Abstract
Background
The number of people undergoing voluntary HIV testing has abruptly decreased since 2020. The geographical heterogeneity of HIV infection and the impact of COVID-19 on the diagnosis of HIV at regional level are important to understand. This study aimed to estimate the HIV incidence by geographical region and understand how the COVID-19 pandemic influenced diagnosis of HIV.
Methods
We used an extended back-calculation method to reconstruct the epidemiological dynamics of HIV/AIDS by geographical region. We used eight regions: Tokyo, the capital of Japan, Hokkaido plus Tohoku, Kanto plus Koshinetsu (excluding Tokyo), Hokuriku, Tokai, Kinki, Chugoku plus Shikoku, and Kyushu plus Okinawa. Four different epidemiological measurements were evaluated: (i) estimated HIV incidence, (ii) estimated rate of diagnosis, (iii) number of undiagnosed HIV infections, and (iv) proportion of HIV infections that had been diagnosed.
Results
The incidence of HIV/AIDS during the COVID-19 pandemic from 2020 to 2022 increased in all regions except Kanto/Koshinetsu (51.3 cases/year), Tokyo (183.9 cases/year), Hokuriku (1.0 cases/year), and Tokai (43.1 cases/year). The proportion of HIV infections that had been diagnosed only exceeded 90% in Tokyo (91.7%, 95% confidence interval (CI): 90.6, 93.3), Kanto/Koshinetsu (91.0%, 95% CI: 87.3, 97.8), and Kinki (92.5%, 95% CI: 90.4, 95.9). The proportion of infections that had been diagnosed was estimated at 83.3% (95% CI: 75.1, 98.7) in Chugoku/Shikoku and 80.5% (95% CI: 73.9, 91.0) in Kyusyu/Okinawa.
Conclusions
In urban regions with major metropolitan cities, including Tokyo, Kinki, and Kanto/Koshinetsu, the number of undiagnosed HIV infections is substantial. However, the proportion of undiagnosed infections was estimated to be smaller than in other regions. The diagnosed proportion was the lowest in Kyusyu/Okinawa (80.5%), followed by Chugoku/Shikoku and Hokkaido/Tohoku. The level of diagnosis in those regional prefectures may have been more influenced and damaged by the COVID-19 pandemic than in urban settings.
Keywords: Community, Heterogeneity, AIDS, Incidence, Statistical estimation, Geographical variations
1. Background
Acquired immunodeficiency syndrome (AIDS), caused by infection with human immunodeficiency virus (HIV), is an immunocompromising disorder characterized by opportunistic infections. As of the end of 2022, a total of 20,003 HIV diagnoses and 8983 AIDS cases had been notified to the government among Japanese nationals. Of these, 38.1% of HIV cases and 24.6% of AIDS cases had been reported from Tokyo, the capital of Japan (The Committee of AIDS Trends et al.). Preventive treatment is widely recognized as the main intervention in HIV and AIDS. A test-and-treat strategy has therefore been underway, with strong efforts made to diagnose infected individuals early, provide antiretroviral therapy, and suppress the viral load (Granich et al., 2009, 2017). If the viral load can be suppressed below 200 copies/ml for more than 6 months so that viral levels are effectively undetectable, HIV-infected individuals are believed not to be able to cause secondary transmission, i.e., the virus is untransmissible. This fact, referred to as U = U, provides a way to successfully control HIV/AIDS in the future (Okoli et al., 2021). UNAIDS has advocated the massage ‘Undetectable = Untransmutable’ (UNAIDS, 2018): i.e., U=U plays a significant epidemiological role, increasing the number of testing and treatment, and ensures that HIV of infected individuals under treatment become undetectable, thereby bringing HIV epidemic under control.
Pre-exposure prophylaxis (PreP) can also prevent 99% of HIV infections via sexual contact (Nair et al., 2023). This is not yet covered by health insurance in Japan, but is already provided in several areas of Japan, especially urban areas, with the cost partly subsidized by a research grant from the Ministry of Health, Labour and Welfare of Japan.
The benefits of HIV diagnosis for both individuals and populations are increasingly clear, and the joint United Nations program on HIV/AIDS (UNAIDS) has therefore promoted the global initiative of “90–90-90” by 2020. This set out goals in care cascades to achieve 90% of people living with HIV knowing their HIV status, 90% of people diagnosed with HIV having access to antiretroviral therapy, and 90% of people receiving antiretroviral therapy having suppressed viral loads (UNAIDS). The year 2020 has already passed, but another extended goal of 95-95-95 has been set for the year 2030, aiming to end the HIV/AIDS epidemic globally.
Nationally, Japan has 380 designated hospitals for the treatment of HIV/AIDS. The country promotes ongoing treatment and follow-up among infected individuals, and meticulous medical support means that it has achieved the second two parts of the 90-90-90 goal, i.e., access to therapy and viral suppression (Iwamoto et al., 2017). However, the focal host of infection in Japan has been men having sex with men, and therefore obstacles have impeded open and casually accessible voluntary HIV testing, especially in remote areas. As of the end of 2017, the proportion of HIV infections that had been diagnosed among Japanese nationals was estimated at 80.3% (95% CI: 78.7%, 82.0%) (Nishiura, 2019). This figure therefore falls short of the first 90% of the 90-90-90 goal. Even at the end of 2022, the proportion of diagnosed infections was estimated at only 89.3% for the whole of Japan (Nishiura et al., 2024).
Alongside the need to improve HIV diagnosis rates in Japan, COVID-19 may also have affected diagnosis and treatment of HIV since 2020. Voluntary HIV testing is mainly managed by local public health centers in Japan. During the pandemic, the healthcare workers in those centers had to move to working on contact tracing and arranging hospital beds for severe COVID-19 cases. The number of voluntary HIV tests therefore decreased abruptly from 2020 (Ejima et al., 2021). The geographical heterogeneities of COVID-19 transmission and the heterogeneous back-up of human resources at public health centers mean that the impact of COVID-19 could have varied across geographical regions. Testing at public health centers is only one option for HIV testing; however, the change in focus could have affected the prognosis of infected individuals during the pandemic. Japan is one of the high-income nations that has seen very limited mortality from COVID-19, but the suppression policy has continued for a long time. This policy influenced delivery of services at public health centers until May 2023, when the legal category of COVID-19 as a notifiable disease was downgraded to category V, the same as seasonal influenza.
Public health centers have therefore been partly relieved of their duty to find hospital beds for all severe COVID-19 cases; however, the geographical heterogeneity of HIV infection and the impact of COVID-19 on the diagnosis of HIV at regional levels should be understood. This study therefore aimed to estimate HIV incidence by geographical region and understand how the COVID-19 pandemic influenced the diagnosis of HIV.
2. Methods
2.1. Epidemiological datasets
Infectious Disease Control Law designates HIV/AIDS as category V notifiable disease in Japan. Physicians are mandated to report all diagnosed infections to the government via local public health centers. We analyzed published surveillance data up to the end of 2022, which is openly announced by the Committee of AIDS Trends (The Committee of AIDS Trends et al.) at the Ministry of Health, Labour and Welfare. Every 3 months, newly diagnosed HIV infections and AIDS cases are reported by region and nationality (i.e., Japanese or non-Japanese). We analyzed data from Japanese nationals because analyzing data from non-Japanese nationals would have required us to account for mobility. Among both Japanese and non-Japanese nationals by the end of 2022, there had been a cumulative total of 23,863 HIV infections and 10,558 AIDS cases. HIV infection was detected using the antibody screening method (i.e., enzyme-linked immunosorbent assay, particle aggregation, and immunochromatography) followed by antibody confirmatory testing (i.e., Western blot method and immunofluorescence assay) or antigen detection testing, including virus isolation and polymerase chain reaction. AIDS was diagnosed when individuals met the clinical diagnostic criteria: (i) confirmed HIV infection and (ii) the presence of one of 23 indicator diseases representing opportunistic infections or tumors. From the surveillance records, we analyzed the yearly incidence dataset of HIV diagnoses and AIDS cases from 1985 to 2022, stratified by eight geographical regions (Tokyo, the capital of Japan, and seven other regions: Hokkaido plus Tohoku, Kanto plus Koshinetsu (excluding Tokyo), Hokuriku, Tokai, Kinki, Chugoku plus Shikoku, and Kyushu plus Okinawa). The geographical classification follows the Doshu system, a system of regional administrative units, each composed of several prefectures.
We carried out an extended back-calculation by region. Brookmeyer and Gail (Brookmeyer and Gail, 1986, 1988) firstly invented a method to estimate the HIV incidence using AIDS incidence data. Wand et al. (Wand et al., 2009). extended the back-calculation model that accounts for the HIV incidence rate and the age of infection, while Ndawinz et al. (Ndawinz et al., 2011). applied a model focusing on the time from infection to diagnosis. To use both the HIV diagnoses and AIDS incidence datasets, Aalen et al. (Aalen et al., 1997). Proposed a model that describes the progression of HIV infection. Generalizing such a multistate nature of infection process that involves both diagnosis and progression, Nishiura (Nishiura, 2019) employed a McKendrick partial differential equation to describe the HIV diagnosis and AIDS incidence using two integral equations.
The incubation period distribution was assumed to be known to allow an explicit estimation. As the baseline assumption, a median incubation period of 10.0 years was adopted and then allowed to vary from 7.5 to 12.3 years. The median of 10.0 years was derived from a published estimate among hemophiliac patients (Aalen et al., 1997), and the ranges were taken from a cohort dataset in San Francisco (Brookmeyer & Goedert, 1989) and a multicenter AIDS cohort study (Boldsen et al., 1988). The discrete nature of the data meant that we handled the incubation period distribution as a discrete distribution, fs of length s years, and used a discrete Weibull distribution. Parameter values for the scale parameter η and shape parameter k were η = 11.6 and k = 2.5 for the median of 10 years, η = 10.0 and k = 1.3 for the median of 7.5 years, and η = 14.3 and k = 2.5 for the median of 12.3 years.
2.2. Extended back-calculation model for regional data
Let denote the number of undiagnosed HIV infections in region , the annual HIV diagnosis rate, the annual rate of developing AIDS, the calendar time, and the infection age. The dynamics of HIV diagnosis and AIDS onset are described as follows:
| (1) |
| (2) |
By solving equation (1) using the boundary condition (2), we obtain
| (3) |
We extended the solution of the McKendrick equation (Nishiura, 2019) to geographically stratified data in region i, and described the incidence of HIV diagnoses ui(t) and AIDS cases ai(t) as:
| (4) |
where αi(t) is the time-dependent hazard rate of HIV diagnosis in region i, ρ(τ) is the hazard of illness onset as a function of the time elapsed since infection τ (derived from the incubation period distribution), and λi(t) is the HIV incidence at calendar time t in region i. αi(t) and λi(t) were dealt with as geographically dependent because of the geographical heterogeneity of transmission and diagnosis. The first sub-equation of (4) calculates the number of new HIV diagnoses in year by summing the number of people newly diagnosed as HIV infected in each year and multiplying it to the probability that they remain undiagnosed and do not develop AIDS until year . Similarly, the second sub-equation of (4) calculates the number of new AIDS incidence in year t by multiplying the hazard of developing AIDS in year to the probability of survival (i.e. undiagnosed and not developing AIDS) by year t. However, the illness onset hazard ρ(τ) is independent of geographical region. Migration of infected individuals was discarded because we had no migration data for infected individuals. Equation (4) was formulated in a continuous time scale. To be compatible with discrete data, we discretized the model to:
| (5) |
Using model (5), we estimated the incidence of infection in region i in year t, λt,i, to reconstruct the epidemic curve of HIV infection, and also αt,i to understand the spatial heterogeneity of diagnosis by region i. Products in equation (5) correspond to discrete survival probability compared with continuous survival functions in integral equations in (4). The product of the hazard of not diagnosed annually and the hazard of not newly developing AIDS annually is further multiplied by the HIV diagnosis rate in the first sub-equation of (5) and by the hazard rate of developing AIDS in the second sub-equation of (5). Both λt,i and αt,i were dealt with as step functions of year t, expressed as a piecewise constant model (changing every 4 years). To model the COVID-19 pandemic from 2020, the last 3 years (2020–2022) were dealt with as an independent interval for 3 years, so that they were explicitly separated from 2019 and earlier.
If the new HIV infection is described by a non-homogeneous Poisson process, the resulting AIDS cases should also be sufficiently captured by a Poisson distribution. The likelihood function to estimate parameters was:
| (6) |
where rt,i and wt,i are the observed number of HIV diagnoses and AIDS cases in year t in region i. Maximum likelihood estimates of parameters were obtained by minimizing the negative logarithm of equation (6). The 95% confidence interval (CI) of parameters was derived from the profile likelihood. The 95% CI of model estimates (e.g., the number of undiagnosed HIV infections and the proportion of infections that were diagnosed) was derived using a parametric bootstrap method. Following the epidemiological reconstruction of the epidemic, the proportion of HIV infections that had been diagnosed was calculated as by region i where x denotes the undiagnosed number of HIV infections calculated as:
| (7) |
Once the epidemiological dynamics had been reconstructed, four different epidemiological measurements were evaluated by geographical region: (i) the estimated HIV incidence (i.e. the number of new infections), (ii) estimated rate of diagnosis, (iii) number of undiagnosed HIV infections, and (iv) proportion of HIV infections that had been diagnosed.
2.3. Ethical considerations
We analyzed data that were publicly available (The Committee of AIDS Trends et al.). The open dataset analyzed was deidentified before the analysis, and we therefore did not have to obtain ethical approval.
3. Results
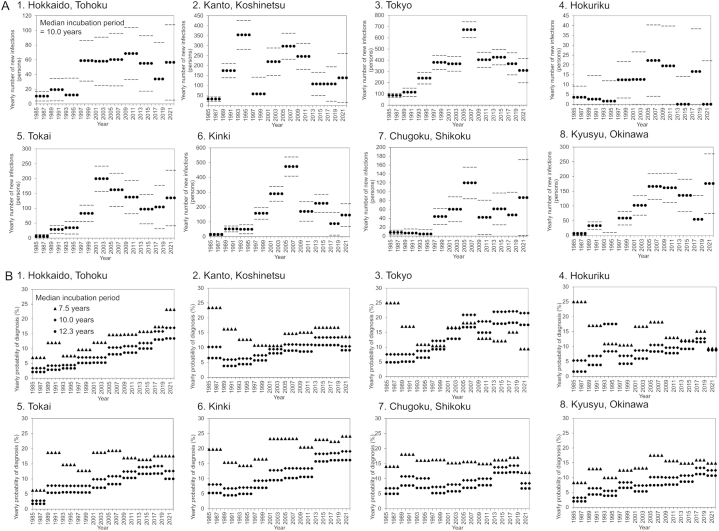
Fig. 1 shows the estimated yearly incidence of HIV infections λ and yearly rate of diagnosis α by region to the end of 2022. Overall, the number of new HIV infections has been nationally decreasing since 2019. However, there was an increase in the expected incidence during the COVID-19 pandemic from 2020 to 2022 (Fig. 1A) in all regions except Kanto/Koshinetsu (51.3 cases/year, 95% CI: 0, 151.4), Tokyo (183.9 cases/year, 95% CI: 82.0, 285.9), Hokuriku (1.0 cases/year 95% CI: 0, 32.8), and Tokai (43.1 cases/year 95% CI: 0, 117.6), but the confidence intervals were wide and therefore the increase was not significant. In Kyusyu and Okinawa, there were 60.3 cases per year (95% CI: 0, 142.5) for the period from 2017 to 2019, and this jumped to 158.0 cases per year (95% CI: 57.3, 258.7) for the period from 2020 to 2022. Assuming a median incubation period of 10.0 years, only Hokkaido/Tohoku (17.6%, 95% CI: 9.9%, 25.4%), and Kinki (20.1%, 95% CI: 14.9%, 25.4%) maintained an upward trend in yearly diagnoses to 2022. Other regions showed a decreasing trend in diagnoses during the pandemic, especially Chugoku/Shikoku, with an estimated yearly rate of 10.3% (95% CI: 5.2%, 15.3%) from 2020 to 2022 (Fig. 1B).
Fig. 1.
Estimated regional HIV incidence and the rate of diagnosis in Japan, 2022.
(A) Maximum likelihood estimates of the yearly incidence of HIV infections by region at the end of 2022. For all panes, dense dots represent the maximum likelihood estimates, and gray dots indicate the 95% confidence intervals with the assumed median incubation period of 10.0 years (derived from profile likelihood). (B) Yearly probability of diagnosis by region at the end of 2022 using different median incubation periods of 7.5, 10.0, and 12.3 years.
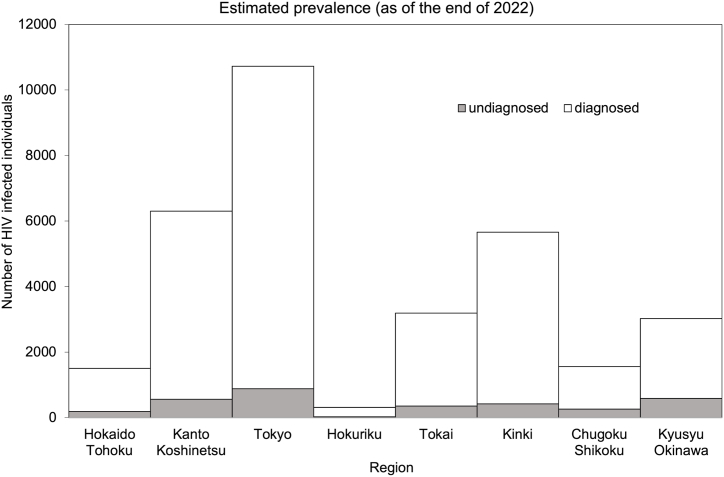
Fig. 2 shows a regional comparison of undiagnosed and diagnosed HIV infections as of the end of 2022. By region, Tokyo has the largest number of both undiagnosed and diagnosed HIV infections. Overall, 886.7 infections were estimated to be undiagnosed, followed by Kyusyu/Okinawa at 588.8 and Kanto/Koshinetsu at 567.1. As of the end of 2022, the three geographic regions with the highest proportion of undiagnosed individuals were Kyusyu/Okinawa (19.5%), Chugoku/Shikoku (16.7%), and Hokkaido/Tohoku (12.5%).
Fig. 2.
Estimated regional prevalence of diagnosed and undiagnosed HIV infections in Japan.
Regional comparison of undiagnosed versus diagnosed HIV infections as of the end of 2022. Gray bars show undiagnosed infections, and white bars diagnosed infections.
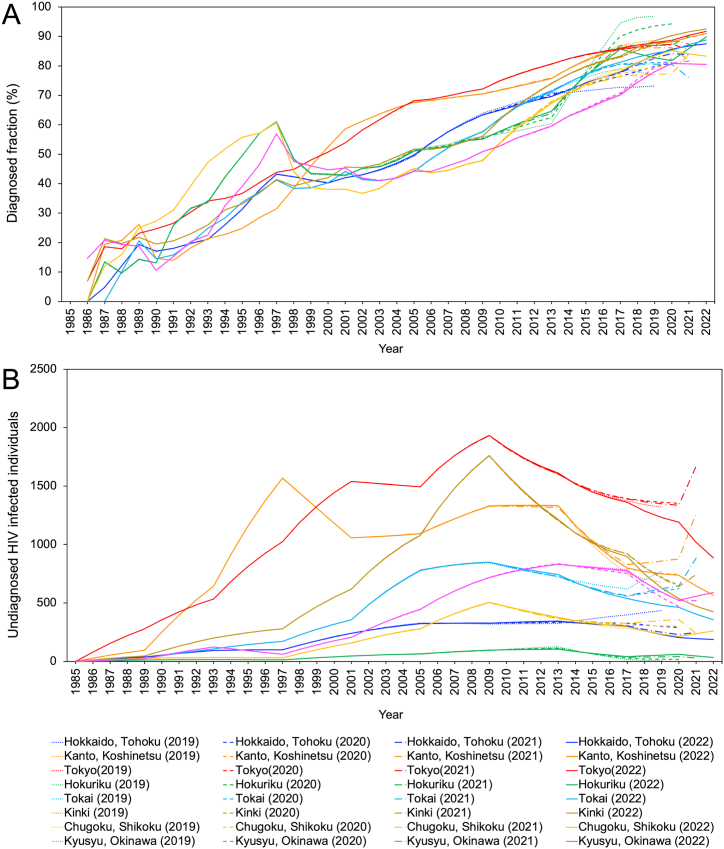
Fig. 3 shows the trends in regional diagnosis rates and the number of undiagnosed HIV infections with a real-time sequential update of estimates from 2019. The proportion of infections that had been diagnosed (Fig. 3A) shows an overall increase in all regions. However, using data to the end of 2021, a decreasing trend was observed in Tokai, Kanto/Koshinetsu, Kinki, Tokyo, and Hokkaido/Tohoku. The decreasing trend disappeared in most regions when we analyzed the data up to the end of 2022. However, Chugoku/Shikoku continued to show a decline, reaching 83.2% at the end of 2022, and the estimate for Kyusyu/Okinawa was 80.8% in 2020 but slightly decreased to 80.5% by the end of 2022 (Fig. 3A).
Fig. 3.
Real time estimates of regional rate of diagnosis and the number of undiagnosed HIV infections, Japan, 2019–2022.
(A) Real time estimates of the rate of diagnosis by region from 2019 to 2022. The dotted line shows estimates up to the end of 2019, the dash-dotted line 2020, the dashed line 2021, and the solid line 2022. The colors represent different regions: blue for Hokkaido/Tohoku, orange for Kanto/Koshinetsu (excluding Tokyo), red for Tokyo, green for Hokuriku, cyan for Tokai, brown for Kinki, yellow for Chugoku/Shikoku, and pink for Kyusyu/Okinawa. (B) Real time estimates for the number of undiagnosed HIV infections by region from 2019 to 2022.
All regions showed a monotonously decreasing trend in the number of undiagnosed infections by the year 2020. However, analysing the data up to the end of 2021, Tokyo, Kanto/Koshinetsu, Tokai, Kinki, and Hokkaido/Tohoku all showed an increasing trend. Analysing the data up to the end of 2022, Tokyo had 886.7 undiagnosed infections, Kanto/Koshinetsu had 567.1, and Hokkaido/Tohoku had 188.3, returning to a decreasing trend. There were 261.3 undiagnosed infections estimated in Chugoku/Shikoku and 588.8 in Kyusyu/Okinawa, both showing an increasing trend to the end of 2022 (Fig. 3B).
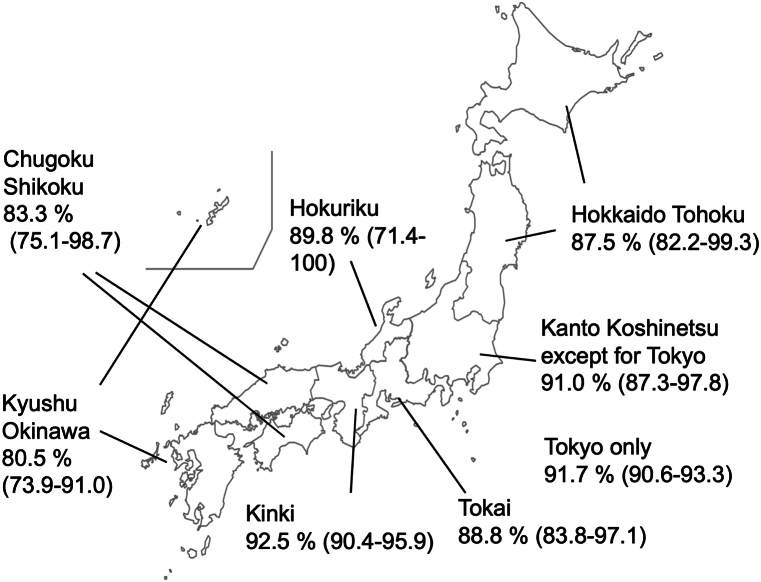
Fig. 4 shows a map of Japan with the estimated proportion of HIV infections that had been diagnosed by the end of 2022. When AIDS cases were included in the computation, the diagnosis rates exceeded 90% only in Tokyo (91.7%, 95% CI: 90.6, 93.3), Kanto/Koshinetsu (91.0%, 95% CI: 87.3, 97.8), and Kinki (92.5%, 95% CI: 90.4, 95.9). The diagnosed proportions in Chugoku/Shikoku and Kyusyu/Okinawa were estimated as only 83.3% (95% CI: 75.1, 98.7) and 80.5% (95% CI: 73.9, 91.0).
Fig. 4.
Diagnosed proportions of HIV infection by region in Japan at the end of 2022.
Proportion of diagnosed HIV infections by region in Japan with 95% confidence intervals derived using the bootstrap method.
4. Discussion
This study reconstructed the epidemiological dynamics of HIV/AIDS in Japan by geographical region, including during the COVID-19 pandemic. We used four different evaluation metrics: (i) HIV incidence, (ii) yearly rate of diagnosis, (iii) number of undiagnosed HIV infections, and (iv) proportion of HIV infections that had been diagnosed. We found that the epidemiology of HIV/AIDS was highly heterogeneous across regions. In a few specific regions with major metropolitan cities, including Tokyo, Kinki (including Osaka), and Kanto/Koshinetsu (including Kanagawa), the number of undiagnosed HIV infections was substantial, but the proportion undiagnosed was estimated to be smaller than other regions. The urban regions were also relatively resilient to the pandemic, but we found a decrease in the rate of diagnosis and increase in the number of undiagnosed infections in both Chugoku/Shikoku and Kyusyu/Okinawa during the COVID-19 pandemic. To our knowledge, this is the first study to clearly show the geographical variations in the incidence and diagnosis rate of HIV in Japan during the pandemic.
The number of new HIV infections was estimated to have continuously decreased over time before the COVID-19 pandemic. However, an upward change in the incidence from 2020 to 2022 was seen in all of Japan except for Tokyo, Kanto/Koshinetsu, and Tokai. In Kyusyu/Okinawa, the number of new infections increased by 97.7 people from 2017 to 2019 to 2020–2022, which is a substantial increment compared with other regions (Fig. 1).
The annual rate of diagnosis was also steadily increasing and improving over the course of time before COVID-19. In Tokyo and Kinki, the diagnosis rate did not decrease during the pandemic and the level of hazard from 2020 to 2022 was comparable to the pre-pandemic period. However, the diagnosis rate decreased in Kanto/Koshinetsu, Tokai, Hokuriku, Chugoku/Shikoku, and Kyusyu/Okinawa.
Tokyo, Kyushu/Okinawa, and Kanto/Koshinetsu have the highest number of undiagnosed HIV infections (Fig. 2), even though the number undiagnosed was declining before the pandemic. During the COVID-19 pandemic, the number of undiagnosed cases in Chugoku/Shikoku and Kyusyu/Okinawa increased, which was an alarming and unforeseen situation. Both these regions also showed both an increasing trend in HIV incidence and a decreasing trend in the annual rate of diagnosis. Tokyo, Kanto/Koshinetsu, and Kinki achieved a high proportion of HIV cases that had been diagnosed, and this exceeded 90% by the end of 2022. The diagnosed proportion was the lowest in Kyusyu/Okinawa (80.5%), followed by Chugoku/Shikoku and Hokkaido/Tohoku.
Summing up these findings, two important points can be taken from this study. First, since before the pandemic period, metropolitan areas have benefited from better (or to be more precise more accessible) opportunities for diagnosis (and therefore possibly better clinical outcomes) of HIV infection, perhaps especially among high risk people. The incidence therefore kept decreasing strongly in Tokyo, Kanto/Koshinetsu, and Kinki. Second, in response to COVID-19, the geographical disparities in HIV diagnosis rates increased, revealing new regional differences of concern. COVID-19 disproportionately affected urban prefectures with metropolitan areas, including Tokyo and Osaka, during the pre-vaccination period up to 2021. However, the diagnostic performance in major metropolitan regions was maintained even during the pandemic. It seems that the diagnostic rate in regional prefectures may have been more influenced and damaged by COVID-19.
The incidence also seems to be geographically heterogeneous. Yearly HIV incidence in the latest time period was estimated to have been increasing in Hokkaido/Tohoku, Kinki, Chugoku/Shikoku, and Kyusyu/Okinawa, especially when using the real-time dataset up to the end of 2021. This increase implies that the opportunity for secondary transmission was maintained even during the COVID-19 pandemic when opportunities for contact with new people were greatly restricted (Muñoz & Xu, 1996). Indeed, considering that 95.7% of HIV/AIDS notifications in Japan were for men (Japan Broadcasting Corporation & Nakamura, 2021), sexual contact between men in some regions could have been maintained independently of the restricted social sectors.
Notably, the rate of HIV diagnosis was largely maintained in urban areas despite the greatly reduced testing volume at public health centers during the COVID-19 pandemic. There are two possible explanations for this. First, those at low risk may have been vulnerable to the reduced testing volume at public health centers. However, the data imply that people at high risk of infection tended to continue to be tested, especially in urban prefectures. Individual clinics in Tokyo that offer a rapid testing service did not experience reduced numbers of tests or positive tests compared with before the pandemic. Second, even during the COVID-19 pandemic, public health centers tried their best to maintain the testing service, for example by reducing the number of testing opportunities or limiting the assigned number of people at public health centers. A specialized testing center (Higashi Shinjuku testing consultation center in Tokyo), known to be more accessible to the high risk group than ordinary testing centers, ran a regular testing service even during the COVID-19 pandemic, so that it could continue to offer this service to people at high risk. This effort should be commended for successfully sustaining the service.
Despite these results, our study has shown that regional prefectures were perhaps more vulnerable to the pressure of the COVID-19 pandemic. In fact, the number of public health centers has declined from 845 in 1996 to 469 in 2020, and public health centers are chronically in short of manpower (AIDS Prevention Information Network. et al.; Japanese Association of Public Health Center and Directors, 2023). Even before the pandemic, both the proportion of HIV infections that were diagnosed and the number of undiagnosed infected individuals have been higher in urban areas than remote areas. The limited number of peers in remote settings may mean that HIV testing is less accessible in these areas. Comprehensive approaches to lowering barriers for testing among high risk groups in regional settings are likely to be the key to success during the post-vaccination (post-COVID) future. We therefore aim to carry out an epidemiological study that explores specific regions with a limited proportion of diagnosed individuals, e.g. Kyushu/Okinawa, Chugoku/Shikoku, and Hokkaido/Tohoku.
Our findings are broadly consistent with published evidence from other countries, where the incidence of sexually transmitted infections increased during the COVID-19 pandemic (Ministry of Health et al., 2023). The transmission of COVID-19 was intense in Nigeria, and routine care of HIV-infected individuals was affected (Soriano et al., 2023). However, geographical areas that were considered to be resilient were able to provide essential public health services in South Africa (Habila et al., 2023). The public health response to COVID-19 was substantial in Kenya, but there was no apparent adverse impact of COVID-19 on HIV care and treatment (Heunis et al., 2023). In Asia, adherence to antiretroviral therapy was lowered in the Philippines during the pandemic (Kimanga et al., 2023), and care providers found it difficult to maintain good mental health of patients in Vietnam (Joves et al., 2023). In this situation, having a trust-based doctor‒patient relationship established through HIV follow-up was considered critical (Matsumoto et al., 2023) and possible alternative options for follow-up, including telemedicine, played a role in maintaining the service and providing continuing care (Penot et al., 2023).
This study had four technical limitations that must be discussed. First, the validity of the extended back-calculation method depends on the assumption of a known incubation period distribution. To address the uncertainty, additional estimates using biomarkers, including CD4 count, are necessary. Second, surveillance data from cases are subject to misclassification bias. For instance, some of the diagnosed cases reported as HIV infections could have already satisfied the definition of AIDS (e.g., esophageal candidiasis was present but not checked via endoscopy). Third, mobility of humans was not explicitly taken into account, and people who were mobile across regions were not reflected in the estimates. Fourth, regional infections may be under-ascertained because of the limited opportunities to undertake testing at healthcare facilities. This diagnostic bias may not be captured by the differential diagnostic effort by geographic regions (mathematically absorbed by the rate of diagnosis α in our model), and may have remained unobserved.
5. Conclusions
Despite the technical limitations, we believe that we successfully reconstructed the epidemiological dynamics of HIV/AIDS by geographical regions in Japan. We were able to estimate HIV incidence, yearly rate of diagnosis, number of undiagnosed HIV infections and proportion of diagnosed HIV infections. Urban regions with major metropolitan cities, including Tokyo, Kinki, and Kanto/Koshinetsu, had substantial numbers of undiagnosed HIV infections, but a smaller undiagnosed proportion than other regions. However, a decrease in the rate of diagnosis and increase in the number of undiagnosed infections were seen in Chugoku/Shikoku and Kyusyu/Okinawa during the COVID-19 pandemic. The diagnosed proportion was the lowest in Kyusyu/Okinawa (80.5%), followed by Chugoku/Shikoku and Hokkaido/Tohoku. Diagnosis rates may therefore have been more influenced and damaged by the COVID-19 pandemic in those regions than in urban areas.
Ethics approval and consent to participate
We analyzed data that are publicly available (The Committee of AIDS Trends et al.). The open dataset that we analyzed was deidentified before the analysis, and ethical approval was therefore not required.
Consent for publication
Not applicable.
Availability of data and materials
All data generated or analyzed during this study are included in the published article and its additional files (Additional_data.xlsx).
Funding
This study was supported by Health and Labour Sciences Research Grants (21HB1002 and 22HB1002). TS received financial support from Yuai Welfare Foundation in Japan. HN received funding from Health and Labour Sciences Research Grants (20CA2024, 20HA2007, 21HB1002, 21HA2016, and 23HA2005); the Japan Agency for Medical Research and Development (JP23fk0108685 and JP23fk0108612); JSPS KAKENHI (21H03198 and 22K19670); the Environment Research and Technology Development Fund (JPMEERF20S11804) of the Environmental Restoration and Conservation Agency of Japan, Kao Health Science Research, the Daikin GAP Fund of Kyoto University, and the Japan Science and Technology Agency SICORP program (JPMJSC20U3 and JPMJSC2105); and the RISTEX program for Science of Science, Technology and Innovation Policy (JPMJRS22B4). The funders had no role in the study design, data collection, and analysis, decision to publish, or preparation of the manuscript.
CRediT authorship contribution statement
Hiroshi Nishiura: Writing – review & editing, Writing – original draft, Supervision, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. Seiko Fujiwara: Writing – review & editing, Writing – original draft, Visualization, Methodology, Investigation, Formal analysis, Data curation. Akifumi Imamura: Writing – review & editing, Validation. Takuma Shirasaka: Writing – review & editing, Validation.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank the local governments, public health centers, and institutes for surveillance for assistance with laboratory testing, epidemiological investigations, and data collection. We thank Melissa Leffler, MBA, from Edanz (https://jp.edanz.com/ac) for editing a draft of the manuscript.
Handling Editor: Dr Yijun Lou
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Abbreviations
- COVID-19
coronavirus disease 2019
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
- HIV
Human immunodeficiency virus
- AIDS
Acquired immunodeficiency syndrome
- CI
confidence intervals
References
- Aalen O.O., Farewell V.T., De Angelis D., Day N.E., Gill O.N. A markov model for HIV disease progression including the effect of HIV diagnosis and treatment: Application to AIDS prediction in england and wales. Statistics in Medicine. 1997;16(19):2191–2210. doi: 10.1002/(sici)1097-0258(19971015)16:19<2191::aid-sim645>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- AIDS Prevention Information Network. (n.d.). Annual Report on the Status of HIV/AIDS in Japan. Retrieved August 18, 2023, from https://api-net.jfap.or.jp/status/japan/nenpo.html.
- Boldsen J.L., Jensen J.L., Sogaard J., Sorensen M. On the incubation time distribution and the Danish AIDS data. Journal of the Royal Statistical Society: Series A. 1988;151(1):42–43. doi: 10.2307/2982183. [DOI] [Google Scholar]
- Brookmeyer R., Gail M.H. Minimum size of the acquired immunodeficiency syndrome (AIDS) epidemic in the United States. Lancet (London, England) 1986;2(8519):1320–1322. doi: 10.1016/s0140-6736(86)91444-3. [DOI] [PubMed] [Google Scholar]
- Brookmeyer R., Gail M.H. A method for obtaining short-term projections and lower bounds on the size of the AIDS epidemic. Journal of the American Statistical Association. 1988;83(402):301–308. doi: 10.1080/01621459.1988.10478599. [DOI] [Google Scholar]
- Brookmeyer R., Goedert J.J. Censoring in an epidemic with an application to hemophilia-associated AIDS. Biometrics. 1989;45(1):325–335. doi: 10.2307/2532057. [DOI] [PubMed] [Google Scholar]
- Ejima K., Koizumi Y., Yamamoto N., Rosenberg M., Ludema C., Bento A.I., Yoneoka D., Ichikawa S., Mizushima D., Iwami S. HIV testing by public health centers and municipalities and new HIV cases during the COVID-19 pandemic in Japan. Journal of Acquired Immune Deficiency Syndromes. 2021;87(2):e182–e187. doi: 10.1097/QAI.0000000000002660. 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granich R.M., Gilks C.F., Dye C., Williams B.G. Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV transmission: A mathematical model. Lancet (London, England) 2009;373(9657):48–57. doi: 10.1016/S0140-6736(08)61697-9. [DOI] [PubMed] [Google Scholar]
- Granich R., Williams B., Montaner J., Zuniga J.M. 90-90-90 and ending AIDS: Necessary and feasible. Lancet (London, England) 2017;390(10092):341–343. doi: 10.1016/S0140-6736(17)31872-X. [DOI] [PubMed] [Google Scholar]
- Habila M.A., Obeng-Kusi M., Ali M.J., Magaji F.A., Shambe I.H., Daru P.H., Jacobs E.T., Madhivanan P., Sagay A.S., Musa J. The impact of the COVID-19 pandemic on routine HIV care and cervical cancer screening in North-Central Nigeria. BMC Women's Health. 2023;23(1):640. doi: 10.1186/s12905-023-02782-6. 2023 Nov 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heunis C., Chikobvu P., Muteba M., Kigozi-Male G., Engelbrecht M., Mushori P. Impact of COVID-19 on selected essential public health services - lessons learned from a retrospective record review in the Free State, South Africa. BMC Health Services Research. 2023;23(1):1244. doi: 10.1186/s12913-023-10166-7. 2023 Nov 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto A., Taira R., Yokomaku Y., Koibuchi T., Rahman M., Izumi Y., Tadokoro K. The HIV care cascade: Japanese perspectives. PLoS One. 2017;12(3) doi: 10.1371/journal.pone.0174360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Japanese Association of Public Health Center Directors Number and trend of public health center establishments. 2023. http://www.phcd.jp/03/HCsuii/ n.d.
- Joves P.J.M., Matulac M.O., Pagcatipunan R.S., Jr. Barriers to antiretroviral medication adherence in people living with HIV (PLHIV) at the time of the COVID-19 pandemic in the Philippines. Travel Medicine and Infectious Disease. 2023;8(10):461. doi: 10.3390/tropicalmed8100461. 2023 Sep. 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimanga D.O., Makory V.N.B., Hassan A.S., Ngari F., Ndisha M.M., Muthoka K.J., Odero L., Omoro G.O., Aoko A., Ng'ang'a L. Impact of the COVID-19 pandemic on routine HIV care and antiretroviral treatment outcomes in Kenya: A nationally representative analysis. PLoS One. 2023;18(11) doi: 10.1371/journal.pone.0291479. 2023 Nov 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto S., Nagai M., Tran L.K., Yamaoka K., Nguyen H.D.T., Tanuma J., Pham T.N., Oka S., Van Tran G. Multicenter observational survey on psychosocial and behavioral impacts of COVID-19 in people living with HIV in Northern Vietnam. Scientific Reports. 2023;13(1) doi: 10.1038/s41598-023-47577-9. 2023 Nov 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ministry of Health, Labor and Welfare Number of public health centers by establishing entity. 2023. https://www.mhlw.go.jp/content/10900000/000765311.pdf n.d.
- Muñoz A., Xu J. Models for the incubation of AIDS and variations according to age and period. Statistics in Medicine. 1996;15(21–22):2459–2473. doi: 10.1002/(sici)1097-0258(19961130)15:22<2459::aid-sim464>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Nair G., Celum C., Szydlo D., Brown E.R., et al. REACH Protocol Team . The lancet. HIV. Advance online publication; 2023. Adherence, safety, and choice of the monthly dapivirine vaginal ring or oral emtricitabine plus tenofovir disoproxil fumarate for HIV pre-exposure prophylaxis among african adolescent girls and young women: A randomised, open-label, crossover trial. S2352-3018(23)00227-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Japan Broadcasting Corporation. Nakamura K. Things to Be cautious about when lifting restrictions and easing self-restraint measures in response to COVID-19. 2021. https://www.nhk.or.jp/kaisetsu-blog/700/456372.html
- Ndawinz J.D., Costagliola D., Supervie V. New method for estimating HIV incidence and time from infection to diagnosis using HIV surveillance data: Results for France. AIDS. 2011;25(15):1905–1913. doi: 10.1097/QAD.0b013e32834af619. [DOI] [PubMed] [Google Scholar]
- Nishiura H. Estimating the incidence and diagnosed proportion of HIV infections in Japan: A statistical modeling study. PeerJ. 2019;7 doi: 10.7717/peerj.6275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiura H., Fujiwara S., Imamura A., Shirasaka T. HIV incidence before and during the COVID-19 pandemic in Japan. Mathematical Biosciences and Engineering: MBE. 2024;21(4):4874–4885. doi: 10.3934/mbe.2024215. [DOI] [PubMed] [Google Scholar]
- Okoli C., Van de Velde N., Richman B., Allan B., Castellanos E., Young B., Brough G., Eremin A., Corbelli G.M., Mc Britton M., Hardy W.D., de Los Rios P. Undetectable equals untransmittable (U = U): Awareness and associations with health outcomes among people living with HIV in 25 countries. Sexually Transmitted Infections. 2021;97(1):18–26. doi: 10.1136/sextrans-2020-054551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penot P., Chateauneuf J., Auperin I., Cordel H., Letembet V.A., Bottero J., Cailhol J. Socioeconomic impact of the COVID-19 crisis and early perceptions of COVID-19 vaccines among immigrant and nonimmigrant people living with HIV followed up in public hospitals in Seine-Saint-Denis, France. PLoS One. 2023;18(10) doi: 10.1371/journal.pone.0276038. 2023 Oct 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano V., Blasco-Fontecilla H., Gallego L., Fernández-Montero J.V., Mendoza C., Barreiro P. Rebound in sexually transmitted infections after the COVID-19 pandemic. AIDS Reviews. 2023;26(3):127–135. doi: 10.24875/AIDSRev.230000. 2023. [DOI] [PubMed] [Google Scholar]
- The Committee of AIDS Trends, Ministry of Health, Labour and Welfare. HIV/AIDS in Japan. Tokyo, Ministry of Health, Labour and Welfare. https://api-net.jfap.or.jp/status/japan/nenpo.html.
- UNAIDS. An ambitious treatment target to help end the AIDS epidemic. https://www.unaids.org/sites/default/files/media_asset/90-90-90_en.pdf.
- UNAIDS Undetectable = untransmittable. 2018. https://www.unaids.org/en/resources/presscentre/featurestories/2018/july/undetectable-untransmittable
- Wand H., Wilson D., Yan P., Gonnermann A., McDonald A., Kaldor J., Law M. Characterizing trends in HIV infection among men who have sex with men in Australia by birth cohorts: Results from a modified back-projection method. Journal of the International AIDS Society. 2009;12:19. doi: 10.1186/1758-2652-12-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in the published article and its additional files (Additional_data.xlsx).