Abstract
Background:
Interleukin (IL)-6 is a major inflammatory cytokine that predicts mortality after out-of-hospital cardiac arrest (OHCA). Targeted temperature management (TTM) is associated with improved all-cause mortality in patients with OHCA. However, the effect of TTM on IL-6 production remains unclear. This study investigated whether TTM has additional anti-inflammatory effects after OHCA.
Methods:
This prospective cohort study included a total of 141 hospitalized patients with OHCA who were treated between January 2015 and June 2023. The study was conducted in the intensive care unit of China Medical University Hospital, Taichung. Postcardiac arrest care included TTM or the control approach (no TTM). The primary outcomes included the 90-day mortality rate and neurologic outcomes after OHCA. Differences between the TTM and control groups were examined using Student t test, chi-square test, and Kaplan–Meier survival curve analysis. Multivariate analysis of variance model was used to examine interaction effects.
Results:
Plasma IL-6 and IL-6/soluble IL-6 receptor complex levels were measured at 6 and 24 hours after resuscitation. IL-6 and IL-6/soluble IL-6 receptor complex production was lower in the TTM group than in the control group (−50.0% vs +136.7%, P < .001; +26.3% vs +102.40%, P < .001, respectively). In addition, the 90-day mortality rate and poor neurologic outcomes were lower in the TTM group than in the control group (36.8% vs 63.0%, relative risk 0.39, 95% confidence interval 0.24–0.64, P < .001; 65.5% vs 81.5%, relative risk 0.80, 95% confidence interval 0.66–0.98, P = .04).
Conclusion:
TTM improves both the mortality rate and neurologic outcomes in patients resuscitated from OHCA, possibly by reducing IL-6-induced proinflammatory responses.
Keywords: IL-6, inflammatory, mortality rate, neurologic outcome, out-of-hospital cardiac arrest, targeted temperature management
1. Introduction
Most of the out-of-hospital cardiac arrest (OHCA) cases (70–85%) are of cardiac origin and arise from coronary heart disease, particularly acute myocardial infarction.[1,2] Despite prompt interventions, such as early cardiopulmonary resuscitation, rapid defibrillation, ambulance transfer, and advanced life support, only approximately 25% of patients are admitted for postcardiac arrest care in hospitals.[1]
Patients resuscitated from OHCA, particularly those with persistent cardiogenic shock complicated by acute myocardial infarction or pulseless ventricular dysrhythmia, often receive emergent coronary reperfusion, vasopressors, inotropes, intra-aortic balloon pump, or extracorporeal membrane oxygenation support.[3] Despite these interventions, high mortality rates and poor neurologic outcomes persist. Thus, studies focusing on the development of new therapeutic strategies are needed.
OHCA triggers excessive inflammatory responses, such as the recruitment of inflammatory cells through damage-associated molecular patterns, Toll-like receptor activity, and interleukin (IL)-1β signaling.[4,5] The circulating level of IL-6, a cytokine produced downstream of IL-1β, is a reliable, independent predictor of 30-day mortality in patients with cardiac arrest.[6] Accumulating evidence suggests that the production of IL-6 and the IL-6/soluble IL-6 receptor trans-signaling complex initiates a proinflammatory cascade through their direct binding to membrane-bound glycoprotein 130.[7] Moreover, the sequential organ failure assessment (SOFA) score provides valuable prognostic information on severe multiple organ failure.[8,9] Patients with OHCA have high levels of IL-6, which is associated with increased risks of multiorgan failure and mortality.[10]
In 2015, the American Heart Association recommended that comatose OHCA survivors receive targeted temperature management (TTM) at 32–36 °C for at least 24 hours to improve their mortality and neurologic outcomes. However, the mechanisms underlying the anti-inflammatory effects of TTM in patients with OHCA and its associated predictors have not been studied in detail in clinical practice. This study aimed to investigate whether TTM has anti-inflammatory effects after OHCA.
2. Methods
2.1. Study design and patients
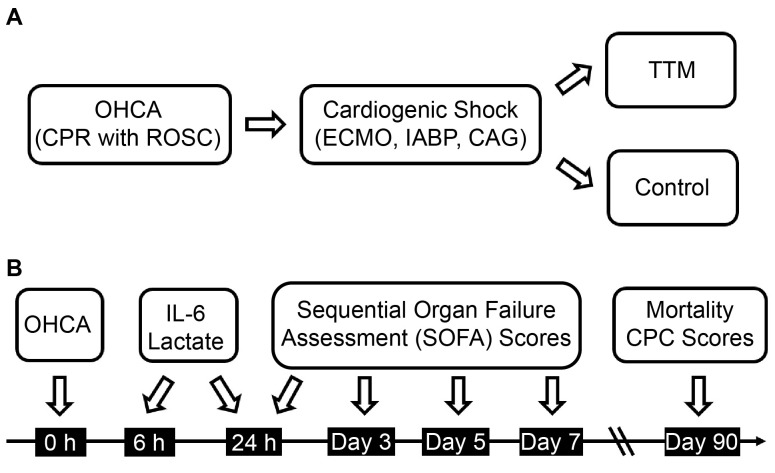
This prospective cohort study (registered at ClinicalTrials.gov, NCT02633358) investigated the effect of TTM on IL-6-induced inflammation in patients resuscitated from OHCA compared to the control (no TTM). The study was conducted in the intensive care unit of China Medical University Hospital, Taichung, Taiwan, between January 2015 and June 2023. Written informed consent was provided by the family members owing to the patients’ lack of consciousness.
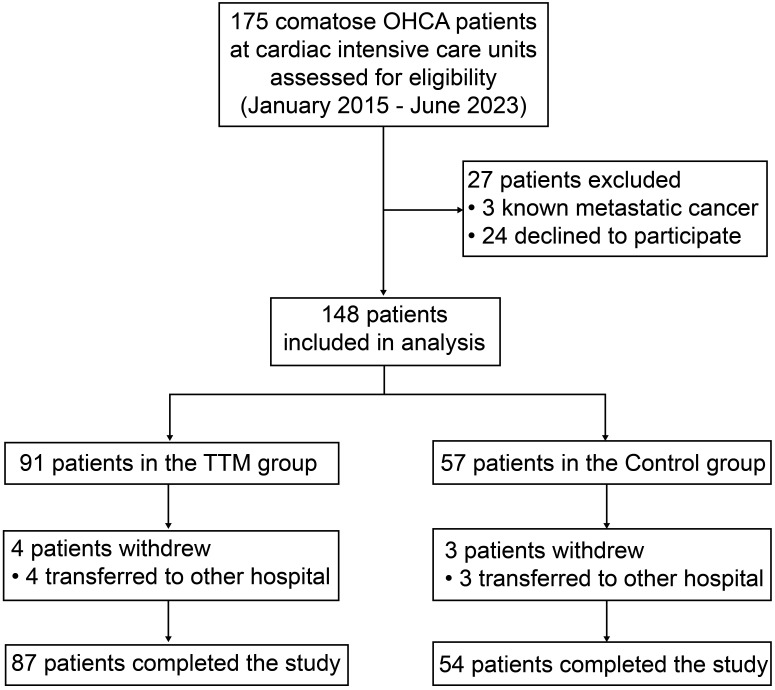
This study included hospitalized patients with OHCA aged ≥ 18 years who achieved a return of spontaneous circulation after cardiopulmonary resuscitation. The study design is depicted in Supplemental Digital Content 1, http://links.lww.com/MD/N632. Exclusion criteria were pregnancy, metastatic cancer, and surrogate decision-makers’ refusal to participate in the clinical trial. The CONSORT flow diagram is displayed in Supplementary Digital Content 2, http://links.lww.com/MD/N633.
Assuming a 90% probability of reduction in mortality from 65% in the control group to 35% in the experimental group, with a 5% level difference, we recruited 141 patients (control group, n = 54; experimental group, n = 87) to this study, ensuring an adequate sample size for statistical analysis. We also used the standard error of the mean to correct the data.
2.2. TTM protocol
The TTM protocol employed in this study was adapted from the method proposed by Scirica et al with minor modifications.[11] Briefly, TTM was started within 6 hours after resuscitation. In addition to traditional ice blankets, various cooling devices were used, including automatic core temperature control devices (such as the Arctic Sun from Medivance or the Thermogard XP from Zoll Medical).
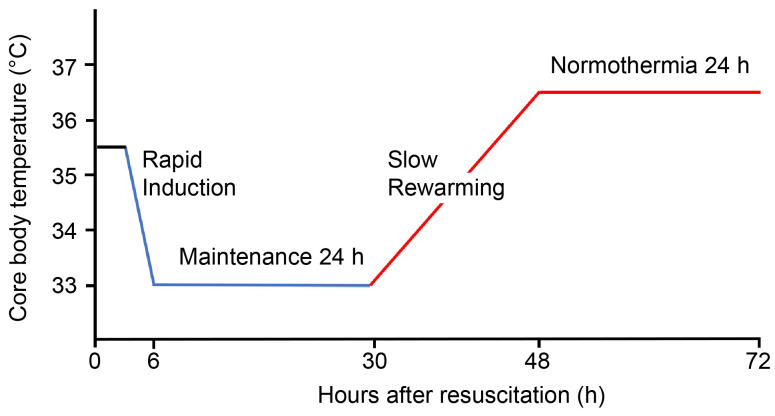
The protocol comprised 4 stages. The first stage involved rapid induction to reach the target core temperature of 33 °C within 2 hours. Intravenous fentanyl (for analgesia) and midazolam (for sedation) were administered to maintain a bedside shivering assessment scale score of 0 and a Richmond Agitation Sedation Scale score of −4 or −5. The second stage focused on maintaining the core temperature at 33 to 34 °C for 24 hours, with continuous monitoring of blood electrolytes and maintenance of sinus rhythm. We maintained adequate blood pressure (mean arterial pressure of 60–90 mm Hg), blood gas (arterial oxygen pressure 70–100 mm Hg), fasting blood glucose level (110–180 mg/dL), and body volume status in the patients. The third stage involved slow rewarming between 0.05 °C and 0.25 °C per hour. The fourth stage encompassed maintaining normothermia at 36 to 37 °C for 24 hours to prevent fever. The core body temperature was measured using an esophageal thermometer. Moreover, continuous electroencephalogram monitoring was performed throughout the study. The TTM protocol is presented in Supplemental Digital Content 3, http://links.lww.com/MD/N634.
2.3. Blood sampling protocol and Immunoassays
At 6 and 24 hours after resuscitation, peripheral venous blood samples were collected into blood collection tubes containing ethylenediaminetetraacetic acid to prevent clotting. To obtain plasma, these tubes were immediately placed on melted ice and centrifuged at 4 °C and 450 × g for 25 minutes (5810R centrifuge, Eppendorf AG, Hamburg, Germany). Immediately after centrifugation, plasma was stored at −70 °C until further analysis. Levels of IL-6, soluble IL-6 receptor, Il-6/soluble IL-6 receptor complex, and soluble glycoprotein 130 were determined by sandwich enzyme-linked immunosorbent assay following the manufacturer protocols (R&D Systems Inc.; absorbance of each well: 450 nm by Synergy H4 microplate reader). A 4-parameter log standard curve was used to calculate the results.
2.4. Assessment of SOFA score and neurologic outcomes
We used the SOFA score to assess the severity of organ failure. The score reflects the status of each organ system, including the cardiovascular, respiratory, central nervous system, liver, kidney, and coagulation. Cerebral performance category (CPC) scores were used to assess neurologic outcomes after cardiac arrest. A CPC score of 1 or 2 indicates a favorable neurologic outcome (satisfactory recovery, mild, and moderate disability), whereas CPC scores of 3 to 5 indicate poor neurologic outcomes (severe disability, coma, persistent vegetative state, and brain death).
2.5. Statistical analyses
General statistical analyses were performed, such as Student t test, chi-square test, and Kaplan-Meier survival curve, to examine the differences between the TTM and control (no TTM) groups. The paired t test was used to compare data at 2 time points. Interaction effects, which represent the combined effects of 2 groups over time, were analyzed using the multivariate analysis of variance model. A Cox regression model was employed to account for potential confounding factors, such as age, sex, initial cardiac rhythm, cardiopulmonary resuscitation time, and coronary heart disease. The analyses were performed using the Excel analysis tool, SAS version 9.4, or SPSS statistical software version 22.0. A 2-tailed P < .05 was considered statistically significant.
3. Results
3.1. Baseline characteristics and initial procedures
A total of 141 patients (mean age: 61 years; men: 79%) who underwent OHCA resuscitation between January 2015 and June 2023 were included in the TTM and control groups. The baseline characteristics of the patients are presented in Table 1. No differences in age distribution, sex, or medical history were observed between the groups. Approximately 62% of the participants had a shockable rhythm at the initial assessment (pulseless ventricular tachycardia or fibrillation), with a cardiopulmonary resuscitation duration of approximately 23 minutes. Coronary heart disease was the primary etiology in patients with OHCA of cardiac origin (74%). In addition, patients with acute myocardial infarction (57%) underwent urgent percutaneous coronary reperfusion. Patients resuscitated from OHCA with cardiogenic shock (41%) required higher-dose vasopressors. Approximately 29% of patients received additional intra-arterial balloon pump support, and 34% received extracorporeal membrane oxygenation support (Table 1).
Table 1.
Baseline characteristics of the study participants and initial procedures.
| TTM (n = 87) | Control (n = 54) | P value | ||
|---|---|---|---|---|
| Age (yr), mean ± SEM | 59.8 ± 1.5 | 62.5 ± 1.9 | 0.25 | |
| Male, n (%) | 70 (80.5) | 42 (77.8) | 0.70 | |
| Medical history, n (%) | Hypertension | 38 (43.7) | 22 (40.7) | 0.60 |
| Diabetes mellitus | 28 (32.2) | 17 (31.5) | 0.93 | |
| Chronic heart failure | 24 (27.6) | 15 (27.8) | 0.98 | |
| Coronary artery disease | 22 (25.3) | 12 (22.2) | 0.68 | |
| End-stage renal disease | 16 (18.4) | 10 (18.5) | 0.98 | |
| Cerebrovascular disease | 7 (8.0) | 3 (5.6) | 0.58 | |
| Chronic obstructive pulmonary disease | 2 (2.3) | 1 (1.9) | 0.86 | |
| Initial cardiac rhythm, n (%) | Shockable | 55 (63.2) | 33 (61.1) | 0.80 |
| Non-shockable | 32 (36.8) | 21 (38.9) | 0.80 | |
| Initial core body temperature (°C), mean ± SEM | 35.7 ± 0.1 | 35.6 ± 0.1 | 0.55 | |
| CPR time (min), mean ± SEM | 21.6 ± 2.3 | 23.8 ± 3.1 | 0.29 | |
| Cardiogenic shock, n (%) | 36 (41.4) | 22 (40.7) | 0.94 | |
| CHD, n (%) | Coronary angiography | 66 (75.9) | 39 (72.2) | 0.63 |
| Left main disease | 10 (11.5) | 4 (7.4) | 0.43 | |
| Triple vessel disease | 33 (37.9) | 21 (38.9) | 0.91 | |
| AMI, n (%) | Percutaneous coronary intervention | 50 (57.5) | 30 (55.6) | 0.82 |
| ST-elevation MI | 31 (35.6) | 18 (33.3) | 0.78 | |
| Non-ST-elevation MI | 19 (21.8) | 12 (22.2) | 0.96 | |
| Intra-aortic balloon pump, n (%) | 26 (29.9) | 15 (27.8) | 0.79 | |
| Extracorporeal membrane oxygenation, n (%) | 28 (32.2) | 20 (37.0) | 0.55 | |
| Blood biochemistry parameters, mean ± SEM | hs-CRP (mg/L) | 1.6 ± 0.2 | 2.2 ± 0.3 | 0.18 |
| Fasting glucose (mg/dL) | 339.5 ± 17.6 | 324.3 ± 23.8 | 0.70 | |
| Total cholesterol (mg/dL) | 158.0 ± 6.3 | 135.8 ± 6.5 | 0.08 | |
| Triglyceride (mg/dL) | 91.6 ± 5.0 | 115.9 ± 8.6 | 0.06 | |
| LDL-C (mg/dL) | 98.1 ± 5.8 | 80.0 ± 5.2 | 0.10 | |
| HDL-C (mg/dL) | 35.7 ± 1.3 | 32.2 ± 1.6 | 0.21 | |
| Albumin (g/dL) | 3.1 ± 0.1 | 2.8 ± 0.1 | 0.09 | |
| NT pro-BNP (pg/mL) | 7699.2 ± 1059.0 | 9569.1 ± 1204.4 | 0.62 | |
| LVEF (%), mean ± SEM | 36.3 ± 1.8 | 32.3 ± 2.3 | 0.17 | |
AMI = acute myocardial infarction, CHD = coronary heart disease, CPR = cardiopulmonary resuscitation, HDL-C = high-density lipoprotein cholesterol, hs-CRP = high-sensitivity C-reactive protein, LDL-C = low-density lipoprotein cholesterol, LVEF = left ventricular ejection fraction, MI = myocardial infraction, NT pro-BNP = N-terminal pro-brain natriuretic peptide, SEM = standard error of the mean, TTM = targeted temperature management.
Initially, the patients developed heart failure with stress hyperglycemia, low high-density lipoprotein cholesterol levels, hypoalbuminemia, and reduced ejection fraction (Table 1). Twenty-four hours after resuscitation, aspartate aminotransferase, creatine kinase, creatine kinase-MB isoenzyme, troponin-I, and neutrophils were significantly elevated, whereas hemoglobin and platelets were decreased (all P < .001; Table 2).
Table 2.
Clinical biochemistry parameters and blood count during the first 24 h after resuscitation.
| After resuscitation | TTM (n = 87) | Control (n = 54) | P value | |
|---|---|---|---|---|
| Liver and kidney function markers, mean ± SEM | ||||
| AST (IU/L) | 0 h | 456.1 ± 77.8 | 255.4 ± 35.7 | .32 |
| 24 h | 977.2 ± 204.8 | 967.6 ± 208.8 | .99 | |
| 24-h difference | +114.3%, P < .001† | +278.9%, P < .001† | .16* | |
| ALT (IU/L) | 0 h | 211.8 ± 47.2 | 113.0 ± 13.4 | .41 |
| 24 h | 291.0 ± 78.2 | 140.9 ± 17.6 | .45 | |
| BUN (mg/dL) | 0 h | 35.1 ± 2.9 | 40.7 ± 3.7 | .25 |
| 24 h | 32.7 ± 2.4 | 41.4 ± 3.9 | .05 | |
| Creatinine (mg/dL) | 0 h | 2.2 ± 0.2 | 2.5 ± 0.3 | .40 |
| 24 h | 2.2 ± 0.2 | 2.4 ± 0.3 | .60 | |
| Muscle and cardiac markers, mean ± SEM | ||||
| CK (IU/L) | 0 h | 434.4 ± 50.5 | 350.9 ± 56.4 | .29 |
| 24 h | 3923.4 ± 635.8 | 3732.9 ± 735.7 | .86 | |
| 24-h difference | +803.2%, P < .001† | +963.8%, P < .001† | .92* | |
| CK-MB (ng/mL) | 0 h | 28.9 ± 4.8 | 25.7 ± 4.6 | .66 |
| 24 h | 230.2 ± 37.3 | 197.1 ± 43.9 | .57 | |
| 24-h difference | +696.5%, P < .001† | +666.9%, P < .001† | .61* | |
| Troponin-I (ng/mL) | 0 h | 1.5 ± 0.4 | 1.8 ± 0.6 | .67 |
| 24 h | 30.7 ± 3.6 | 27.7 ± 4.0 | .59 | |
| 24-h difference | +1946.7%, P < .001† | +1438.9%, P < .001† | .65* | |
| Blood count, mean ± SEM | ||||
| WBC (K/μL) | 0 h | 13.9 ± 0.6 | 13.8 ± 0.5 | .95 |
| 24 h | 14.9 ± 0.8 | 13.2 ± 0.5 | .11 | |
| Neutrophils (K/μL) | 0 h | 8.4 ± 0.5 | 8.5 ± 0.3 | .90 |
| 24 h | 12.7 ± 0.7 | 11.2 ± 0.5 | .11 | |
| 24-h difference | +51.2%, P < .001† | +31.8%, P < .001† | .12* | |
| Hemoglobin (g/dL) | 0 h | 12.5 ± 0.3 | 12.1 ± 0.3 | .36 |
| 24 h | 11.6 ± 0.3 | 10.8 ± 0.3 | .07 | |
| 24-h difference | −7.2%, P < .001† | −10.7%, P < .001† | .31* | |
| Platelets (K/μL) | 0 h | 186.5 ± 7.9 | 166.2 ± 10.9 | .13 |
| 24 h | 152.1 ± 7.3 | 129.5 ± 9.1 | .06 | |
| 24-h difference | −18.4%, P < .001† | −22.1%, P < .001† | .85† | |
24-h difference = (level at 24 hours after resuscitation—level at resuscitation)/level at resuscitation, P value = differences between the 2 groups using Student t test.
ALT = alanine aminotransferase, AST = aspartate aminotransferase, BUN = blood urea nitrogen, CK = creatine kinase, CK-MB = creatine kinase-MB isoenzyme, MANOVA = multivariate analysis of variance, SEM = standard error of the mean, TTM = targeted temperature management, WBC = white blood cells.
P value = interaction effect, differences between the 2 groups, and over time using the MANOVA model.
P value = time differences using the paired t test.
3.2. IL-6, IL-6/soluble IL-6 receptor complex, and lactate clearance rate
The IL-6 levels at 6 hours after resuscitation were similar between the 2 groups; however, the IL-6 levels differed between the groups 24 hours after resuscitation. The IL-6 production rate between 6 and 24 hours after resuscitation was considerably lower in the TTM group than in the control group (−50.0% vs +136.7%, P < .001), and similar results were noted for the IL-6/soluble IL-6 receptor complex (+26.3% vs +102.4%, P < .001). In addition, a significant interaction between temperature management and time was noted for IL-6 and IL-6/soluble IL-6 receptor complex (P for interaction < .001). However, soluble IL-6 receptor and soluble glycoprotein 130 levels did not significantly differ between the groups. Lactate levels at 6 hours after resuscitation were similar in both groups. However, lactate levels at 24 hours after resuscitation differed between the groups. The lactate clearance rate between 6 and 24 hours was considerably higher in the TTM group than in the control group (−59.5% vs −36.2%, P = .03; Table 3).
Table 3.
Comparison of IL-6 and IL-6/sIL-6R complex production and lactate clearance between the targeted temperature management (TTM) and control groups.
| After resuscitation | TTM (n = 87), mean ± SEM | Control (n = 54), mean ± SEM | P value | |
|---|---|---|---|---|
| Core body temperature (°C) | 6 h | 35.9 ± 0.1 | 36.1 ± 0.2 | .51 |
| 24 h | 33.3 ± 0.1 | 36.6 ± 0.2 | < .001 | |
| IL-6 (pg/mL) | 6 h | 2020.5 ± 177.9 | 1952.4 ± 147.9 | .22 |
| 24 h | 1011.2 ± 252.4 | 4621.5 ± 726.4 | < .001 | |
| 18-h difference | −50.0%, P < .001† | +136.7%, P < .001† | < .001* | |
| sIL-6R (ng/mL) | 6 h | 40.0 ± 1.6 | 34.9 ± 1.7 | .28 |
| 24 h | 37.5 ± 1.3 | 34.8 ± 1.9 | .27 | |
| 18-h difference | −6.3%, P = .11† | −0.3%, P = .07† | .85* | |
| IL-6/sIL-6R complex (pg/mL) | 6 h | 164.8 ± 7.8 | 173.6 ± 13.3 | .20 |
| 24 h | 208.1 ± 16.1 | 351.4 ± 27.8 | < .001 | |
| 18-h difference | +26.3%, P = .004† | +102.4%, P < .001† | < .001* | |
| sgp130 (ng/mL) | 6 h | 240.0 ± 20.7 | 198.4 ± 32.8 | .42 |
| 24 h | 254.7 ± 21.1 | 222.9 ± 38.0 | .23 | |
| 18-h difference | +6.1%, P = .48† | +12.3%, P = .75† | .83* | |
| Lactate (mmol/L) | 6 h | 11.6 ± 0.7 | 12.7 ± 0.8 | .32 |
| 24 h | 4.7 ± 0.5 | 8.1 ± 1.0 | .001 | |
| 18-h difference | −59.5%, P < .001† | −36.2%, P < .001† | .03* |
18-h difference = (level at 24 hours after resuscitation—level at 6 hours after resuscitation)/level at 6 hours after resuscitation. P value = differences between the 2 groups using Student t test.
IL-6 = interleukin-6, IL-6/sIL-6R = interleukin-6/soluble interleukin-6 receptor, MANOVA = multivariate analysis of variance, SEM = standard error of the mean, sgp130 = soluble glycoprotein 130, sIL-6R = soluble interleukin-6 receptor.
P value = interaction effect, differences between the 2 groups, and over time using the MANOVA model.
P value = time differences using paired t test.
3.3. IL-6 levels and SOFA score
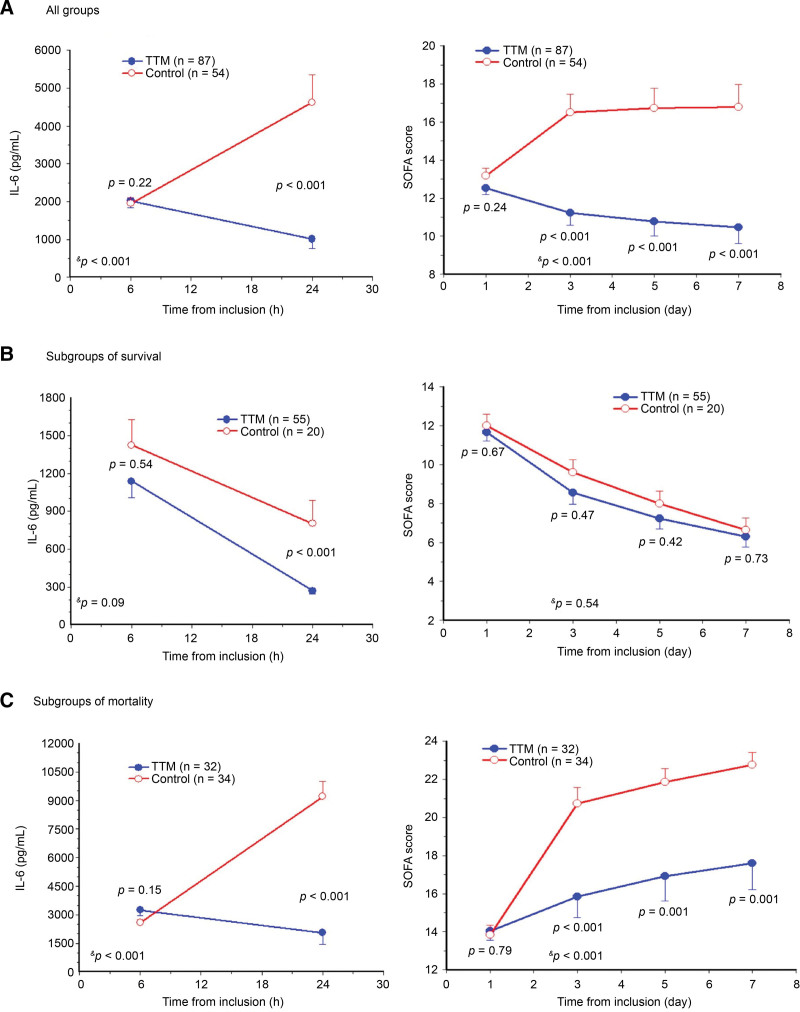
The IL-6 levels at 6 hours after resuscitation were similar between the TTM and control groups (2020.5 vs 1952.4 pg/mL, P = .22). However, the IL-6 level at 24 hours after resuscitation was lower in the TTM group than in the control group (1011.2 vs 4621.5 pg/mL, P < .001). A significant interaction between temperature management and time (−50.0% vs + 136.7%, P < .001; Fig. 1A, left) was observed. The SOFA score was similar between the groups on day 1 (12.5 vs 13.2, P = .24) but markedly lower in the TTM group than in the control group on days 3 (11.2 vs 16.5, P < .001), 5 (10.8 vs 16.7, P < .001), and 7 (10.5 vs 16.8, P < .001). Also, a significant interaction between temperature management and time (P < .001; Fig. 1A, right) was observed.
Figure 1.
Circulating interleukin (IL)-6 predicts the sequential organ failure assessment (SOFA) score in patients with out-of-hospital cardiac arrest. (A) Analyses of the 2 groups revealed that targeted temperature management (TTM) resulted in a noticeable reduction in IL-6 levels and SOFA score. (B) Subgroup analyses of survival showed that TTM reduced IL-6 levels and SOFA score in both groups. (C) Subgroup analyses of mortality indicated that TTM prolonged life expectancy with a relatively lower SOFA score resulting from a moderate decrease in IL-6 levels. P value = differences between the 2 groups using Student t test. &P value = interaction effect, differences between the 2 groups, and over time using the multivariate analysis of variance model.
Subgroup analyses for 90-day survival revealed low IL-6 levels (< 1000 pg/mL) at 24 hours after resuscitation and low SOFA scores (< 10) on days 3, 5, and 7 in the TTM and control groups (Fig. 1B). On the contrary, subgroup analyses for 90-day mortality revealed high IL-6 levels (> 2000 pg/mL) at 6 hours after resuscitation and high SOFA scores (> 14) on days 3, 5, and 7 in the TTM and control groups (Fig. 1C).
3.4. Mortality rate and neurologic outcomes
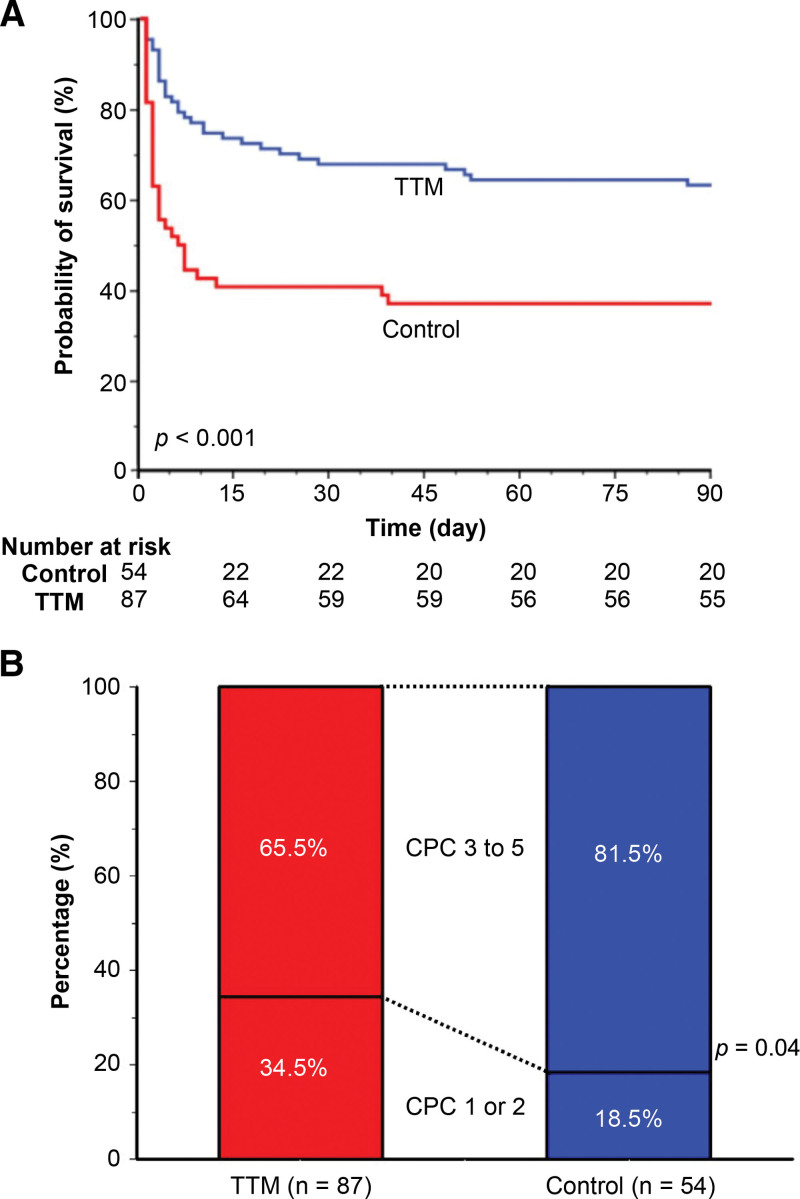
The 90-day mortality rate of the TTM group was significantly lower than that of the control group (36.8% vs 63.0%, relative risk 0.39; 95% CI 0.24–0.64, P < .001; Table 4; Fig. 2A). Not only TTM but also an initial shockable rhythm and a short duration of cardiopulmonary resuscitation (≤ 10 minutes) were associated with a lower mortality rate compared to the control. The proportion of patients with poor neurologic outcomes (CPC scores, 3–5) was 65.5% in the TTM group and 81.5% in the control group (relative risk 0.80, 95% CI 0.66–0.98, P = .04; Table 4; Fig. 2B).
Table 4.
Ninety-day mortality rate-adjusted covariables using Cox regression model and neurologic outcomes between the TTM and control groups.
| Mortality-adjusted covariables using Cox regression model, n (%) | Relative risk (95% CI) | P value | |||
|---|---|---|---|---|---|
| TTM vs Control | 32/87 (36.8) | 34/54 (63.0) | 0.39 (0.24–0.64) | < .001 | |
| Initial shockable rhythm vs initial non-shockable rhythm | 30/88 (34.1) | 36/53 (67.9) | 0.36 (0.22–0.60) | < .001 | |
| CPR time ≤ 10 min vs > 10 min | 17/50 (34.0) | 49/91 (53.8) | 0.53 (0.30–0.94) | .03 | |
| Age < 75 years vs ≥ 75 years | 53/120 (44.2) | 13/21 (61.9) | 0.72 (0.39–1.35) | .31 | |
| Male vs female | 51/112 (45.5) | 15/29 (51.7) | 1.00 (0.53–1.88) | 1.00 | |
| CHD vs non-CHD | 49/104 (47.1) | 17/37 (45.9) | 1.04 (0.58–1.86) | .91 | |
| Neurologic outcomes, n (%) | TTM (n = 87) | Control (n = 54) | Relative risk (95% CI) | P value | |
|---|---|---|---|---|---|
| Poor neurologic outcomes | 57 (65.5) | 44 (81.5) | 0.80 (0.66–0.98) | .04 | |
| CPC scores | 1 | 19 (21.8) | 6 (11.1) | ||
| 2 | 11 (12.6) | 4 (7.4) | |||
| 3 | 8 (9.2) | 3 (5.6) | |||
| 4 | 17 (19.5) | 7 (13.0) | |||
| 5 | 32 (36.8) | 34 (63.0) | |||
CHD = coronary heart disease, CI = confidence interval, CPC = cerebral performance category, CPR = cardiopulmonary resuscitation, TTM = targeted temperature management.
Figure 2.
Survival rates and neurological outcomes measured using cerebral performance category (CPC) scores 90 days after out-of-hospital cardiac arrest. (A) Survival rate of the targeted temperature management (TTM) group was higher than that of the control group. (B) Favorable neurologic outcomes (CPC score 1 or 2) were greater in the TTM group than in the control group.
4. Discussion
The results of this study revealed that TTM not only improved mortality and neurologic outcomes but also reduced the production of IL-6 and IL-6/sIL-6R complex in patients with OHCA. A possible explanation is that inhibition of IL-6 production by TTM attenuates the systemic inflammatory response triggered by myocardial ischemia and reperfusion injury after cardiac arrest.[12]
OHCA triggers a series of systemic inflammatory responses mediated by the inflammatory cytokine IL-6.[13] In this study, the IL-6 and IL-6/soluble IL-6 receptor complex production rates between 6 and 24 hours were lower in the TTM group than in the control group (–50.0% vs +136.7%, P < .001; +26.3% vs +102.4%, P < .001, respectively). No differences in soluble IL-6 receptor and soluble glycoprotein 130 levels were observed between and within the 2 groups. Recent TTM studies have indicated that TTM at 33–34 °C or 36–37 °C resulted in better mortality and neurologic outcomes compared with no TTM.[14,15] The difference in IL-6 production rate between 6 and 24 hours after resuscitation can be attributed to the higher incidence of fever and more temperature fluctuations in the control group than in the TTM group. Fever significantly increases cerebral metabolism rate and oxygen consumption. Besides, IL-6 is associated with systemic inflammatory responses, particularly fever.[16] Thus, TTM might inhibit IL-6 production by preventing fever, thereby reducing inflammatory responses.
The SOFA score and serial IL-6 levels have been used to predict prognosis in critically ill patients for over a decade.[6] TTM is associated with a reduction in IL-6 levels, which consequently lowers the SOFA score.[13,17] In this study, IL-6 levels > 2000 pg/mL at 6 hours after resuscitation predicted poor outcomes, and IL-6 levels < 1000 pg/mL at 24 hours after resuscitation predicted favorable outcomes. Thus, TTM reduces IL-6 levels, as well as SOFA score and rate of poor prognosis. In this study, the 90-day mortality rate of the TTM group was significantly lower than that of the control group. Furthermore, the TTM group had significantly fewer 90-day poor neurologic outcomes (CPC scores of 3, 4, and 5) than did the control group. The improvements in mortality and neurologic outcomes following TTM observed in this study are similar to those in other studies.[18,19]
Although TTM has become a standard practice in postcardiac arrest care to prevent fever in recent years, TTM at 33–34 °C (therapeutic hypothermia) is associated with many severe complications. Therapeutic hypothermia is likely to result in hypokalemia, slow heart rate, QT prolongation, and polymorphic ventricular arrhythmias, such as torsade de pointes.[15] In addition, it can hinder proper management of cerebral hemorrhage or other major bleeding events because of impaired coagulation and platelet dysfunction.[20] Recently, TTM at 36–37 °C (controlled normothermia) has become popular in minimizing these complications; similar results were achieved in most patients (70–85%) who had not undergone shock status, and this approach resulted in improved survival and neurologic outcomes.[15,21] However, survival and neurologic outcomes after controlled normothermia were slightly inferior to those after therapeutic hypothermia in patients experiencing shock or those requiring high doses of vasopressors.[22,23] This difference could be attributed to more severe inflammation in patients who experience cardiac shock and more pronounced anti-inflammatory effects of therapeutic hypothermia compared to those of controlled normothermia.
5. Limitations
This study has some limitations. First, the number of patients with OHCA enrolled in this study was too small to accurately predict real-world conditions. Second, nearly 41% of patients with cardiac shock required prolonged vasopressor support, but only 34% received more stable circulatory support, such as extracorporeal membrane oxygenation. Such management may directly affect the patient prognosis, resulting in a less favorable outcome. Third, although TTM improves clinical survival and neurologic outcomes, this study primarily used TTM at 33 to 34 °C (therapeutic hypothermia). Thus, it is crucial to explore and differentiate between indications for therapeutic hypothermia (33–34 °C) and controlled normothermia (36–37 °C). Fourth, several studies have attempted to pharmacologically mitigate inflammation in acute myocardial infarction or pulseless ventricular arrhythmias using IL-1 receptor antagonists or anti-IL-6 receptor monoclonal antibodies.[24,25] Anti-IL-6 receptor antibodies significantly reduced systemic inflammation and myocardial injury markers but with no additional beneficial effect on survival or neurologic outcomes.[26] In this study, considerable improvement in neurologic outcomes was noted when IL-6 levels were > 2000 pg/mL within the first 6 hours. Thus, determining whether to target IL-6 levels to guide the choice between therapeutic hypothermia and controlled normothermia is a valuable area for future research.
6. Conclusions
TTM inhibits IL-6 production and exerts anti-inflammatory effects, thereby improving mortality and neurologic outcomes in patients resuscitated from OHCA. An IL-6 level > 2000 pg/mL at 6 hours after OHCA predicts poor neurologic outcomes and the need for more intensive care.
Acknowledgments
The authors thank Hsuan-Ching Wang, the head nurse at the Cardiac Intensive Care Unit, and Yi-Chun Chou, the head nurse at the Stroke and Neurology Critical Care Unit, for providing clinical support.
Author contributions
Conceptualization: Da-Long Chen, Yu-Kai Lin, Po-Yen Ko, Jen-Jyh Lin, Chih-Yang Huang.
Data curation: Da-Long Chen, Yu-Kai Lin, Po-Yen Ko, Jen-Jyh Lin.
Formal analysis: Da-Long Chen, Chih-Yang Huang.
Investigation: Da-Long Chen, Chih-Yang Huang.
Methodology: Da-Long Chen.
Writing – original draft: Da-Long Chen.
Visualization: Chih-Yang Huang.
Supervision: Guei-Jane Wang, Kuan-Cheng Chang.
Writing – review & editing: Guei-Jane Wang, Kuan-Cheng Chang.
Supplementary Material
Abbreviations:
- CPC
- cerebral performance category
- IL
- interleukin
- OHCA
- out-of-hospital cardiac arrest
- SOFA
- sequential organ failure assessment
- TTM
- targeted temperature management
This study was approved by the Institutional Review Board of China Medical University Hospital, Taichung, Taiwan, on October 26, 2015 (approval number: CMUH104-REC3-058). This study was registered with ClinicalTrials.gov (registration number: NCT02633358). As this study was a prospective cohort study, informed consent was signed by the decision-makers before the study.
The authors have no conflicts of interest to disclose.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Supplemental Digital Content is available for this article.
How to cite this article: Chen D, Lin Y, Ko P, Lin J, Huang C, Wang G, Chang K-C. Effect of targeted temperature management on systemic inflammatory responses after out-of-hospital cardiac arrest: A prospective cohort study. Medicine 2024;103:38(e39780).
References
- [1].McNally B, Robb R, Mehta M, et al.; Centers for Disease Control and Prevention. Out-of-hospital cardiac arrest surveillance - cardiac arrest registry to enhance survival (CARES), United States, October 1, 2005-December 31, 2010. MMWR Surveill Summ. 2011;60:1–19. [PubMed] [Google Scholar]
- [2].Loma-Osorio P, Aboal J, Sanz M, et al. Clinical characteristics and vital and functional prognosis of out-of-hospital cardiac arrest survivors admitted to five cardiac intensive care units. Rev Esp Cardiol (Engl Ed). 2013;66:623–8. [DOI] [PubMed] [Google Scholar]
- [3].Wang CH, Chou NK, Becker LB, et al. Improved outcome of extracorporeal cardiopulmonary resuscitation for out-of-cardiac arrest - a comparison with that for extracorporeal rescue for in-hospital cardiac arrest. Resuscitation. 2014;85:1219–24. [DOI] [PubMed] [Google Scholar]
- [4].Vaahersalo J, Skrifvars MB, Pulkki K, et al.; FINNRESUSCI Laboratory Study Group. Admission interleukin-6 is associated with post resuscitation organ dysfunction and predicts long-term neurological outcome after out-of-hospital ventricular fibrillation. Resuscitation. 2014;85:1573–9. [DOI] [PubMed] [Google Scholar]
- [5].Fang L, Moore XL, Dart AM, Wang LM. Systemic inflammatory response following acute myocardial infarction. J Geriatr Cardiol. 2015;12:305–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Chong JY, Ahn HJ, Park JS, et al. Interleukin-6 as a potential predictor of neurologic outcomes in cardiac arrest survivors who underwent target temperature management. J Emerg Med. 2020;59:828–35. [DOI] [PubMed] [Google Scholar]
- [7].Rose-John S. IL-6 trans-signaling via the soluble IL-6 receptor: importance for the pro-inflammatory activities of IL-6. Int J Biol Sci. 2012;8:1237–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Elabbassi W, Aila FA, Chowdhury MA, et al. The impact of severity of initial illness, determined by SOFA score, and presence of anemia on outcomes among patients requiring extra corporal membrane oxygenation (ECMO) support: a single center experience. Indian Heart J. 2017;69:762–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Cour M, Bresson D, Hernu R, Argaud L. SOFA score to assess the severity of the post-cardiac arrest syndrome. Resuscitation. 2016;102:110–5. [DOI] [PubMed] [Google Scholar]
- [10].Bro-Jeppesen J, Kjaergaard J, Wanscher M, et al. Systemic inflammatory response and potential prognostic implications after out-of-hospital cardiac arrest: a substudy of the target temperature management trial. Crit Care Med. 2015;43:1223–32. [DOI] [PubMed] [Google Scholar]
- [11].Scirica BM. Therapeutic hypothermia after cardiac arrest. Circulation. 2013;127:244–50. [DOI] [PubMed] [Google Scholar]
- [12].Hausenloy DJ, Yellon DM. Myocardial ischemia-reperfusion injury: a neglected therapeutic target. J Clin Invest. 2013;123:92–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bro-Jeppesen J, Kjaergaard J, Stammet P, et al.; TTM-Trial Investigators. Predictive value of interleukin-6 in post-cardiac arrest patients treated with targeted temperature management at 33 °C or 36 °C. Resuscitation. 2016;98:1–8. [DOI] [PubMed] [Google Scholar]
- [14].Casamento A, Minson A, Radford S, et al. A comparison of therapeutic hypothermia and strict therapeutic normothermia after cardiac arrest. Resuscitation. 2016;106:83–8. [DOI] [PubMed] [Google Scholar]
- [15].Dankiewicz J, Cronberg T, Lilja G, et al.; TTM2 Trial Investigators. Hypothermia versus normothermia after out-of-hospital cardiac arrest. N Engl J Med. 2021;384:2283–94. [DOI] [PubMed] [Google Scholar]
- [16].Eskilsson A, Mirrasekhian E, Dufour S, Schwaninger M, Engblom D, Blomqvist A. Immune-induced fever is medicated by IL-6 receptors on brain endothelial cells coupled to STAT3-dependent induction of brain endothelial prostaglandin synthesis. J Neurosci. 2014;34:15957–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Peberdy MA, Andersen LW, Abbate A, et al. National Post Arrest Research Consortium (NPARC) Investigators. Inflammatory markers following resuscitation from out-of-hospital cardiac arrest-a prospective multicenter observational study. Resuscitation. 2016;103:117–24. [DOI] [PubMed] [Google Scholar]
- [18].van der Wal G, Brinkman S, Bisschops LL, et al. Influence of mild therapeutic hypothermia after cardiac arrest on hospital mortality. Crit Care Med. 2011;39:84–8. [DOI] [PubMed] [Google Scholar]
- [19].Wang CJ, Yang SH, Lee CH, Lin RL, Peng MJ, Wu CL. Therapeutic hypothermia application vs standard support care in post resuscitated out-of-hospital cardiac arrest patients. Am J Emerg Med. 2013;31:319–25. [DOI] [PubMed] [Google Scholar]
- [20].Polderman KH. Hypothermia and coagulation. Crit Care. 2012;16:A20. [Google Scholar]
- [21].Nielsen N, Wetterslev J, Cronberg T, et al. TTM Trial Investigators. Targeted temperature management at 33°C versus 36°C after cardiac arrest. N Engl J Med. 2013;369:2197–206. [DOI] [PubMed] [Google Scholar]
- [22].Lascarrou JB, Merdji H, Le Gouge A, et al. CRICS-TRIGGERSEP Group. Targeted temperature management for cardiac arrest with nonshockable rhythm. N Engl J Med. 2019;381:2327–37. [DOI] [PubMed] [Google Scholar]
- [23].Düring J, Annborn M, Cariou A, et al. Influence of temperature management at 33 °C versus normothermia on survival in patients with vasopressor support after out-of-hospital cardiac arrest: a post hoc analysis of the TTM-2 trial. Crit Care. 2022;26:231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].de Jesus NM, Wang L, Lai J, et al. Antiarrhythmic effects of interleukin 1 inhibition after myocardial infarction. Heart Rhythm. 2017;14:727–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kleveland O, Kunszt G, Bratlie M, et al. Effect of a single dose of the interleukin-6 receptor antagonist tocilizumab on inflammation and troponin T release in patients with non-ST-elevation myocardial infarction: a double-blind, randomized, placebo-controlled phase 2trial. Eur Heart J. 2016;37:2406–13. [DOI] [PubMed] [Google Scholar]
- [26].Meyer MAS, Wiberg S, Grand J, et al. Treatment effects of interleukin-6 receptor antibodies for modulating the systemic inflammatory response after out-of-hospital cardiac arrest (The IMICA Trial): a double-blinded, placebo-controlled, single-center, randomized, clinical trial. Circulation. 2021;143:1841–51. [DOI] [PMC free article] [PubMed] [Google Scholar]