It has been clear for a number of years that small DNA tumor viruses such as simian virus 40 (SV40) and papillomavirus interact with cell cycle control pathways during lytic replication in a way that promotes entry into the S phase of the cell cycle. Since these viruses do not code for their own DNA polymerase or other accessory factors that support DNA replication, this strategy is a means of subverting the cell cycle control machinery to support viral DNA replication. In contrast to these viruses, herpesviruses contain a much greater genetic complexity that encodes a viral DNA polymerase, as well as accessory factors involved in generating nucleotide pools, etc. Indeed, herpesviruses have evolved a distinct viral replication strategy and, unlike SV40 and papillomavirus, herpesviruses do not require an S-phase environment to support viral replication. Further, numerous studies from several different herpesvirus systems have provided unifying evidence that these viruses encode factors that elicit a cell cycle block, thereby actively preventing entry into S phase. The conserved nature of this function across different members of the herpesvirus family suggests that it is an integral aspect of the herpesvirus replication strategy. As discussed here, regulation of the cell cycle during herpesvirus DNA replication has evolved as a complex series of interactions involving multiple viral factors, further implying an important role for this function in the life cycle of the virus.
The interaction between herpesviruses and the cell cycle regulatory machinery is even more interesting, however. While viral factors elicit cell cycle arrest signaling, some viral factors also activate certain cell cycle regulatory pathways that would normally promote cell cycle progression. These cell cycle-promoting functions also appear to be important since inhibition of these pathways inhibits viral replication. Therefore, it appears that although herpesviruses elicit cell cycle arrest, their complex interactions with the cell cycle regulatory machinery likely evolved to poise the cell in a precise cell cycle position which most favors viral replication.
Although a scattering of publications prior to 1995 provided hints into the interaction between herpesviruses and cell cycle control pathways, accumulating interest from several different laboratories over the past 5 or 6 years has begun to provide general underlying themes into this issue as well as elucidate some of the details of these interactions. These recent studies have been carried out in a largely independent fashion with alpha-, beta-, and gammaherpesvirus systems (primarily herpes simplex virus [HSV], cytomegalovirus [CMV], and Epstein-Barr virus [EBV]). The purpose of this review is to bridge the current understanding of virus-cell cycle interactions for these three herpesvirus lytic replication systems. To this end, I have provided a fairly detailed summary of the existing experimental data for each of these herpesvirus systems, which will hopefully serve as a tool to apprise investigators of related progress outside of their respective disciplines. At the end of this review, I have commented on some of the common strategies utilized by these viruses to achieve efficient cell synchronization during lytic replication.
HERPES SIMPLEX VIRUS
In 1988, de Bruyn and Knipe (15) provided evidence that infection with HSV leads to a block in cellular DNA synthesis. Approximately 10 years later, Dargan and Subak-Sharpe (14) showed that infection of cells with L particles (naturally produced HSV virions that do not contain any viral DNA) blocks cell growth without inducing apoptosis. Since the lack of cell accumulation occurred without any apparent apoptosis, this suggested the presence of a cell cycle inhibitory factor within the HSV virion. Further evidence that infection by HSV leads to inhibition of cell cycle progression came from molecular studies addressing alterations in a key set of cell cycle regulatory transcription factor complexes, the E2F family of complexes (33). That study found that HSV infection leads to the nuclear accumulation of the repressive form of E2F (which suppresses progression into S phase). More specifically, the study demonstrated an accumulation of E2F factors complexed with the retinoblastoma tumor suppressor protein, pRb, which inhibits E2F-mediated activation of S-phase-specific gene expression. In addition, the study found that the E2F family member, E2F4, is translocated into the nucleus following infection, where it is complexed with another member of the pRb family, p107. That work supported the idea that HSV infection elicits molecular changes in cell cycle regulatory circuits that are consistent with cells exiting the cell cycle and arresting in G1.
At around the same time, two other groups reported that the HSV-encoded immediate-early transcription factor, infected cell protein 0 (ICP0), can specifically elicit a cell cycle arrest (21, 28). (As mentioned above, L particles carry the capacity to block cell cycle progression [14]. Although ICP0 is an immediate-early protein, it has also been shown to be a virion component [47]. Therefore, the growth arrest activity identified in L particles by Dargan and Subak-Sharpe [14] may be attributable, at least in part, to ICP0.) Hobbs and DeLuca (21) showed that infection of serum-starved HEL cells with an HSV mutant that only expresses ICP0 blocks their ability to enter S phase following readdition of serum. Lomonte and Everett (28) showed that transfection of asynchronously growing HEp-2 cells with an ICP0 expression plasmid resulted in an enrichment of cells in G1. Both groups also showed that infection of cells synchronized in G2 caused an ICP0-dependent G2/M arrest, suggesting that ICP0 also has the capacity to activate a G2/M checkpoint. Microarray analysis following infection with the ICP0-expressing mutant virus revealed induction of the tumor suppressor protein, p53 (which is known to signal both G1 and G2 checkpoints), as well as several genes that are known downstream targets of p53 (p21, gadd45, and mdm2) (21). Unexpectedly, all three of the p53 target genes can also be induced by ICP0 in p53 −/− cells. This observation indicated that, although the ICP0-mediated induction of p53 may contribute to the induction of p21, gadd45, and mdm2, ICP0 also activates these genes through a p53-independent pathway. Therefore, ICP0 signals more than one checkpoint pathway (i.e., a p53-dependent pathway and one or more p53-independent pathways).
Despite their observation that ICP0 signals checkpoint functions leading to a cell cycle arrest, Lomonte and Everett (28) also showed that an ICP0-defective mutant virus still elicits growth arrest. This result indicated that there is another HSV-encoded factor(s) that also induces cellular growth arrest signaling. Consistent with this observation, another immediate-early HSV gene, ICP27, has also been implicated in HSV-mediated growth arrest (D. Knipe, personal communication). Therefore, HSV encodes at least two distinct proteins that can induce a cell cycle arrest.
Two other reports have more thoroughly addressed the initial findings by de Bruyn and Knipe (15), showing that infection with whole intact HSV leads to a cell cycle block. Ehmann et al. (17) and Song et al. (41) showed that HSV infection blocks progression of cells into S phase following release from serum starvation. In addition, these groups also showed that HSV infection actively arrests cycling cells (in G1). These studies also addressed changes in the key cell cycle-regulatory kinase complex family, cyclin-cyclin-dependent kinases (cyclin-cdks), following infection with HSV. The cyclin-cdk complexes, cyclinE-cdk2 and cyclinD-cdk4, are involved in phosphorylating pRb in late G1. Phosphorylated pRb then dissociates from E2F, thereby promoting E2F-dependent activation of S-phase-specific gene expression (and therefore, progression of cells into S phase). As such, these cyclin-cdk complexes are required for G1 to S phase transition. Song et al. (41) showed that infection of CV-1 cells leads to a loss of pRb phosphorylation. Further, this group showed that infection with HSV inhibits induction of cyclins D1 and D3 following readdition of serum in serum-starved cell cultures. This study also showed that HSV infection inhibits the formation of new electrophoretic forms of cdk2 and cdk4 which typically accompany restimulation of serum-starved cultures. The report by Ehmann et al. (17) showed similar effects on cyclins and cdks. Specifically this group reported that infection of quiescent human lung cells suppressed serum-induced cyclin D-cdk4 and cyclin D-cdk6 activity as well as cyclin E-cdk2 activity. These changes were also accompanied by a loss of cyclin E and a failure of cdk2 to translocate into the nucleus.
Together, the studies described above provide convincing evidence that HSV alters the cell cycle regulatory machinery in a way that promotes a block in G0 or G1 and that this likely occurs, in part, through the downregulation of cyclin-cdk complexes involved in the G1/S phase transition. Despite these observations, however, other groups have provided evidence that G1/S phase cyclins are required for the HSV lytic replication cycle. Early studies showed that a cellular factor(s) from cells in G1/S phase supported the viral replication cycle (9). Ralph et al. (35) then showed that immediate-early genes are specifically activated when cells are released from a serum starvation-induced growth arrest. A follow-up study showed that the cdk inhibitors, roscovitine and olomoucine, block immediate-early transcription and viral replication in asynchronously growing cells (24, 39). In a separate series of experiments, Kawaguchi et al. (27) showed that ICP0 interacts with, and stabilizes, cyclin D3. (Note that although ICP0 stabilizes cyclin D3, infection with wild-type virus suppresses the level of cyclin D3. This indicates that in addition to the stabilizing activity of ICP0, HSV also encodes a counteracting down-regulatory activity. Both activities may work together to modulate cyclin D3 levels to a critical homeostatic level.) Van Sant et al. (44) went on to show that a single amino acid mutation in ICP0 that abrogates ICP0's ability to interact with cyclin D3 impaired the ability of a corresponding mutant virus to replicate in serum-deprived/arrested cells but not in proliferating cells. This suggested that (i) cyclin D3 is required for viral DNA replication and (ii) although the level of cyclin D3 in proliferating cells is sufficient to support viral replication, ICP0 was required to induce cyclin D3 in G0-arrested cells.
In contrast to the studies by Ehmann et al. (17) and Song et al. (41) showing that HSV infection suppresses cyclin-cdk function, a study by Hossain et al (22) reported that infection of serum-starved CV-1 cells results in (i) no change in cdk4 activity, (ii) an induction of cyclin A (but not of cyclin E), and (iii) a transient induction of cdk2 activity. Although these results appear on the surface to be at odds with the results of Ehmann et al. (17) and Song et al. (41), there is an important difference in the experimental design of the experiments that may explain these seemingly discrepant results. While infection of quiescent cells in the Ehmann et al. (17) and Song et al. (41) studies was carried out in the presence of fresh serum, Hossain et al. (22) infected quiescent cells in the presence of spent media. As a result, the Ehmann and Song experiments specifically addressed the ability of HSV to suppress serum-stimulated induction of cyclin-cdk function while the Hossain study addressed whether HSV can activate cyclin-cdk function above the uninduced serum-starved background levels. Together these results suggest that HSV may only partially suppress the high-level cyclin-cdk activity that occurs during serum stimulation to levels that block the G1/S phase transition but not to basal serum starvation levels. Although HSV blocks cellular DNA replication, it may not simply “shut down” the cell cycle but, instead, may modulate cyclin-cdk function to specific levels that allow activation of the cell in a way that helps support efficient viral DNA replication.
CYTOMEGALOVIRUS
Like HSV, CMV has clearly been shown by several groups to block the G1/S phase transition in several different cell systems (8, 16, 29, 38). Moreover, two distinct CMV-encoded proteins have been shown to cause cell growth arrest, the tegument protein, UL69 (20, 30) (a homologue of the HSV ICP27 protein which has also been shown to cause growth arrest), and the immediate-early gene product, IE2 (45) (IE2 has also been shown to have cell growth-promoting activity in some systems [see below]). Nevertheless, on the surface, the CMV-related cell cycle literature appears even more conflicted than the HSV literature, because although CMV encodes these cell cycle arrest functions, it elicits a fairly robust series of molecular events that typically occur during activation of cell proliferation (at least in uninfected cells). In this section, I will first discuss the cell cycle-promoting activities associated with CMV. I will then address the growth arrest data in more detail and discuss how the interplay between these diametrically opposed activities may play out during CMV lytic replication.
In 1990 and 1991, studies by Albrecht's group (3, 4) showed that CMV infection activates expression of the cellular proto-oncogenes fos, jun, and myc. Other studies have demonstrated that a CMV-induced G1 cell cycle arrest coincides with an unexpected induction of cyclin E levels—with a corresponding induction of cyclin E-associated kinase activity (8, 23). Evidence that the induction of cyclin E-cdk2 activity is important for the viral replication cycle was reported by Bresnahan et al. (7), who showed that either roscovitine or a cdk2 dominant-negative inhibitor blocks viral DNA replication.
The observed induction of cyclin E-cdk2 activity is unexpected since cyclin E-cdk2 activity typically spikes during a narrow window corresponding to the G1/S transition and this is thought to play a regulatory role in transiting this checkpoint. Even more striking, however, is the observation that in cells arrested by CMV, there is also an accompanying induction of the G2/M-specific cyclin, cyclin B, along with a corresponding activation of cyclin B-dependent kinase activity (16, 23, 38). In addition to these events, two other studies have shown that the CMV-encoded immediate-early proteins IE1 and IE2 bind p107 (34) and pRb (19), respectively, and inactivate their ability to repress E2F-dependent transcription. (Interestingly, IE2 does not bind pRb when pRb is phosphorylated [19]. Therefore, the CMV-induced cyclin E-cdk2 activity likely inhibits the interaction between pRb and IE2. Moreover, IE2 has specifically been shown to induce the cyclin E promoter, which likely occurs through E2F elements [6], indicating that IE2 itself is involved in regulating cyclin E-cdk2 activity, which in turn regulates IE2's interaction with pRb. It appears then, that IE2 has tapped into an existing feed-forward mechanism to disrupt its functional interaction with pRb following the completion of certain signaling events [e.g. induction of cyclin E expression through activation of its E2F elements].)
Based on these studies, it is clear that although CMV blocks cell cycle progression, it has also integrated itself into certain aspects of cell cycle activation signaling. Moreover, it has been reported that in certain cell systems, CMV infection can specifically elicit cell cycle progression (1, 40). Two other groups (10, 32) have shown that introduction of either of the immediate-early CMV-encoded genes, IE1 or IE2, into serum-starved cells using an adenovirus transduction system can induce cell cycling. How can this data be reconciled with the preponderance of other studies showing that infection with CMV blocks cell cycle progression? The ability of individual CMV genes to elicit cell cycle progression may simply occur through their cell cycle-promoting activities in the absence of the restraining functions of other virally encoded growth inhibitory factors. On the other hand, the reports showing activation of cell proliferation by the whole virus require a more sophisticated explanation. It is possible that the conflicting nature of the different signaling events does not always lead to cell growth arrest but, instead, the outcome on cell proliferation may depend on the genetic background of the cell and/or the particular configuration of the cell cycle regulatory machinery in distinct cell systems. Such issues may help explain CMV's association with the cell proliferative disorder, coronary restinosis (42), despite its general ability to induce growth arrest in other tissues.
The ability of IE1 and IE2 to bind to p107 and pRb and activate E2F-mediated transcription is consistent with their capacity to promote the G1/S phase transition. In contrast to the results reported by Murphy et al. (32), however, Wiebusch and Hagemeier (45) have shown that expression of IE2 blocks cell cycle progression in G1 in a variety of cell lines. Other investigators have shown that IE1 (31) and IE2 (5, 31, 42) induce the expression of p53. Speir et al. (42) showed that IE2 interacts directly with p53 and that this interaction may play a role in stabilizing the protein. Unexpectedly, Speir et al. (42) showed that IE2 blocks p53's ability to activate transcription. Despite this, however, Bonin and McDougall (5) showed that p53's checkpoint function is not altered and that it retains the ability to induce p21 and mdm2. This indicates that although IE2 blocks p53's transactivation function, p53 retains the ability to activate a checkpoint through a novel mechanism. Together, these data show that not only does IE2 have a cell cycle-promoting function mediated through its interaction with pRb, but it also activates checkpoint signaling through its induction of p53. This may, at least in part, explain how IE2 can elicit opposite outcomes on cell cycle progression observed in different systems.
As mentioned above, in addition to IE2, the CMV-encoded viral capsid protein UL69 also induces a G1 arrest when introduced into proliferating cells (30). Further, UL69 was shown to be important in the context of the whole virus, since a viral mutant that does not express UL69 is impaired in its ability to induce growth arrest. Therefore, like HSV, CMV also encodes more than one factor that plays a role in fully achieving growth arrest during lytic replication. Although only one EBV-encoded gene product, the immediate-early transcription factor Zta, has thus far been shown to play a role in eliciting cell growth arrest (11), EBV also encodes a UL69/ICP27 homologue, BMLF1, and it will be important to determine whether this protein has a growth arrest function during EBV lytic replication. Nevertheless, previous studies have already shown that Zta itself elicits cell growth arrest signaling through the interaction with more than one distinct cell growth arrest pathway (36) (see below). It appears, then, that in the same way that small DNA tumor viruses interact with a multitude of cell cycle control points in their effort to elicit cell proliferation, a similar picture is emerging for the growth arrest functions of herpesviruses during lytic replication. Despite this, the accumulating data for CMV indicate that the virus may have also evolved with mechanisms to activate the cell. As such, the virus may block cellular DNA replication while at the same time activating certain cell cycle pathways that poise the cell in an environment that more effectively generates energy and resources to support high-level viral DNA replication.
EPSTEIN-BARR VIRUS
There are a couple of important distinctions between EBV and HSV or CMV that should be addressed prior to discussing the relationship between EBV lytic replication and the cell cycle. First, EBV-encoded genes that are expressed during the latency phase of the life cycle have potent cell cycle-promoting activity, and expression of this set of genes accounts for the ability of EBV to elicit growth transformation following infection of naive B lymphocytes. The association between EBV and a growing panel of human cancers is likewise attributable to the activities of one or more of these latency-associated genes. In contrast, lytic EBV gene expression is mutually exclusive of latency-associated gene expression and, to date, there is little evidence that lytic gene products play a role in EBV-associated proliferative disorders or cancers.
A second, more practical experimental distinction between EBV and HSV or CMV should also be pointed out. While full lytic replication can be attained by infecting certain cell types with CMV and HSV, this kind of efficient lytic replication system does not exist for EBV. Instead, the lytic EBV replication cycle is typically studied by exposing latently infected cells to agents that induce a switch from the latent to the lytic gene expression program. This approach is somewhat problematic for specifically analyzing the functional role of EBV-encoded genes in altering the cell cycle, since it is difficult to distinguish between effects elicited by the virus and those that are induced by the treatment itself. Nevertheless, treatment of latently infected cells with most lytic cycle-inducing agents causes a G0/G1 arrest (18, 25, 37). Although it has been difficult to attribute growth arrest functions to EBV-encoded genes through this approach, these studies have suggested that the converse is true, since treatment of latently infected cells with some of these agents induces growth arrest prior to detectable expression of immediate-early genes (18, 25, 37).
Some of the early evidence that EBV replicates in growth-arrested tissues came from in vivo observations that EBV replication occurs principally in the more differentiated layers of the oral epithelium (2, 46, 48). Again, in this system, it was difficult to determine whether the virus responds to growth arrest (and/or differentiation) signals or whether the virus specifically induces growth arrest. Nevertheless, other studies have shown that the promoter for the immediate-early gene product, Zta, is responsive to epithelial differentiation signaling (26), further supporting the notion that growth arrest signaling can induce the EBV lytic cycle.
The first demonstration that EBV gene expression can induce growth arrest (11) utilized the ability of the EBV immediate-early transactivator, Zta, to induce the EBV lytic replication cycle (13) in the absence of any other external stimuli. The introduction of a Zta expression vector into EBV-positive cells therefore provided a means of initiating the lytic cascade in the absence of other signaling events. Using this approach, it was found that transfection of a Zta expression vector into an EBV-positive epithelial cell system induced a G0/G1 cell cycle arrest (11). Further, this study showed that the Zta gene itself can induce growth arrest, since the introduction of a Zta expression vector into several different EBV-negative cell lines similarly blocked cell cycle progression.
Subsequent analyses of Zta-mediated growth arrest showed that Zta interacts at multiple distinct points in the cell cycle regulatory machinery (36). Like the CMV-encoded IE2 protein, Zta interacts with p53 and inhibits its transactivation function (50). Further, Zta stabilizes p53 (11; unpublished data), and despite inhibiting its transactivation function (36, 50), Zta does not block p53-mediated induction of p21 (36). Therefore, like the CMV IE2 protein, Zta induces a transactivation-independent p53 checkpoint function(s). Moreover, like IE2, Zta also signals p21 induction through a p53-independent mechanism (36). Zta also induces another cyclin-dependent kinase inhibitor, p27, and induction of this cdk inhibitor occurs in part through a pathway that is independent of p21 and p53 (11, 36). Therefore, Zta interacts with multiple distinct control points in the cell cycle regulatory machinery, each of which likely contributes to its cell growth arrest function.
Another EBV-encoded lytic transactivator, Rta, has been shown to interact with pRb (49). This interaction does not disrupt pRb's ability to interact with E2F in vitro (49), indicating that unlike the interaction between IE2 and pRb, the association of Rta with pRb may not promote activation of E2F function (19). Despite this observation, however, it was found that induction of the lytic cycle in the cell line Akata by anti-immunoglobulin (Ig) treatment leads to the dissociation of pRb-E2F1 complexes (49). Since Rta binds pRb in conjunction with E2F1 in vitro, the dissociation of pRb and E2F1 following anti-Ig treatment may occur through the action of another viral lytic protein or it may be the direct result of anti-Ig signaling. Another study showed that by using an adenovirus-Rta transduction system, Rta can induce the expression of E2F1 (43), supporting a possible cell cycle-activating function of Rta. This provides an initial suggestion that, like CMV and HSV, EBV may induce certain cell cycle activation pathways despite its ability to elicit a cell cycle arrest.
CONCLUSIONS
It is clear from the studies discussed above that HSV, CMV, and EBV have evolved to replicate primarily in growth-arrested cells. This property may have evolved initially as a means to simply relieve the virus from a requirement for an S-phase environment to support viral replication. The loss of an S-phase dependency may have then led to the evolution of mechanisms to actively regulate cell cycle restriction points to prevent competition with the cellular DNA replication machinery for nucleotide pools. This would likely have a significant impact on the efficiency of viral replication and may be the driving evolutionary force that forges this issue as a common theme among different members of the herpesvirus family.
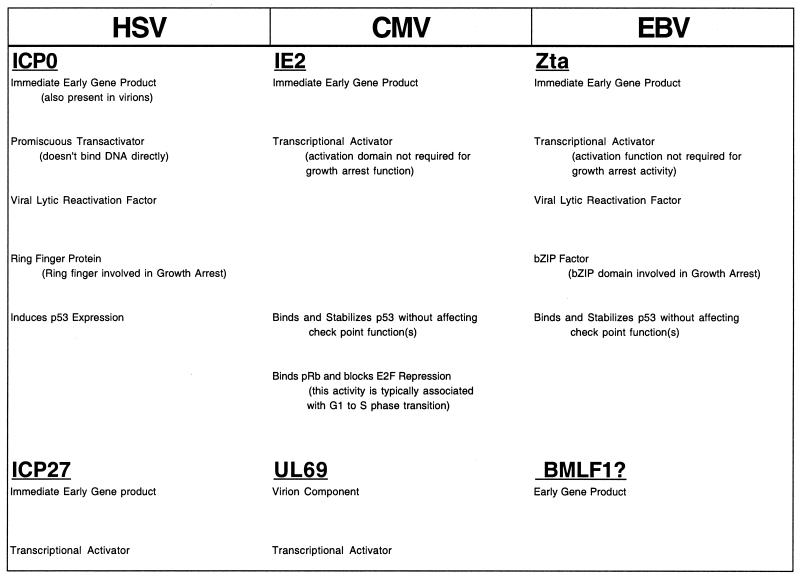
Mechanistically, there are several similarities in the manner through which growth arrest is effected by these three viruses. For example, the mechanisms utilized by all three viruses to elicit growth arrest is sophisticated in that they signal growth arrest through multiple cell cycle regulatory pathways. Further, CMV and HSV (and perhaps EBV) encode more than one gene that is involved in promoting cell growth arrest (Fig. 1). All three of these viruses have tapped into G0/G1 checkpoint functions, and they all encode immediate-early proteins that specifically induce the expression and function of p53 (Fig. 1). The CMV-encoded IE2 and the EBV-encoded Zta proteins have both been shown to bind and stabilize p53. Further, these interactions induce p53's checkpoint function despite inactivating its ability to activate transcription. The evolution of functional interactions between ICP0, IE2, and Zta and p53 may have occurred through a convergent mechanism, since there is no apparent homology between these three viral proteins. This implicates p53 as a particularly effective target in mediating growth arrest during the lytic replication phase of these viruses. ICP0 and Zta, however, have also been shown to activate p53-independent checkpoint functions, indicating that other cellular factors are also important for the induction of virus-mediated growth arrest.
FIG. 1.
Viral growth arrest factors. BMLF1 is not shown to have growth arrest activity, but it is homologous to UL69 and ICP27.
Although all of the viral proteins that have been implicated in eliciting cell growth arrest are transcription factors, the transactivation functions of Zta (12, 36) and IE2 (45) are not essential for activity (Fig. 1). Further, the RING finger domain of ICP0, which is a key motif in mediating protein-protein interactions, has been shown to be essential for eliciting growth arrest (28). This suggests that the transcriptional activation functions of these proteins may be less important for inducing cell growth arrest than direct interactions with key cell cycle control factors.
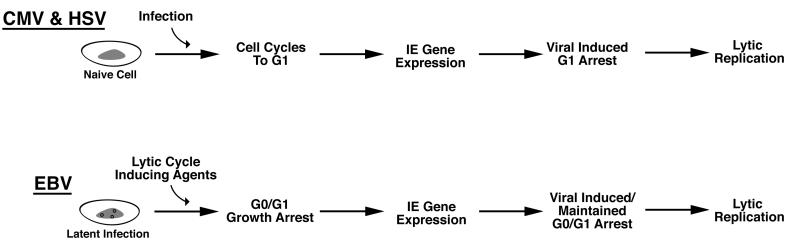
Synchrony in G1 appears to be an early step in the lytic cycle for each of these viruses (Fig. 2). All of the virally encoded growth arrest genes discussed here are either virion components (UL69 and ICP0) and/or immediate-early genes (IE2, ICP0, and Zta). The utilization of very early functioning viral factors to elicit growth arrest makes sense to help ensure that cells are arrested at the appropriate point of G0 or G1, prior to engaging in viral DNA replication. This would help ensure that cellular DNA replication is blocked prior to the onset of viral DNA replication to avoid competition for resources. Nevertheless, since synchronization can take a relatively long time due to the length of the cell cycle, herpesviruses appear to have also adapted an additional strategy that helps ensure that growth arrest occurs before the initiation of viral DNA replication. Specifically, immediate-early gene expression for each of these viruses has evolved to be responsive to G0/G1 signaling events (Table 1) so that these genes are expressed immediately prior to the G0 or G1 checkpoint at which the respective gene product functions. The promoter for the Zta gene has been shown to be responsive to G0 growth arrest signals (18, 25, 26, 37). Salvant et al. (38) have shown that immediate-early CMV gene expression does not occur until the infected cell has progressed to the G1 phase of the cell cycle. Last, Jordan et al. (24) have provided evidence that activation of HSV immediate-early genes by the virion protein VP16 cannot occur without G1 cyclin-dependent kinases. This linkage of virus-sensing mechanisms to the activation of viral cell cycle regulatory factors, then, elicits the appropriate cell cycle staging prior to the onset of viral DNA replication (Fig. 2), thereby increasing replication efficiency.
FIG. 2.
Models for temporal course of viral and cell cycle events during herpesvirus lytic replication.
TABLE 1.
Evidence for cell cycle regulation of immediate-early gene expression
| Virus | Evidence linking reactivation to the cell cycle |
|---|---|
| HSV | Roscovitine blocks immediate-early gene expression |
| CMV | Roscovitine and cdk2 dominant-negative mutant block DNA replication |
| Cells infected in S or G2/M progress to G1 before expressing immediate-early genes | |
| Autorepression of IE2 is relieved by pRb binding, which only occurs when pRb is hypophosphorylated | |
| EBV | Agents that activate immediate-early gene expression cause G0/G1 growth arrest |
In addition to activating cell checkpoint functions, there is evidence that each of these viruses simultaneously induces certain cell cycle activation signals. Although the role for this activity is unclear, there is mounting evidence that these interactions have evolved as integral aspects of lytic replication, since disruption of these signaling pathways inhibits progression of the viral replication cycle. It is likely that a key purpose behind blocking cellular DNA replication may be to prevent competition for nucleotide pools. On the other hand, an “activated” cell would likely be a better setting for viral replication than a cell that has exited the cell cycle (i.e., a G0 cell), since there would likely be more significant energy generation and greater production of other resources that may support viral replication. It appears, then, that these viruses have evolved mechanisms to block cellular DNA while at the same time activating pathways that either mimic entry into the cell cycle or elicit a partial cell cycle progression. As such, these viruses appear to have evolved highly sophisticated interactions with the cell cycle regulatory machinery in a way that supports efficient viral replication.
ACKNOWLEDGMENTS
I thank Gareth Inman, Martin Allday, Roger Everett, Thomas Shenk, Cathy Flemington, and David Knipe for helpful discussions and for sharing unpublished information.
This work was supported by National Institutes of Health grant GM48045.
REFERENCES
- 1.Albrecht T, Nachtigal M, St. Jeor S, Rapp F. Induction of cellular DNA synthesis and increased mitotic activity in Syrian hamster embryo cells abortively infected with human cytomegalovirus. J Gen Virol. 1976;30:167–177. doi: 10.1099/0022-1317-30-2-167. [DOI] [PubMed] [Google Scholar]
- 2.Becker J, Leser U, Marschall M, Langford A, Jilg W, Gelderblom H, Reichart P, Wolf H. Expression of proteins encoded by Epstein-Barr virus trans-activator genes depends on differentiation of epithelial cells in oral hairy leukoplakia. Proc Natl Acad Sci USA. 1991;88:8332–8336. doi: 10.1073/pnas.88.19.8332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boldogh I, AbuBakar S, Albrecht T. Activation of proto-oncogenes: an immediate early event in human cytomegalovirus infection. Science. 1990;247:561–564. doi: 10.1126/science.1689075. [DOI] [PubMed] [Google Scholar]
- 4.Boldogh I, AbuBakar S, Deng C, Albrecht T. Transcriptional activation of cellular oncogenes fos, jun, and myc by human cytomegalovirus. J Virol. 1991;65:1568–1571. doi: 10.1128/jvi.65.3.1568-1571.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonin L, McDougall J. Human cytomegalovirus IE2 86-kilodalton protein binds p53 but does not abrogate G1 checkpoint function. J Virol. 1997;71:5861–5870. doi: 10.1128/jvi.71.8.5861-5870.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bresnahan W, Albrecht T, Thompson E. The cyclin E promoter is activated by human cytomegalovirus 86-kDa immediate early protein. J Biol Chem. 1998;273:22075–22082. doi: 10.1074/jbc.273.34.22075. [DOI] [PubMed] [Google Scholar]
- 7.Bresnahan W, Boldogh I, Chi P, Thompson E, Albrecht T. Inhibition of cellular Cdk2 activity blocks human cytomegalovirus replication. Virology. 1997;231:239–247. doi: 10.1006/viro.1997.8489. [DOI] [PubMed] [Google Scholar]
- 8.Bresnahan W, Boldogh P, Thompson E, Albrecht T. Human cytomegalovirus inhibits cellular DNA synthesis and arrests productively infected cells in late G1. Virology. 1996;224:150–160. doi: 10.1006/viro.1996.0516. [DOI] [PubMed] [Google Scholar]
- 9.Cai W, Schaffer P. A cellular function can enhance gene expression and plating efficiency of a mutant defective in the gene for ICP0, a transactivating protein of herpes simplex virus type 1. J Virol. 1991;65:4078–4090. doi: 10.1128/jvi.65.8.4078-4090.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Castillo J, Yurochko A, Kowalik T. Role of human cytomegalovirus immediate-early proteins in cell growth control. J Virol. 2000;74:8028–8037. doi: 10.1128/jvi.74.17.8028-8037.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cayrol C, Flemington E. The Epstein-Barr virus bZIP transcription factor Zta causes G0/G1 cell cycle arrest through induction of cyclin-dependent kinase inhibitors. EMBO J. 1996;15:2748–2759. [PMC free article] [PubMed] [Google Scholar]
- 12.Cayrol C, Flemington E K. G0/G1 growth arrest mediated by a region encompassing the bZIP domain of the Epstein-Barr virus transactivator Zta. J Biol Chem. 1996;271:31799–31802. doi: 10.1074/jbc.271.50.31799. [DOI] [PubMed] [Google Scholar]
- 13.Countryman J, Jensen H, Seibel R, Wolf H, Miller G. Polymorphic proteins encoded within BZLF1 of defective and standard Epstein-Barr viruses disrupt latency. J Virol. 1987;61:3672–3679. doi: 10.1128/jvi.61.12.3672-3679.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dargan D, Subak-Sharpe J. The effects of herpes simplex virus type I L-particles on virus entry, replication, and the infectivity of naked herpesvirus DNA. Virology. 1997;239:378–388. doi: 10.1006/viro.1997.8893. [DOI] [PubMed] [Google Scholar]
- 15.de Bruyn K, Knipe D. Formation of DNA replication structures in herpes virus-infected cells requires a viral DNA binding protein. Cell. 1988;55:857–868. doi: 10.1016/0092-8674(88)90141-9. [DOI] [PubMed] [Google Scholar]
- 16.Dittmer D, Mocarski E. Human cytomegalovirus infection inhibits G1/S transition. J Virol. 1997;71:1629–1634. doi: 10.1128/jvi.71.2.1629-1634.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ehmann G, McLean T, Bachenheimer S. Herpes simplex virus type 1 infection imposes a G1/S block in asynchronously growing cells and prevents G1 entry in quiescent cells. Virology. 2000;267:335–349. doi: 10.1006/viro.1999.0147. [DOI] [PubMed] [Google Scholar]
- 18.Fukuda M, Satoh T, Takanashi M, Hirai K, Ohnishi E, Sairenji T. Inhibition of cell growth and Epstein-Barr virus reactivation by CD40 stimulation in Epstein-Barr virus-transformed B cells. Viral Immunol. 2000;13:215–229. doi: 10.1089/vim.2000.13.215. [DOI] [PubMed] [Google Scholar]
- 19.Hagemeier C, Caswell R, Hayhurst G, Sinclair J, Kouzarides T. Functional interaction between the HCMV IE2 transactivator and the retinoblastoma protein. EMBO J. 1994;13:2897–2903. doi: 10.1002/j.1460-2075.1994.tb06584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hayashi M, Blankenship C, Shenk T. Human cytomegalovirus UL69 protein is required for efficient accumulation of infected cells in the G1 phase of the cell cycle. Proc Natl Acad Sci USA. 2000;97:2692–2696. doi: 10.1073/pnas.050587597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hobbs W, DeLuca N. Perturbation of cell cycle progression and cellular gene expression as a function of herpes simplex virus ICP0. J Virol. 1999;73:8245–8255. doi: 10.1128/jvi.73.10.8245-8255.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hossain A, Holt T, Ciacci-Zanella J, Jones C. Analysis of cyclin-dependent kinase activity after herpes simplex virus type 2 infection. J Gen Virol. 1997;78:3341–3348. doi: 10.1099/0022-1317-78-12-3341. [DOI] [PubMed] [Google Scholar]
- 23.Jault F, Jault J, RRuchti F, Fortunato E, Clark C, Corbeil J, Richman D, Spector D. Cytomegalovirus infection induces high levels of cyclins, phosphorylated RB, and p53, leading to cell cycle arrest. J Virol. 1995;69:6697–6704. doi: 10.1128/jvi.69.11.6697-6704.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jordan R, Schang L, Schaffer P. Transactivation of herpes simplex virus type 1 immediate-early gene expression by virion-associated factors is blocked by an inhibitor of cyclin-dependent protein kinases. J Virol. 1999;73:8843–8847. doi: 10.1128/jvi.73.10.8843-8847.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kanamori M, Tajima M, Satoh Y, Hoshikawa Y, Miyazawa Y, Okinaga K, Kurata T, Sairenji T. Differential effects of TPA on cell growth and Epstein-Barr virus reactivation in epithelial cell lines derived from gastric tissues and B cell line Raji. Virus Genes. 2000;20:117–225. doi: 10.1023/a:1008110312661. [DOI] [PubMed] [Google Scholar]
- 26.Karimi L, Crawford D, Speck S, Nicholson L. Identification of an epithelial cell differentiation responsive region within the BZLF1 promoter of the Epstein-Barr virus. J Gen Virol. 1995;76:759–765. doi: 10.1099/0022-1317-76-4-759. [DOI] [PubMed] [Google Scholar]
- 27.Kawaguchi Y, Van Sant C, Roizman B. Herpes simplex virus 1 α regulatory protein ICP0 interacts with and stabilizes the cell cycle regulator cyclin D3. J Virol. 1997;71:7328–7336. doi: 10.1128/jvi.71.10.7328-7336.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lomonte P, Everett R. Herpes simplex virus type 1 immediate-early protein Vmw110 inhibits progression of cells through mitosis and from G1 into S phase of the cell cycle. J Virol. 1999;73:9456–9467. doi: 10.1128/jvi.73.11.9456-9467.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu M, Shenk T. Human cytomegalovirus infection inhibits cell cycle progression at multiple points, including the transition from G1 to S. J Virol. 1996;70:8850–8857. doi: 10.1128/jvi.70.12.8850-8857.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu M, Shenk T. Human cytomegalovirus UL69 protein induces cells to accumulate in G1 phase of the cell cycle. J Virol. 1999;73:676–683. doi: 10.1128/jvi.73.1.676-683.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muganda P, Mendoza O, Hernandez J, Qian Q. Human cytomegalovirus elevates levels of the cellular protein p53 in infected fibroblasts. J Virol. 1994;68:8028–8034. doi: 10.1128/jvi.68.12.8028-8034.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murphy E, Streblow D, Nelson J, Stinski M. The human cytomegalovirus IE86 protein can block cell cycle progression after inducing transition into the S phase of permissive cells. J Virol. 2000;74:7108–7118. doi: 10.1128/jvi.74.15.7108-7118.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Olgiate J, Ehmann G, Vidyarthi S, Hilton M, Bachenheimer S. Herpes simplex virus induces intracellular redistribution of E2F4 and accumulation of E2F pocket protein complexes. Virology. 1999;258:257–270. doi: 10.1006/viro.1999.9755. [DOI] [PubMed] [Google Scholar]
- 34.Poma E, Kowalik T, Zhu L, Sinclair J, Huang E. The human cytomegalovirus IE1–72 protein interacts with the cellular p107 protein and relieves p107-mediated transcriptional repression of an E2F-responsive promoter. J Virol. 1996;70:7867–7877. doi: 10.1128/jvi.70.11.7867-7877.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ralph W, Jr, Cabatingan M, Schaffer P. Induction of herpes simplex virus type 1 immediate-early gene expression by a cellular activity expressed in Vero and NB41A3 cells after growth arrest release. J Virol. 1994;68:6871–6882. doi: 10.1128/jvi.68.11.6871-6882.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rodriguez A, Armstrong M, Dwyer D, Flemington E. Genetic dissection of cell growth arrest functions mediated by the Epstein-Barr virus lytic gene product, Zta. J Virol. 1999;73:9029–9038. doi: 10.1128/jvi.73.11.9029-9038.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rodriguez A, Jung E J, Flemington E K. Cell cycle analysis of Epstein-Barr virus-infected cells following treatment with lytic cycle-inducing agents. J Virol. 2001;75:4482–4489. doi: 10.1128/JVI.75.10.4482-4489.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salvant B S, Fortunato E A, Spector D H. Cell cycle dysregulation by human cytomegalovirus: influence of the cell cycle phase at the time of infection and effects on cyclin transcription. J Virol. 1998;72:3729–3741. doi: 10.1128/jvi.72.5.3729-3741.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schang L, Phillips J, Schaffer P. Requirement for cellular cyclin-dependent kinases in herpes simplex virus replication and transcription. J Virol. 1998;72:5626–5637. doi: 10.1128/jvi.72.7.5626-5637.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sinclair J, Baillie J, Bryant L, Caswell R. Human cytomegalovirus mediates cell cycle progression through G1 into early S phase in terminally differentiated cells. J Gen Virol. 2000;81:1553–1563. doi: 10.1099/0022-1317-81-6-1553. [DOI] [PubMed] [Google Scholar]
- 41.Song B, Liu J, Yeh K, Knipe D. Herpes simplex virus infection blocks events in G1 phase of the cell cycle. Virology. 2000;267:326–334. doi: 10.1006/viro.1999.0146. [DOI] [PubMed] [Google Scholar]
- 42.Speir E, Modali R, Huang E, Leon M, Shawl F, Finkel T, Epstein S. Potential role of human cytomegalovirus and p53 interaction in coronary restenosis. Science. 1994;265:391–394. doi: 10.1126/science.8023160. [DOI] [PubMed] [Google Scholar]
- 43.Swenson J, Mauser A, Kaufmann W, Kenney S. The Epstein-Barr virus protein BRLF1 activates S phase entry through E2F1 induction. J Virol. 1999;73:6540–6550. doi: 10.1128/jvi.73.8.6540-6550.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Van Sant C, Kawaguchi Y, Roizman B. A single amino acid substitution in the cyclin D binding domain of the infected cell protein no. 0 abrogates the neuroinvasiveness of herpes simplex virus without affecting its ability to replicate. Proc Natl Acad Sci USA. 1999;96:8184–8189. doi: 10.1073/pnas.96.14.8184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wiebusch L, Hagemeier C. Human cytomegalovirus 86-kilodalton IE2 protein blocks cell cycle progression in G1. J Virol. 1999;73:9274–9283. doi: 10.1128/jvi.73.11.9274-9283.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wolf H, Haus M, Wilmes E. Persistence of Epstein-Barr virus in the parotid gland. J Virol. 1984;51:795–798. doi: 10.1128/jvi.51.3.795-798.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yao F, Courtney R. Association of ICP0 but not ICP27 with purified virions of herpes simplex virus type 1. J Virol. 1992;66:2709–2716. doi: 10.1128/jvi.66.5.2709-2716.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Young L S, Lau R, Rowe M, Niedobotek G, Packham G, Shanahan F, Rowe D T, Greenspan D, Greenspan J S, Rickinson A B, Farrell P J. Differentiation-associated expression of the Epstein-Barr virus BZLF1 transactivator protein in oral hairy leukoplakia. J Virol. 1991;65:2868–2874. doi: 10.1128/jvi.65.6.2868-2874.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zacny V, Wilson J, Pagano J. The Epstein-Barr virus immediate-early gene product, BRLF1, interacts with the retinoblastoma protein during the viral lytic cycle. J Virol. 1998;72:8043–8051. doi: 10.1128/jvi.72.10.8043-8051.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang Z, Gutsch D, Kenney S. Functional and physical interaction between p53 and BZLF1: implications for Epstein-Barr virus latency. J Virol. 1994;14:1929–1938. doi: 10.1128/mcb.14.3.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]