Abstract
OBJECTIVES:
Continuous renal replacement therapy (CRRT) and shock are both associated with high morbidity and mortality in the ICU. Adult data suggest renoprotective effects of vasopressin vs. catecholamines (norepinephrine and epinephrine). We aimed to determine whether vasopressin use during CRRT was associated with improved kidney outcomes in children and young adults.
DESIGN:
Secondary analysis of Worldwide Exploration of Renal Replacement Outcomes Collaborative in Kidney Disease (WE-ROCK), a multicenter, retrospective cohort study.
SETTING:
Neonatal, cardiac, PICUs at 34 centers internationally from January 1, 2015, to December 31, 2021.
PATIENTS/SUBJECTS:
Patients younger than 25 years receiving CRRT for acute kidney injury and/or fluid overload and requiring vasopressors. Patients receiving vasopressin were compared with patients receiving only norepinephrine/epinephrine. The impact of timing of vasopressin relative to CRRT start was assessed by categorizing patients as: early (on or before day 0), intermediate (days 1–2), and late (days 3–7).
INTERVENTIONS:
None.
MEASUREMENTS AND MAIN RESULTS:
Of 1016 patients, 665 (65%) required vasopressors in the first week of CRRT. Of 665, 248 (37%) received vasopressin, 473 (71%) experienced Major Adverse Kidney Events at 90 days (MAKE-90) (death, renal replacement therapy dependence, and/or > 125% increase in serum creatinine from baseline 90 days from CRRT initiation), and 195 (29%) liberated from CRRT on the first attempt within 28 days. Receipt of vasopressin was associated with higher odds of MAKE-90 (adjusted odds ratio [aOR], 1.80; 95% CI, 1.20–2.71; p = 0.005) but not liberation success. In the vasopressin group, intermediate/late initiation was associated with higher odds of MAKE-90 (aOR, 2.67; 95% CI, 1.17–6.11; p = 0.02) compared with early initiation.
CONCLUSIONS:
Nearly two-thirds of children and young adults receiving CRRT required vasopressors, including over one-third who received vasopressin. Receipt of vasopressin was associated with more MAKE-90, although earlier initiation in those who received it appears beneficial. Prospective studies are needed to understand the appropriate timing, dose, and subpopulation for use of vasopressin.
Keywords: acute kidney injury, dialysis, vasoactives, vasopressor, Worldwide Exploration of Renal Replacement Outcomes Collaborative in Kidney Disease
KEY POINTS
Question: In children requiring vasopressors on continuous renal replacement therapy (CRRT), does vasopressin use, and timing of vasopressin initiation, associate with CRRT liberation or kidney outcomes?
Findings: Two-thirds of patients required vasopressors, of whom 37% received vasopressin. Patients who received vasopressin had worse outcomes; however, early vasopressin initiation (day 0 of CRRT or sooner) reduced odds of Major Adverse Kidney Events at 90 days compared with later initiation.
Meaning: The use of vasopressors including vasopressin is common in children requiring CRRT, although it remains unclear when and in whom vasopressin may be most beneficial. Prospective studies are needed to understand the optimal timing, dose, and subpopulation for vasopressin use.
Acute kidney injury (AKI) is common in critically ill children and associated with poor outcomes, particularly when continuous renal replacement therapy (CRRT) is prescribed (1–3). Children treated with CRRT suffer mortality rates up to 64% (4–7), and survivors are at increased risk for ongoing dialysis dependence and chronic kidney disease (8–10). Children with shock requiring vasopressors experience equally high rates of morbidity and mortality (11, 12). Recently, the multinational Worldwide Exploration of Renal Replacement Outcomes Collaborative in Kidney Disease (WE-ROCK) demonstrated that critically ill children receiving CRRT commonly require vasoactive medications (7, 10, 13), although little is known about outcomes in this vulnerable population (14–16). Given the independently poor prognosis of pediatric AKI requiring CRRT and shock requiring vasopressors, a better understanding of patients with coincidence of these problems is required to identify management strategies that promote survival and kidney recovery.
Catecholaminergic agents (i.e., epinephrine and norepinephrine) are typically the first-line vasopressor therapy in the pediatric population (17, 18). Through stimulation of adrenergic receptors, these medications may have untoward effects, including tachyarrhythmias, hyperglycemia, lactic acidosis, increased catabolism, and myocardial oxygen consumption, particularly when used at high doses (17, 19). Conversely, vasopressin—a common adjunctive vasopressor—is a peptide hormone that augments blood pressure via its direct vasoconstrictor effects mediated by V1 receptor (20). While catecholamines cause renal afferent arteriolar vasoconstriction decreasing glomerular filtration rate (GFR), vasopressin binds V1a receptors on efferent arterioles, increasing GFR and producing a diuretic effect (21). Taken together, it has been hypothesized that use of vasopressin in patients with shock may improve outcomes and mitigate AKI (18, 22). Several adult studies have demonstrated renoprotective effects with vasopressin compared with catecholamines (23–25), namely lower progression to severe AKI, CRRT requirement, and mortality in patients requiring vasopressors. Such data have not been reported in children.
Given these knowledge gaps, we sought to examine if vasopressin use, and timing of vasopressin initiation during CRRT, were associated with successful CRRT liberation and Major Adverse Kidney Events at 90 days (MAKE-90) in a large, international, contemporary cohort of critically ill children and young adults requiring vasopressors while receiving CRRT for AKI and/or fluid overload. We hypothesized that vasopressin administration during CRRT would be associated with increased odds of successful CRRT liberation and lower odds of MAKE-90 and that these effects would be greatest in those who received vasopressin earlier in the CRRT course.
METHODS
Study Design and Patient Selection
We performed a planned secondary analysis of the WE-ROCK international registry, a multicenter (26), international (nine countries) retrospective study of critically ill children and young adults 0–25 years who received CRRT for AKI and/or fluid overload from January 1, 2015, to December 31, 2021. Patients with previous dialysis dependence, concurrent extracorporeal membrane oxygenation use, and those receiving CRRT for another indication were excluded. Patients were included in our analysis if they received vasopressor therapy with norepinephrine, epinephrine, and/or vasopressin at any time during the first week of CRRT. Given our intention was to focus on vasodilatory states, other vasoactive medications (i.e., milrinone, dobutamine) were not collected or analyzed. Given the WE-ROCK study design, daily data (including receipt of vasoactives) was not available beyond CRRT day 7; granular hemodynamic data were also not collected as part of the original study. Complete methodological details have been previously published (27). Each center received local Institutional Review Board approval for the study with a waiver of informed consent (Supplementary Appendix 1, http://links.lww.com/CCX/B401), and the study was performed in accordance with the ethical standards of the 1964 Declaration of Helsinki and later amendments. This study is reported following Strengthening the Reporting of Observational Studies in Epidemiology reporting guidelines (Supplementary Appendix 2, http://links.lww.com/CCX/B401).
Subgroup Definitions
To assess for associations between vasopressin use and outcomes, patients were first categorized into one of two groups based on vasopressor exposure during the first week of CRRT: 1) patients who received vasopressin at any time (with or without norepinephrine/epinephrine; “vasopressin group”) and 2) patients who did not receive any vasopressin (i.e., only received norepinephrine and/or epinephrine; “no vasopressin group”). As vasopressin is not typically used as a first-line vasopressor (17, 28), we did not evaluate patients who received vasopressin alone as the numbers were small (n = 15). To assess for associations between timing of vasopressin initiation and outcomes, the vasopressin group was further subcategorized into three groups: 1) early initiation (CRRT day 0 or before CRRT), 2) intermediate initiation (CRRT days 1–2), or 3) late initiation (CRRT days 3–7). Finally, to assess for an association between vasopressin dose and outcomes, the vasopressin group was dichotomized into “low-dose” (≤ 0.5 mU/kg/min) and “high-dose” (> 0.5 mU/kg/min) using the median dose received in the first week of CRRT, extrapolated from an adult “low-dose” threshold of 0.03 U/min (25, 29).
Quantification of Vasoactive Exposure
Vasopressor exposure was quantified in the 24 hours before CRRT initiation and daily for the first 7 days of CRRT using the Vasoactive-Inotropic Score (VIS), as has been previously defined and validated (30). Vasopressin exposure was also quantified by assessing the fraction of total vasoactive load (by VIS) contributed by vasopressin each day (Supplemental Methods, http://links.lww.com/CCX/B401). Trend in VIS was characterized by a daily percent change in VIS for each day of CRRT (Supplemental Methods, http://links.lww.com/CCX/B401).
Other Definitions
The patients' severity of illness was quantified at ICU admission using the Pediatric Risk of Mortality III score (31, 32) and in the 24 hours before CRRT initiation using the Pediatric Logistic Organ Dysfunction-2 (PELOD-2) score (31, 33). Patients were classified as having sepsis if they were being treated for a known or suspected infection and met at least two Systemic Inflammatory Response Syndrome criteria at CRRT initiation (34). Baseline serum creatinine (SCr) was defined as the lowest SCr (mg/dL) within 90 days before admission; if unknown, a value was imputed using body surface area and an estimated GFR of 100 mL/min/1.73 m2, as previously validated (26). Percent fluid balance from ICU admission to CRRT initiation was calculated using cumulative fluid input and output methodology (Supplemental Methods, http://links.lww.com/CCX/B401) (4, 35, 36).
Outcomes
The primary outcomes were CRRT liberation success and MAKE-90. Liberation success was defined as no need for any renal replacement therapy (RRT) modality for greater than or equal to 72 hours after the first attempt within 28 days of initiation. MAKE-90 was defined as a composite of any of the following at 90 days from CRRT initiation: 1) death, 2) RRT dependence, or 3) persistent kidney dysfunction (> 125% increase in SCr from baseline). Secondary outcomes included CRRT duration, 28-day ventilator-free and ICU-free days (28–days requiring invasive mechanical ventilation or days in the ICU, respectively, with patients who died within 28 d assigned “0”), in-hospital mortality, and the percentage of CRRT days requiring vasopressor support during the first week using the following formula:
Statistical Analysis
Data were summarized as frequencies (%) for categorical variables and median (interquartile range) for continuous variables. Categorical variables were compared using chi-square or Fisher exact tests and continuous variables using Wilcoxon rank-sum or Kruskal-Wallis tests, as appropriate. Comparisons were first made between the no vasopressin group vs. vasopressin group to assess for differences in demographics, clinical features, and outcomes. Logistic mixed-effects regression modeling was used to assess for the independent association between the receipt of vasopressin and primary outcomes, after accounting for a priori identified potential confounders including severity of illness by PELOD-2, vasoactive burden by VIS, the presence of preexisting comorbidities, and urine output before CRRT initiation. The model also included a site-specific random intercept to account for clustering of patients within centers. Sensitivity analyses were also performed in patients with sepsis to assess for potential differences in this unique population and in only those who received CRRT more proximate to ICU admission (i.e., ≤ 2 d) to reduce population heterogeneity.
Subsequent comparisons focused on solely the vasopressin group. First, comparisons were made between the early vasopressin, intermediate vasopressin, and late vasopressin groups to assess differences in demographics, clinical features, and outcomes. A sensitivity analysis was also performed examining only patients who survived until day 7 of CRRT or successfully liberated from CRRT before day 7 to control for the potential impact of early mortality. Adjusting for the same a priori identified confounders above, logistic mixed-effects regression modeling was used to assess for an independent association between timing of vasopressin initiation and outcomes. Finally, comparisons were similarly made between those receiving low-dose and high-dose vasopressin. A p value of 0.05 was considered statistically significant. All statistical analyses were performed using R, Version 4.3.1 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
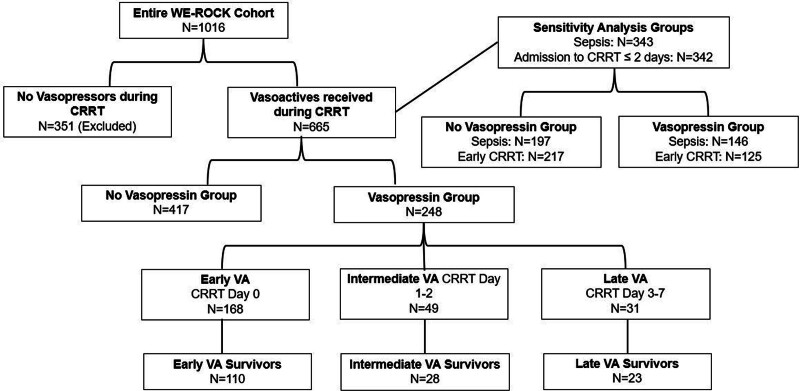
Of 1016 patients, 351 were excluded because they did not receive vasopressors during the first week of CRRT, leaving 665 patients (65%) (Fig. 1). Among these 665 patients, median age was 8.2 years (1.5–14.9 yr), the most common indication for ICU admission was shock, infection, or major trauma (n = 271, 41%) including 343 (52%) with sepsis, and 547 (82%) had at least one comorbidity (Table 1). At CRRT initiation, 526 (79%) were requiring vasopressors and 635 (96%) were receiving invasive mechanical ventilation (Table 1). Liberation from CRRT was successful on the first attempt within 28 days in 195 patients (29%) and MAKE-90 occurred in 473 patients (71%), due to 90-day mortality in 327 (49%), persistent kidney dysfunction in 102 (15%), and RRT dependence in 44 (7%) (Table 1).
Figure 1.
Consolidated Standards of Reporting Trials flow diagram. CRRT = continuous renal replacement therapy, VA = vasopressin, WE-ROCK = Worldwide Exploration of Renal Replacement Outcomes Collaborative in Kidney Disease.
TABLE 1.
Demographic, Clinical Characteristics, and Outcomes of Children Requiring Vasopressors While Receiving Continuous Renal Replacement Therapy Who Received Vasopressin Compared With Those Who Did Not
| Variable | All (n = 665) | No Vasopressin (n = 417) | Vasopressin (n = 248) | p |
|---|---|---|---|---|
| Demographics | ||||
| Age, yr | 8.2 (1.5–14.9) | 7.0 (1.3–14.7) | 9.6 (2.1–15.3) | 0.14 |
| Sex, female (%) | 302 (45) | 186 (45) | 116 (47) | 0.59 |
| Admission weight, kg | 24 (11–53) | 22 (10–53) | 30 (12–53) | 0.14 |
| Admission diagnosis, n (%) | 0.15 | |||
| Shock/infection/major trauma | 271 (41) | 158 (38) | 113 (46) | |
| Respiratory failure | 150 (23) | 96 (23) | 54 (22) | |
| Post-surgical/minor trauma | 30 (4.5) | 17 (4.1) | 13 (5.2) | |
| Primary cardiac | 98 (15) | 63 (15) | 35 (14) | |
| Other | 116 (17) | 83 (20) | 33 (13) | |
| Comorbidities, n (%) | 0.58 | |||
| 0 | 118 (18) | 74 (18) | 44 (18) | |
| 1 | 328 (49) | 205 (49) | 123 (50) | |
| 2 | 140 (21) | 93 (22) | 47 (19) | |
| > 2 | 79 (12) | 45 (11) | 34 (14) | |
| Pediatric Risk of Mortality III | 15 (11–19) | 14 (10–19) | 16 (11–20) | 0.11 |
| Sepsis, n (%) | 343 (52) | 197 (47) | 146 (59) | 0.004 |
| CRRT initiation data | ||||
| Time from ICU admission to CRRT, d | 2 (1–7) | 2 (1–6) | 2 (1–9) | 0.24 |
| Pediatric Logistic Organ Dysfunction -2 | 7 (4–9) | 6 (3–9) | 7 (4–10) | 0.002 |
| Receipt of vasopressors, n (%) | 526 (79) | 303 (73) | 223 (90) | < 0.001 |
| Pre-CRRT Vasoactive-Inotropic Score | 12 (3–30) | 8 (0–23) | 22 (10–39) | < 0.001 |
| Mechanical ventilation, n (%) | 635 (96) | 395 (95) | 240 (97) | 0.14 |
| %Fluid balance from ICU admission | 9 (3–21) | 8 (3–18) | 11 (5–26) | 0.003 |
| Urine output 24 hr prior, mL/kg/hr | 0.51 (0.13–1.2) | 0.52 (0.12–1.3) | 0.45 (0.13–1.1) | 0.39 |
| Time to first negative fluid balance, d | 1 (0–1) | 1 (0–1) | 1 (0–2) | 0.158 |
| Outcomes | ||||
| 28-d CRRT liberation status, n (%) | < 0.001 | |||
| Liberated | 195 (29) | 142 (34) | 53 (21) | |
| Liberation not attempted | 306 (46) | 172 (41) | 134 (54) | |
| Reinstituted | 164 (25) | 103 (25) | 61 (25) | |
| CRRT duration, d | 7 (4–16) | 7 (4–16) | 7 (3–16) | 0.27 |
| %Days 1–7 of CRRT requiring vasopressors | 75 (40–100) | 63 (38–100) | 100 (63–100) | < 0.001 |
| 28-d ventilator-free days | 0 (0–28) | 6 (0–28) | 0 (0–19) | < 0.001 |
| 28-d ICU-free days | 0 (0–3) | 0 (0–6) | 0 (0–0) | < 0.001 |
| In-hospital mortality, n (%) | 330 (50) | 185 (44) | 145 (58) | < 0.001 |
| Major Adverse Kidney Events at 90 d, n (%) | 473 (71) | 276 (68) | 197 (79) | 0.001 |
| 90-d mortality | 327 (49) | 185 (44) | 142 (57) | 0.001 |
| Persistent kidney dysfunction | 102 (15) | 63 (15) | 39 (16) | 0.83 |
| Renal replacement therapy dependence | 44 (7) | 28 (7) | 16 (6) | 0.89 |
CRRT = continuous renal replacement therapy.
The vasopressin group consisted of all patients who received vasopressin and/or norepinephrine/epinephrine, whereas the no vasopressin group consisted of patients receiving norepinephrine/epinephrine without vasopressin. Continuous variables are reported as median (interquartile range). p values were obtained using Pearson χ2 test, Wilcoxon rank-sum test, or Fisher exact test, as appropriate.
Demographics, Clinical Features, and Outcomes by Receipt of Vasopressin
Two hundred forty-eight patients (37%) received vasopressin in the first week of CRRT (vasopressin group). A summary of the demographics, clinical characteristics, and outcomes of the cohort by receipt of vasopressin are included in Table 1. Patients in the vasopressin group more frequently had sepsis, had higher pre-CRRT PELOD-2 score, more commonly required vasopressors before CRRT with higher VIS at CRRT start, and had greater %fluid balance at CRRT initiation. On each day of CRRT (days 0–7), patients in the vasopressin group had higher median VIS compared with the no vasopressin group, although the median %VIS accounted for by vasopressin was less than 20% for each day (Supplemental Table 1, http://links.lww.com/CCX/B401). The vasopressin group required vasopressors on a higher percentage of CRRT days in the first week (100% [63–100%] vs. 63% [38–100%]; p < 0.001), had six fewer ventilator-free days (p < 0.001), were less likely to liberate successfully form CRRT on the first attempt (21% vs. 34%, p < 0.001), and had more MAKE-90 (79% vs. 68%; p = 0.001) driven by higher mortality (57% vs. 44%; p = 0.001) (Table 1). On multivariable logistic regression analysis, receipt of vasopressin was not associated with liberation success but was associated with higher odds of MAKE-90 (adjusted odds ratio [aOR], 1.80; 95% CI, 1.20–2.71; p = 0.005) (Table 2). Sensitivity analyses performed only in patients with sepsis (n = 343) (Supplemental Table 2, http://links.lww.com/CCX/B401) and in those who started CRRT less than or equal to 2 days from ICU admission (n = 342) (Supplemental Table 3, http://links.lww.com/CCX/B401) demonstrated similar findings to the whole cohort.
TABLE 2.
Multivariable Analyses Examining the Association Between A Priori Selected Demographic and Clinical Variables and the Primary Outcomes of Successful Continuous Renal Replacement Therapy Liberation and Development of Major Adverse Kidney Events at 90 Days in Children Requiring Vasopressors While Receiving Continuous Renal Replacement Therapy
| Variable | Adjusted OR Liberation Success (95% CI) | p | Adjusted OR Major Adverse Kidney Events at 90 d (95% CI) | p |
|---|---|---|---|---|
| Entire cohort | ||||
| Receipt of vasopressin, yes | 0.59 (0.38–1.06) | 0.082 | 1.80 (1.20–2.71) | 0.005 |
| Pre-CRRT UOP | 1.14 (1.01–1.30) | 0.038 | 0.87 (0.78–0.97) | 0.014 |
| Pre-CRRT PELOD-2 | 1.01 (0.95–1.08) | 0.71 | 1.06 (1.01–1.12) | 0.023 |
| Pre-CRRT VIS | 1.01 (1.00–1.02) | 0.074 | 1.00 (0.99–1.00) | 0.39 |
| Comorbidities, yes | 0.55 (0.31–0.96) | 0.035 | 3.35 (2.16–5.21) | 0.023 |
| Vasopressin group only | ||||
| Timing of vasopressin initiation | ||||
| Early vasopressin | ||||
| Intermediate/late vasopressin | 1.36 (0.52–3.59) | 0.53 | 2.67 (1.17–6.11) | 0.02 |
| Pre-CRRT UOP | 1.46 (0.86–2.48) | 0.16 | 0.93 (0.62–1.39) | 0.73 |
| Pre-CRRT PELOD-2 | 0.97 (0.88–1.07) | 0.55 | 1.08 (1.00–1.15) | 0.044 |
| Pre-CRRT VIS | 1.01 (0.99–1.03) | 0.30 | 1.00 (0.99–1.01) | 0.98 |
| Comorbidities, yes | 0.58 (0.21–1.58) | 0.28 | 2.33 (1.08–5.04) | 0.032 |
CRRT = continuous renal replacement therapy, OR = odds ratio, PELOD-2 = Pediatric Logistic Organ Dysfunction-2, UOP = urine output, VIS = Vasoactive-Inotropic Score.
Multivariable logistic regression performed with above a priori selected variables using mixed-effects logistic regression to adjust for center.
Association of Timing of Vasopressin Initiation With Outcomes
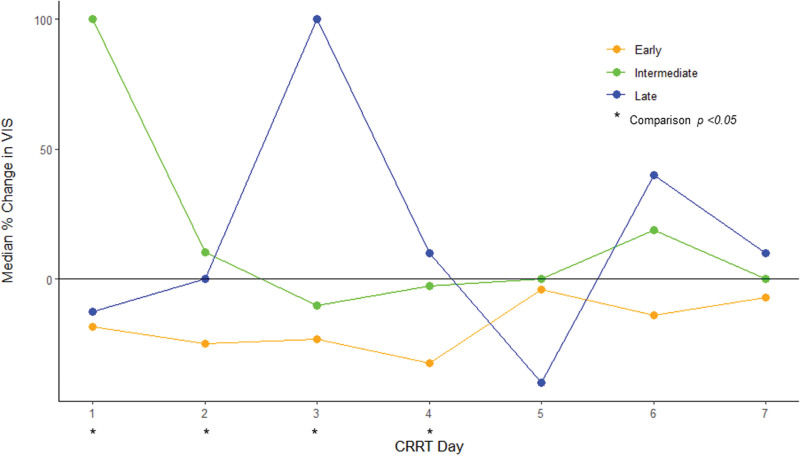
In the vasopressin group (n = 248), a majority had early vasopressin initiation (n = 168, 68%), whereas 49 (20%) had intermediate vasopressin initiation, and 31 (12%) had late vasopressin initiation (Table 3; and Supplemental Fig. 1, http://links.lww.com/CCX/B401). Early vasopressin patients initiated CRRT closest to ICU admission, had the lowest urine output in the 24 hours before CRRT initiation, were most likely to need vasoactives pre-CRRT, and had the highest VIS at CRRT start, compared with the intermediate and late vasopressin initiation groups (Table 3). There were no differences in other demographic variables or severity of illness markers between groups (Table 3). Only the early vasopressin group consistently reduced its vasoactive requirement each day based on daily VIS %Change (Fig. 2; and Supplemental Table 4, http://links.lww.com/CCX/B401). Early vasopressin patients were more likely to successfully liberate from CRRT (24% vs. 16% vs. 16%; p = 0.022), had shorter CRRT duration (6 d [2–15 d] vs. 7 d [4–18 d] vs. 9 d [5–16 d]; p = 0.045), and less MAKE-90 (75% vs. 92% vs. 84%; p = 0.03) compared with intermediate and late vasopressin patients (Table 3). Similar findings were seen on a sensitivity analysis excluding patients who died during the first week of CRRT (Supplemental Tables 5–7 and Supplemental Fig. 2, http://links.lww.com/CCX/B401). On multivariable logistic regression, intermediate or late vasopressin initiation was associated with higher odds of MAKE-90 (aOR, 2.67; 95% CI, 1.17–6.11; p = 0.02) compared with early vasopressin initiation (Table 2). Conversely, timing of vasopressin initiation was not associated with CRRT liberation success (Table 2). Similar findings were seen in the sensitivity analysis cohort (Supplemental Table 7, http://links.lww.com/CCX/B401).
TABLE 3.
Demographic, Clinical Characteristics, and Outcomes of Children Requiring Vasopressors While Receiving Continuous Renal Replacement Therapy Based on Timing of Vasopressin Administration
| Variable | Early Vasopressin (n = 168) | Intermediate Vasopressin (n = 49) | Late Vasopressin (n = 31) | p |
|---|---|---|---|---|
| Demographics | ||||
| Age, yr | 9.9 (2.5–15.7) | 6.6 (1.1–14.2) | 10.9 (2.0–17.3) | 0.31 |
| Sex, female (%) | 76 (45) | 25 (51) | 15 (48) | 0.76 |
| Admission weight, kg | 31 (13–60) | 20 (10–47) | 38 (13–54) | 0.14 |
| Admission diagnosis, n (%) | 0.043 | |||
| Shock/infection/major trauma | 87 (52) | 18 (37) | 8 (26) | |
| Respiratory failure | 30 (18) | 14 (29) | 10 (32) | |
| Post-surgical/minor trauma | 9 (5) | 3 (6) | 1 (3) | |
| Primary cardiac | 24 (14) | 5 (10) | 6 (20) | |
| Other | 18 (11) | 9 (18) | 6 (19) | |
| Comorbidities, n (%) | 0.15 | |||
| 0 | 33 (20) | 7 (14) | 4 (13) | |
| 1 | 88 (52) | 25 (51) | 10 (32) | |
| 2 | 27 (16) | 9 (18) | 11 (35) | |
| > 2 | 20 (12) | 8 (16) | 6 (19) | |
| Pediatric Risk of Mortality III | 16 (12–21) | 15 (8–18) | 13 (11–17) | 0.06 |
| Sepsis, n (%) | 102 (61) | 28 (57) | 16 (52) | 0.62 |
| CRRT initiation data | ||||
| Time from ICU admission to CRRT, d | 2 (1–7) | 4 (1–13) | 5 (2–11) | 0.02 |
| Pediatric Logistic Organ Dysfunction-2 | 8.0 (5.0–10.0) | 7.0 (4.0–10.0) | 5.0 (3–9.5) | 0.20 |
| Receipt of vasopressors, n (%) | 160 (95) | 37 (76) | 26 (84) | < 0.001 |
| Pre-CRRT Vasoactive-Inotropic Score | 30 (15–46) | 10 (2–25) | 6 (3–17) | < 0.001 |
| Mechanical ventilation, n (%) | 162 (96) | 47 (98) | 31 (100) | 0.85 |
| %Fluid balance from ICU admission | 11 (50–28) | 11 (4–18) | 13 (5–26) | 0.71 |
| Urine output 24 hr prior, mL/kg/hr | 0.38 (0.10–0.98) | 0.41 (0.16–0.99) | 0.87 (0.41–1.4) | 0.034 |
| Time to first negative fluid balance, d | 1 (0–2) | 1 (0–2) | 1 (0–1) | 0.22 |
| Outcomes | ||||
| 28-d CRRT liberation status, n (%) | 0.022 | |||
| Liberated | 40 (24) | 8 (16) | 5 (16) | |
| Liberation not attempted | 80 (48) | 36 (73) | 18 (58) | |
| Reinstituted | 48 (29) | 5 (10) | 8 (26) | |
| CRRT duration, d | 6 (2–15) | 7 (4–18) | 9 (5–16) | 0.045 |
| %Days 1–7 of CRRT requiring vasopressors | 100 (63–100) | 100 (75–100) | 83 (56–88) | 0.02 |
| 28-d ventilator-free days | 0 (0–21) | 0 (0–0) | 0 (0–12) | 0.017 |
| 28-d ICU-free days | 0 (0,0) | 0 (0,0) | 0 (0,0) | 0.025 |
| In-hospital mortality, n (%) | 92 (55) | 34 (69) | 19 (61) | 0.18 |
| Major Adverse Kidney Events at 90 d, n (%) | 126 (75) | 45 (92) | 26 (84) | 0.030 |
| 90-d mortality | 88 (52) | 35 (71) | 19 (61) | 0.053 |
| Persistent kidney dysfunction | 30 (18) | 4 (8) | 5 (16) | 0.26 |
| Renal replacement therapy dependence | 8 (5) | 6 (12) | 2 (6) | 0.17 |
CRRT = continuous renal replacement therapy.
Early vasopressin group initiated vasopressin on day 0 of CRRT, intermediated vasopressin group initiated vasopressin on days 1–2 of CRRT, and late vasopressin group initiated vasopressin on days 3–7 of CRRT. Continuous variables are reported as median (interquartile range). p values were obtained using Pearson χ2 test, Wilcoxon rank-sum test, or Fisher exact test, as appropriate.
Figure 2.
Median daily percent change in Vasoactive-Inotropic Score (VIS) by timing of vasopressin initiation (early, intermediate, or late) in all patients receiving vasopressin (VA group). CRRT = continuous renal replacement therapy.
Association of Vasopressin Dose With Outcomes
Two hundred forty-five of 248 patients in the vasopressin group had dosing information available, with 99 (40%) receiving low-dose vasopressin and 146 (60%) receiving high-dose vasopressin (Table 4). Patients who received high-dose vasopressin were younger and more likely to have at least one comorbidity (Table 4). There were no differences in pre-CRRT severity of illness or other CRRT initiation data between groups (Table 4). Patients who received high-dose vasopressin were more likely to never have a CRRT liberation attempt by 28 days (63% vs. 41%; p = 0.002) and required vasopressors for a higher percentage of CRRT days in the first week (100% [75–100%] vs. 88% [61–100%]; p = 0.019). While there was no difference in MAKE-90 between low-dose and high-dose vasopressin groups, the high-dose vasopressin group had higher 90-day mortality (67% vs. 43%; p < 0.001) but lower RRT dependence at 90 days in survivors (3% vs. 11%; p = 0.02).
TABLE 4.
Demographic, Clinical Characteristics, and Outcomes of Children Requiring Vasopressors While Receiving Continuous Renal Replacement Therapy Based on Median Dose of Vasopressin Received
| Variable | Low-Dose Vasopressin (Median Dose ≤ 0.5 mU/kg/min) (n = 99) | High-Dose Vasopressin (> 0.5 mU/kg/min) (n = 146) | p |
|---|---|---|---|
| Demographics | |||
| Age, yr | 11.1 (4.0–15.9) | 6.8 (1.4–14.9) | 0.042 |
| Sex, female (%) | 46 (46) | 68 (47) | 0.99 |
| Admission weight, kg | 35 (16–54) | 22 (11–53) | 0.033 |
| Admission diagnosis, n (%) | 0.39 | ||
| Shock/infection/major trauma | 44 (44) | 67 (46) | |
| Respiratory failure | 21 (21) | 32 (22) | |
| Post-surgical/minor trauma | 3 (3) | 10 (7) | |
| Primary cardiac | 15 (15) | 20 (14) | |
| Other | 16 (16) | 17 (12) | |
| Comorbidities, n (%) | 0.042 | ||
| 0 | 22 (22) | 21 (14) | |
| 1 | 38 (38) | 83 (57) | |
| 2 | 23 (23) | 24 (16) | |
| > 2 | 16 (16) | 18 (12) | |
| Pediatric Risk of Mortality III | 16 (11–20) | 16 (11–20) | 0.40 |
| Sepsis, n (%) | 57 (58) | 87 (60) | 0.75 |
| CRRT initiation data | |||
| Time from ICU admission to CRRT, d | 2 (1–8) | 3 (1–9) | 0.12 |
| Pediatric Logistic Organ Dysfunction-2 | 7 (5–10) | 7 (4–10) | 0.50 |
| Receipt of vasopressors, n (%) | 91 (92) | 129 (88) | 0.37 |
| Pre-CRRT Vasoactive-Inotropic Score | 20 (10–33) | 25 (10–42) | 0.21 |
| Mechanical ventilation, n (%) | 94 (95) | 143 (99) | 0.12 |
| Urine output 24 hr prior, mL/kg/hr | 0.51 (0.16–1.06) | 0.40 (0.11–1.07) | 0.65 |
| Time to first negative fluid balance, d | 1 (0–2) | 1 (0–1) | 0.25 |
| Outcomes | |||
| 28-d CRRT liberation status, n (%) | 0.002 | ||
| Liberated | 23 (23) | 28 (19) | |
| Liberation not attempted | 41 (41) | 92 (63) | |
| Reinstituted | 35 (35) | 26 (18) | |
| CRRT duration, d | 10 (4–17) | 7 (2–13) | 0.09 |
| %Days 1–7 of CRRT requiring vasopressors | 88 (61–100) | 100 (75–100) | 0.019 |
| 28-d ventilator-free days | 0 (0–27) | 0 (0–7) | 0.002 |
| 28-d ICU-free days | 0 (0–1) | 0 (0–0) | 0.09 |
| In-hospital mortality, n (%) | 46 (46) | 98 (67) | 0.001 |
| Major Adverse Kidney Events at 90 d, n (%) | 74 (75) | 122 (84) | 0.091 |
| 90-d mortality | 43 (43) | 98 (67) | < 0.001 |
| Persistent kidney dysfunction | 20 (20) | 19 (13) | 0.13 |
| Renal replacement therapy dependence | 11 (11) | 5 (3) | 0.02 |
CRRT = continuous renal replacement therapy.
Three of 248 patients who received vasopressin excluded from this analysis due to missing dose data (n = 245). Continuous variables are reported as median (interquartile range). p values were obtained using Pearson χ2 test, Wilcoxon rank-sum test, or Fisher exact test, as appropriate.
DISCUSSION
In this secondary analysis of the large, multinational, multicenter WE-ROCK study of children and young adults receiving CRRT for AKI and/or fluid overload, we found that nearly two-thirds required vasopressors in the first week of CRRT. One in three of these patients received vasopressin, with most (67%) initiating vasopressin early (i.e., on day 0 of CRRT or before). Patients who received vasopressin more commonly had sepsis, were sicker by PELOD-2 and VIS at time of CRRT initiation, and generally suffered worse outcomes, including lower rates of CRRT liberation and higher rates of MAKE-90, than those who received only catecholamines (norepinephrine/epinephrine). The associations between higher odds of MAKE-90 and receipt of vasopressin persisted on multivariable regressions, a finding that requires further exploration. However, when only those receiving vasopressin were examined, early administration (i.e., on day 0 of CRRT or before) appeared to be associated with improved outcomes, including higher rates of successful CRRT liberation, shorter CRRT duration, and lower odds of MAKE-90. Finally, while receipt of high-dose vasopressin (> 0.5 mU/kg/min) was generally associated with worse outcomes, those who received high-dose vasopressin and survived had lower RRT dependence at 90 days, a finding warranting further exploration.
In this study, children and young adults requiring CRRT and concomitant vasopressor medication had worse outcomes if they received vasopressin, a result that is contradictory to our original hypothesis and the adult literature (23–25). The reasons for this discrepancy are unclear, although it is possible that uncaptured differences in illness severity and patient heterogeneity played a significant role. This hypothesis is supported by a pre- and post-Vasopressin and Septic Shock Trial (VASST) propensity-matched retrospective study in adults (37). In this study, patients receiving vasopressin vs. norepinephrine in the pre-VASST period were sicker (i.e., higher illness severity scores, more organ dysfunction, higher norepinephrine burden) but did have higher mortality even after matching for illness severity. However, these differences were not seen in the post-trial period after the same matching technique was applied, presumably secondary to a more thoughtful and targeted approach to vasopressin use informed by VASST that was not captured by the dataset (37). Furthermore, Bhatraju et al (38) demonstrated in a post hoc analysis of VASST that only one specific subphentoype of sepsis-associated AKI, characterized by better renal function, lower rates of sepsis, and lower vasopressor burden, had improved outcomes with receipt of vasopressin, while no benefit was seen in the remaining apparently sicker patients. These data are relevant given that our vasopressin group had higher rates of sepsis and greater vasoactive burden at time of CRRT initiation than the no vasopressin group, although a sensitivity analysis examining only sepsis patients demonstrated similar findings to the entire cohort. Last, it remains possible that vasopressin only has benefit before CRRT, as its potential renoprotective effects were all demonstrated in cohorts where vasopressin was started before CRRT initiation (23, 25). Regardless of the reason, our data combined with small prospective pediatric studies of vasopressin showing nonsignificant yet divergent mortality outcomes (39, 40), highlight the need for further, rigorous study in more homogenous populations to identify the patient population (if any) in whom use of vasopressin improves outcomes.
In patients receiving vasopressin, our data suggest that earlier initiation is associated with improved outcomes, consistent with previous adult literature (23–25). In this cohort, intermediate/late vasopressin initiation was independently associated with higher odds of MAKE-90, even though patients with early vasopressin initiation appeared sicker at time of CRRT start. The benefit of early vasopressin has been highlighted in adult studies, as only these patients have had lower progression to severe AKI, less need for CRRT, and lower mortality compared with those receiving norepinephrine (23, 25). More recently, a study of adults with septic shock who received vasopressin showed that survivors were started on vasopressin at a lower lactate concentration, lower norepinephrine-equivalent dose, and at an earlier time point in sepsis (41). There are multiple purported reasons for the benefit seen with early vasopressin administration. Vasopressin is a hormone with relative deficiency in shock states, so exogenous administration may simply restore levels necessary for adequate vascular tone (20). Low vasopressin levels are associated with coronary, cerebral, and pulmonary vasodilation, leading to blood redirection to critical organs affected in shock states, but potentially decreasing renal perfusion (20). As catecholamines further reduce renal blood flow via afferent arteriolar vasoconstriction, an indirect benefit of vasopressin may come from its ability to decrease catecholamine burden (42); indeed, in this study, we did see that early vasopressin patients were the only ones to consistently reduce their vasoactive requirement (which was largely driven by catecholamines) each day of CRRT and also noted that receipt of high-dose vasopressin (which may have been catecholamine sparing) was associated with lower RRT dependence at 90 days. Ultimately, while not definitive, these data provide some preliminary evidence to suggest that if vasopressin is to be used in children and young adults receiving CRRT, introducing it early in the CRRT course may be most beneficial. Conversely, although some literature does suggest higher doses of vasopressin may improve hemodynamics (29), these data are less robust and further work is needed to assess for potential dose-dependent effects of vasopressin on outcomes.
Taken together, these data are hypothesis generating and highlight the need for future studies investigating the timing and ideal patient population for use of vasopressin in children receiving CRRT and requiring vasopressors. Specifically, future studies are needed to investigate the role of early vasopressin when catecholamine levels are still relatively low (41). Importantly, predictive enrichment (i.e., identifying the specific AKI subphenotype) may define a cohort most likely to benefit from vasopressin treatment (43), and existing prognostic enrichment tools for AKI and/or CRRT such as the Renal Angina Index could identify patients appropriate for enrollment before CRRT initiation (5, 44, 45). As noted above, pediatric studies are also needed to evaluate the effects of duration and dosing of vasopressin on outcomes, incorporating strategies that have been successful in adult populations (23–25).
This study has several strengths. It is the first large, multicenter, international study examining the demographics, clinical characteristics, and outcomes of critically ill children and young adults requiring vasopressors while receiving CRRT and the association between receipt of vasopressin and outcomes. The main limitations of our study come from its retrospective nature and the heterogeneity of the population that was unable to be accounted for. While we attempted to control for the latter through several sensitivity analyses whose results ultimately were consistent with the entire cohort, this heterogeneity likely contributed to the unexpected results demonstrated. Furthermore, both groups could have received catecholamines, and thus it is possible the adverse effects of epinephrine/norepinephrine offset any beneficial effects of vasopressin in those patients. We were unable to control for when and in what context vasopressin was used, nor do we have granular hemodynamic data to categorize the etiology of vasopressor requirement, which will need to be addressed in future studies. We only evaluated patients with severe AKI who were already requiring CRRT, which may be a time point beyond which vasopressin is helpful with regard to renoprotection. Finally, we only looked at vasopressin, norepinephrine, and epinephrine as an attempt to focus on vasodilatory states; however, nearly 15% of our patients had a primary cardiac diagnosis and given the coincidence of myocardial dysfunction in pediatric septic shock (46), it is possible that inadvertent inclusion of those with cardiogenic shock biased the results.
CONCLUSIONS
In a large, international, multicenter study of children and young adults requiring CRRT for AKI and/or fluid overload, nearly two-thirds required vasopressors in the first week of CRRT, with one in three receiving vasopressin. Receipt of vasopressin appears to be independently associated with higher rates of MAKE-90; however, earlier initiation in those receiving vasopressin appears beneficial. Future studies are needed to better understand the ideal subpopulation for use, dose, and timing of vasopressin initiation in these patients.
Supplementary Material
APPENDIX
The Consortia-on behalf of the Worldwide Exploration of Renal Replacement Outcomes Collaborative in Kidney Disease (WE-ROCK) Investigators: The following individuals served as collaborators and investigators for the WE-ROCK studies. They collaborated in protocol development and review, data analysis, and participated in drafting or review of the article, and their names should be citable by PubMed.
Emily Ahern, CPNP, DNP (Children’s Hospital Colorado, University of Colorado School of Medicine, Aurora, CO), Ayse Akcan Arikan, MD (Baylor College of Medicine, Texas Children’s Hospital, Houston, TX), Issa Alhamoud, MD (University of Iowa Stead Family Children’s Hospital, Carver College of Medicine, Iowa City, IA), Rashid Alobaidi, MD, MSc (Univeristy of Alberta, Edmonton, AB, Canada), Pilar Anton-Martin, MD, PhD (Le Bonheur Children’s Hospital, Memphis, TN), Shanthi S. Balani, MD (University of Minnesota, Minneapolis, MN), Matthew Barhight, MD, MS (Ann and Robert H. Lurie Children’s Hospital of Chicago, Chicago, IL), Abby Basalely, MD, MS (Cohen Children’s Medical Center, Zucker School of Medicine, New Hyde Park, NY), Amee M. Bigelow, MD, MS (Nationwide Children’s Hospital, The Ohio State University College of Medicine, Columbus, OH), Gabriella Bottari, MD (Bambino Gesù Children Hospital, IRCCS, Rome, Italy), Andrea Cappoli, MD (Bambino Gesù Children Hospital, IRCCS, Rome, Italy), Eileen A. Ciccia, MD (Washington University School of Medicine, St. Louis Children’s Hospital, St. Louis, MO), Michaela Collins, BA (Cincinnati Children’s Hospital Medical Center, University of Cincinnati College of Medicine, Cincinnati, OH), Denise Colosimo, MD (Meyer Children’s Hospital, IRCCS, Florence, Italy), Gerard Cortina, MD (Medical University of Innsbruck, Innsbruck, Austria), Mihaela A. Damian, MD, MPH (Stanford University School of Medicine, Palo Alto, CA), Sara De la Mata Navazo, MD (Gregorio Marañón University Hospital, School of Medicine, Madrid, Spain), Gabrielle DeAbreu, MD (Cohen Children’s Medical Center, Zucker School of Medicine, New Hyde Park, NY), Akash Deep, MD (King’s College Hospital, London, United Kingdom), Kathy L. Ding, BS (University of Colorado, School of Medicine, Aurora, CO), Kristin J. Dolan, MD (Baylor College of Medicine, Texas Children’s Hospital, Houston, TX), Sarah N. Fernandez Lafever, MD, PhD (Gregorio Marañón University Hospital, School of Medicine, Madrid, Spain), Dana Y. Fuhrman, DO, MS (University of Pittsburgh Medical Center Children’s Hospital of Pittsburgh, Pittsburgh, PA), Ben Gelbart, MBBS (Royal Children’s Hospital, University of Melbourne, Murdoch Children’s Research Institute, Melbourne, VIC, Australia), Katja M. Gist, DO, MSc (Cincinnati Children’s Hospital Medical Center, University of Cincinnati College of Medicine, Cincinnati, OH), Stephen M. Gorga, MD, MSc (University of Michigan Medical School, C.S. Mott Children’s Hospital, Ann Arbor, MI), Francesco Guzzi, MD (Santo Stefano Hospital, Prato, Italy), Isabella Guzzo, MD (Bambino Gesù Children Hospital, IRCCS, Rome, Italy), Taiki Haga, MD (Osaka City General Hospital, Osaka, Japan), Elizabeth Harvey, MD (Hospital for Sick Children, Toronto, ON, Canada), Denise C. Hasson, MD (NYU Langone Health, Hassenfeld Children’s Hospital, New York, NY), Taylor Hill-Horowitz, BS (Cohen Children’s Medical Center, Zucker School of Medicine, New Hyde Park, NY), Haleigh Inthavong, BS, MS (Baylor College of Medicine, Texas Children’s Hospital, Houston, TX), Catherine Joseph, MD (Baylor College of Medicine, Texas Children’s Hospital, Houston, TX), Ahmad Kaddourah, MD, MS (Sidra Medicine and Weil Cornel Medicine, Qatar, Doha, Qatar), Aadil Kakajiwala, MD, MSCI (Children’s National Hospital, Washington, DC), Aaron D. Kessel, MD, MS (Cohen Children’s Medical Center, Zucker School of Medicine, New Hyde Park, NY), Sarah Korn, DO (Westchester Medical Center, Westchester, NY), Kelli A. Krallman, BSN, MS (Cincinnati Children’s Hospital Medical Center, University of Cincinnati College of Medicine, Cincinnati, OH), David M. Kwiatkowski, MD, Msc (Lucile Packard Children’s Hospital, Palo Alto, CA), Jasmine Lee, MSc (Hospital for Sick Children, Toronto, ON, Canada), Laurance Lequier, MD (Univeristy of Alberta, Edmonton, AB, Canada), Tina Madani Kia, BS (Univeristy of Alberta, Edmonton, AB, Canada), Kenneth E. Mah, MD, MS (Stanford University School of Medicine, Palo Alto, CA), Eleonora Marinari, MD (Bambino Gesù Children Hospital, IRCCS, Rome, Italy), Susan D. Martin, MD (Golisano Children’s Hospital at University of Rochester Medical Center, Rochester, NY), Shina Menon, MD (Seattle Children’s Hospital, University of Washington, Seattle, WA), Tahagod H. Mohamed, MD (Nationwide Children’s Hospital, The Ohio State University College of Medicine, Columbus, OH), Catherine Morgan, MD, MSc (Univeristy of Alberta, Edmonton, AB, Canada), Theresa A. Mottes, APRN (Ann and Robert H. Lurie Children’s Hospital of Chicago, Chicago, IL), Melissa A. Muff-Luett, MD (University of Nebraska Medical Center, Children’s Hospital & Medical Center, Omaha, NE), Siva Namachivayam, MBBS (Royal Children’s Hospital, University of Melbourne, Murdoch Children’s Research Institute, Melbourne, VIC, Australia), Tara M. Neumayr, MD (Washington University School of Medicine, St. Louis Children’s Hospital, St. Louis, MO), Jennifer Nhan Md, MS (Children’s National Hospital, Washington, DC), Abigail O’Rourke, MD (Cohen Children’s Medical Center, Zucker School of Medicine, New Hyde Park, NY), Nicholas J. Ollberding, PhD (Cincinnati Children’s Hospital Medical Center, University of Cincinnati College of Medicine, Cincinnati, OH), Matthew G. Pinto, MD (Maria Fareri Children’s Hospital at Westchester Medical Center, Valhalla, NY), Dua Qutob, MD (Sidra Medicine and Weil Cornel Medicine, Qatar, Doha, Qatar), Valeria Raggi, MD (Bambino Gesù Children Hospital, IRCCS, Rome, Italy), Stephanie Reynaud, MD (IWK Health, Dalhousie University, Halifax, NS, Canada), Zaccaria Ricci, MD (Meyer Children’s Hospital, IRCCS, Florence, Italy), Zachary A. Rumlow, DO (University of Iowa Stead Family Children’s Hospital, Carver College of Medicine, Iowa City, IA), María J. Santiago Lozano, MD, PhD (Gregorio Marañón University Hospital, School of Medicine, Madrid, Spain), Emily See, MBBS (Royal Children’s Hospital, University of Melbourne, Murdoch Children’s Research Institute, Melbourne, VIC, Australia), David T. Selewski, MD, MSCR (Medical University of South Carolina, Charleston, SC), Carmela Serpe, MSc, PhD (Bambino Gesù Children Hospital, IRCCS, Rome, Italy), Alyssa Serratore, RN, MsC (Royal Children’s Hospital, University of Melbourne, Murdoch Children’s Research Institute, Melbourne, VIC, Australia), Ananya Shah, BS (University of Colorado, School of Medicine, Aurora, CO), Weiwen V. Shih, MD (Children’s Hospital Colorado, University of Colorado School of Medicine, Aurora, CO; University of Colorado, School of Medicine, Aurora, CO), H. Stella Shin, MD (Children’s Healthcare of Atlanta, Emory University, Atlanta, GA), Cara L. Slagle, MD (Cincinnati Children’s Hospital Medical Center, University of Cincinnati College of Medicine, Cincinnati, OH), Sonia Solomon, DO (Maria Fareri Children’s Hospital at Westchester Medical Center, Valhalla, NY), Danielle E. Soranno, MD (Indiana University School of Medicine, Riley Hospital for Children, Indianapolis, IN), Rachana Srivastava, MD (Mattel Children’s Hospital at UCLA, Los Angeles, CA), Natalja L. Stanski, MD (Cincinnati Children’s Hospital Medical Center, University of Cincinnati College of Medicine, Cincinnati, OH), Michelle C. Starr, MD, MPH (Indiana University School of Medicine, Riley Hospital for Children, Indianapolis, IN), Erin K. Stenson, MD (Children’s Hospital Colorado, University of Colorado School of Medicine, Aurora, CO; University of Colorado, School of Medicine, Aurora, CO), Amy E. Strong, MD, MSCE (University of Iowa Stead Family Children’s Hospital, Carver College of Medicine, Iowa City, IA), Susan A. Taylor, MSc (King’s College Hospital, London, United Kingdom), Sameer V. Thadani, MD (Baylor College of Medicine, Texas Children’s Hospital, Houston, TX), Amanda M. Uber, DO (University of Nebraska Medical Center, Children’s Hospital & Medical Center, Omaha, NE), Brynna Van Wyk, ARNP, MSN (University of Iowa Stead Family Children’s Hospital, Carver College of Medicine, Iowa City, IA), Tennille N. Webb, MD, MSPH (Children’s of Alabama/University of Alabama at Birmingham, Birmingham, AL), Huaiyu Zang, PhD (Cincinnati Children’s Hospital Medical Center, University of Cincinnati College of Medicine, Cincinnati, OH), Emily E. Zangla, DO (University of Minnesota, Minneapolis, MN), Michael Zappitelli, MD, MSc (Hospital for Sick Children, Toronto, ON, Canada).
The follow individuals contributed to data collection, cleaning and management: T. Christine E. Alvarez, MHI, RN (Cincinnati Children’s Hospital Medical Center, Cincinnati, OH), Elizabeth Bixler, BS (Baylor College of Medicine, Texas Children’s Hospital, Houston, TX), Erica Blender Brown, MA, CRA (Medical University of South Carolina, Charleston, SC), Cheryl L. Brown, BS (Cincinnati Children’s Hospital Medical Center, Cincinnati, OH), Ambra Burrell, BA (Nationwide Children’s Hospital, Columbus, OH), Anwesh Dash, BS (University of Tennessee Health Science Center College of Medicine, Memphis, TN), Jennifer L. Ehrlich, RN, MHA (University of Iowa Stead Family Children’s Hospital, Carver College of Medicine, Iowa City, IA), Simrandeep Farma, HBSc (Hospital for Sick Children, Toronto, ON, Canada), Kim Gahring, RN, BSN, CCRN (Children’s Hospital Colorado, Aurora, CO), Barbara Gales, RN (Mattel Children Hospital at UCLA, Los Angeles, CA), Madison R. Hilgenkamp (University of Nebraska Medical Center, Children’s Hospital & Medical Center, Omaha, NE), Sonal Jain, MS (Seattle Children’s Hospital, Seattle, WA), Kate Kanwar, BA, MS (Nationwide Children’s Hospital, Columbus, OH), Jennifer Lusk, BSN, RN, CCRN (Children’s Hospital Colorado, Aurora, CO), Christopher J. Meyer, BA AA (Cincinnati Children’s Hospital Medical Center, Cincinnati, OH), Katherine Plomaritas, BSN, RN (University of Michigan, C.S. Mott Children’s Hospital, Ann Arbor, MI), Joshua Porter, BS (University of Tennessee Health Science Center College of Medicine, Memphis, TN), Jessica Potts, BSN, RN (Children’s of Alabama/University of Alabama at Birmingham, Birmingham, AL), Alyssa Serratore, BNurs, GDipNP(PIC), RN, MsC (Royal Children’s Hospital, Melbourne, VIC, Australia), Elizabeth Schneider, BS (University of Tennessee Health Science Center College of Medicine, Memphis, TN), Vidushi Sinha, BS (University of Tennessee Health Science Center College of Medicine, Memphis, TN), P. J. Strack, RN, BSN, CCRN (Children’s Mercy Hospital, Kansas City, MO), Sue Taylor, RN (King’s College Hospital, London, United Kingdom), Katherine Twombley, MD (Medical University of South Carolina, Charleston, SC), Brynna Van Wyk, MSN, ARNP, CPNP (University of Iowa Stead Family Children’s Hospital, Carver College of Medicine, Iowa City, IA), Samantha Wallace, MS (Indiana University School of Medicine, Riley Hospital for Children, Indianapolis, IN), Janet Wang, BS (University of Tennessee Health Science Center College of Medicine, Memphis, TN), Megan Woods, BS (University of Tennessee Health Science Center College of Medicine, Memphis, TN), Marcia Zinger, RN (Cohen Children’s Medical Center, New Hyde Park, NY), Alison Zong, BS (University of Tennessee Health Science Center College of Medicine, Memphis, TN).
Footnotes
All authors were involved in conceptualization, resources, and writing—review and editing. Drs. Hasson, Stanski, Gist, Seo, and Ollberding were involved in methodology. Drs. Hasson, Stanski, Gist, Seo, and Ollberding were involved in formal analysis and investigation. Drs. Hasson, Stanski, Gist, and Seo were involved in writing—original draft preparation. Dr. Gist was involved in funding acquisition. Drs. Stanski and Gist were involved in supervision.
Data cleaning and management supported in kind by the Cincinnati Children’s Hospital Medical Center (CCHMC) Heart Institute Research Core. Statistical analyses supported by internal CCHMC funding (principal investigator to Dr. Gist).
Dr. Stanski received funding from the National Institute of General Medical Sciences (K23GM151444-01). Dr. Gist is a consultant for BioPorto Diagnostics and Potrero Medical; she received funding from the Gerber Foundation. Dr. Stenson received funding from the National Institute of Child Health and Development (K12HD047349). Dr. Ollberding serves as a consultant for SeaStar Medical. The remaining authors have disclosed that they do not have any potential conflicts of interest.
De-identified summary data are available through the Worldwide Exploration of Renal Replacement Outcomes Collaborative in Kidney Disease (WE-ROCK) collaborative. Data dictionaries, in addition to study protocol and statistical analysis plans will be made available upon request. More information about the process and available data can be obtained by contacting the corresponding author (to Dr. Hasson). The data from the WE-ROCK collaborative will be made available to researchers who provide a methodologically sound proposal for use in achieving the goals of the approved proposal following an application process and execution of a data-use agreement as required by the Institutional Review Board at the Cincinnati Children’s Hospital Medical Center as part of the approval of this collaborative study.
The Consortia-on behalf of the Worldwide Exploration of Renal Replacement Outcomes Collaborative in Kidney Disease (WE-ROCK) Investigators are listed in the Appendix.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
Contributor Information
JangDong Seo, Email: jangdong.seo@cchmc.org.
Erin K. Stenson, Email: erin.stenson@childrenscolorado.org.
Aaron Kessel, Email: akessel@northwell.edu.
Taiki Haga, Email: beasties@live.jp.
Sara LaFever, Email: sarahlafever@gmail.com.
Maria Jose Santiago, Email: mjsantiagolozano@gmail.com.
Matthew Barhight, Email: mbarhight@luriechildrens.org.
David Selewski, Email: selewski@musc.edu.
Zaccaria Ricci, Email: zaccaria.ricci@meyer.it.
Nicholas J. Ollberding, Email: nicholas.ollberding@cchmc.org.
Natalja L. Stanski, Email: natalja.stanski@cchmc.org.
Emily Ahern, Children’s Hospital Colorado, University of Colorado School of Medicine, Aurora, CO.
Ayse Akcan Arikan, Baylor College of Medicine, Texas Children’s Hospital, Houston, TX.
Issa Alhamoud, University of Iowa Stead Family Children’s Hospital, Carver College of Medicine, Iowa City, IA.
Rashid Alobaidi, Univeristy of Alberta, Edmonton, AB, Canada.
Pilar Anton-Martin, Le Bonheur Children’s Hospital, Memphis, TN.
Shanthi S. Balani, University of Minnesota, Minneapolis, MN.
Matthew Barhight, Ann and Robert H. Lurie Children’s Hospital of Chicago, Chicago, IL.
Abby Basalely, Cohen Children’s Medical Center, Zucker School of Medicine, New Hyde Park, NY.
Amee M. Bigelow, Nationwide Children’s Hospital, The Ohio State University College of Medicine, Columbus, OH.
Gabriella Bottari, Bambino Gesù Children Hospital, IRCCS, Rome, Italy.
Andrea Cappoli, Bambino Gesù Children Hospital, IRCCS, Rome, Italy.
Eileen A. Ciccia, Washington University School of Medicine, St. Louis Children’s Hospital, St. Louis, MO.
Michaela Collins, Cincinnati Children’s Hospital Medical Center, University of Cincinnati College of Medicine, Cincinnati, OH.
Denise Colosimo, Meyer Children’s Hospital, IRCCS, Florence, Italy.
Gerard Cortina, Medical University of Innsbruck, Innsbruck, Austria.
Mihaela A. Damian, Stanford University School of Medicine, Palo Alto, CA.
Sara De la Mata Navazo, Gregorio Marañón University Hospital, School of Medicine, Madrid, Spain.
Gabrielle DeAbreu, Cohen Children’s Medical Center, Zucker School of Medicine, New Hyde Park, NY.
Akash Deep, King’s College Hospital, London, United Kingdom.
Kathy L. Ding, University of Colorado, School of Medicine, Aurora, CO.
Kristin J. Dolan, Baylor College of Medicine, Texas Children’s Hospital, Houston, TX.
Sarah N. Fernandez Lafever, Gregorio Marañón University Hospital, School of Medicine, Madrid, Spain.
Dana Y. Fuhrman, University of Pittsburgh Medical Center Children’s Hospital of Pittsburgh, Pittsburgh, PA.
Ben Gelbart, Royal Children’s Hospital, University of Melbourne, Murdoch Children’s Research Institute, Melbourne, VIC, Australia.
Katja M. Gist, Cincinnati Children’s Hospital Medical Center, University of Cincinnati College of Medicine, Cincinnati, OH.
Stephen M. Gorga, University of Michigan Medical School, C.S. Mott Children’s Hospital, Ann Arbor, MI.
Francesco Guzzi, Santo Stefano Hospital, Prato, Italy.
Isabella Guzzo, Bambino Gesù Children Hospital, IRCCS, Rome, Italy.
Taiki Haga, Osaka City General Hospital, Osaka, Japan.
Elizabeth Harvey, Hospital for Sick Children, Toronto, ON, Canada.
Denise C. Hasson, NYU Langone Health, Hassenfeld Children’s Hospital, New York, NY.
Taylor Hill-Horowitz, Cohen Children’s Medical Center, Zucker School of Medicine, New Hyde Park, NY.
Haleigh Inthavong, Baylor College of Medicine, Texas Children’s Hospital, Houston, TX.
Catherine Joseph, Baylor College of Medicine, Texas Children’s Hospital, Houston, TX.
Ahmad Kaddourah, Sidra Medicine and Weil Cornel Medicine, Qatar, Doha, Qatar.
Aadil Kakajiwala, Children’s National Hospital, Washington, DC.
Aaron D. Kessel, Cohen Children’s Medical Center, Zucker School of Medicine, New Hyde Park, NY.
Sarah Korn, Westchester Medical Center, Westchester, NY.
Kelli A. Krallman, Cincinnati Children’s Hospital Medical Center, University of Cincinnati College of Medicine, Cincinnati, OH.
David M. Kwiatkowski, Lucile Packard Children’s Hospital, Palo Alto, CA.
Jasmine Lee, Hospital for Sick Children, Toronto, ON, Canada.
Laurance Lequier, Univeristy of Alberta, Edmonton, AB, Canada.
Tina Madani Kia, Univeristy of Alberta, Edmonton, AB, Canada.
Kenneth E. Mah, Stanford University School of Medicine, Palo Alto, CA.
Eleonora Marinari, Bambino Gesù Children Hospital, IRCCS, Rome, Italy.
Susan D. Martin, Golisano Children’s Hospital at University of Rochester Medical Center, Rochester, NY.
Shina Menon, Seattle Children’s Hospital, University of Washington, Seattle, WA.
Tahagod H. Mohamed, Nationwide Children’s Hospital, The Ohio State University College of Medicine, Columbus, OH.
Catherine Morgan, Univeristy of Alberta, Edmonton, AB, Canada.
Theresa A. Mottes, Ann and Robert H. Lurie Children’s Hospital of Chicago, Chicago, IL.
Melissa A. Muff-Luett, University of Nebraska Medical Center, Children’s Hospital & Medical Center, Omaha, NE.
Siva Namachivayam, Royal Children’s Hospital, University of Melbourne, Murdoch Children’s Research Institute, Melbourne, VIC, Australia.
Tara M. Neumayr, Washington University School of Medicine, St. Louis Children’s Hospital, St. Louis, MO.
Jennifer Nhan Md, Children’s National Hospital, Washington, DC.
Abigail O’Rourke, Cohen Children’s Medical Center, Zucker School of Medicine, New Hyde Park, NY.
Nicholas J. Ollberding, Cincinnati Children’s Hospital Medical Center, University of Cincinnati College of Medicine, Cincinnati, OH.
Matthew G. Pinto, Maria Fareri Children’s Hospital at Westchester Medical Center, Valhalla, NY.
Dua Qutob, Sidra Medicine and Weil Cornel Medicine, Qatar, Doha, Qatar.
Valeria Raggi, Bambino Gesù Children Hospital, IRCCS, Rome, Italy.
Stephanie Reynaud, IWK Health, Dalhousie University, Halifax, NS, Canada.
Zaccaria Ricci, Meyer Children’s Hospital, IRCCS, Florence, Italy.
Zachary A. Rumlow, University of Iowa Stead Family Children’s Hospital, Carver College of Medicine, Iowa City, IA.
María J. Santiago Lozano, Gregorio Marañón University Hospital, School of Medicine, Madrid, Spain.
Emily See, Royal Children’s Hospital, University of Melbourne, Murdoch Children’s Research Institute, Melbourne, VIC, Australia.
David T. Selewski, Medical University of South Carolina, Charleston, SC.
Carmela Serpe, Bambino Gesù Children Hospital, IRCCS, Rome, Italy.
Alyssa Serratore, Royal Children’s Hospital, University of Melbourne, Murdoch Children’s Research Institute, Melbourne, VIC, Australia.
Ananya Shah, University of Colorado, School of Medicine, Aurora, CO.
Weiwen V. Shih, Children’s Hospital Colorado, University of Colorado School of Medicine, Aurora, CO; University of Colorado, School of Medicine, Aurora, CO.
H. Stella Shin, Children’s Healthcare of Atlanta, Emory University, Atlanta, GA.
Cara L. Slagle, Cincinnati Children’s Hospital Medical Center, University of Cincinnati College of Medicine, Cincinnati, OH.
Sonia Solomon, Maria Fareri Children’s Hospital at Westchester Medical Center, Valhalla, NY.
Danielle E. Soranno, Indiana University School of Medicine, Riley Hospital for Children, Indianapolis, IN.
Rachana Srivastava, Mattel Children’s Hospital at UCLA, Los Angeles, CA.
Natalja L. Stanski, Cincinnati Children’s Hospital Medical Center, University of Cincinnati College of Medicine, Cincinnati, OH.
Michelle C. Starr, Indiana University School of Medicine, Riley Hospital for Children, Indianapolis, IN.
Erin K. Stenson, Children’s Hospital Colorado, University of Colorado School of Medicine, Aurora, CO; University of Colorado, School of Medicine, Aurora, CO.
Amy E. Strong, University of Iowa Stead Family Children’s Hospital, Carver College of Medicine, Iowa City, IA.
Susan A. Taylor, King’s College Hospital, London, United Kingdom.
Sameer V. Thadani, Baylor College of Medicine, Texas Children’s Hospital, Houston, TX.
Amanda M. Uber, University of Nebraska Medical Center, Children’s Hospital & Medical Center, Omaha, NE.
Brynna Van Wyk, University of Iowa Stead Family Children’s Hospital, Carver College of Medicine, Iowa City, IA.
Tennille N. Webb, Children’s of Alabama/University of Alabama at Birmingham, Birmingham, AL.
Huaiyu Zang, Cincinnati Children’s Hospital Medical Center, University of Cincinnati College of Medicine, Cincinnati, OH.
Emily E. Zangla, University of Minnesota, Minneapolis, MN.
Michael Zappitelli, Hospital for Sick Children, Toronto, ON, Canada).
E. Alvarez, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH.
Elizabeth Bixler, Baylor College of Medicine, Texas Children’s Hospital, Houston, TX.
Erica Blender Brown, Medical University of South Carolina, Charleston, SC.
Cheryl L. Brown, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH.
Ambra Burrell, Nationwide Children’s Hospital, Columbus, OH.
Anwesh Dash, University of Tennessee Health Science Center College of Medicine, Memphis, TN.
Jennifer L. Ehrlich, University of Iowa Stead Family Children’s Hospital, Carver College of Medicine, Iowa City, IA.
Simrandeep Farma, Hospital for Sick Children, Toronto, ON, Canada.
Kim Gahring, Children’s Hospital Colorado, Aurora, CO.
Barbara Gales, Mattel Children Hospital at UCLA, Los Angeles, CA.
Madison R. Hilgenkamp, (University of Nebraska Medical Center, Children’s Hospital & Medical Center, Omaha, NE
Sonal Jain, Seattle Children’s Hospital, Seattle, WA.
Kate Kanwar, Nationwide Children’s Hospital, Columbus, OH.
Jennifer Lusk, Children’s Hospital Colorado, Aurora, CO.
Christopher J. Meyer, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH.
Katherine Plomaritas, University of Michigan, C.S. Mott Children’s Hospital, Ann Arbor, MI.
Joshua Porter, University of Tennessee Health Science Center College of Medicine, Memphis, TN.
Jessica Potts, Children’s of Alabama/University of Alabama at Birmingham, Birmingham, AL.
Alyssa Serratore, Royal Children’s Hospital, Melbourne, VIC, Australia.
Elizabeth Schneider, University of Tennessee Health Science Center College of Medicine, Memphis, TN.
Vidushi Sinha, University of Tennessee Health Science Center College of Medicine, Memphis, TN.
P. J. Strack, Children’s Mercy Hospital, Kansas City, MO.
Sue Taylor, King’s College Hospital, London, United Kingdom.
Katherine Twombley, Medical University of South Carolina, Charleston, SC.
Brynna Van Wyk, University of Iowa Stead Family Children’s Hospital, Carver College of Medicine, Iowa City, IA.
Samantha Wallace, Indiana University School of Medicine, Riley Hospital for Children, Indianapolis, IN.
Janet Wang, University of Tennessee Health Science Center College of Medicine, Memphis, TN.
Megan Woods, University of Tennessee Health Science Center College of Medicine, Memphis, TN.
Marcia Zinger, Cohen Children’s Medical Center, New Hyde Park, NY.
Alison Zong, University of Tennessee Health Science Center College of Medicine, Memphis, TN).
Collaborators: Emily Ahern, Ayse Akcan Arikan, Issa Alhamoud, Rashid Alobaidi, Pilar Anton-Martin, Shanthi S. Balani, Matthew Barhight, Abby Basalely, Amee M. Bigelow, Gabriella Bottari, Andrea Cappoli, Eileen A. Ciccia, Michaela Collins, Denise Colosimo, Gerard Cortina, Mihaela A. Damian, Sara De la Mata Navazo, Gabrielle DeAbreu, Akash Deep, Kathy L. Ding, Kristin J. Dolan, Sarah N. Fernandez Lafever, Dana Y. Fuhrman, Ben Gelbart, Katja M. Gist, Stephen M. Gorga, Francesco Guzzi, Isabella Guzzo, Taiki Haga, Elizabeth Harvey, Denise C. Hasson, Taylor Hill-Horowitz, Haleigh Inthavong, Catherine Joseph, Ahmad Kaddourah, Aadil Kakajiwala, Aaron D. Kessel, Sarah Korn, Kelli A. Krallman, David M. Kwiatkowski, Jasmine Lee, Laurance Lequier, Tina Madani Kia, Kenneth E. Mah, Eleonora Marinari, Susan D. Martin, Shina Menon, Tahagod H. Mohamed, Catherine Morgan, Theresa A. Mottes, Melissa A. Muff-Luett, Siva Namachivayam, Tara M. Neumayr, Jennifer Nhan Md, Abigail O’Rourke, Nicholas J. Ollberding, Matthew G. Pinto, Dua Qutob, Valeria Raggi, Stephanie Reynaud, Zaccaria Ricci, Zachary A. Rumlow, María J. Santiago Lozano, Emily See, David T. Selewski, Carmela Serpe, Alyssa Serratore, Ananya Shah, Weiwen V. Shih, H. Stella Shin, Cara L. Slagle, Sonia Solomon, Danielle E. Soranno, Rachana Srivastava, Natalja L. Stanski, Michelle C. Starr, Erin K. Stenson, Amy E. Strong, Susan A. Taylor, Sameer V. Thadani, Amanda M. Uber, Brynna Van Wyk, Tennille N. Webb, Huaiyu Zang, Emily E. Zangla, Michael Zappitelli, T. Christine, E. Alvarez, Elizabeth Bixler, Erica Blender Brown, Cheryl L. Brown, Ambra Burrell, Anwesh Dash, Jennifer L. Ehrlich, Simrandeep Farma, Kim Gahring, Barbara Gales, Madison R. Hilgenkamp, Sonal Jain, Kate Kanwar, Jennifer Lusk, Christopher J. Meyer, Katherine Plomaritas, Joshua Porter, Jessica Potts, Alyssa Serratore, Elizabeth Schneider, Vidushi Sinha, P. J. Strack, Sue Taylor, Katherine Twombley, Brynna Van Wyk, Samantha Wallace, Janet Wang, Megan Woods, Marcia Zinger, and Alison Zong
REFERENCES
- 1.Kaddourah A, Basu RK, Bagshaw SM, et al. ; AWARE Investigators: Epidemiology of acute kidney injury in critically ill children and young adults. N Engl J Med 2017; 376:11–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Riley AA, Watson M, Smith C, et al. : Pediatric continuous renal replacement therapy: Have practice changes changed outcomes? A large single-center ten-year retrospective evaluation. BMC Nephrol 2018; 19:268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sutherland SM, Zappitelli M, Alexander SR, et al. : Fluid overload and mortality in children receiving continuous renal replacement therapy: The prospective pediatric continuous renal replacement therapy registry. Am J Kidney Dis 2010; 55:316–325 [DOI] [PubMed] [Google Scholar]
- 4.Goldstein SL, Currier H, Graf Cd, et al. : Outcome in children receiving continuous venovenous hemofiltration. Pediatrics 2001; 107:1309–1312 [DOI] [PubMed] [Google Scholar]
- 5.Basu RK, Zappitelli M, Brunner L, et al. : Derivation and validation of the renal angina index to improve the prediction of acute kidney injury in critically ill children. Kidney Int 2014; 85:659–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hayes LW, Oster RA, Tofil NM, et al. : Outcomes of critically ill children requiring continuous renal replacement therapy. J Crit Care 2009; 24:394–400 [DOI] [PubMed] [Google Scholar]
- 7.Gist KM, Menon S, Anton-Martin P, et al. ; WE-ROCK Investigators: Time to continuous renal replacement therapy initiation and 90-day major adverse kidney events in children and young adults. JAMA Netw Open 2024; 7:e2349871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heung M, Chawla LS: Predicting progression to chronic kidney disease after recovery from acute kidney injury. Curr Opin Nephrol Hypertens 2012; 21:628–634 [DOI] [PubMed] [Google Scholar]
- 9.Clark EG, Bagshaw SM: Unnecessary renal replacement therapy for acute kidney injury is harmful for renal recovery. Semin Dial 2015; 28:6–11 [DOI] [PubMed] [Google Scholar]
- 10.Fuhrman DY, Stenson EK, Alhamoud I, et al. ; WE-ROCK Investigators: Major adverse kidney events in pediatric continuous kidney replacement therapy. JAMA Netw Open 2024; 7:e240243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Proulx F, Fayon M, Farrell CA, et al. : Epidemiology of sepsis and multiple organ dysfunction syndrome in children. Chest 1996; 109:1033–1037 [DOI] [PubMed] [Google Scholar]
- 12.Watson RS, Carcillo JA, Linde-Zwirble WT, et al. : The epidemiology of severe sepsis in children in the United States. Am J Respir Crit Care Med 2003; 167:695–701 [DOI] [PubMed] [Google Scholar]
- 13.Stenson EK, Alhamoud I, Alobaidi R, et al. ; WE-ROCK Investigators: Factors associated with successful liberation from continuous renal replacement therapy in children and young adults: Analysis of the worldwide exploration of renal replacement outcomes collaborative in Kidney Disease Registry. Intensive Care Med 2024; 50:861–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cortina G, McRae R, Hoq M, et al. : Mortality of critically ill children requiring continuous renal replacement therapy: Effect of fluid overload, underlying disease, and timing of initiation. Pediatr Crit Care Med 2019; 20:314–322 [DOI] [PubMed] [Google Scholar]
- 15.Russell JA, Walley KR, Singer J, et al. ; VASST Investigators: Vasopressin versus norepinephrine infusion in patients with septic shock. N Engl J Med 2008; 358:877–887 [DOI] [PubMed] [Google Scholar]
- 16.Joannidis M, Druml W, Forni LG, et al. : Prevention of acute kidney injury and protection of renal function in the intensive care unit: Update 2017: Expert opinion of the Working Group on Prevention, AKI section, European Society of Intensive Care Medicine. Intensive Care Med 2017; 43:730–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weiss SL, Peters MJ, Alhazzani W, et al. : Surviving sepsis campaign international guidelines for the management of septic shock and sepsis-associated organ dysfunction in children. Pediatr Crit Care Med 2020; 21:e52–e106 [DOI] [PubMed] [Google Scholar]
- 18.Jha A, Zilahi G, Rhodes A: Vasoactive therapy in shock. BJA Educ 2021; 21:270–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jentzer JC, Coons JC, Link CB, et al. : Pharmacotherapy update on the use of vasopressors and inotropes in the intensive care unit. J Cardiovasc Pharmacol Ther 2015; 20:249–260 [DOI] [PubMed] [Google Scholar]
- 20.Holmes CL, Patel BM, Russell JA, et al. : Physiology of vasopressin relevant to management of septic shock. Chest 2001; 120:989–1002 [DOI] [PubMed] [Google Scholar]
- 21.Edwards RM, Trizna W, Kinter LB: Renal microvascular effects of vasopressin and vasopressin antagonists. Am J Physiol 1989; 256:F274–F278 [DOI] [PubMed] [Google Scholar]
- 22.Annane D, Ouanes-Besbes L, de Backer D, et al. : A global perspective on vasoactive agents in shock. Intensive Care Med 2018; 44:833–846 [DOI] [PubMed] [Google Scholar]
- 23.Gordon AC, Mason AJ, Thirunavukkarasu N, et al. ; VANISH Investigators: Effect of early vasopressin vs norepinephrine on kidney failure in patients with septic shock: The VANISH randomized clinical trial. JAMA 2016; 316:509–518 [DOI] [PubMed] [Google Scholar]
- 24.Hajjar LA, Vincent JL, Barbosa Gomes Galas FR, et al. : Vasopressin versus norepinephrine in patients with vasoplegic shock after cardiac surgery: The VANCS randomized controlled trial. Anesthesiology 2017; 126:85–93 [DOI] [PubMed] [Google Scholar]
- 25.Gordon AC, Russell JA, Walley KR, et al. : The effects of vasopressin on acute kidney injury in septic shock. Intensive Care Med 2010; 36:83–91 [DOI] [PubMed] [Google Scholar]
- 26.Zappitelli M, Parikh CR, Akcan-Arikan A, et al. : Ascertainment and epidemiology of acute kidney injury varies with definition interpretation. Clin J Am Soc Nephrol 2008; 3:948–954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Menon S, Krallman KA, Arikan AA, et al. ; WE-ROCK Investigators: Worldwide exploration of renal replacement outcomes collaborative in kidney disease (WE-ROCK). Kidney Int Rep 2023; 8:1542–1552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Evans L, Rhodes A, Alhazzani W, et al. : Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Intensive Care Med 2021; 47:1181–1247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Torgersen C, Dünser MW, Wenzel V, et al. : Comparing two different arginine vasopressin doses in advanced vasodilatory shock: A randomized, controlled, open-label trial. Intensive Care Med 2010; 36:57–65 [DOI] [PubMed] [Google Scholar]
- 30.Gaies MG, Gurney JG, Yen AH, et al. : Vasoactive–inotropic score as a predictor of morbidity and mortality in infants after cardiopulmonary bypass*. Pediatr Crit Care Med 2010; 11:234–238 [DOI] [PubMed] [Google Scholar]
- 31.Gonçalves J-P, Severo M, Rocha C, et al. : Performance of PRISM III and PELOD-2 scores in a pediatric intensive care unit. Eur J Pediatr 2015; 174:1305–1310 [DOI] [PubMed] [Google Scholar]
- 32.Pollack MM, Patel KM, Ruttimann UE: PRISM III: An updated Pediatric Risk of Mortality score. Crit Care Med 1996; 24:743–752 [DOI] [PubMed] [Google Scholar]
- 33.Leteurtre S, Duhamel A, Salleron J, et al. ; Groupe Francophone de Réanimation et d’Urgences Pédiatriques (GFRUP): PELOD-2: An update of the pediatric logistic organ dysfunction score. Crit Care Med 2013; 41:1761–1773 [DOI] [PubMed] [Google Scholar]
- 34.Goldstein B, Giroir B, Randolph A; International Consensus Conference on Pediatric Sepsis: International pediatric sepsis consensus conference: Definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med 2005; 6:2–8 [DOI] [PubMed] [Google Scholar]
- 35.Selewski DT, Barhight MF, Bjornstad EC, et al. : Fluid assessment, fluid balance, and fluid overload in sick children: A report from the Pediatric Acute Disease Quality Initiative (ADQI) conference. Pediatr Nephrol 2023; 39:955–979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goldstein SL, Akcan-Arikan A, Alobaidi R, et al. ; Pediatric ADQI Collaborative: Consensus-based recommendations on priority activities to address acute kidney injury in children: A modified Delphi consensus statement. JAMA Netw Open 2022; 5:e2229442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Russell JA, Wellman H, Walley KR: Vasopressin versus norepinephrine in septic shock: A propensity score matched efficiency retrospective cohort study in the VASST coordinating center hospital. J Intensive Care 2018; 6:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bhatraju PK, Zelnick LR, Herting J, et al. : Identification of acute kidney injury subphenotypes with differing molecular signatures and responses to vasopressin therapy. Am J Respir Crit Care Med 2019; 199:863–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Choong K, Bohn D, Fraser DD, et al. ; Canadian Critical Care Trials Group: Vasopressin in pediatric vasodilatory shock: A multicenter randomized controlled trial. Am J Respir Crit Care Med 2009; 180:632–639 [DOI] [PubMed] [Google Scholar]
- 40.Kumar A, Ghotra GS, Raj S, et al. : Low-dose vasopressin and renal perfusion in pediatric cardiac surgery. Ann Card Anaesth 2023; 26:309–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sacha GL, Lam SW, Wang L, et al. : Association of catecholamine dose, lactate, and shock duration at vasopressin initiation with mortality in patients with septic shock. Crit Care Med 2022; 50:614–623 [DOI] [PubMed] [Google Scholar]
- 42.Dünser MW, Hasibeder WR: Sympathetic overstimulation during critical illness: Adverse effects of adrenergic stress. J Intensive Care Med 2009; 24:293–316 [DOI] [PubMed] [Google Scholar]
- 43.Odum JD, Wong HR, Stanski NL: A precision medicine approach to biomarker utilization in pediatric sepsis-associated acute kidney injury. Front Pediatr 2021; 9:632248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Basu RK, Kaddourah A, Goldstein SL; AWARE Study Investigators: Assessment of a renal angina index for prediction of severe acute kidney injury in critically ill children: A multicentre, multinational, prospective observational study. Lancet Child Adolesc Health 2018; 2:112–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stenson EK, Brinton JT, Beil L, et al. : Modification of the Renal Angina Index for identifying the need for renal replacement therapy in critically ill pediatric patients. J Clin Nephrol 2020; 4:070–076 [Google Scholar]
- 46.Wheeler DS, Wong HR, Zingarelli B: Pediatric sepsis—part I: “Children are not small adults!”. Open Inflamm J 2011; 4:4–15 [DOI] [PMC free article] [PubMed] [Google Scholar]