Abstract
Portal vein thrombosis (PVT) is a common thrombotic complication of cirrhosis. It can lead to variceal bleeding and bowel ischemia and also complicate liver transplantation. Identifying the possible risk factors associated with PVT can aid in identifying patients at high risk, enabling their screening and potentially preventing PVT through the rational use of anticoagulants. This study focuses on examining the clinical characteristics of PVT in cirrhotic patients and identifying the clinical and biochemical factors that are linked to the development of PVT. Consecutive hospitalized cirrhotic patients between 2015 and 2023 were identified through the hospital’s computerized medical records based on the Tenth Revision of the International Statistical Classification of Diseases and Related Health Problems (ICD-10) coding system and retrospectively analyzed. 928 individuals were included in this study; 783 (84.3%) without PVT and 145 (15.7%) with benign PVT. Hepatitis B virus (HBV) was significantly more common in the PVT group (P-value = .02), while alcohol and primary sclerosing cholangitis (PSC) were less common in this group (P-value = .01 and .02, respectively). Hepatocellular carcinoma (HCC) (P-value < .01), ascites (P-value = .01), and spontaneous bacterial peritonitis (SBP) (P-value = .02) were more common in the PVT group. Patients with PVT had a higher international normalized ratio (INR) level (P-value = .042) and lower plasma albumin (P-value = .01). No differences were identified in white blood cell, hemoglobin, platelet, and bilirubin levels. However, patients with PVT had higher model for end-stage liver disease (MELD) (P-value = .01) and Child-Pugh scores (P-value = .03). This study demonstrated a higher likelihood of PVT presence in cirrhotic patients with advanced age, HBV, and HCC, along with ascites, SBP, splenomegaly, hypoalbuminemia, elevated INR, and a higher MELD score. Nevertheless, additional research endeavors are necessary to accurately ascertain and validate supplementary risk factors within a broader demographic.
Keywords: cirrhosis, portal vein thrombosis, PVT, risk factor, thromboembolism
1. Introduction
Although rare in the general population, portal vein thrombosis (PVT) is more frequent in cirrhotic patients.[1] Due to the advancements in accurate and noninvasive liver imaging techniques, the detection of PVT in cirrhotic patients has become more frequent. According to a recent meta-analysis, the pooled prevalence of PVT in individuals with cirrhosis is reported to be 14%.[2] However, it is essential to note that the estimated prevalence can range from as low as 0.6% to as high as 26%.[3,4] The variations in prevalence rates observed across different studies may be attributed to differences in participant characteristics and diagnostic approaches utilized.
PVT is typically identified as an incidental discovery in the majority of patients, although in certain cases, it manifests as the deterioration of chronic liver disease. The exact relationship between PVT and liver cirrhosis, whether it is a consequence or an aggravating factor, or both, is still not fully understood.[5] The development of PVT is a complex process involving multiple factors. While more than 1 risk factor is commonly identified, it is rare to find a single factor in cirrhosis patients. The primary cause of PVT is structural damage to the liver due to a decrease in blood flow through the portal vein. Additionally, damage to the vessel wall and increased blood clotting play significant roles. Other factors, such as hormonal therapy, malignancies, and inherited or acquired thrombotic risk factors, also contribute to the development of PVT.[6] Complications of PVT can include hyperdynamic circulation, variceal hemorrhage, and intestinal ischemia.[7] Furthermore, PVT can complicate liver transplants and make the procedure technically more challenging.[8]
The study focuses on examining the clinical characteristics of PVT in cirrhotic patients receiving care at a tertiary care hospital in Tehran, Iran, as well as identifying the clinical and biochemical factors linked to the development of PVT. This knowledge can significantly alter the screening and treatment approach for these patients by providing a clear rationale for the use of anticoagulation therapy.
2. Methods
2.1. Study protocol
This study was a retrospective analysis undertaken at Taleghani Hospital, a tertiary care unit for hepatobiliary disorders, in Tehran, Iran. Ethics committee approval is not necessary for retrospective studies at Shahid Beheshti Medical University. Patients diagnosed with liver cirrhosis from March 2015 to September 2023 were identified through the hospital’s computerized medical records based upon the Tenth Revision of the International Statistical Classification of Diseases and Related Health Problems (ICD-10) coding system http://www.who.int/classifications/icd/en/.
Patients with cirrhosis regardless of the duration or cause of the disease, who were above 18 were enrolled in the study. Patients with the following conditions were excluded: liver transplantation, splenectomy, budd-chiari syndrome, polycythemia vera, essential thrombocytosis, history of venous thromboembolism or thrombophilia, history of transjugular intrahepatic portosystemic shunt, pregnancy, malignant PVT, immunocompromised patients and patients receiving anticoagulant agents. Patients with incomplete clinical data were also excluded.
The diagnosis of PVT was established when solid material was present within the main portal vein and its branches, along with a filling defect observed during the Doppler study, further confirmation was obtained through contrast-enhanced computed tomography (CT) or magnetic resonance imaging (MRI). The diagnosis of benign PVT was established by an experienced radiologist by the following criteria: no pulsation in Doppler, non-enhancement at the arterial phase, absence of disruption of the vein walls, and a PV diameter <20 mm. Patients who did not exhibit the characteristic features of chronic PVT, such as collateral circulation (portal cavernous transformation) or portal hypertension, were deemed as having acute PVT.
The following data were extracted from the medical records: demographic data, etiology of cirrhosis, clinical presentation, comorbidities, portal hypertension complications, and blood biochemistry.
2.2. Statistical analysis
The STATA version 17.0 (StataCorp, College Station, Lakeway, TX, USA) was utilized for all the analyses conducted in this study. Continuous variables were compared with the Student t test or Mann–Whitney U test. Categorical variables were compared with Pearson chi-square or Fisher exact tests. Binary logistic regression was used to determine univariate and multivariate odds ratios (ORs) and confidence intervals (CIs) for the dependent variable (with/without PVT groups). A 2-tailed P-value < .05 was considered statistically significant.
3. Results
3.1. Baseline characteristics
A total of 928 individuals were included in this study: 783 (84.4%) without PVT and 145 (15.6%) with PVT. The mean age of patients was 55.8 ± 14.7, and patients with PVT were 3 years older on average (P-value = .01). Patients with PVT had a higher body mass index (BMI) but it was not statistically significant (P-value = .78).
3.2. Etiology and comorbidities
Cryptogenic cirrhosis was the most common etiology (21.6%), followed by nonalcoholic steatohepatitis (NASH) (20.2%), hepatitis B virus (HBV) (15%), hepatitis C virus (HCV) (12.5%), primary sclerosing cholangitis (PSC) (9.2%), autoimmune hepatitis (AIH) (7.7%), alcohol (5.6%) and other less common causes, respectively. HBV was significantly more common in the PVT group (P-value = .02), while alcohol and PSC were less common (P-value = .01 and .02, respectively). There was no significant difference in terms of comorbidities between the 2 groups, except ulcerative colitis (UC) which was more common in patients without PVT (P-value = .005).
3.3. Cirrhosis complications and related events
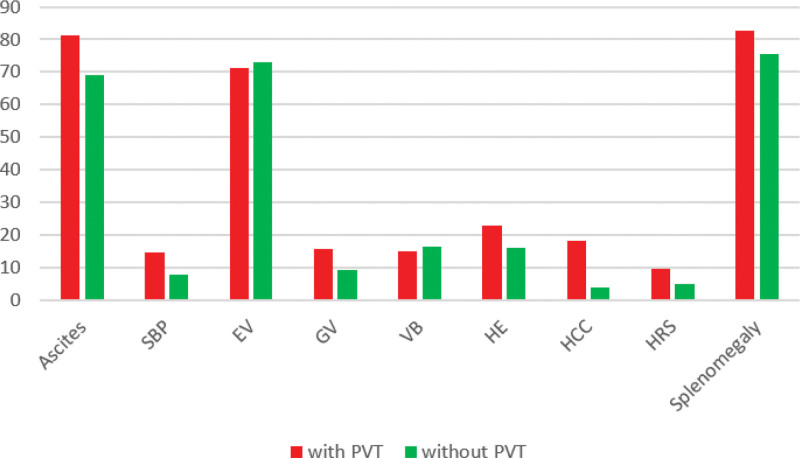
Regarding cirrhosis complications, ascites, spontaneous bacterial peritonitis (SBP), hepatocellular carcinoma (HCC), and hepatorenal syndrome (HRS) were significantly more common in the PVT group (P-value < .05). Gastric varices and encephalopathy were relatively more common in the PVT group (P-value = .054 and .068, respectively). No differences were identified in esophageal varices, variceal bleeding, endoscopic variceal band ligation (EVBL), and spleen size (Fig. 1).
Figure 1.
Percent frequencies of cirrhosis complications based on the presence of PVT. Abbreviations: EV: esophageal varices, GV: gastric varices, HCC: hepatocellular carcinoma, HE: hepatic encephalopathy, HRS: hepatorenal syndrome, SBP: spontaneous bacterial peritonitis, VB: variceal bleeding.
3.4. Blood biochemistry
Patients with PVT had higher international normalized ratio (INR) (P-value = .042) and lower plasma albumin (P-value = .01) levels. No differences were identified in white blood cell (WBC), hemoglobin, platelet, and bilirubin levels. However, patients with PVT had higher MELD (P-value = .01) and Child-Pugh scores (P-value = .03). No significant difference was observed in platelet-to-lymphocyte ratio (PLR) and neutrophil-to-lymphocyte (NLR) ratio as inflammatory markers. Table 1 provides the details of the descriptive analysis of the groups.
Table 1.
Baseline characteristics of the patients.
| Variable | Total (n = 928) | Without PVT (n = 783) | With PVT (n = 145) | P-value |
|---|---|---|---|---|
| Age | 55.8 ± 14.7 | 55.3 ± 15 | 58.3 ± 13.3 | .01 |
| Sex (male/ female) (%) | 560 (60.3%)/ 368 (39.7%) | 470 (60%)/ 313 (40%) | 90 (62.1%)/ 55 (37.9%) | .64 |
| BMI | 22.96 ± 5.56 | 22.94 ± 5.57 | 23.22 ± 6.00 | .78 |
| Etiology (%) | ||||
| HBV | 139 (15%) | 108 (13.8%) | 31 (21.4%) | .02 |
| HCV | 116 (12.5%) | 94 (12%) | 22 (15.2%) | .29 |
| Alcohol | 52 (5.6%) | 50 (6.4%) | 2 (1.4%) | .01 |
| NASH | 187 (20.2%) | 163 (20.9%) | 24 (16.5%) | .23 |
| AIH | 71 (7.7%) | 65 (8.3%) | 6 (4.1%) | .08 |
| PBC | 9 (1%) | 9 (1.5%) | 0 | .37 |
| PSC | 85 (9.2%) | 79 (10.1%) | 6 (4.1%) | .02 |
| Wilson | 12 (1.3%) | 12 (1.6%) | 0 | .23 |
| Cryptogenic | 200 (21.6%) | 164 (21%) | 36 (24.9%) | .3 |
| Others | 28 (3%) | 26 (3.3%) | 2 (1.4%) | .29 |
| Unknown | 24 (2.6%) | 8 (1.1%) | 16 (11%) | <.01 |
| Comorbidities (%) | ||||
| Diabetes mellitus | 294 (32.3%) | 253 (32.7%) | 41 (32.8%) | .97 |
| Hypertension | 175 (19.4%) | 146 (18.8%) | 29 (23.2%) | .25 |
| Hypothyroidism | 58 (6.4%) | 46 (5.9%) | 12 (9.6%) | .12 |
| Cardiovascular disease | 138 (15.3%) | 120 (15.5%) | 18 (14.4%) | .75 |
| Ulcerative colitis | 66 (7.3%) | 64 (8.3%) | 2 (1.6%) | .005 |
| Malignancy | 46 (5.1%) | 37 (4.8%) | 9 (7.2%) | .25 |
| Hyperlipidemia | 42 (4.7%) | 39 (5%) | 3 (2.4%) | .25 |
| COPD | 21 (2.3%) | 19 (2.4%) | 2 (1.6%) | .76 |
| Cerebral vascular accident | 16 (1.8%) | 14 (1.8%) | 2 (1.6%) | 1 |
| Chronic kidney disease | 33 (3.7%) | 30 (3.9%) | 3 (2.4%) | .6 |
| Cirrhosis complications (%) | ||||
| Ascites | ||||
| No | 260 (29.2%) | 236 (31%) | 24 (18.6%) | <.01 |
| Mild | 185 (20.8%) | 161 (21.1%) | 24 (18.6%) | |
| Moderate | 180 (20.2%) | 151 (19.8%) | 29 (22.5%) | |
| Severe | 266 (29.8%) | 214 (28.1%) | 52 (40.3%) | |
| SBP | 76 (8.8%) | 61 (8%) | 15 (14.7%) | .02 |
| Esophageal varices | ||||
| No | 200 (27.4%) | 167 (27.1%) | 33 (28.9%) | .65 |
| F1 | 144 (19.7%) | 118 (19.2%) | 26 (22.8%) | |
| F2 | 148 (20.3%) | 125 (20.3%) | 23 (20.2%) | |
| F3 | 238 (32.6%) | 206 (33.4%) | 32 (28.1%) | |
| Gastric varices | 73 (10.3%) | 57 (9.4%) | 16 (15.7%) | .054 |
| Variceal bleeding | 147 (16.3%) | 129 (16.5%) | 18 (15%) | .68 |
| EVBL | 594 (64%) | 505 (64.5%) | 89 (61.4%) | .47 |
| Encephalopathy | 148 (17%) | 123 (16.1%) | 25 (23.1%) | .068 |
| Hepatocellular carcinoma | 49 (5.6%) | 30 (3.9%) | 19 (18.5%) | <.01 |
| Hepatorenal syndrome | 48 (5.6%) | 38 (5%) | 10 (9.8%) | .046 |
| Spleen size | ||||
| ≤12 cm | 173 (23.2%) | 152 (24.4%) | 21 (17.2%) | .195 |
| 12 to 20 cm | 487 (65.3%) | 403 (64.6%) | 84 (68.9%) | |
| ≥20 cm | 86 (11.5%) | 69 (11%) | 17 (13.9%) | |
| Blood biochemistry | ||||
| White blood cell | 6.70 ± 4.61 | 6.68 ± 4.47 | 6.79 ± 5.38 | .39 |
| Hemoglobin | 10.4 ± 2.32 | 10.4 ± 2.32 | 10.4 ± 2.32 | .50 |
| Platelets | 127.2 ± 95.74 | 128.6 ± 96.46 | 118.6 ± 91.13 | .86 |
| INR | 1.48 ± 0.68 | 1.47 ± 0.69 | 1.58 ± 0.63 | .042 |
| Total bilirubin | 5.25 ± 7.3 | 5.2 ± 7.27 | 5.58 ± 7.52 | .29 |
| Albumin | 3.31 ± 0.62 | 3.33 ± 0.62 | 3.18 ± 0.54 | .01 |
| Child-Pugh score | 8.67 ± 0.07 | 8.62 ± 2.03 | 9.05 ± 1.76 | .03 |
| MELD score | 15.86 ± 6.76 | 15.65 ± 6.76 | 17.16 ± 6.63 | .01 |
| PLR | 120.88 ± 78.1 | 123.11 ± 79.47 | 90.49 ± 1.38 | .3 |
| NLR | 2.92 ± 1.94 | 2.95 ± 1.97 | 2.52 ± 1.52 | .53 |
Abbreviations: AIH = autoimmune hepatitis, BMI = body mass index, COPD = chronic obstructive pulmonary disease, EVBL = endoscopic variceal band ligation, HBV = hepatitis B virus, HCV = hepatitis C virus, INR = international normalized ratio, MELD = model for end-stage liver disease, NASH = nonalcoholic steatohepatitis, NLR = neutrophil-to-lymphocyte ratio, PBC = primary biliary cholangitis, PLR = platelet-to-lymphocyte ratio, PSC = primary sclerosing cholangitis, PVT = portal vein thrombosis, SBP = spontaneous bacterial peritonitis.
3.5. Univariate and multivariate analysis
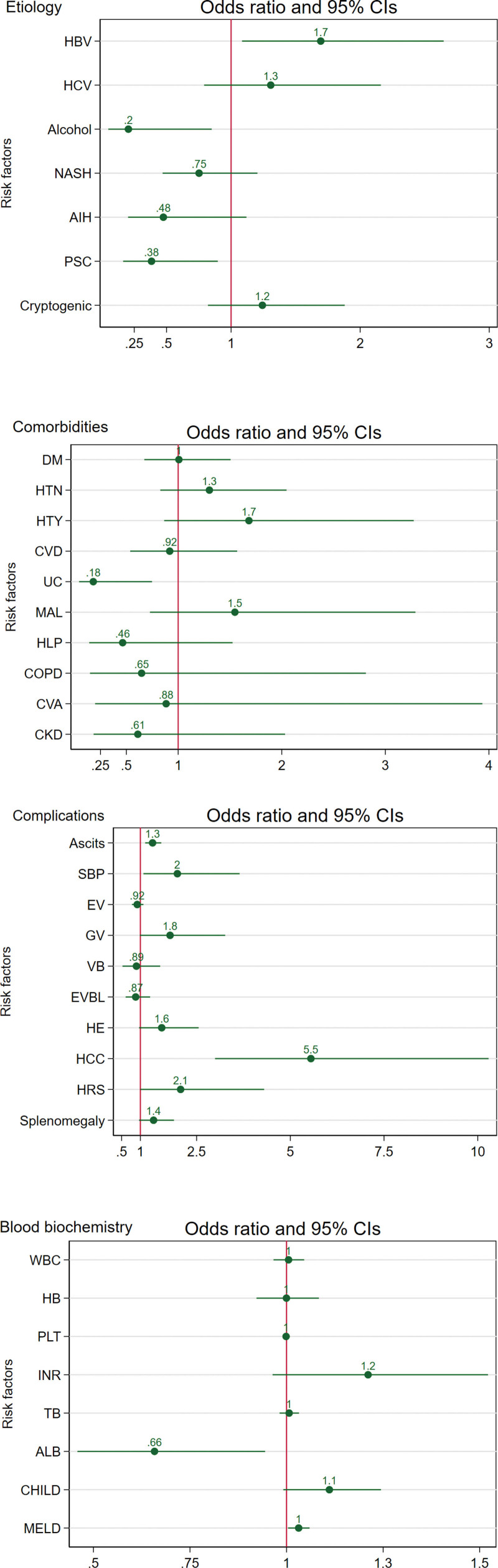
Figure 2 and Table S1, Supplemental Digital Content, http://links.lww.com/MD/N603 show the univariate analysis results for possible risk factors of PVT, and Table S2, Supplemental Digital Content, http://links.lww.com/MD/N604 shows the multivariate analysis results. Higher age was associated with PVT (OR = 1.01, 95% CI: 1.00–1.03), while no association was identified for sex. Univariate analysis identified HBV, ascites, SBP, HCC, and hypoalbuminemia as the potential risk factors of PVT and alcohol, PSC, and UC as the potential protective factors of PVT. However, multivariate analysis revealed that UC (OR = 0.13, 95% CI: 0.02–0.77), and not PSC (OR = 1.39, 95% CI: 0.46–4.14), has a protective effect for PVT. Moreover, after adjusting for age, sex, and etiology, multivariate analysis suggested splenomegaly and higher INR levels as the risk factors of PVT and no significant effect for SBP and UC (P-value > .05).
Figure 2.
Forest plots of univariate analysis of probable risk factors of PVT. Abbreviations: AIH: autoimmune hepatitis, ALB: albumin, CHILD: Child-Pugh score, CKD: chronic kidney disease, COPD: chronic obstructive pulmonary disease, CVA: cerebral vascular accident, CVD: cardiovascular disease, DM: diabetes mellitus, EV: esophageal varice, EVBL: endoscopic variceal band ligation, GV: gastric varice, HB: hemoglobin, HBV: hepatitis B virus, HCC: hepatocellular carcinoma, HCV: hepatitis C virus, HE: hepatic encephalopathy, HLP: hyperlipidemia, HRS: hepatorenal syndrome, HTN: hypertension, HTY: hypothyroidism, INR: international normalized ratio, MAL: malignancy, MELD: model for end-stage liver disease, NASH: nonalcoholic steatohepatitis, PBC: primary biliary cholangitis, PLT: platelet, PSC: primary sclerosing cholangitis, SBP: spontaneous bacterial peritonitis, TB: total bilirubin, UC: ulcerative colitis, VB: variceal bleeding, WBC: white blood cell.
4. Discussion
The occurrence of PVT is approximately 1% among the overall population.[9] Genetic mutations, venous infestations (including schistosomiasis), hepatic vascular abnormalities, and cirrhosis are the other risk factors of PVT.[10] Cirrhosis stands as the primary risk factor for PVT, with a reported prevalence of 28% among individuals diagnosed with PVT.[9] Recent research has indicated that individuals with cirrhosis not only experience a decrease in the production of clotting factors by the liver but also suffer from a significant deficiency in natural anticoagulants.[11,12] This deficiency in natural anticoagulants may serve as a compensatory mechanism to counteract the bleeding tendency caused by the lack of pro-coagulants. Studies have shown a higher amount of factor VIII and a lower amount of protein C in cirrhotic patients compared to the healthy controls. Protac-induced coagulation inhibition percentage (PICI%) assay, which evaluates the function of the protein C anticoagulant system, is significantly lower in cirrhotic patients compared to the healthy controls.[13] Moreover, reduced clearance of activated factors, quantitative and qualitative platelet defects hyperfibrinolysis, increased intravascular coagulation, reduced blood flow, and endothelial damage contribute to the pathophysiology of this event in cirrhosis.[14,15]
PVT was found among 15.6% of patients in this study. This finding aligns with Abdel-Razik et al, who reported a prevalence of 17.9% of de-novo PVT in cirrhotic patients.[16] A recent meta-analysis estimated the pooled prevalence of PVT in cirrhotic patients at 14%.[2] On the other hand, of the 254 patients with PVT, Ogren et al found cirrhosis in 28% of the autopsies.[17] However, it should be noted that the true incidence of PVT in cirrhosis could be higher due to the challenges associated with diagnosing intrahepatic thrombosis, which may go unnoticed unless thrombosis is detected in the main portal vein. Moreover, most patients with chronic PVT are asymptomatic, and PVT is an accidental finding when they go under imaging for other reasons.
Current guidelines recommend color Doppler ultrasound as the primary screening tool for PVT and CT scan or MRI for diagnosis.[18,19] In this study, color Doppler ultrasound was the primary diagnostic tool for PVT, and further confirmation was obtained through a CT scan or MRI. Doppler ultrasonography is commonly utilized as a primary screening method for detecting thrombus in the portal system due to its cost-effectiveness, non-reliance on intravenous contrast material, and convenience for repeated examinations.[20] Nevertheless, its sensitivity is contingent on the operator, ranging from 60% to 100%.[21] CT is now available worldwide and becoming more popular because of superior accuracy and detailed information in confirming the presence and location of thrombus and in identifying diverse abdominal etiologies linked to PVT.
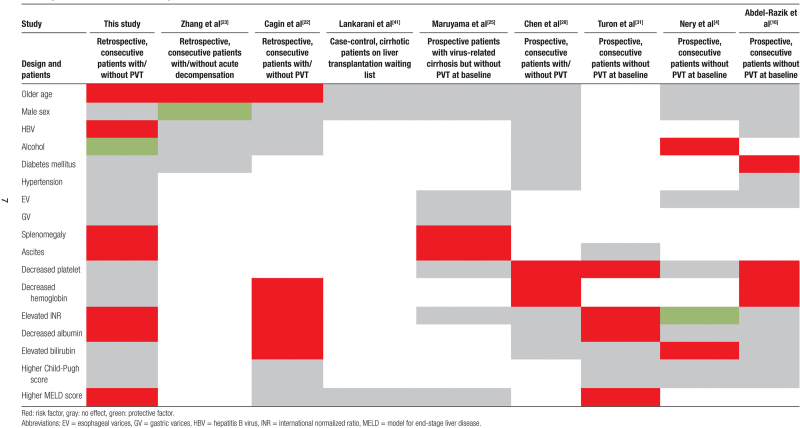
The progression of PVT is impacted by a variety of factors. Within our research, we incorporated established potential risk factors of PVT into both univariate and multivariate logistic analysis. Older age, HBV as etiology, splenomegaly and ascites as portal hypertension consequences, elevated INR and lower albumin as biochemical factors, HCC, and MELD score were associated significantly with the PVT group than the non-PVT. Older age was identified as an independent risk factor for PVT development in our patients. This finding is in line with Zhang et al and Cagin et al, who reported age as a risk factor for PVT.[22,23] Lertpipopmetha et al reported HBV-associated cirrhosis and HCC as the major risk factors of PVT in the Southeast Asian population.[24] Our study, which was performed in Iran, a Middle-east Asian country, revealed the same result with a high prevalence of HBV and HCC in cirrhotic patients with PVT.
Regarding portal hypertension consequences, in a prospective study, Maruyama et al followed up cirrhotic patients without PVT at baseline. They found that patients with PVT had larger spleen size and more severe ascites than those without PVT. However, there were no significant differences between the 2 groups in esophageal and gastric varices as the other portal hypertension consequences.[25] Our findings are in agreement with this study. They did not investigate the differences in SBP and encephalopathy. However, our study did not identify SBP and encephalopathy as the potential risk factors for PVT.
Recent studies, with a relatively small population, suggested that endoscopic variceal treatment may increase the risk of PVT in liver cirrhosis.[26,27] However, our study did not identify EVBL as a potential risk factor for PVT. The small may have distorted the results of these studies. Nevertheless, it should be noted that even if EVBL increases the risk of PVT, this should not be interpreted that it should be avoided in patients with esophageal varices, as esophageal varices bleeding is a life-threatening event in patients with cirrhosis; rather, thrombosis prophylaxis may be considered in patients who undergo endoscopic variceal treatment. Further studies with large populations are needed to investigate the association of EVBL and PVT, and screening for and prophylaxis of PVT after EVBL in patients with liver cirrhosis.
A notable correlation between reduced platelet count and PVT is generally anticipated due to their same etiology in cirrhotic patients.[28] Nevertheless, the analysis carried out in this study revealed no statistically significant differences in platelet levels across the groups. The findings of previous studies corroborate the outcomes of our study.[4,25] We did not identify any association between hemoglobin level and PVT development. However, some studies reported a lower level of hemoglobin as a risk factor for PVT development in cirrhotic patients.[16,22] The reason behind this discovery can be linked to the existence of splenomegaly and hypersplenism in cirrhotic patients, which are associated with increased breakdown of red blood cells and subsequently reduced hemoglobin levels. Nevertheless, none of these studies consider spleen size a potential confounding factor.
Child-Pugh and MELD are 2 scoring systems used to assess the severity of liver disease and the prognosis of the patient.[29] Several laboratory markers are employed in these scoring systems, including albumin, bilirubin, and INR. It is known that increased bilirubin, elevated INR, and decreased plasma albumin levels parallel the stages of cirrhosis. Serum albumin level could indicate the overall hepatic function and reserve; elevated serum bilirubin is associated with deterioration in hepatic and portal venous flow. In this study, we identified lower plasma albumin levels and elevated INR as the risk factors of PVT development, while no association was found for bilirubin levels. Multiple studies have verified the association of hypoalbuminemia and PVT development.[30,31] Cagin et al, in a retrospective study, and Nery et al, in a prospective study, identified a significant association between increased serum bilirubin and PVT.[4,22]
Results on INR as a potential risk factor of PVT development are more controversial. In agreement with Cagin et al and Turon et al, we found a significantly increased INR level in patients with PVT.[22,31] Conversely, Nery et al found a significantly lower INR level in the PVT group.[4] While an elevated INR level in the general population is typically linked to a higher likelihood of bleeding, this correlation may not hold true within cirrhotic patients. Cirrhotic patients not only experience decreased production of clotting factors in the liver but also exhibit a significant deficiency in natural anticoagulants. This deficiency in anticoagulants may offset the increased risk of bleeding due to the lack of pro-coagulants.[32]
Two recent studies investigated the role of inflammatory markers in predicting PVT in cirrhotic patients. They found that inflammatory markers, including D-dimer, PLR, and NLR are significantly higher than cirrhotic patients without PVT.[33,34] These studies are distinguished by the larger patient population with PVT, which enhances its strength compared to our study. Nevertheless, it is important to note that both of these studies employed an unclear matching method, which can introduce selection bias. More studies with large populations and clear methodology are needed to shed light on this field.
Unexpectedly, alcoholic liver disease and UC, which were previously identified as risk factors for thrombosis, were found to be linked to a reduced risk of PVT in this study. Despite Nery et al’s findings of a high prevalence of PVT in alcoholic patients,[4] this study found a significantly lower incidence of PVT in alcoholic patients compared to other etiologies. The variation in the prevalence of alcoholic cirrhosis between the 2 studies (39.2% vs 5.6%) could account for the differing outcomes observed. In Iran, being an Islamic country, the consumption of alcohol is strictly forbidden. Additionally, alcohol consumption is socially stigmatized, leading patients to potentially deny their intake and attribute their cirrhosis to other causes. However, recent studies attributed a protective effect for alcohol in venous thromboembolism.[35,36] In line with this, a nationwide United States cohort study with a population of 1.9 million alcoholic cirrhosis patients reported patients with alcoholic cirrhosis have a lower risk of PVT (OR = 0.76) and venous thromboembolism (OR = 0.69) compared to nonalcoholic cirrhotic patients.[37]
UC is associated with an increased risk of thromboembolic events, and it is recommended that hospitalized patients with UC receive prophylactic anticoagulants.[38,39] Although there are reports of increased risk of PVT in hospitalized patients with UC,[40] Surprisingly, we found a lower incidence of PVT in cirrhotic patients with UC. However, we found no study assessing the risk of PVT in patients with UC and concomitant cirrhosis in the literature. Further studies are needed to confirm our findings.
Numerous investigations have explored potential risk factors for thrombosis in individuals with cirrhosis, with the most recent findings outlined in Table 2. In general, various discrepancies exist among these studies regarding the risk factors associated with PVT. These variations may stem from differences in study populations, diagnostic techniques, and, notably, research design and methodologies.
Table 2.
Summary of recent studies assessed the potential risk factors of PVT.
The role of PVT in the progression of liver cirrhosis is a matter of concern. In a prospective study involving 1243 cirrhotic patients without PVT at baseline with a mean follow-up time of 47 months, it was observed that PVT was not independently associated with the progression of liver disease or mortality. This study revealed that complete and permanent portal venous occlusion was only present in a small minority of patients with PVT. Therefore, the authors proposed that the development of PVT serves as a marker rather than a direct cause of liver disease progression.[4] Furthermore, 2 recent prospective studies have demonstrated no significant difference in survival rate between patients with and without PVT.[22,23] However, a prior study had reported an increased mortality rate in patients with PVT.[42]
To the best of our knowledge, this is the first study that investigates the risk factors of port vein thrombosis with such a wide range of possible variables. However, the current study had some limitations. First, the data utilized in this investigation were acquired exclusively from a single center, encompassing a comparatively limited number of subjects in some etiologies. Second, information regarding portal vein velocity, and degree of obstruction was not present in our database; therefore, this study could not evaluate the influence of portal venous system characteristics on thrombosis. Moreover, despite examining the impact of comorbidities on PVT, the inability to access sufficient data hindered the investigation of the influence of the severity of the underlying disease and its level of control on PVT. Although we excluded patients receiving anticoagulants to avoid potential confounding factors, due to the lack of data, we could not investigate the confounding effect of other drugs such as nonselective beta-blockers, which previous studies have shown can be associated with an increased risk of PVT.[43] Finally, due to the retrospective nature of the study, it was not feasible to conduct a follow-up on the patients to evaluate the impact of PVT on the mortality of cirrhotic patients.
5. Conclusions
PVT is a common thrombotic complication of cirrhosis. Identifying the possible risk factors associated with PVT can aid in identifying patients at high risk, enabling their screening and potentially preventing PVT through the rational use of anticoagulants. This study demonstrated a higher likelihood of PVT presence in cirrhotic patients with advanced age, HBV, and HCC, along with ascites, SBP, splenomegaly, hypoalbuminemia, elevated INR, and a higher MELD score. Nevertheless, additional research endeavors are necessary to ascertain and validate supplementary risk factors within a broader demographic more accurately.
Author contributions
Conceptualization: Erfan Arabpour, Behzad Hatami, Leila Pasharavesh, Amir Hassan Rabbani, Saba Zarean Shahraki, Mohammad reza Zali.
Data curation: Erfan Arabpour, Behzad Hatami, Leila Pasharavesh, Amir Hassan Rabbani, Mahmoud Amiri.
Formal analysis: Erfan Arabpour.
Investigation: Behzad Hatami, Mahmoud Amiri.
Methodology: Erfan Arabpour, Behzad Hatami, Saba Zarean Shahraki, Mohammad reza Zali.
Resources: Amir Hassan Rabbani, Mahmoud Amiri.
Software: Erfan Arabpour.
Supervision: Behzad Hatami.
Writing – original draft: Erfan Arabpour, Behzad Hatami, Saba Zarean Shahraki.
Writing – review & editing: Behzad Hatami, Leila Pasharavesh, Amir Hassan Rabbani, Mahmoud Amiri, Mohammad reza Zali.
Supplementary Material
Abbreviations:
- AIH
- autoimmune hepatitis
- ALB
- albumin
- BMI
- body mass index
- CHILD
- Child-Pugh score
- CI
- confidence interval
- CKD
- chronic kidney disease
- COPD
- chronic obstructive pulmonary disease
- CT
- computed tomography
- CVA
- cerebral vascular accident
- CVD
- cardiovascular disease
- DM
- diabetes mellitus
- EV
- esophageal varice
- EVBL
- endoscopic variceal band ligation
- GV
- gastric varice
- HB
- hemoglobin
- HBV
- hepatitis B virus
- HCC
- hepatocellular carcinoma
- HCV
- hepatitis C virus
- HE
- hepatic encephalopathy
- HLP
- hyperlipidemia
- HRS
- hepatorenal syndrome
- HTN
- hypertension
- HTY
- hypothyroidism
- ICD-10
- the Tenth Revision of the International Statistical Classification of Diseases and Related Health Problems
- INR
- international normalized ratio
- MAL
- malignancy
- MELD
- model for end-stage liver disease
- MRI
- magnetic resonance imaging
- NASH
- nonalcoholic steatohepatitis
- NLR
- neutrophil-to-lymphocye ratio
- OR
- odds ratio
- PBC
- primary biliary cholangitis
- PLR
- platelet-to-lymphocyte ratio
- PLT
- platelet
- PSC
- primary sclerosing cholangitis
- PV
- portal vein thrombosis
- PVT
- portal vein thrombosis
- SBP
- spontaneous bacterial peritonitis
- TB
- total bilirubin
- UC
- ulcerative colitis
- VB
- variceal bleeding
- WBC
- white blood cell
The authors have no funding and conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Supplemental Digital Content is available for this article.
How to cite this article: Arabpour E, Hatami B, Pasharavavesh L, Rabbani AH, Zarean Shahraki S, Amiri M, Zali MR. Clinical characteristics and predictors of benign portal vein thrombosis in patients with liver cirrhosis: A retrospective single-center study. Medicine 2024;103:38(e39823).
Contributor Information
Erfan Arabpour, Email: erfanarabpour1999@gmail.com.
Leila Pasharavavesh, Email: l.pasharavesh@gmail.com.
Amir Hassan Rabbani, Email: amirhassanrabbani92@gmail.com.
Saba Zarean Shahraki, Email: Saba.zarean@gmail.com.
Mahmoud Amiri, Email: Mahmood.amiri18@yahoo.com.
References
- [1].Prakash S, Bies J, Hassan M, Mares A, Didia SC. Portal vein thrombosis in cirrhosis: a literature review. Front Med (Lausanne). 2023;10:1134801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Pan J, Wang L, Gao F, et al. Epidemiology of portal vein thrombosis in liver cirrhosis: a systematic review and meta-analysis. Eur J Intern Med. 2022;104:21–32. [DOI] [PubMed] [Google Scholar]
- [3].Tsochatzis EA, Senzolo M, Germani G, Gatt A, Burroughs AK. Systematic review: portal vein thrombosis in cirrhosis. Aliment Pharmacol Ther. 2010;31:366–74. [DOI] [PubMed] [Google Scholar]
- [4].Nery F, Chevret S, Condat B, et al. Groupe d'Etude et de Traitement du Carcinome Hépatocellulaire. Causes and consequences of portal vein thrombosis in 1,243 patients with cirrhosis: results of a longitudinal study. Hepatology. 2015;61:660–7. [DOI] [PubMed] [Google Scholar]
- [5].Mantaka A, Augoustaki A, Kouroumalis EA, Samonakis DN. Portal vein thrombosis in cirrhosis: diagnosis, natural history, and therapeutic challenges. Ann Gastroenterol. 2018;31:315–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Odriozola A, Puente A, Cuadrado A, et al. Portal vein thrombosis in the setting of cirrhosis: a comprehensive review. J Clin Med. 2022;11:6435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Trebicka J, Strassburg CP. Etiology and complications of portal vein thrombosis. Viszeralmedizin. 2014;30:375–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ozer A, Aktas H, Yilmaz TU, et al. Liver transplant in patients with portal vein thrombosis: the experience of 55 patients. Exp Clin Transplant. 2019;8. [DOI] [PubMed] [Google Scholar]
- [9].Kumar A, Sharma P, Arora A. portal vein obstruction–epidemiology, pathogenesis, natural history, prognosis and treatment. Aliment Pharmacol Ther. 2015;41:276–92. [DOI] [PubMed] [Google Scholar]
- [10].del Carmen Manzano-Robleda M, Barranco-Fragoso B, Uribe M, Méndez-Sánchez N. Portal vein thrombosis: what is new? Ann Hepatol. 2015;14:20–7. [PubMed] [Google Scholar]
- [11].Violi F, Pignatelli P, Castellani V, Carnevale R, Cammisotto V. Gut dysbiosis, endotoxemia and clotting activation: a dangerous trio for portal vein thrombosis in cirrhosis. Blood Rev. 2023;57:100998. [DOI] [PubMed] [Google Scholar]
- [12].Zanetto A, Northup P, Roberts L, Senzolo M. Hemostasis in cirrhosis: understanding destabilising factors during acute decompensation. J Hepatol. 2023;78:1037–47. [DOI] [PubMed] [Google Scholar]
- [13].El Bokl MA, Shawky A, Riad GS, et al. Procoagulant versus anticoagulant factors in cirrhotic patients. Arab J Gastroenterol. 2014;15:123–9. [DOI] [PubMed] [Google Scholar]
- [14].Campello E, Spiezia L, Gavasso S, et al. Endothelial damage of the portal vein is associated with heparin-like effect in advanced stages of cirrhosis. Thromb Haemost. 2020;120:1173–81. [DOI] [PubMed] [Google Scholar]
- [15].Islam R, Kundu S, Jha SB, et al. Cirrhosis and coagulopathy: mechanisms of hemostasis changes in liver failure and their management. Cureus. 2022;14:e23785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Abdel-Razik A, Mousa N, Elhelaly R, Tawfik A. De-novo portal vein thrombosis in liver cirrhosis: risk factors and correlation with the Model for End-stage Liver Disease scoring system. Eur J Gastroenterol Hepatol. 2015;27:585–92. [DOI] [PubMed] [Google Scholar]
- [17].Ögren M, Bergqvist D, Björck M, Acosta S, Eriksson H, Sternby NH. Portal vein thrombosis: prevalence, patient characteristics and lifetime risk: a population study based on 23 796 consecutive autopsies. World J Gastroenterol. 2006;12:2115–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Northup PG, Garcia-Pagan JC, Garcia-Tsao G, et al. Vascular liver disorders, portal vein thrombosis, and procedural bleeding in patients with liver disease: 2020 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2021;73:366–413. [DOI] [PubMed] [Google Scholar]
- [19].Simonetto DA, Singal AK, Garcia-Tsao G, Caldwell SH, Ahn J, Kamath PS. ACG clinical guideline: disorders of the hepatic and mesenteric circulation. Am J Gastroenterol. 2020;115:18–40. [DOI] [PubMed] [Google Scholar]
- [20].Koratala A, Reisinger N. Venous excess Doppler ultrasound for the nephrologist: pearls and pitfalls. Kidney Med. 2022;4:100482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Chawla YK, Bodh V. Portal vein thrombosis. J Clin Exp Hepatol. 2015;5:22–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Cagin YF, Bilgic Y, Berber I, et al. The risk factors of portal vein thrombosis in patients with liver cirrhosis. Exp Ther Med. 2019;17:3189–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Zhang Y, Xu B-y, Wang X-b, et al. Prevalence and clinical significance of portal vein thrombosis in patients with cirrhosis and acute decompensation. Clin Gastroenterol Hepatol. 2020;18:2564–2572. e2561. [DOI] [PubMed] [Google Scholar]
- [24].Lertpipopmetha K, Auewarakul CU. High incidence of hepatitis B infection-associated cirrhosis and hepatocellular carcinoma in the Southeast Asian patients with portal vein thrombosis. BMC Gastroenterol. 2011;11:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Maruyama H, Okugawa H, Takahashi M, Yokosuka O. De novoportal vein thrombosis in virus-related cirrhosis: predictive factors and long-term outcomes. Am J Gastroenterol. 2013;108:568–74. [DOI] [PubMed] [Google Scholar]
- [26].Wang L, Guo X, Shao X, et al. Association of endoscopic variceal treatment with portal venous system thrombosis in liver cirrhosis: a case–control study. Therap Adv Gastroenterol. 2022;15:17562848221087536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Wang L, Guo X, Xu X, et al. Association of portal venous system thrombosis with endoscopic variceal treatment: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2021;32:125–31. [DOI] [PubMed] [Google Scholar]
- [28].Chen H, Trilok G, Wang F, Qi X, Xiao J, Yang C. A single hospital study on portal vein thrombosis in cirrhotic patients-clinical characteristics & risk factors. Indian J Med Res. 2014;139:260–6. [PMC free article] [PubMed] [Google Scholar]
- [29].Ruf A, Dirchwolf M, Freeman RB. From Child-Pugh to MELD score and beyond: taking a walk down memory lane. Ann Hepatol. 2022;27:100535. [DOI] [PubMed] [Google Scholar]
- [30].Gîrleanu I, Trifan A, Cojocariu C, Sîngeap A-M, Sfarti C, Stanciu C. The risk of thrombotic events in patients with liver cirrhosis. Rev Med Chir Soc Med Nat Iasi. 2012;116:991–6. [PubMed] [Google Scholar]
- [31].Turon F, Driever EG, Baiges A, et al. Predicting portal thrombosis in cirrhosis: a prospective study of clinical, ultrasonographic and hemostatic factors. J Hepatol. 2021;75:1367–76. [DOI] [PubMed] [Google Scholar]
- [32].Flores B, Trivedi HD, Robson SC, Bonder A. Hemostasis, bleeding and thrombosis in liver disease. J Transl Sci. 2017;3:10.15761/JTS.1000182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Nie G-L, Yan J, Li Y, et al. Predictive model for non-malignant portal vein thrombosis associated with cirrhosis based on inflammatory biomarkers. World J Gastrointest Oncol. 2024;16:1213–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Xing Y, Tian Z, Jiang Y, et al. A practical nomogram based on systemic inflammatory markers for predicting portal vein thrombosis in patients with liver cirrhosis. Ann Med. 2022;54:302–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Chen M, Ji M, Chen T, Hong X, Jia Y. Alcohol consumption and risk for venous thromboembolism: a meta-analysis of prospective studies. Front Nutr. 2020;7:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Pomp ER, Rosendaal FR, Doggen CJ. Alcohol consumption is associated with a decreased risk of venous thrombosis. Thromb Haemost. 2008;99:59–63. [DOI] [PubMed] [Google Scholar]
- [37].Fan X, Huang X, Hershman M, et al. Portal vein thrombosis prevalence and mortality among alcoholic cirrhosis in a nationwide inpatient cohort. Eur J Gastroenterol Hepatol. 2020;32:1160–7. [DOI] [PubMed] [Google Scholar]
- [38].Nguyen GC, Bernstein CN, Bitton A, et al. Consensus statements on the risk, prevention, and treatment of venous thromboembolism in inflammatory bowel disease: Canadian Association of Gastroenterology. Gastroenterology. 2014;146:835–48.e6. [DOI] [PubMed] [Google Scholar]
- [39].Olivera PA, Zuily S, Kotze PG, et al. International consensus on the prevention of venous and arterial thrombotic events in patients with inflammatory bowel disease. Nat Rev Gastroenterol Hepatol. 2021;18:857–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Maconi G, Bolzacchini E, Dell’Era A, Russo U, Ardizzone S, de Franchis R. Portal vein thrombosis in inflammatory bowel diseases: a single-center case series. J Crohns Colitis. 2012;6:362–7. [DOI] [PubMed] [Google Scholar]
- [41].Lankarani KB, Homayon K, Motevalli D, Heidari ST, Alavian SM, Malek-Hosseini SA. Risk factors for portal vein thrombosis in patients with cirrhosis awaiting liver transplantation in Shiraz, Iran. Hepat Mon. 2015;15:e26407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Englesbe MJ, Kubus J, Muhammad W, et al. Portal vein thrombosis and survival in patients with cirrhosis. Liver Transpl. 2010;16:83–90. [DOI] [PubMed] [Google Scholar]
- [43].Xu X, Guo X, De Stefano V, et al. Nonselective beta-blockers and development of portal vein thrombosis in liver cirrhosis: a systematic review and meta-analysis. Hepatol Int. 2019;13:468–81. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.