ABSTRACT
Background and objectives
EUS is an important modality for diagnosis and assessment of gastrointestinal (GI) subepithelial lesions. However, EUS is invasive and operator-dependent and requires sedation in most cases. The newly developed ultrasound capsule endoscopy (USCE) system, with both white-light and ultrasound imaging modalities, is a minimally invasive method for superficial and submucosal imaging of the esophagus. This animal study aimed to evaluate the feasibility and efficacy of the USCE system for upper GI tract and small bowel scanning.
Methods
Three Bama miniature pigs were selected to scan their esophagus, stomach, small bowel, and simulated submucosal lesions. USCE was performed first, followed by EUS. The feasibility of USCE was measured by obtaining ultrasound images of normal GI walls and submucosal lesions under the guidance of optical viewing. The efficacy of USCE was evaluated by comparing tissue structures and lesion features shown on ultrasound images obtained with both instruments.
Results
Under the optical mode of USCE, the GI tract was well visualized, and all simulated lesions were located. Clear ultrasound images of normal GI tract and submucosal lesions were acquired. Ultrasound images of the esophagus, stomach, and small bowel were characterized by differentiated multilayer structures on USCE, which was consistent with the structures displayed on EUS. And the visualization of submucosal lesions, using both USCE and EUS, was characterized by a hypoechoic and well-demarcated mass in the layer of submucosa.
Conclusions
This animal study indicated the feasibility and potential clinical efficacy of this USCE for simultaneous optical mucosal visualization and transmural ultrasound imaging of upper GI tract and small bowel, providing possibility of using this technology for a wider range of GI tract.
Key words: Ultrasound, Capsule endoscopy, EUS, Esophagus, Stomach, Small bowel
INTRODUCTION
EUS incorporates both ultrasonography and endoscopy technologies, providing high-resolution real-time images of the intramural gastrointestinal (GI) tract and extramural structures.[1] Since its development, EUS allows visualization of previously inaccessible submucosal regions of the GI tract.[2] EUS has been established as an irreplaceable modality for diagnosis and staging of GI subepithelial lesions.[3,4] However, EUS has limitations. EUS is invasive and uncomfortable under unsedated situations, leading to low patient compliance. Although sedation can improve patient comfort, its cost has been a major concern, as well potential anesthesia-related adverse events.[5] Besides, EUS is considered to be among the most challenging procedures in GI endoscopy. Extensive and effective training is of great importance as operator technique is central to diagnostic accuracy of EUS.[6] In addition, the equipped unit of EUS is expensive. Thus EUS has not been widely adopted in primary hospitals because of limited medical resources and lack of local expertise.[7]
These limitations of EUS suggest that there is room for improvement in GI tract diagnostics. Capsule endoscopy (CE) offers a more patient-friendly alternative to conventional endoscopy, with good tolerance and no need for sedation, which has been widely applied in clinical practice.[8–11] In recent years, the development of CE in diagnosis of GI diseases continues to evolve, from single white-light imaging modality to other imaging modalities, such as optical coherence tomography (OCT),[12,13] X-ray imaging,[14] and ultrasonography techniques, for subsurface evaluation of GI tract. Several groups have proposed the concept of ultrasound capsule endoscopy (USCE) and explored the feasibility of USCE for ultrasound imaging of the GI tract.[15–17] However, owing to technical constraints, most USCEs contain single ultrasound scanning modality, enabling only submucosal imaging of GI tract. A swallowable USCE, combining both ultrasonography and endoscopy technologies, has not been realized.
Following the example of clinical utility set by EUS, a novel USCE system (Huiwei Medical Technology, Co, Ltd, Taizhou, China) has been developed for visualization of the esophagus. This USCE system implements dual-imaging modalities, namely, white-light imaging and ultrasound scanning, in a tethered capsule that can be swallowed and is reusable. Our group has successfully conducted USCE in a pilot, single-center study, demonstrating that the capsule offers a minimally invasive and simple procedure for the diagnosis of esophageal subepithelial lesions with lower cost and better patient tolerance.[18] In the current study, to further investigate the potential use of this USCE system in GI tract diagnostics, we aimed to test the feasibility and efficacy of USCE for superficial and submucosal imaging of the esophagus, stomach, and small bowel in a porcine model.
METHODS
Characteristics of USCE
The USCE system consists of a tethered ultrasound endoscopic capsule with a connector, an imaging platform, and a computer workstation[18] [Figure 1A and 1B]. The capsule has a diameter of 13 mm and a length of 31 mm, which is comparable to the size of a video endoscopy capsule and a 1.35-mm-diameter, 1.3-m-long, flexible tether that connects it to the imaging platform for power supply and signal transmission. The electronics enclosed in the capsule include a CMOS camera and 5 light-emitting diodes for optical viewing, a high-frequency ultrasound transducer for ultrasound signal generation and reception, and a rotation motor to drive the transducer for a 360° B-mode scan [Figure 1C]. The imaging platform is responsible for light generation, collection of information from the capsule, and image processing. The computer workstation is installed with the UltraCapsule software, which could be switched between different interfaces to display the real-time optical or ultrasonic images or both of them. The ultrasonic frequency of the capsule is 40 MHz, and it provides an axial (depth) resolution of 80 μm (tissue) in tissue. Optical images are captured and recorded at 30 frames per second with a resolution of 1280 × 720.
Figure 1.

Devices of the USCE system. A, Computer workstation with installed UltraCapsule software and imaging platform. B, Capsule with a flexible cable and connector. C, Overall design of the capsule. USCE: ultrasound capsule endoscopy.
This USCE system is portable, which was designed to be mass produced, easily maintained, and deployed to various sites. The capsule can be reused, and standard high-level disinfection is conducted between procedures.
EUS device
The EUS system was provided by Olympus Optical, Co, Ltd (Tokyo, Japan). This system consists of an Olympus UM-3R miniprobe scanner and an imaging unit. The frequency of the ultrasonic transducer inside the miniprobe is 20 MHz, which can probe 60-μm axial (depth) resolutions in tissue.
Animal experiments
Three Bama pigs were selected for the endoscopic procedures in this study. They first underwent USCE, followed by EUS immediately. Porcine models were used because of the similarity of their GI tract with the human GI tract in terms of physiology and histological structure.[19] The study protocol including animal experiments was approved by the ethics committee of Changhai Hospital.
Three healthy juvenile male Bama mini-pigs (5–6 months old, 25–30 kg; Department of Animal Science and Technology, Naval Medical University) were housed in individually ventilated cages at the animal rearing cabin of Naval Medical University under controlled environmental conditions (temperature 22°C ± 2°C; relative humidity 60%–70%). The pigs were fed with sugar water for 3 days and fasted the night before the experiment to obtain a relatively empty proximal intestine.
The experiments were carried out under general anesthesia. After anesthesia, an endoscopist performed the esophagogastroduodenoscopy (EGD) to mark 2 simulated lesions in 2 different locations in the upper GI tract, including the lower esophagus and gastric body, by injecting 0.9% saline and methylene blue mixture into the submucosa to gain a liquid pad with a diameter of about 1 cm. Then, an artificial stoma was surgically created on the pig’s lateral side to directly access the small bowel, and another simulated lesion was marked approximately 80–100 cm from the distal end of the pylorus in the jejunum.
Both USCE and EUS were carried out under anesthesia. The USCE procedure was performed by an endoscopist with an experience of >500 cases of EUS operation. First, the capsule was attached to EGD with an endoscopic snare. The capsule was delivered to the stomach under endoscopic placement. Then, the lighting source of EGD was turned off, and the optical viewing of EGD was replaced by the optical mode of USCE. The endoscopist slowly observed the upper GI tract (including the duodenum) according to conventional EGD procedure and located the simulated lesions. The performance of USCE was tested at specified locations, including the normal lower esophagus and gastric body, as well as the simulated lesions. Once the locations were arrived or located, the capsule was switched to ultrasound mode, and ultrasound images were obtained. After visualization of the upper GI tract, EGD with the capsule was removed. The capsule was inserted directly into the small bowel through the artificial stoma; both optical and ultrasound images of normal small bowel and simulated lesion were captured.
After USCE procedures, the endoscopist performed EUS procedures to obtain optical and ultrasound images of identical specified locations (lower esophagus, gastric body, small bowel, and the 3 simulated lesions).
Once the experiments were completed, the animals were given excessive anesthesia euthanasia.
Outcomes
The primary goal of this animal study was the feasibility and efficacy of USCE in the upper GI tract and small bowel. The feasibility of USCE was defined as the ability to obtain clear ultrasound images of specified locations of normal GI tract and simulated lesions under the guidance of optical viewing. The efficacy of USCE was evaluated by comparing layered histologic structures of normal GI tract and characteristics of simulated lesions presented on each group of ultrasound images by USCE and EUS.
The scanned images of all locations were monitored in real-time procedures, and high-quality ultrasound images were selected by the researcher. High-quality ultrasound images were defined as clearly recognizable tissue layers presented on them. Each group of images obtained by USCE and EUS contained both optical and ultrasound images at the same location. To compare USCE with EUS results, 2 endoscopists with more than 5 years of EUS experience, who were blinded to the USCE and EUS procedures, evaluated and marked structure layers of ultrasound images independently.
RESULTS
Feasibility of USCE
The USCE procedures were successfully performed on the 3 pigs. Under optical viewing, the mucosal surface of esophagus, stomach, and small bowel could be clearly visualized, and all the simulated lesions could be recognized, and then ultrasound scanning could be completed. Representative optical images of normal esophagus, stomach, small bowel, and simulated lesions are presented in Figures 2-4. At designated spots of normal GI tract (lower esophagus, gastric body, small bowel) and simulated lesions, clear ultrasound images with recognizable structure layers have been acquired in all 3 pigs.
Figure 2.
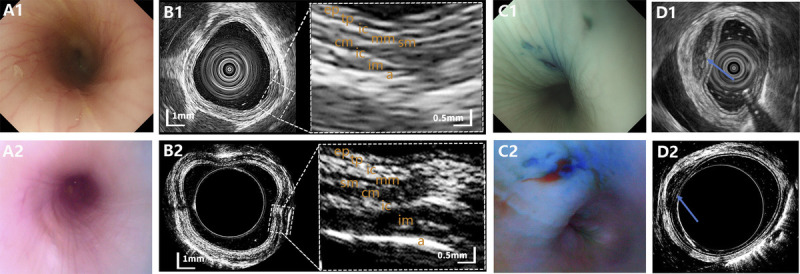
Representative images of normal esophagus and esophageal lesion observed on EUS and USCE, with no. 1 and 2 standing for EUS and USCE, respectively. A, Optical images of normal esophagus. B, Ultrasound images of normal esophagus. C, Optical images of esophageal lesion. D, Ultrasound images of esophageal lesion. USCE: ultrasound capsule endoscopy.
Figure 4.
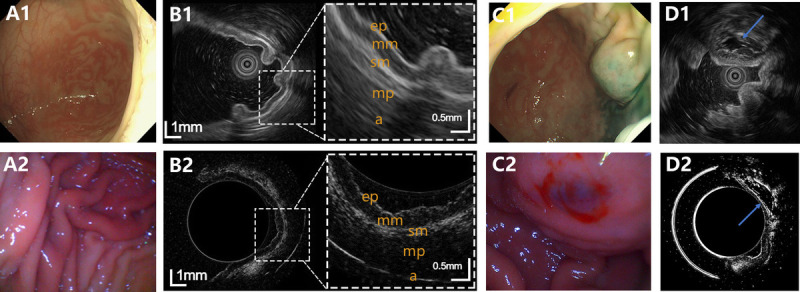
Representative images of normal small bowel and small bowel lesion observed on EUS and USCE, with no. 1 and 2 standing for EUS and USCE, respectively. A, Optical images of normal small bowel. B, Ultrasound images of normal small bowel. C, Optical images of small bowel lesion. D, Ultrasound images of small bowel lesion. USCE: ultrasound capsule endoscopy.
Efficacy of USCE
For ultrasound scanning of the esophagus, stomach, and small bowel, paired ultrasound images of high-quality obtained from USCE and EUS were compared. Representative optical and ultrasound images of normal GI walls and simulated lesions acquired by EUS and USCE are shown in Figures 2-4.
As for esophagus imaging, the structure of the normal esophageal walls was shown as 9 clear and recognizable layers on USCE, including epithelial, lamina propria, interface between lamina propria and muscularis mucosa, muscularis mucosa, submucosa, muscularis propria superficial layer, intramuscular connective tissue, muscularis propria deep layer, and adventitia. The 9-layered structure on USCE was consistent with the structure displayed on EUS [Figure 2B]. For visualization of the simulated subepithelial lesions in the esophagus under the ultrasound mode, the simulated lesions were characterized by a recognizable, hypoechoic, and well-demarcated mass in the layer of submucosa using both USCE and EUS [Figure 2D].
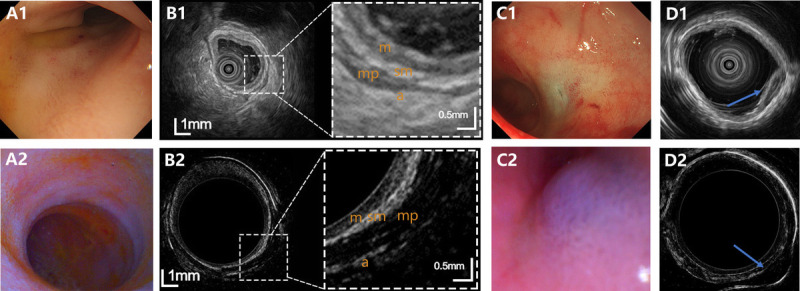
As for stomach imaging, the normal gastric walls was characterized by a 5-layer structure on USCE, including epithelial, mucosa, submucosa, muscularis propria, and adventitia, which was consistent with the structure displayed on EUS [Figure 3B]. The simulated subepithelial lesions in the stomach were all characterized by a hypoechoic and well-demarcated mass in the layer of submucosa under the ultrasound mode of both USCE and EUS [Figure 3D].
Figure 3.
Representative images of normal stomach and gastric lesion observed on EUS and USCE, with no. 1 and 2 standing for EUS and USCE, respectively. A, Optical images of normal stomach. B, Ultrasound images of normal stomach. C, Optical images of gastric lesion. D, Ultrasound images of gastric lesion. USCE: ultrasound capsule endoscopy.
As for small bowel imaging, clear 4-layered differentiation of the lumen wall structure was shown on USCE, including mucosa, submucosa, muscularis propria, and adventitia. The sublayers of the small bowel on USCE were consistent with those displayed on EUS [Figure 4B]. In the small intestine, the simulated subepithelial lesions under USCE were characterized by a hypoechoic and well-demarcated mass originating from submucosa, which were in agreement with those of EUS [Figure 4D].
DISCUSSION
In this study, our group used a novel USCE system with the dual modalities of optical viewing and ultrasound scanning performed simultaneously to observe the upper GI tract and small bowel of experimental animals, to evaluate its technical feasibility and clinical usefulness. This in vivo animal study demonstrated that the USCE was able to view GI tract in the optical mode and obtain high-quality and informative ultrasound images from the esophagus, stomach, and small bowel, highlighting the possibility of using this technology for a wider range of GI tract indications with further improvement.
In this study, both white-light and US imaging modalities of USCE for visualization of the esophagus, stomach, and small bowel were evaluated. Under the optical mode, the capsule could observe the mucosal surface of the upper GI tract and small bowel clearly, and all simulated lesions could be located. Under the ultrasound mode, specified locations were scanned, and layered structures of normal GI walls could be differentiated by the high-frequency scanning of USCE, which were consistent with those on EUS. In addition, the characteristics and layer of simulated lesions could also be recognized. Although the movement of the capsule in the stomach and small bowel was dependent on EGD manipulation or direct access to the small bowel, the technical feasibility of this USCE for both superficial and submucosal imaging of the upper GI tract and small bowel is validated, offering possibility that the capsule could be used in different positions of the GI tract.
In prior studies of other USCEs, ultrasound scanning of esophagus and small bowel has been explored in in vivo or in vitro animal studies. However, the majority of previous USCEs contained only the ultrasound transducer for ultrasound imaging, without the function of optical viewing.[15,16,20–22] The only USCE with dual imaging modalities is too big (39 × 21 mm) to be swallowed.[23] Besides, all the prior studies were still in the proof-of-concept stage for new capsule development. Compared with previous USCEs, current USCE has several advantages: (1) it contains both functions of white-light imaging and ultrasound scanning; (2) it is small (13 × 31 mm) to be swallowed by human subjects, which has been proved in our previous study[18]; (3) it has demonstrated feasibility and safety in human subjects for diagnosis of esophageal diseases.[18] Our study explores further underlying application of this USCE system in stomach and small intestine. To our largest knowledge, this is the first study to demonstrate the feasibility of an USCE for both superficial and submucosal imaging of the upper GI tract and small bowel in a porcine model.
Further technical improvement of this USCE needs to be achieved to support its controllable movement and practical application in the stomach and small bowel, such as magnetic manipulation of USCE in the stomach or wireless USCE in the small bowel. EUS is used to evaluate subepithelial lesions in the esophagus, stomach, duodenum, and colon.[1] For small bowel visualization, CE, enteroscopy, and device-assisted enteroscopy have represented an important innovation in the last decade and opened the so-called “SB black-box,” limited to the visualization of the tissue surface.[24] However, ultrasound scanning also plays an important role in small bowel diseases, such as inflammatory bowel diseases.[25,26] Therefore, the expanded examination range of GI tract by USCE, from esophagus to upper GI tract and small bowel, would make significant sense. The next important step in this process would be to tackle the issue of capsule movement in the stomach and small bowel. New solutions are needed to make further progress, and further human studies are warranted.
In conclusion, this article adds information that this USCE is feasible and useful to observe the upper GI surface and small bowel and obtain subsurface information, opening up the possibility of using this technology for a wider range of GI tract. Future studies are merited to improve and validate this technology.
Acknowledgment
Thanks to HuiweiMedical Technology, Co, Ltd (Taizhou, China), for providing capsules and technical support. The company had no role in the design of the study, the collection, analysis, or interpretation of data or preparing the manuscript.
Source of Funding
This study is supported by grants from the “Ten Thousand Plan”—National High Level Talents Special Support Plan (to Z. Liao).
Author Contributions
Zhuan Liao and Zhao-Shen Li did the study concept and design. Yi-Zhi Chen, Xiao-Ou Qiu, Lei Wang, and Xi Jiang conducted the study. Yi-Zhi Chen, Xiao-Ou Qiu, Lei Wang, and Xi Jiang, and Jing-Song Xia acquired the data. Yi-Zhi Chen, Xiao-Ou Qiu, Lei Wang, and Xi Jiang analyzed and interpreted the data. Yi-Zhi Chen, Xiao-Ou Qiu, and Xi Jiang drafted the manuscript. All authors critically revised the manuscript for important intellectual content. Zhuan Liao obtained funding. All authors gave final approval of the manuscript.
Footnotes
Received: 10 February 2024; Accepted: 5 June 2024.
Y.-Z. C., X.-O. Q., L. W., and X. J. contributed equally to this work.
Contributor Information
Yi-Zhi Chen, Email: chenyizhiyz@163.com.
Xiao-Ou Qiu, Email: qqqxo277@163.com.
Lei Wang, Email: onerain@126.com.
Xi Jiang, Email: jiangxi_stella@126.com.
Xiao-Ju Su, Email: xjsuch@163.com.
Jing-Song Xia, Email: xiajingsong@smmu.edu.cn.
Conflicts of Interest
Zhao-Shen Li is an Honorary Editor-in-Chief of the journal. This article was subject to the journal's standard procedures, with peer review handled independently of the editor and his research group.
References
- 1.Sooklal S, Chahal P. Endosc Ultrasound. Surg Clin North Am 2020;100(6):1133–1150. doi: 10.1016/j.suc.2020.07.003. [DOI] [PubMed] [Google Scholar]
- 2.Vanella G Bronswijk M Arcidiacono PG, et al. Current landscape of therapeutic EUS: changing paradigms in gastroenterology practice. Endosc Ultrasound 2023;12(1):16–28 doi: 10.4103/eus-d-21-00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simons-Linares CR, Chahal P. Advances in interventional endoscopic ultrasound (EUS): a technical review. J Clin Gastroenterol 2020;54(7):579–590 doi: 10.1097/mcg.0000000000001337. [DOI] [PubMed] [Google Scholar]
- 4.Maluf-Filho F Dotti CM Halwan B, et al. An evidence-based consensus statement on the role and application of endosonography in clinical practice. Endoscopy 2009;41(11):979–987 doi: 10.1055/s-0029-1215192. [DOI] [PubMed] [Google Scholar]
- 5.Inadomi JM, Gunnarsson CL, Rizzo JA, Fang H. Projected increased growth rate of anesthesia professional-delivered sedation for colonoscopy and EGD in the United States: 2009 to 2015. Gastrointest Endosc 2010;72(3):580–586 doi: 10.1016/j.gie.2010.04.040. [DOI] [PubMed] [Google Scholar]
- 6.Bekkali NL, Johnson GJ. Training in ERCP and EUS in the UK anno 2017. Frontline Gastroenterol 2017;8(2):124–128 doi: 10.1136/flgastro-2016-100771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wan S, Chen Y, Xiao Y, Zhao Q, Li M, Wu S. Spatial analysis and evaluation of medical resource allocation in China based on geographic big data. BMC Health Serv Res 2021;21(1):1084 doi: 10.1186/s12913-021-07119-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pennazio M Rondonotti E Despott EJ, et al. Small-bowel capsule endoscopy and device-assisted enteroscopy for diagnosis and treatment of small-bowel disorders: European Society of Gastrointestinal Endoscopy (ESGE) guideline—update 2022. Endoscopy 2023;55(1):58–95 doi: 10.1055/a-1973-3796. [DOI] [PubMed] [Google Scholar]
- 9.Jiang B Pan J Qian YY, et al. Clinical guideline on magnetically controlled capsule gastroscopy (2021 edition). J Dig Dis 2023;24(2):70–84 doi: 10.1111/1751-2980.13173. [DOI] [PubMed] [Google Scholar]
- 10.Sharma VK, Eliakim R, Sharma P, Faigel D. ICCE consensus for esophageal capsule endoscopy. Endoscopy 2005;37(10):1060–1064 doi: 10.1055/s-2005-870311. [DOI] [PubMed] [Google Scholar]
- 11.Cortegoso Valdivia P Skonieczna-Żydecka K Elosua A, et al. Indications, detection, completion and retention rates of capsule endoscopy in two decades of use: a systematic review and meta-analysis. Diagnostics (Basel, Switzerland) 2022;12(5):1105 doi: 10.3390/diagnostics12051105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dong J Grant C Vuong B, et al. Feasibility and safety of tethered capsule endomicroscopy in patients with barrett's esophagus in a multi-center study. Clin Gastroenterol Hepatol. 2022;20(4):756–765.e3. doi: 10.1016/j.cgh.2021.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gora MJ Quénéhervé L Carruth RW, et al. Tethered capsule endomicroscopy for microscopic imaging of the esophagus, stomach, and duodenum without sedation in humans (with video). Gastrointest Endosc 2018;88(5):830–840.e3 doi: 10.1016/j.gie.2018.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gluck N Half EE Bieber V, et al. Novel prep-less X-ray imaging capsule for colon cancer screening: a feasibility study. Gut 2019;68(5):774–775 doi: 10.1136/gutjnl-2018-316127. [DOI] [PubMed] [Google Scholar]
- 15.Qiu Y Huang Y Zhang Z, et al. Ultrasound capsule endoscopy with a mechanically scanning micro-ultrasound: a porcine study. Ultrasound Med Biol 2020;46(3):796–804 doi: 10.1016/j.ultrasmedbio.2019.12.003. [DOI] [PubMed] [Google Scholar]
- 16.Lee JH, Traverso G, Ibarra-Zarate D, Boning DS, Anthony BW. Ex vivo and in vivo imaging study of ultrasound capsule endoscopy. J Med Devices 2020;14(2):021005 doi: 10.1115/1.4046352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cox BF Stewart F Lay H, et al. Ultrasound capsule endoscopy: sounding out the future. Ann Transl Med 2017;5(9):201 doi: 10.21037/atm.2017.04.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qiu XO Jiang X Chen YZ, et al. New US capsule endoscopy for superficial and submucosal imaging of the esophagus: the first-in-human study. Gastrointest Endosc 2023;98(4):642–652 doi: 10.1016/j.gie.2023.06.015. [DOI] [PubMed] [Google Scholar]
- 19.Swindle MM, Makin A, Herron AJ, Clubb FJ, Jr., Frazier KS. Swine as models in biomedical research and toxicology testing. Vet Pathol 2012;49(2):344–356 doi: 10.1177/0300985811402846. [DOI] [PubMed] [Google Scholar]
- 20.Lay HS, Cox BF, Seetohul V, Demore CEM, Cochran S. Design and simulation of a ring-shaped linear array for microultrasound capsule endoscopy. IEEE Trans Ultrason Ferroelectr Freq Control 2018;65(4):589–599 doi: 10.1109/tuffc.2018.2794220. [DOI] [PubMed] [Google Scholar]
- 21.Wang X Seetohul V Chen R, et al. Development of a mechanical scanning device with high-frequency ultrasound transducer for ultrasonic capsule endoscopy. IEEE Trans Med Imaging 2017;36(9):1922–1929 doi: 10.1109/tmi.2017.2699973. [DOI] [PubMed] [Google Scholar]
- 22.Lay HS Cummins G Cox BF, et al. In-vivo evaluation of microultrasound and thermometric capsule endoscopes. IEEE Trans Biomed Eng 2019;66(3):632–639 doi: 10.1109/tbme.2018.2852715. [DOI] [PubMed] [Google Scholar]
- 23.Norton JC Slawinski PR Lay HS, et al. Intelligent magnetic manipulation for gastrointestinal ultrasound. Sci Robot 2019;4(31): doi: 10.1126/scirobotics.aav7725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tontini GE Manfredi G Orlando S, et al. Endoscopic ultrasonography and small-bowel endoscopy: present and future. Digest Endosc 2019;31(6):627–643 doi: 10.1111/den.13429. [DOI] [PubMed] [Google Scholar]
- 25.de Voogd F van Wassenaer EA Mookhoek A, et al. Intestinal ultrasound is accurate to determine endoscopic response and remission in patients with moderate to severe ulcerative colitis: a longitudinal prospective cohort study. Gastroenterology 2022;163(6):1569–1581 doi: 10.1053/j.gastro.2022.08.038. [DOI] [PubMed] [Google Scholar]
- 26.Dolinger MT Aronskyy I Kellar A, et al. Determining the accuracy of intestinal ultrasound scores as a pre-screening tool in Crohn's disease clinical trials. Am J Gastroenterol 2023; doi: 10.14309/ajg.0000000000002632. [DOI] [PubMed] [Google Scholar]


