Abstract
Hearing loss significantly affects communication, social interactions, and the overall quality of life. The bone-anchored hearing aid (BAHA) is an implantable system that bypasses the outer and middle ear to directly stimulate the cochlea through bone conduction. This study aimed to compare hearing performance and subjective auditory ability improvements between transcutaneous and percutaneous BAHA devices using audiological assessments and Speech, Spatial and Qualities of Hearing Scale.
This cross-sectional prospective study included 29 participants aged 14 to 69 years who had used BAHA for at least 6 months. Both Cochlear Baha System’s percutaneous (connect) and transcutaneous (attract) implants were evaluated. Audiological assessments involved pure-tone audiometry, speech recognition threshold, and free-field (FF) audiometry, while subjective auditory ability was measured using the Turkish Speech, Spatial and Qualities of Hearing scale (Tr-SSQ).
Significant improvements in FF audiometry averages and speech recognition thresholds were observed with BAHA compared to without BAHA (P < .001). Both implant types provided similar FF averages, speech audiometry results, and Tr-SSQ outcomes, with no significant differences between them. Tr-SSQ scores showed substantial satisfaction, indicating significant improvements in speech perception, spatial perception, and hearing quality with BAHA (P < .001).
The findings align with previous research, demonstrating that BAHA is a reliable and effective solution for hearing rehabilitation. The study also emphasized the importance of using both audiological test results and daily hearing function scales to comprehensively evaluate the benefits of hearing rehabilitation in real-world environments. In conclusion, BAHA, regardless of the implant type, can provide predictable and lasting improvements in hearing thresholds and daily hearing abilities, making it a valuable option for patients with conductive hearing loss.
Keywords: audiology, bone-anchored hearing aid, hearing function, Speech, Spatial and Qualities of Hearing Scale
1. Introduction
Hearing loss is a prevalent condition that significantly affects individuals’ communication skills, social interactions, and the overall quality of life (QoL).[1] The bone-anchored hearing aid (BAHA) is an implantable hearing rehabilitation system that has been in use for over 4 decades.[2] These devices bypass the outer and middle ear by directly stimulating the cochlea via bone conduction (BC), and there are 2 main types: percutaneous and transcutaneous implants.[3,4] BC implants can also be categorized as active and passive systems.[5]
Active percutaneous implants, such as Baha Connect, involve an abutment penetrating the skin to connect directly to the implant. On the other hand, passive transcutaneous systems, such as the Baha Attract, deliver the acoustic stimulus through closed skin and utilize a magnet located under the skin to hold the sound processor in place on the implant screw, greatly reducing skin and soft tissue complications compared to the Baha Connect.[5–10] The processor of these devices adjusts across multiple power levels based on the patient’s BC thresholds.[3] The decision between percutaneous and transcutaneous BAHA implants depends on various factors, including clinical recommendations and individual patient needs. Both types have their own advantages and potential drawbacks, making it essential to thoroughly evaluate their performance and impact on patients’ lives.[11]
The success of hearing devices depends not only on hearing improvement but also on patient satisfaction. The patient’s satisfaction with the rehabilitation directly affects the willingness to use the device, therapy compliance, and success. Therefore, the critical aim of hearing rehabilitation should be to increase the QoL and patient satisfaction, as well as the improve hearing perception.[1] Evaluating the benefits of BAHA through both audiological tests and overall quality of hearing scales is crucial.[12–14] The Speech, Spatial and Qualities of Hearing Scale (SSQ) is a widely used self-reported measure of daily hearing function for individuals with hearing impairment. The SSQ designed encompasses questions related to listening capabilities in various practical daily situations categorized into speech, spatial, and qualities of hearing.[15]
This study aimed to compare the audiological performance and hearing difficulties across various domains subjective auditory ability contributions of transcutaneous and percutaneous BAHA types using audiological data and SSQ. By utilizing both audiological data and subjective auditory abilities, we aim to deliver valuable insights that could guide clinical decisions and enhance patient outcomes in the realm of auditory rehabilitation.
2. Methods
2.1. Study design
The single-centered, cross-sectional, prospective study included individuals fitted with BAHA who received regular follow-ups. Data were collected between July 2022 and May 2024. In this study, the contributions of transcutaneous and percutaneous BAHA types to audiologic performance and subjective auditory ability were compared.
2.2. Subject
Participants meeting BAHA application criteria were included after providing informed consent. The Cochlear Baha System (Cochlear Ltd, Sydney, Australia) was implanted percutaneously (connect) or transcutaneously (attract) in all enrolled patients. The study cohort comprised individuals aged 14 to 69 years who had regularly used BAHA in 1 ear for at least 6 months.
Exclusions encompassed those with single-sided deafness, unilateral conductive hearing loss, inability to reliably respond to questionnaires, and concurrent neurological or genetic disorders.
The sample size was determined using the G-Power 3.1 (Universitat Dusseldorf, Germany) statistical tool. A review of the literature found a significant difference with an effect size (Cohen d) of 1.31 in audiological assessment of percutaneous vs transcutaneous BAHAs: a pilot study.[7] In this study, it was hypothesized that a comparable discrepancy would be observed between the hearing and subjective auditory ability outcomes between the percutaneous and transcutaneous groups. Accordingly, a sample size of 17 participants per group was calculated as necessary to detect the aforementioned difference with 95% power and 5% type 1 error rate. Forty-four participants (22 participants for each group) were enrolled in our study. However, 2 users did not attend follow-up visits regularly in the transcutaneous group. Five users who did not attend follow-up visits regularly, 7 users who were not old enough or had the capacity to respond to the scale, and 1 user with single-sided deafness in the percutaneous group were excluded from the study. Therefore, our study was completed with 29 participants (20 participants in the transcutaneous group and 9 participants in the percutaneous group).
Audio processors used included Baha 5, Baha 5 Power, Baha 5 SuperPower, and BAHA 6 max selected based on clinical indications.
2.3. Audiological evaluations
Pure-tone audiometry was performed with a Madsen Astera 2 clinical audiometer (GN Otometrics A/S, 2630 Taastrup, Denmark) using Telephonics TDH 39 P headphones for air conduction (AC) and B71 vibrator for BC measurement. Three different pure-tone audiometry average (PTA) and free-field test average (FFA) methods were used to assess the auditory performance of subjects. These methods, categorized as PTA1 (arithmetic means of pure-tone audiometry thresholds at 0.5-, 1-, and 2-kHz frequencies), PTA2 (arithmetic means of pure-tone audiometry thresholds at 1-, 2-, and 4-kHz frequencies), PTA3 (arithmetic means of pure-tone audiometry thresholds at 0.5-, 1-, 2-, and 4-kHz frequencies), FFA1 (arithmetic means of free-field [FF] audiometry thresholds at 0.5-, 1-, and 2-kHz frequencies), FFA2 (arithmetic means of FF audiometry thresholds at 1-, 2-, and 4-kHz frequencies), and FFA3 (arithmetic means of FF audiometry thresholds at 0.5-, 1-, 2-, and 4-kHz frequencies) for PTA and FF threshold average, respectively, were chosen because they allow a comprehensive assessment of the frequency bands critical for speech intelligibility. Unaided assessments included AC (0.25–8 kHz) and BC (0.5–4 kHz) hearing thresholds, pure-tone audiometry arithmetic averages (PTA1, PTA2, and PTA3) across specified frequencies (0.5, 1, and 2 kHz; 1, 2, and 4 kHz; and 0.5, 1, 2, and 4 kHz), and measurements of air-bone gaps and speech recognition threshold (SRT) test. The SRT test was performed in quiet with supra-aural headphones and in sound field with loudspeakers. The tests performed with BAHA included FF threshold test and SRT test (in quiet) in FF. FF (sound field) threshold tests were assessed under a quiet condition using warble tones emitted from a loudspeaker at 1 m in front of the participant (0° azimuth) between 250- and 8000-Hz frequencies, both with and without BAHA. All tests were conducted in a soundproof chamber using a Madsen Astera2 clinical audiometer (GN Otometrics A/S, 2630 Taastrup, Denmark,). The FF test thresholds and average of FF were calculated using the same frequencies (0.25–8 kHz) and method as the pure-tone audiometry threshold and average. After performing the FF threshold tests, FFAs were calculated. The arithmetic means of the FF thresholds at 0.5, 1, and 2 kHz; 1, 2, and 4 kHz; and 0.5, 1, 2, and 4 kHz were defined as FFA1, FFA2, and FFA3, respectively.
2.4. Turkish SSQ
The SSQ is specifically developed to assess a variety of hearing difficulties across various domains.[15] The SSQ consists of 3 subscales: perception of speech, spatial perception, and qualities of hearing. The first subscale, “speech perception,” evaluates the ability to recognize, discriminate, and follow the sounds of speech. The second subscale, known as “spatial perception,” presents data on the ability to determine the location, distance, and spatial mobility of the heard sound. The third subscale, “qualities of hearing,” includes items related to the identifiability of simultaneous sounds experienced in everyday life and quantifies clarity, naturality, intelligibility, and hearing effort.[15,16] Participants were assessed using the Turkish SSQ (Tr-SSQ) to evaluate satisfaction with BAHA implantation.[16] This 49-item questionnaire measured various aspects of hearing skills, such as speech and environmental noise discrimination, orientation, and positioning, both with and without BAHA. Sections on speech perception, spatial perception, and hearing quality were scored on a scale from 0 to 10, where a score of 10 indicated perfect performance and 0 indicated no performance in the described situation.
2.5. Statistical analysis
Statistical analysis was conducted using the IBM SPSS software, version 24. Data normality was assessed with the Kolmogorov-Smirnov test. Nonparametric tests were applied for data that did not follow a normal distribution (P < .05). Analyses were performed with a 95% confidence interval, considering P < .05 as statistically significant. Results were presented as mean ± standard deviation for continuous variables. Categorical variables were summarized using frequency and percentage. The Wilcoxon rank-sum test was used to compare BAHA results within groups. The Mann–Whitney U test was employed to compare audiological and Tr-SSQ results between the percutaneous and transcutaneous BAHA groups. The Spearman correlation test was used to examine relationships between audiological results, Tr-SSQ, and demographic characteristics.
2.6. Ethics committee approval
The study received approval from the Ethics Committee of Dokuz Eylul University (protocol number: 2022/28-20). Informed consent was obtained from the participants aged 18 years and above, as well as from parents of participants under 18 years of age. This study adhered to the ethical standards outlined in the 1964 Declaration of Helsinki and its subsequent revisions.
3. Results
3.1. Sample profiles
A total of 29 participants (13 females and 16 males) with a mean age of 28.00 ± 16.21 years were included in the study. No significant correlations or differences were found between age, gender, and Tr-SSQ score averages (P > .05). Demographic characteristics of BAHA users are provided in Table 1. Two percutaneous BAHA users underwent transcutaneous implant placement due to skin problems such as the development of dense granulation tissues on the abutment and epithelialization. In this study, one of the patients was a transcutaneous implant user for 4 years and the other for 5 years, and therefore, these participations were included in the transcutaneous group.
Table 1.
Demographic characteristic of the BAHA users.
| Age (yr), mean ± SD (min–max) | 28.00 ± 16.21 (14–69) |
| Side of implanted ear (n) | |
| Right | 18 |
| Left | 11 |
| Implant type of BAHA (n) | |
| Percutaneous | 9 |
| Transcutaneous | 20 |
| Audio processor type of BAHA | |
| Baha 5 | 13 |
| Baha 5 P | 6 |
| Baha 5 SP | 3 |
| Baha 6 max | 7 |
| Indication for BAHA (n) | |
| Atresia | 10 |
| Chronic otitis media | 14 |
| Tympanosclerosis | 1 |
| External auditory canal stenosis | 4 |
BAHA = bone-anchored hearing aid, max = maximum, min = minimum, n = number or participants, SD = standard deviation.
3.2. Audiological evaluation
The preimplantation audiological results, including AC and BC PTA values, SRT, and air-bone gaps of BAHA users, are presented in Table 2. There was no statistical difference between percutaneous and transcutaneous users in terms of preimplantation audiological results (P > .05). No statistically significant difference was observed between all BAHA 5 sound processors and BAHA 6 max in terms of audiological test results, speech reception thresholds, and FF thresholds performed with BAHA (P > .05).
Table 2.
Preimplantation pure-tone audiometry and SRT results of participants.
| Mean ± SD (min–max) (dB) | P values | ||
|---|---|---|---|
| Transcutaneous (n = 20) | Percutaneous (n = 9) | ||
| AC PTA1 | 55.88 ± 11.55 (37 to 70) | 54.26 ± 11.91 (40 to 73) | > .05 |
| AC PTA2 | 58.19 ± 13.75 (32 to 85) | 53.81 ± 17.45 (33 to 90) | > .05 |
| AC PTA3 | 59.67 ± 12.21 (36 to 85) | 55.57 ± 17.04 (34 to 82.5) | > .05 |
| BC PTA1 | 16.75 ± 12.12 (−5 to 38) | 15.00 ± 11.55 (2 to 42) | > .05 |
| BC PTA1 | 18.65 ± 12.49 (−5 to 37) | 18.15 ± 14.68 (2 to 43) | > .05 |
| BC PTA3 | 17.77 ± 11.63 (−5 to 36) | 16.56 ± 12.83 (1 to 41) | > .05 |
| ABG 0.5 kHz | 45.50 ± 14.59 (15 to 65) | 45.56 ± 9.17 (35 to 60) | > .05 |
| ABG 1 kHz | 43.75 ± 12.66 (20 to 65) | 42.78 ± 12.53 (30 to 60) | > .05 |
| ABG 2 kHz | 37.50 ± 15.94 (15 to 65) | 32.22 ± 14.81 (15 to 60) | > .05 |
| ABG 4 kHz | 41.50 ± 13.48 (15 to 65) | 35.00 ± 11.18 (15 to 50) | > .05 |
ABG = air-bone gap, AC = air conduction, BC = bone conduction, max = maximum, min = minimum, PTA = pure-tone audiometry average, SD = standard deviation, SRT = speech recognition threshold.
Significant differences were found between the FFAs and SRT results with and without BAHA for all users (Table 3).
Table 3.
SRT, free-field average, and average gain results of participants.
| Mean ± SD (min–max) (dB) | P values | ||
|---|---|---|---|
| Hearing level | Average gain* | ||
| SRT without BAHA | 60.51 ± 11.69 (40–80) | 34.08 | < .001 |
| SRT with BAHA | 26.43 ± 12.39 (10–60) | ||
| FFA1 without BAHA | 60.02 ± 11.65 (38–82) | 34.42 | < .001 |
| FFA1 with BAHA | 25.60 ± 10.70 (10–60) | ||
| FFA2 without BAHA | 59.35 ± 11.98 (38–80) | 25.57 | < .001 |
| FFA2 with BAHA | 33.78 ± 12.32 (17–73) | ||
| FFA3 without BAHA | 59.85 ± 11.19 (42–79) | 26.32 | < .001 |
| FFA3 with BAHA | 33.53 ± 13.58 (15–73) | ||
| Mean | 30.10 | ||
Statistical significance, P < .001.
BAHA = bone-anchored hearing aid, FFA = free-field test average, max = maximum, min = minimum, SD = standard deviation, SRT = speech recognition threshold.
Average gain calculated as mean FFAs and SRT without BAHA minus FFAs and SRT with BAHA.
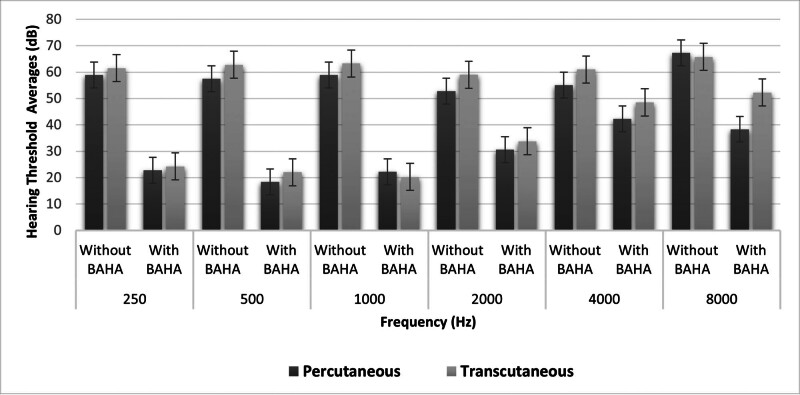
No significant differences were found between transcutaneous and percutaneous BAHA users’ audiological test results (P > .05; Table 4; Fig. 1).
Table 4.
Audiological characteristics of transcutaneous and percutaneous BAHA users.
| Mean ± SD (dB) | P value | ||
|---|---|---|---|
| Transcutaneous (n = 20) | Percutaneous (n = 9) | ||
| SRT without BAHA | 61.53 ± 11.48 | 58.47 ± 12.56 | > .05 |
| SRT with BAHA | 26.50 ± 11.01 | 26.25 ± 16.20 | > .05 |
| FFA1 without BAHA | 61.68 ± 11.40 | 56.33 ± 11.99 | > .05 |
| FFA1 with BAHA | 26.20 ± 12.09 | 24.26 ± 7.11 | > .05 |
| FFA2 without BAHA | 61.08 ± 10.94 | 55.52 ± 13.92 | > .05 |
| FFA2 with BAHA | 34.15 ± 13.24 | 32.96 ± 10.66 | > .05 |
| FFA3 without BAHA | 61.53 ± 10.27 | 56.11 ± 12.85 | > .05 |
| FFA3 with BAHA | 33.35 ± 13.29 | 33.94 ± 15.04 | > .05 |
BAHA = bone-anchored hearing aid, FFA = free-field test average, SD = standard deviation, SRT = speech recognition threshold.
Figure 1.
FF test results of percutaneous and transcutaneous implant users with and without BAHA. BAHA = bone-anchored hearing aid, FF = free field.
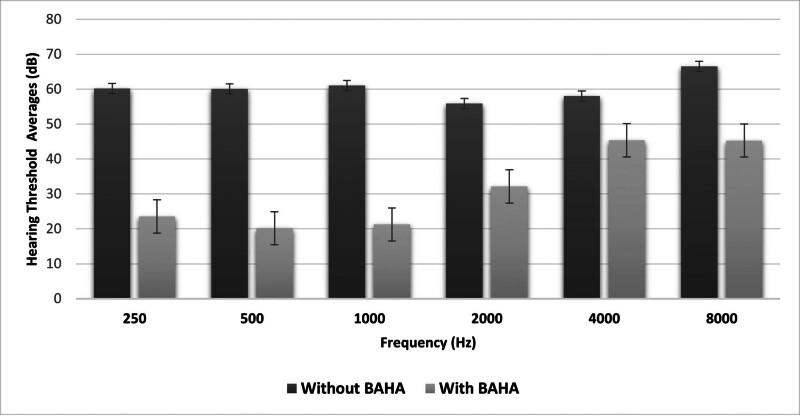
The FF averages of transcutaneous and percutaneous BAHA users with and without BAHA are illustrated in Figure 1. Although the hearing thresholds obtained with the percutaneous implant were better than the transcutaneous implant at frequencies above 2 kHz, no statistically significant difference was observed between frequencies (P > .05; Fig. 1). There was a statistically significant difference between the FF thresholds of all participants with and without BAHA at all frequencies (P < .001). The hearing gain of BAHA users was higher at frequencies between 0.25 and 1 kHz compared to frequencies above 2 kHz (Fig. 2).
Figure 2.
FF test results of all participants with and without BAHA. BAHA = bone-anchored hearing aid, FF = free field.
3.3. Tr-SSQ results
The unaided speech perception, spatial perception, and hearing quality scores of BAHA patients were 1.49 ± 1.12, 1.21 ± 0.86, and 1.96 ± 1.42, respectively. The aided scores were 6.04 ± 1.62, 5.27 ± 2.01, and 6.87 ± 1.70, respectively. The total Tr-SSQ score was 1.55 ± 1.09 without BAHA and 5.95 ± 1.70 with BAHA. There was a statistically significant difference between the participants with and without BAHA scores (P < .001). No statistically significant differences were observed in Tr-SSQ scores between percutaneous and transcutaneous BAHA (P > .05; Table 5). A moderate inverse correlation was observed between the postoperative FFA1 average and the scores for speech perception (r = −0.413, P = .03), spatial perception (r = −0.401, P = .03), and hearing quality (r = −0.459, P = .01). As the FF thresholds of patients decreased, their satisfaction scores increased (Table 5). No statistically significant difference was obtained between all BAHA 5 and BAHA 6 speech processors in terms of Tr-SSQ subscale scores (P > .05).
Table 5.
Tr-SSQ results among percutaneous and transcutaneous BAHA users.
| Tr-SSQ | Mean ± SD (points) | P values | |||
|---|---|---|---|---|---|
| Transcutaneous (n = 20) | Percutaneous (n = 9) | All BAHA users (n = 29) | |||
| Speech perception | Without BAHA | 1.56 ± 1.24 | 1.33 ± 0.86 | 1.49 ± 1.12 | > .05 |
| With BAHA | 5.90 ± 1.72 | 6.34 ± 1.42 | 6.04 ± 1.62 | ||
| Spatial perception | Without BAHA | 1.17 ± 0.90 | 1.32 ± 0.82 | 1.21 ± 0.86 | > .05 |
| With BAHA | 5.02 ± 1.77 | 5.81 ± 2.49 | 5.27 ± 2.01 | ||
| Hearing quality scores | Without BAHA | 1.96 ± 1.57 | 1.96 ± 1.06 | 1.96 ± 1.42 | > .05 |
| With BAHA | 6.67 ± 1.65 | 7.30 ± 1.84 | 6.87 ± 1.70 | ||
| Total Tr-SSQ score | Without BAHA | 1.55 ± 1.19 | 1.55 ± 0.92 | 1.55 ± 1.09 | > .05 |
| With BAHA | 5.73 ± 1.57 | 6.42 ± 1.99 | 5.95 ± 1.70 | ||
BAHA = bone-anchored hearing aid, SD = standard deviation, Tr-SSQ = Turkish Speech Perception, Spatial Perception, Hearing Quality Scale.
4. Discussion
This study aimed to investigate the contributions of BAHA to audiological performance and daily hearing function. We utilized pure-tone audiometry findings, FF tests, and Tr-SSQ. We compared the audiological and patient satisfaction outcomes of transcutaneous implants and percutaneous implants. Several studies have evaluated the effect of BAHA on QoL, but few studies have comparatively investigated the effects of transcutaneous and percutaneous implants on daily hearing quality and audiological parameters.
The outcomes of percutaneous and transcutaneous implants have been compared, with different studies reporting similar outcomes between the 2 devices.[4,7,17] Svagan et al[9] reported that percutaneous implants showed higher satisfaction compared to transcutaneous implants, according to the Glasgow Benefit Inventory (GBI) results. Iseri et al[17] observed no significant differences between percutaneous and transcutaneous implants in terms of GBI and audiological evaluation results. Several studies have shown overall patient satisfaction with BAHA.[3,7,17–20] The Tr-SSQ and audiological results demonstrated both subjective and objective benefits, as well as satisfaction with BAHA. BAHAs provided not only audiological gain but also improved subjective auditory abilities for patients with conductive hearing loss.
QoL scales have frequently indicated that BAHA enhances users’ social interactions, communication skills, and overall life satisfaction. In the literature, self-rated QoL,[18,21] GBI,[4,7,9,13,17,22,23] and Abbreviated Profile of Hearing Aid Benefit[7,20,21,24,25] scales are most commonly used to assess the impact of BAHA devices on quality and hearing performance of life. The SSQ used in our study, however, has been much less frequently employed in evaluating patients with ADHEAR,[26,27] Bonebridge,[1] Osia,[25] and BAHA[28] implants. Our study is one of the few that assesses not only audiological performance but also speech perception, spatial perception, and hearing quality for both types of implants.
In our study, acquired conductive hearing loss due to chronic otitis media was the most common indication for BAHA. The literature has reported similar findings, identifying chronic otitis media as the most frequent indication.[2,3,18]
The Abbreviated Profile of Hearing Aid Benefit questionnaire has been used in a number of studies to assess long-term satisfaction with BAHA devices in people with microtia,[21] congenital aural atresia,[20] and chronic otitis media.[24] These studies indicated that the implant significantly reduced hearing difficulty in various listening conditions.[20,21]
Hirth et al examined patient satisfaction with the BC ADHEAR system in children with unilateral conductive hearing loss using the SSQ questionnaire. Although the unaided SSQ scores were relatively high (total score = 6.5), the results with the BC device (total score = 7.8) were even higher.[26] In our study, which involved individuals with bilateral hearing loss, the aided Tr-SSQ scores (total score = 5.95) showed a much more dramatic increase compared to the unaided scores (total score = 1.55). Although patient satisfaction scores are higher for unilateral losses compared to bilateral losses, satisfaction significantly improves with the use of the device in both cases.
In their study, Svagan et al[9] found that patients with single-sided deafness who received transcutaneous implants scored the lowest in terms of satisfaction. To account for the potential variability in satisfaction outcomes, our study included only individuals with bilateral hearing loss, excluding those with unilateral loss.
Bere et al[29] compared BAHA 5 and BAHA 6 for audiological and hearing quality in their study. The authors found that BAHA 6 improved sound quality and hearing performance compared to BAHA 5. In our study, no significant difference was observed between all users of BAHA 5 and BAHA 6 speech processors in terms of audiological parameters and Tr-SSQ scores. Although our results differ from the study by Bere et al,[29] the fact that the number of participants using BAHA 6 was lower than the number of participants using BAHA 5 and that speech tests in noise were not included in the study may have caused this difference.
Audiological evaluations of BAHA users showed a significant benefit with the device (P = .000). According to the Tr-SSQ results, patients reported difficulty in listening environments encountered without devices (P = .000). An inverse relationship was observed between the degree of hearing loss and Tr-SSQ scores; as hearing impairment increased, Tr-SSQ scores decreased. This finding is consistent with another study by Saroul et al,[19] which also reported that satisfaction decreased as hearing loss increased.
Fuchsmann et al[20] found an average hearing improvement of 33 dB in patients using BAHA. Similarly, our study found that FFA1 had an average hearing improvement of 34 dB. Following the findings of other researchers,[3,8,17] we noted improvement in both FF responses, SRT with BAHA. These improved by an average of 30 (range = 25–34) dB.
Transcutaneous and percutaneous BAHA have advantages over each other; there were no significant differences in terms of satisfaction and audiological outcomes between the patient groups in our study either. In our study, Tr-SSQ scores were moderately inversely correlated with FFA1 in all BAHA users. As hearing thresholds improve, individuals’ speech and spatial perception and hearing quality satisfaction increase.
Skin problems associated with percutaneous BAHA implants have been documented in the literature, including complications such as skin flap necrosis, infection, skin growth over the abutment, osseointegration failure, and implant extrusion. It is essential to note that the transcutaneous system, a non–skin-penetrating BC implant, has demonstrated efficacy with fewer soft tissue complications compared with percutaneous implants.[4,17,30,31] Transcutaneous implants have lower skin complication rates and provide more aesthetic advantages.[4,17] Percutaneous implants provide up to 15 dB gain in hearing, especially at high frequencies.[4]
This aligns with the importance of considering skin reactions and complications when choosing between different types of BAHA implants.
In our study, FF assessments were performed at 0.5-, 1-, 2-, and 4-kHz frequencies. In our clinic, we evaluate our patients at these frequencies in a daily routine. Moreover, in the literature, while some studies included 0.5-, 1-, 2-, and 4-kHz frequencies in the evaluation of BAHA patients,[5,9,20,24,29] similar to our study, some studies added 3 kHz.[17,21,25] In this sense, there is no standard practice in the literature that all clinicians follow.
In conclusion, although both transcutaneous and percutaneous BAHA implants may offer similar audiological outcomes and patient satisfaction, the occurrence of skin problems, as evidenced by patients needing to switch implants due to such issues, underscores the significance of carefully evaluating and managing skin-related complications in the selection of the most suitable BAHA implant type.
5. Limitations
This study has several limitations. First, the sample size was relatively small, which may restrict the generalizability of the findings to broader populations. Second, the study was conducted at a single center, which could introduce bias related to specific clinical practices and patient demographics at that institution. Third, the follow-up periods varied among participants, potentially affecting the consistency of the results over time. Future research should aim to include larger multicenter samples with more uniform follow-up periods and perform audiological assessments under noise conditions (especially including speech in noise tests) to validate and extend these findings.
6. Conclusion
This study aimed to evaluate the long-term hearing performance, functional outcomes, patient-reported hearing abilities, and satisfaction measures after BAHA implantation. Objective audiological tests confirmed the efficacy of BAHA, although the controlled test environments may not fully replicate real-life conditions. Nevertheless, BAHA consistently demonstrated reliable and sustained improvements in hearing, accompanied by high patient satisfaction and significant enhancements across diverse listening environments. Our findings underscore BAHA as a robust solution for hearing rehabilitation, effectively enhancing both auditory capabilities and quality of subjective hearing abilities. Furthermore, our study supports the effectiveness of both transcutaneous and percutaneous implants in conductive hearing loss rehabilitation.
Acknowledgments
The authors express their gratitude to the participants who volunteered to participate in this study.
Author contributions
Conceptualization: Serpil Mungan Durankaya, Yüksel Olgun, Ilayda Kiremitçi, Hande Evin Eskicioğlu, Enis Alpin Güneri, Gülce Kirazli, Selhan Gürkan, Taner Kemal Erdağ, Günay Kirkim.
Data curation: Serpil Mungan Durankaya, Ilayda Kiremitçi, Hande Evin Eskicioğlu, Gülce Kirazli, Selhan Gürkan, Günay Kirkim.
Formal analysis: Serpil Mungan Durankaya, Ilayda Kiremitçi, Gülce Kirazli, Hande Evin Eskicioğlu.
Methodology: Serpil Mungan Durankaya, Yüksel Olgun, Ilayda Kiremitçi, Hande Evin Eskicioğlu, Enis Alpin Güneri, Gülce Kirazli, Selhan Gürkan, Taner Kemal Erdağ.
Visualization: Serpil Mungan Durankaya, Yüksel Olgun, Ilayda Kiremitçi, Hande Evin Eskicioğlu, Gülce Kirazli.
Writing – original draft: Serpil Mungan Durankaya, Yüksel Olgun, Ilayda Kiremitçi, Hande Evin Eskicioğlu, Günay Kirkim.
Writing – review & editing: Serpil Mungan Durankaya, Yüksel Olgun, Ilayda Kiremitçi, Hande Evin Eskicioğlu, Enis Alpin Güneri, Gülce Kirazli, Selhan Gürkan, Taner Kemal Erdağ, Günay Kirkim.
Investigation: Yüksel Olgun, Enis Alpin Güneri, Selhan Gürkan, Taner Kemal Erdağ, Günay Kirkim.
Supervision: Yüksel Olgun, Enis Alpin Güneri, Taner Kemal Erdağ, Günay Kirkim.
Abbreviations:
- AC
- air conduction
- BAHA
- bone-anchored hearing aid
- BC
- bone conduction
- FF
- free field
- FFA
- free-field test average
- GBI
- Glasgow Benefit Inventory
- PTA
- pure-tone audiometry average
- QoL
- quality of life
- SRT
- speech recognition threshold
- SSQ
- Speech, Spatial and Qualities of Hearing Scale,
- Tr-SSQ
- Turkish Speech, Spatial and Qualities of Hearing Scale
The authors have no funding and conflicts of interest to disclose.
Informed voluntary consent forms were obtained from the participants and parents of all children who agreed to participate in the study.
This cross-sectional clinical research was approved by Dokuz Eylul University, Ethical Committee (protocol number: 2022/28-20). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or National Research Committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. Written informed consent was obtained from all individuals above 18 years of age or from the parents of children under 18 years of age included in the study.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
How to cite this article: Mungan Durankaya S, Olgun Y, Kiremitçi I, Evin Eskicioğlu H, Güneri EA, Kirazli G, Gürkan S, Erdağ TK, Kirkim G. The effect of percutaneous and transcutaneous BAHA on hearing and subjective auditory abilities: A comparative study. Medicine 2024;103:38(e39697).
All authors approved the final manuscript as submitted and agreed to be accountable for all aspects of the work. The requirements for authorship stated earlier in this document have been met, and each author believes that the manuscript represents honest work.
Contributor Information
Yüksel Olgun, Email: yuksel.olgun@deu.edu.tr.
Ilayda Kiremitçi, Email: ilayda.krmtc@gmail.com.
Hande Evin Eskicioğlu, Email: hande.evin@deu.edu.tr.
Enis Alpin Güneri, Email: alpin.guneri@deu.edu.tr.
Gülce Kirazli, Email: gulce.kirazli@ege.edu.tr.
Selhan Gürkan, Email: selhangurkan@gmail.com.
Taner Kemal Erdağ, Email: taner.erdag@deu.edu.tr.
Gunay Kirkim, Email: gunay.kirkim@deu.edu.tr.
References
- [1].Irmer C, Volkenstein S, Dazert S, Neumann A. The bone conduction implant BONEBRIDGE increases quality of life and social life satisfaction. Eur Arch Otorhinolaryngol. 2022;279:5555–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Amaral MSAD, Santos FRD, Danieli F, Massuda ET, Reis ACMB, Hyppolito MA. Surgical and audiological results of bone-anchored hearing aids: comparison of two surgical techniques. Braz J Otorhinolaryngol. 2022;88:533–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Azevedo C, Breda M, Ribeiro D, Mar FM, Vilarinho S, Dias L. Functional and patient-reported outcomes of bone-anchored hearing aids (BAHA): a prospective case series study. J Otol. 2023;18:7–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Robinette K, Sims J, Pang B, Babu S. Transcutaneous versus percutaneous bone-anchored hearing aids: a quality of life comparison. Am J Otolaryngol. 2023;44:103758. [DOI] [PubMed] [Google Scholar]
- [5].Zernotti ME, Di Gregorio MF, Galeazzi P, Tabernero P. Comparative outcomes of active and passive hearing devices by transcutaneous bone conduction. Acta Otolaryngol. 2016;136:556–8. [DOI] [PubMed] [Google Scholar]
- [6].Heywood RL, Patel PM, Jonathan DA. Comparison of hearing thresholds obtained with Baha preoperative assessment tools and those obtained with the osseointegrated implant. Ear Nose Throat J. 2011;90:E21–7. [DOI] [PubMed] [Google Scholar]
- [7].Portelli D, Ciodaro F, Loteta S, Alberti G, Bruno R. Audiological assessment with matrix sentence test of percutaneous vs transcutaneous bone-anchored hearing aids: a pilot study. Eur Arch Otorhinolaryngol. 2023;280:4065–72. [DOI] [PubMed] [Google Scholar]
- [8].Sprinzl G, Lenarz T, Hagen R, et al. Long-term, multicenter results with the first transcutaneous bone conduction implant. Otol Neurotol. 2021;42:858–66. [DOI] [PubMed] [Google Scholar]
- [9].Svagan M, Povalej Brzan P, Rebol J. Comparison of satisfaction between patients using percutaneous and transcutaneous bone conduction devices. Otol Neurotol. 2019;40:651–7. [DOI] [PubMed] [Google Scholar]
- [10].Tisch M. Implantable hearing devices. GMS Curr Top Otorhinolaryngol Head Neck Surg. 2017;16:Doc06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Reinfeldt S, Håkansson B, Taghavi H, Eeg-Olofsson M. New developments in bone-conduction hearing implants: a review. Med Devices (Auckl). 2015;8:79–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Gürses E, Türkyilmaz MD, Sennaroğlu G. Evaluation of auditory temporal processing in patients fitted with bone-anchored hearing aids. Eur Arch Otorhinolaryngol. 2020;277:351–9. [DOI] [PubMed] [Google Scholar]
- [13].McLarnon CM, Davison T, Johnson IJ. Bone-anchored hearing aid: comparison of benefit by patient subgroups. Laryngoscope. 2004;114:942–4. [DOI] [PubMed] [Google Scholar]
- [14].Rasmussen J, Olsen SO, Nielsen LH. Evaluation of long-term patient satisfaction and experience with the Baha® bone conduction implant. Int J Audiol. 2012;51:194–9. [DOI] [PubMed] [Google Scholar]
- [15].Gatehouse S, Noble W. The Speech, Spatial and Qualities of Hearing Scale (SSQ). Int J Audiol. 2004;43:85–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kiliç N, Şahin Kamişli GI, Gündüz B, Bayramoğlu I, Kemaloğlu YK. Turkish validity and reliability study of the Speech, Spatial and Qualities of Hearing Scale. Turk Arch Otorhinolaryngol. 2021;59:172–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Iseri M, Orhan KS, Tuncer U, et al. Transcutaneous bone-anchored hearing aids versus percutaneous ones: multicenter comparative clinical study. Otol Neurotol. 2015;36:849–53. [DOI] [PubMed] [Google Scholar]
- [18].Badran K, Bunstone D, Arya AK, Suryanarayanan R, Mackinnon N. Patient satisfaction with the bone-anchored hearing aid: a 14-year experience. Otol Neurotol. 2006;27:659–66. [DOI] [PubMed] [Google Scholar]
- [19].Saroul N, Gilain L, Montalban A, Giraudet F, Avan P, Mom T. Patient satisfaction and functional results with the bone-anchored hearing aid (BAHA). Eur Ann Otorhinolaryngol Head Neck Dis. 2011;128:107–13. [DOI] [PubMed] [Google Scholar]
- [20].Fuchsmann C, Tringali S, Disant F, et al. Hearing rehabilitation in congenital aural atresia using the bone-anchored hearing aid: audiological and satisfaction results. Acta Otolaryngol. 2010;130:1343–51. [DOI] [PubMed] [Google Scholar]
- [21].Fan X, Yang T, Niu X, Wang Y, Fan Y, Chen X. Long-term outcomes of bone conduction hearing implants in patients with bilateral microtia-atresia. Otol Neurotol. 2019;40:998–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Jones SEM, Roplekar-Bance R, Green R, Rae C, Ferguson A, Spielmann PM. Patient-reported outcomes in middle ear and active transcutaneous bone conduction hearing implants. J Int Adv Otol. 2021;17:405–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Topcu MT, Mutlu B, Celik S, Celikgun B, Mutlu A, Kalcioglu MT. Bone-anchored hearing implants: surgical and audiological comparison of different surgical techniques. Int Arch Otorhinolaryngol. 2022;26:e649–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Garcier M, Lavedrine A, Gagneux C, Eluecque T, Bozorg Grayeli A, Bozorg Grayeli A. Bone-anchored and closed skin bonebridge implant in adults: hearing performances and quality of life. Audiol Neurootol. 2021;26:310–6. [DOI] [PubMed] [Google Scholar]
- [25].Kim Y, Choe G, Oh H, Choi BY. A comparative study of audiological outcomes and compliance between the Osia system and other bone conduction hearing implants. Eur Arch Otorhinolaryngol. 2023;280:2217–24. [DOI] [PubMed] [Google Scholar]
- [26].Hirth D, Weiss R, Stöver T, Kramer S. Audiological benefit and subjective satisfaction with the ADHEAR hearing system in children with unilateral conductive hearing loss. Eur Arch Otorhinolaryngol. 2021;278:2781–8. [DOI] [PubMed] [Google Scholar]
- [27].Neumann K, Thomas JP, Voelter C, Dazert S. A new adhesive bone conduction hearing system effectively treats conductive hearing loss in children. Int J Pediatr Otorhinolaryngol. 2019;122:117–25. [DOI] [PubMed] [Google Scholar]
- [28].Pai I, Kelleher C, Nunn T, et al. Outcome of bone-anchored hearing aids for single-sided deafness: a prospective study. Acta Otolaryngol. 2012;132:751–5. [DOI] [PubMed] [Google Scholar]
- [29].Bere Z, Nagy R, Molnar F, Kiss JG, Rovo L. Comparison between Baha® 5 and the new BAHA 6 Max sound processors: results among BAHA attract users. Speech Lang Hear. 2024:1–8. 10.1080/2050571X.2024.2304998. [DOI] [Google Scholar]
- [30].Steehler MW, Larner SP, Mintz JS, Steehler MK, Lipman SP, Griffith S. A comparison of the operative techniques and the postoperative complications for bone-anchored hearing aid implantation. Int Arch Otorhinolaryngol. 2018;22:368–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Yin GD, Zeng X, Li P. Skin reactions caused by bone-anchored hearing aid (BAHA) implantation. J Otol. 2015;10:159–62. [DOI] [PMC free article] [PubMed] [Google Scholar]