Abstract
Purpose
To report a case of disappearance of lens epithelial cells (LECs) detected at the time of treatment for intraocular lens (IOL) dislocation 12 months after cataract surgery.
Observations
A 59-year-old male underwent phacoemulsification with implantation of a posterior chamber acrylic IOL for posterior subcapsular cataract without any complications. Twelve months after cataract surgery, the IOL was dislocated inferiorly from the capsular bag due to rubbing the eye strongly, without capsular deviation. Fibrotic changes around the anterior capsular margin and posterior capsular opacification were not observed. During IOL repositioning, adhesions between the anterior and posterior capsules or zonule weakness were not observed. Six months after repositioning, the in-the-bag IOL dislocated into the anterior chamber because of zonular dialysis caused by strong eye rubbing again. Several days after scleral fixation of the IOL, the intraocular pressure decreased, possibly due to leakage from the wound. On inquiring about details, it was informed that the patient had a habit of sleeping in the prone position, with his face touching the pillow. After discontinuing this habit, his visual status stabilized.
Conclusions and Importance
LEC may rarely disappear 12 months after cataract surgery. Although LEC survival is affected by various factors, minor trauma, such as slight eye rubbing and sleeping in a prone position, may influence IOL stability in the capsular bag and be related to early postoperative LEC disappearance.
Keywords: Lens epithelial cells, Dead bag syndrome, IOL dislocation, Trauma
1. Introduction
Residual lens epithelial cells (LECs) migrate and proliferate after cataract surgery, causing posterior capsule opacification (PCO), the most common complication of cataract surgery.1 Additionally, some LECs around the anterior capsule margin differentiate into myofibroblast-like cells, causing fibrotic changes and contraction of the lens capsule.2 Such secondary LEC proliferation and fibrotic changes commonly occur. Kimura et al. reported a decrease in the size of the capsulorrhexis in all 38 eyes 6 months after surgery.3 Clear removal of LECs mechanically or pharmacologically was attempted to prevent PCO and fibrotic changes at the anterior margin of the capsule; however, this could not be achieved.2
Culp et al. recently reported a new entity of intraocular lens (IOL) dislocation, dead bag syndrome, with histological analysis of 10 cases.4 The capsular bag appeared to be clear, becoming diaphanous and floppy, and unable to support the IOL within it and this occurred from 3.7 to 12.8 years postoperatively. Histopathological examination revealed thinning and/or splitting of capsular bags. LECs are either absent or rare in capsular bags. The etiology of capsular degeneration is unclear, but an absence of secondary proliferation of LECs and fibrotic changes might be related to these changes because LECs are thought to be important in maintaining the capsule.5 However, the mechanism and time course of LECs disappearance are unknown.6 The capsular polishing performed during cataract surgery is perceived to be unrelated.7 It was speculated that LECs may be present at the end of surgery but disappear without forming fibrotic changes.8
Here, we report a case of a clear lens capsule detected during the treatment of an IOL dislocation 12 months after cataract surgery.
2. Case report
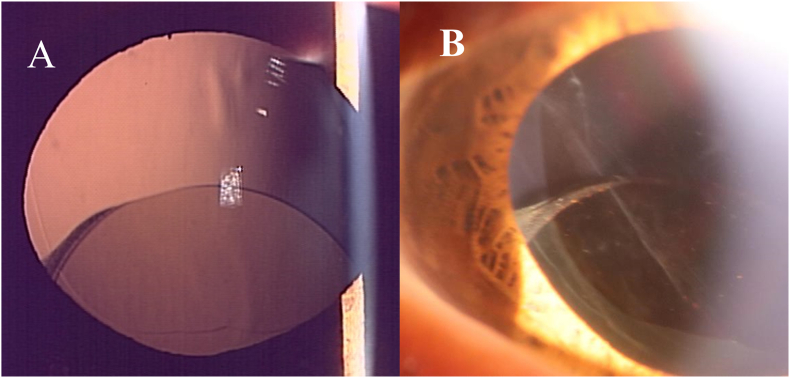
A 59-year-old male visited the Iwate Medical University Hospital with blurred vision in his left eye. The best-corrected visual acuity of the left eye was 20/25 due to a posterior subcapsular cataract. He had undergone phacoemulsification with the implantation of a posterior chamber acrylic IOL (Avansee Preload 1P, Kowa Co., Ltd., Japan) under sub-Tenon's anesthesia using lidocaine in his left eye. LEC removal or topical and/or intracameral injections of antibiotics, anesthetics, and so on were not performed. No complications occurred during the surgery. The patient's visual acuity had returned to 20/20. The immediate postoperative course was uneventful. Twelve months after cataract surgery, he returned to our hospital complaining of sudden irregular vision in the left eye from the previous two days. He worried that he might have rubbed his eyes strongly during sleep. The best-corrected visual acuity of the left eye was 20/20, but slit-lamp examination of the left eye showed an inferiorly dislocated IOL out of the capsular bag (Fig. 1A). The capsular bag had not deviated. Fibrotic changes or LECs were not observed around the anterior capsular margins (Fig. 1B). The central posterior capsule was also clear. We attempted to reposition the IOL. No adhesions were observed between the anterior and posterior capsules during surgery. We checked the zonule, but no obvious weakness was found; therefore, the IOL was inserted into the capsular bag without a capsular tension ring. The patient's complaints of visual disturbances disappeared.
Fig. 1.
Slit-lamp photograph of the left eye. A) Retro-illumination photography image showing inferiorly dislocated IOL out of the bag but no deviation of the lens capsule. B) Completely clear anterior capsular margin.
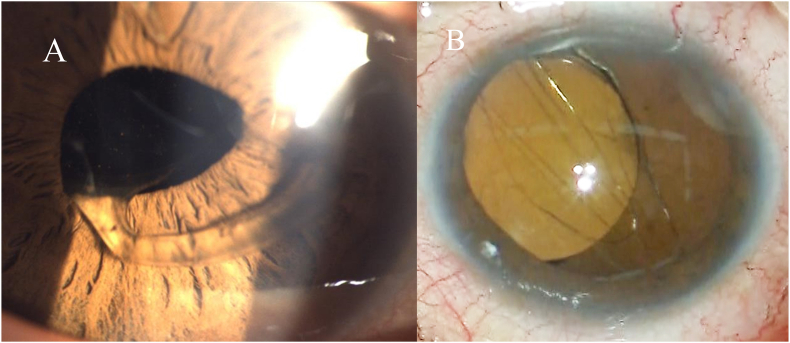
Six months after the second surgery, the patient returned to our hospital with sudden blurring of vision once more. He reported that he rubbed his eyes strongly again while sleeping. The best-corrected visual acuity of the left eye was 20/20, but slit-lamp examination of the left eye revealed that the in-the-bag IOL was partially dislocated into the anterior chamber due to zonular dialysis causing pupillary capture of a haptic (still within the capsular bag, Fig. 2A). The dislocated in-the-bag IOL was removed, and scleral fixation was performed. During removal of the in-the-bag IOL, the lens capsular bag was almost clear without Soemmerring ring formation (Fig. 2B). Visual acuity remained 20/20, and intraocular pressure (IOP) was 18 mmHg. The patient's visual disturbance was reduced. However, several days after the surgery, the patient complained of visual impairment. The IOP was 5 mmHg, and the best-corrected visual acuity of the left eye was 20/40. The decrease in visual acuity was thought to be due to temporal leakage of aqueous humor from the wound. On inquiring about details, the patient revealed a habit of sleeping in the prone position, with his face touching the pillow. The patient was advised to discontinue the prone position. Thereafter, his visual status became stable.
Fig. 2.
A) Slit-lamp photograph of the left eye showing deviation of in-the-bag IOL haptic onto the iris through the pupil and completely clear capsular bag covered IOL haptic. B) Intraoperative image showing temporary dislocation of in-the-bag IOL without Soemmerring's ring and anterior capsular fibrosis.
3. Discussion
In dead capsular syndrome, LECs in the capsular bags are either absent or rare.4 However, the disappearance of LECs after cataract surgery is rare. The etiology of dead capsular syndrome remains unknown. Some patients, reported as dead bag syndrome, originally had a white and intumescent cataract, which led to the speculation that the role of oncotic pressure within the capsular bag might be related to killing LECs.4 Moreno-Montañes et al. also described a similar case of a 37-year-old patient with a completely clear capsular bag 2 years after cataract surgery.9 The patient had a white cataract and a history of Acanthamoeba keratitis. The authors speculated that Acanthamoeba keratitis and its treatment may be related to the killing of LECs. However, the cataract in our case was not white, and there were no infections. We did not perform anything related to LECs survival, such as LEC removal10 or topical and/or intracameral injections of antibiotics, anesthetics, and so on, during the cataract surgery.7
Various factors influence the LEC's survival, including IOL design and material, inflammation, growth factors, and so on.11 In human12,13 and animal14 eyes, separating the anterior and posterior capsule allows aqueous humor to circulate within the capsule and prevents posterior capsule opacification. These factors may have influenced the disappearance of LECs in our case.
In dead bag syndrome, thinning and/or splitting of the lens capsule results in loss of the zonular fibers and IOL dislocation.4 In our case, when the IOL was repositioned within the capsular bag at the time of the first dislocation, an obvious weakness of the zonular fibers was not observed. IOL dislocations occurred after rubbing the eye strongly, suggesting that the dislocation in our case was due to mechanical force and not capsular degeneration.
In an experimental study, ocular trauma was reported to cause rotation of the acrylic IOL with no or insignificant leakage from the incision.15 On the other hand, in another study, the rotation of the IOL in the capsular bag during surgery decreased posterior capsular opacification, which may have been due to the decrease of LECs.16 It was difficult to accurately assess the extent of ocular trauma in our case because the patient rubbed his eye while sleeping. However, rubbing the eye strongly might lead to the IOL dislocation. Moreover, the patient might have sometimes rubbed the eye slightly and had a habit of sleeping in the prone position, with his face possibly touching the pillow. Although these habits do not cause serious damage to the eye, a slight mechanical force on the eye is exerted, which could have affected the stability of the IOL in the capsule bag. These habits may have been related to the early loss of LECs.
A nationwide cohort study in Korea examining the incidence and risk factors for total IOL dislocation found that the incidence of total IOL dislocation peaked at the age of 40–50 in males.17 This may be related to the fact that trauma is a known risk factor and appears to be more common in males. In 10 cases reported as dead bag syndrome,4 cataract surgery was performed at 53.9 years old, and 7 were men, suggesting that this syndrome tended to occur in younger males. This is similar to typical IOL dislocations. More cases are required to reveal the etiology of LEC disappearance after cataract surgery. However, slight trauma may be one of its causes and be related to dead bag syndrome many years later.
4. Conclusions
Herein, we present a case of LECs disappearance 12 months after cataract surgery. The patient had a habit of rubbing his eyes and sleeping in the prone position. Although further study is needed to elucidate the true cause of LEC disappearance, these minor traumas may have influenced the IOL stability in the capsular bag and may be related to early postoperative LEC disappearance.
Patient consent
The patient consented to publication of the case in writing.
Funding
No funding or grant support
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
CRediT authorship contribution statement
Daijiro Kurosaka: Writing – original draft, Supervision, Resources, Investigation, Conceptualization. Kouhei Hashizume: Writing – review & editing, Resources. Hiroyuki Oshima: Resources. Shigenori Miyoshi: Resources. Machi Itamochi: Investigation. Shota Kamei: Investigation.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
None.
References
- 1.Nibourg L.M., Gelens E., Kuijer R., Hooymans J.M., van Kooten T.G., Koopmans S.A. Prevention of posterior capsular opacification. Exp Eye Res. 2015;136:100–115. doi: 10.1016/j.exer.2015.03.011. [DOI] [PubMed] [Google Scholar]
- 2.Fișuș A.D., Findl O. Capsular fibrosis: a review of prevention methods and management. Eye (Lond) 2020;34(2):256–262. doi: 10.1038/s41433-019-0723-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kimura W., Yamanishi S., Kimura T., Sawada T., Ohte A. Measuring the anterior capsule opening after cataract surgery to assess capsule shrinkage. J Cataract Refract Surg. 1998;24(9):1235–1238. doi: 10.1016/s0886-3350(98)80018-7. [DOI] [PubMed] [Google Scholar]
- 4.Culp C., Qu P., Jones J., et al. Clinical and histopathological findings in the dead bag syndrome. J Cataract Refract Surg. 2022;48(2):177–184. doi: 10.1097/j.jcrs.0000000000000742. [DOI] [PubMed] [Google Scholar]
- 5.Danysh B.P., Duncan M.K. The lens capsule. Exp Eye Res. 2009;88(2):151–164. doi: 10.1016/j.exer.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Werner L. The dead bag syndrome. J Cataract Refract Surg. 2022;48(5):517–518. doi: 10.1097/j.jcrs.0000000000000930. [DOI] [PubMed] [Google Scholar]
- 7.Darian-Smith E., Safran S.G., Coroneo M.T. Zonular and capsular bag disorders: a hypothetical perspective based on recent pathophysiological insights. J Cataract Refract Surg. 2023;49(2):207–212. doi: 10.1097/j.jcrs.0000000000001098. [DOI] [PubMed] [Google Scholar]
- 8.Darian-Smith E., Safran S.G., Coroneo M.T. Comment on: clinical and histopathological findings in the dead bag syndrome. J Cataract Refract Surg. 2022;48(7):871–872. doi: 10.1097/j.jcrs.0000000000000960. [DOI] [PubMed] [Google Scholar]
- 9.Moreno-Montañés J., Barrio-Barrio J., De-Nova E., Werner L. Lens epithelial cell death secondary to acanthamoeba keratitis: absence of capsular bag opacification six years after cataract surgery. Case Rep Ophthalmol. 2011;2(3):354–359. doi: 10.1159/000334785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Darian-Smith E., Safran S.G., Coroneo M.T. Lens epithelial cell removal in routine phacoemulsification: is it worth the bother? Am J Ophthalmol. 2022;239:1–10. doi: 10.1016/j.ajo.2022.01.013. [DOI] [PubMed] [Google Scholar]
- 11.Konopińska J., Młynarczyk M., Dmuchowska D.A., Obuchowska I. Posterior capsule opacification: a review of experimental studies. J Clin Med. 2021;10(13) doi: 10.3390/jcm10132847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hara T., Hara T., Narita M., Hashimoto T., Motoyama Y., Hara T. Long-term study of posterior capsular opacification prevention with endocapsular equator rings in humans. Arch Ophthalmol. 2011;129(7):855–863. doi: 10.1001/archophthalmol.2011.38. [DOI] [PubMed] [Google Scholar]
- 13.Pallikaris I.G., Stojanovic N.R., Ginis H.S. A new endocapsular open ring for prevention of anterior and posterior capsule opacification. Clin Ophthalmol. 2016;10:2205–2212. doi: 10.2147/OPTH.S106770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nagamoto T., Tanaka N., Fujiwara T. Inhibition of posterior capsule opacification by a capsular adhesion-preventing ring. Arch Ophthalmol. 2009;127(4):471–474. doi: 10.1001/archophthalmol.2009.63. [DOI] [PubMed] [Google Scholar]
- 15.Pereira F.A., Milverton E.J., Coroneo M.T. Miyake-Apple study of the rotational stability of the Acrysof Toric intraocular lens after experimental eye trauma. Eye (Lond) 2010;24(2):376–378. doi: 10.1038/eye.2009.150. [DOI] [PubMed] [Google Scholar]
- 16.Joshi R.S., Chavan S.A. Rotation versus non-rotation of intraocular lens for prevention of posterior capsular opacification. Indian J Ophthalmol. 2019;67(9):1428–1432. doi: 10.4103/ijo.IJO_1854_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee G.I., Lim D.H., Chi S.A., Kim S.W., Shin D.W., Chung T.Y. Risk factors for intraocular lens dislocation after phacoemulsification: a nationwide population-based cohort study. Am J Ophthalmol. 2020;214:86–96. doi: 10.1016/j.ajo.2020.03.012. [DOI] [PubMed] [Google Scholar]