monocyte aggregates
- P7
post-natal day 7
- β2M
beta 2 microglobulin
- BMM
bone marrow monocytes
- CBC
complete blood count
- PSGL
1 – P-selectin glycoprotein ligand-1
Footnotes
Disclosures: None
References
- 1.Davenport P, Fan HH, Nolton E, Feldman HA, Lorenz V, Canas J, Acosta-Zaldivar M, Yakah W, Arthur C, Martin C, Stowell S, Koehler J, Mager D and Sola-Visner M. Platelet transfusions in a murine model of neonatal polymicrobial sepsis: Divergent effects on inflammation and mortality. Transfusion. 2022. [DOI] [PMC free article] [PubMed]
- 2.Chen C, Wu S, Chen J, Wu J, Mei Y, Han T, Yang C, Ouyang X, Wong MCM and Feng Z. Evaluation of the Association of Platelet Count, Mean Platelet Volume, and Platelet Transfusion With Intraventricular Hemorrhage and Death Among Preterm Infants. JAMA Netw Open. 2022;5:e2237588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sparger KA, Assmann SF, Granger S, Winston A, Christensen RD, Widness JA, Josephson C, Stowell SR, Saxonhouse M and Sola-Visner M. Platelet Transfusion Practices Among Very-Low-Birth-Weight Infants. JAMA Pediatr. 2016;170:687–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gunnink SF, Vlug R, Fijnvandraat K, van der Bom JG, Stanworth SJ and Lopriore E. Neonatal thrombocytopenia: etiology, management and outcome. Expert Rev Hematol. 2014;7:387–95. [DOI] [PubMed] [Google Scholar]
- 5.Baer VL, Lambert DK, Henry E, Snow GL, Sola-Visner MC and Christensen RD. Do platelet transfusions in the NICU adversely affect survival? Analysis of 1600 thrombocytopenic neonates in a multihospital healthcare system. J Perinatol. 2007;27:790–6. [DOI] [PubMed] [Google Scholar]
- 6.Curley A, Stanworth SJ, Willoughby K, Fustolo-Gunnink SF, Venkatesh V, Hudson C, Deary A, Hodge R, Hopkins V, Lopez Santamaria B, Mora A, Llewelyn C, D’Amore A, Khan R, Onland W, Lopriore E, Fijnvandraat K, New H, Clarke P, Watts T and PlaNe TMC. Randomized Trial of Platelet-Transfusion Thresholds in Neonates. N Engl J Med. 2019;380:242–251. [DOI] [PubMed] [Google Scholar]
- 7.Hilt ZT, Pariser DN, Ture SK, Mohan A, Quijada P, Asante AA, Cameron SJ, Sterling JA, Merkel AR, Johanson AL, Jenkins JL, Small EM, McGrath KE, Palis J, Elliott MR and Morrell CN. Platelet-derived beta2M regulates monocyte inflammatory responses. JCI Insight. 2019;4. [DOI] [PMC free article] [PubMed]
- 8.Stolla MC, Catherman SC, Kingsley PD, Rowe RG, Koniski AD, Fegan K, Vit L, McGrath KE, Daley GQ and Palis J. Lin28b regulates age-dependent differences in murine platelet function. Blood Adv. 2019;3:72–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morrell CN, Pariser DN, Hilt ZT and Vega Ocasio D. The Platelet Napoleon Complex-Small Cells, but Big Immune Regulatory Functions. Annu Rev Immunol. 2019;37:125–144. [DOI] [PubMed] [Google Scholar]
- 10.Koupenova M, Livada AC and Morrell CN. Platelet and Megakaryocyte Roles in Innate and Adaptive Immunity. Circ Res. 2022;130:288–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Allen N, Barrett TJ, Guo Y, Nardi M, Ramkhelawon B, Rockman CB, Hochman JS and Berger JS. Circulating monocyte-platelet aggregates are a robust marker of platelet activity in cardiovascular disease. Atherosclerosis. 2019;282:11–18. [DOI] [PubMed] [Google Scholar]
- 12.Koenen RR, von Hundelshausen P, Nesmelova IV, Zernecke A, Liehn EA, Sarabi A, Kramp BK, Piccinini AM, Paludan SR, Kowalska MA, Kungl AJ, Hackeng TM, Mayo KH and Weber C. Disrupting functional interactions between platelet chemokines inhibits atherosclerosis in hyperlipidemic mice. Nat Med. 2009;15:97–103. [DOI] [PubMed] [Google Scholar]
- 13.Koltsova EK, Sundd P, Zarpellon A, Ouyang H, Mikulski Z, Zampolli A, Ruggeri ZM and Ley K. Genetic deletion of platelet glycoprotein Ib alpha but not its extracellular domain protects from atherosclerosis. Thromb Haemost. 2014;112:1252–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hilt ZT, Maurya P, Tesoro L, Pariser DN, Ture SK, Cleary SJ, Looney MR, McGrath K and Morrell C. beta2M Signals Monocytes Through Non-Canonical TGFbeta Receptor Signal Transduction. Circ Res. 2021. [DOI] [PMC free article] [PubMed]
- 15.Scheible KM, Emo J, Yang H, Holden-Wiltse J, Straw A, Huyck H, Misra S, Topham DJ, Ryan RM, Reynolds AM, Mariani TJ and Pryhuber GS. Developmentally determined reduction in CD31 during gestation is associated with CD8+ T cell effector differentiation in preterm infants. Clin Immunol. 2015;161:65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baker-Groberg SM, Lattimore S, Recht M, McCarty OJ and Haley KM. Assessment of neonatal platelet adhesion, activation, and aggregation. J Thromb Haemost. 2016;14:815–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Christensen ST, Pedersen SF, Satir P, Veland IR and Schneider L. The primary cilium coordinates signaling pathways in cell cycle control and migration during development and tissue repair. Curr Top Dev Biol. 2008;85:261–301. [DOI] [PubMed] [Google Scholar]
- 18.Zeeuw van der Laan EAN, van der Velden S, Porcelijn L, Semple JW, van der Schoot CEand Kapur R. Evaluation of Platelet Responses in Transfusion-Related Acute Lung Injury (TRALI). Transfus Med Rev. 2020;34:227–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cognasse F, Payrat JM, Corash L, Osselaer JC and Garraud O. Platelet components associated with acute transfusion reactions: the role of platelet-derived soluble CD40 ligand. Blood. 2008;112:4779–80; author reply 4780–1. [DOI] [PubMed] [Google Scholar]
- 20.Davenport P and Sola-Visner M. Platelets in the neonate: Not just a small adult. Res Pract Thromb Haemost. 2022;6:e12719. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.