Abstract
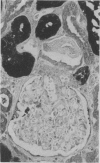
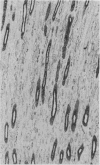
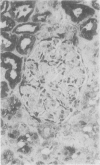
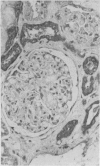
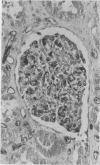
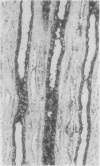
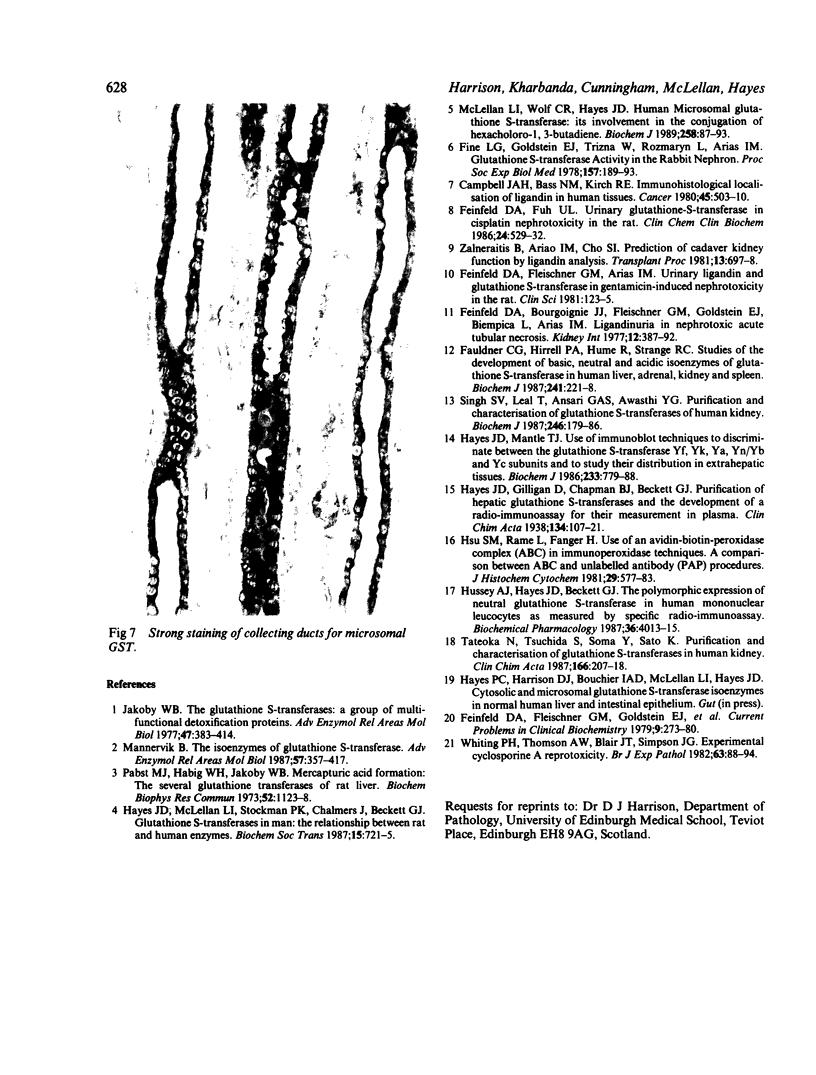
To determine whether the tissue distribution of glutathione S-transferase (GST) isoenzymes could define the precise nature of renal injury, 13 adult kidneys were studied, using specific antibodies raised against purified isoenzymes. Basic GST stained strongly proximal convoluted tubules and some medullary tubules; acidic GST stained strongly distal convoluted tubules and medullary tubules; neutral GST stained similarly to acidic GST, but weaker, and microsomal GST stained glomerular and interstitial endothelium and collecting ducts deep in the medulla, although there was considerable variation in staining intensity among cases. It is suggested that the measurement of these isoenzymes in serum and urine may help to elucidate the localisation of tissue damage, which may be particularly valuable in patients with cyclosporine toxicity following renal transplantation.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Campbell J. A., Bass N. M., Kirsch R. E. Immunohistological localization of ligandin in human tissues. Cancer. 1980 Feb;45(3):503–510. doi: 10.1002/1097-0142(19800201)45:3<503::aid-cncr2820450315>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Faulder C. G., Hirrell P. A., Hume R., Strange R. C. Studies of the development of basic, neutral and acidic isoenzymes of glutathione S-transferase in human liver, adrenal, kidney and spleen. Biochem J. 1987 Jan 1;241(1):221–228. doi: 10.1042/bj2410221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinfeld D. A., Bourgoignie J. J., Fleischner G., Goldstein E. J., Biempica L., Arias I. M. Ligandinuria in nephrotoxic acute tubular necrosis. Kidney Int. 1977 Dec;12(6):387–392. doi: 10.1038/ki.1977.129. [DOI] [PubMed] [Google Scholar]
- Feinfeld D. A., Fleischner G. M., Arias I. M. Urinary ligandin and glutathione-S-transferase in gentamicin-induced nephrotoxicity in the rat. Clin Sci (Lond) 1981 Jul;61(1):123–125. doi: 10.1042/cs0610123. [DOI] [PubMed] [Google Scholar]
- Feinfeld D. A., Fleischner G. M., Goldstein E. J., Levine R. D., Levine S. D., Avram M. M., Arias I. M. Ligandinuria: an indication of tubular cell necrosis. Curr Probl Clin Biochem. 1979;(9):273–280. [PubMed] [Google Scholar]
- Feinfeld D. A., Fuh V. L., Safirstein R. Urinary glutathione-S-transferase in cisplatin nephrotoxicity in the rat. J Clin Chem Clin Biochem. 1986 Aug;24(8):529–532. doi: 10.1515/cclm.1986.24.8.529. [DOI] [PubMed] [Google Scholar]
- Fine L. G., Goldstein E. J., Trizna W., Rozmaryn L., Arias I. M. Glutathione-S-transferase activity in the rabbit nephron: segmental localization in isolated tubules and formation of thiol adducts of ethacrynic acid. Proc Soc Exp Biol Med. 1978 Feb;157(2):189–193. doi: 10.3181/00379727-157-40018. [DOI] [PubMed] [Google Scholar]
- Hayes J. D., Gilligan D., Chapman B. J., Beckett G. J. Purification of human hepatic glutathione S-transferases and the development of a radioimmunoassay for their measurement in plasma. Clin Chim Acta. 1983 Oct 31;134(1-2):107–121. doi: 10.1016/0009-8981(83)90189-4. [DOI] [PubMed] [Google Scholar]
- Hayes J. D., Mantle T. J. Use of immuno-blot techniques to discriminate between the glutathione S-transferase Yf, Yk, Ya, Yn/Yb and Yc subunits and to study their distribution in extrahepatic tissues. Evidence for three immunochemically distinct groups of transferase in the rat. Biochem J. 1986 Feb 1;233(3):779–788. doi: 10.1042/bj2330779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes J. D., McLellan L. I., Stockman P. K., Chalmers J., Beckett G. J. Glutathione S-transferases in man: the relationship between rat and human enzymes. Biochem Soc Trans. 1987 Aug;15(4):721–725. doi: 10.1042/bst0150721. [DOI] [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981 Apr;29(4):577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Hussey A. J., Hayes J. D., Beckett G. J. The polymorphic expression of neutral glutathione S-transferase in human mononuclear leucocytes as measured by specific radioimmunoassay. Biochem Pharmacol. 1987 Nov 15;36(22):4013–4015. doi: 10.1016/0006-2952(87)90472-2. [DOI] [PubMed] [Google Scholar]
- Mannervik B. The isoenzymes of glutathione transferase. Adv Enzymol Relat Areas Mol Biol. 1985;57:357–417. doi: 10.1002/9780470123034.ch5. [DOI] [PubMed] [Google Scholar]
- McLellan L. I., Wolf C. R., Hayes J. D. Human microsomal glutathione S-transferase. Its involvement in the conjugation of hexachlorobuta-1,3-diene with glutathione. Biochem J. 1989 Feb 15;258(1):87–93. doi: 10.1042/bj2580087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabst M. J., Habig W. H., Jakoby W. B. Mercapturic acid formation: the several glutathione transferases of rat liver. Biochem Biophys Res Commun. 1973 Jun 19;52(4):1123–1128. doi: 10.1016/0006-291x(73)90616-5. [DOI] [PubMed] [Google Scholar]
- Singh S. V., Leal T., Ansari G. A., Awasthi Y. C. Purification and characterization of glutathione S-transferases of human kidney. Biochem J. 1987 Aug 15;246(1):179–186. doi: 10.1042/bj2460179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tateoka N., Tsuchida S., Soma Y., Sato K. Purification and characterization of glutathione S-transferases in human kidney. Clin Chim Acta. 1987 Jul 15;166(2-3):207–218. doi: 10.1016/0009-8981(87)90423-2. [DOI] [PubMed] [Google Scholar]
- Whiting P. H., Thomson A. W., Blair J. T., Simpson J. G. Experimental cyclosporin A nephrotoxicity. Br J Exp Pathol. 1982 Feb;63(1):88–94. [PMC free article] [PubMed] [Google Scholar]
- Zalneraitis B., Arias I. M., Cho S. I. Prediction of cadaver kidney function by ligandin analysis. Transplant Proc. 1981 Mar;13(1 Pt 2):697–698. [PubMed] [Google Scholar]