Abstract
Background
Biliary tract cancer (BTC) is one of the most aggressive malignancies and surgery represents the only curative treatment approach. However, even in patients with complete tumor resection 5-year survival rates are below 30%. So far, prognostic markers to assess the outcome of these patients are lacking. We therefore evaluated bone mineral density (BMD) as a prognostic tool in patients receiving surgery for BTC.
methods
76 BTC patients undergoing tumor resection in our clinic (Duesseldorf cohort) as well as an external validation cohort of 34 BTC patients (Cologne cohort) were included. BMD was analyzed at the first lumbar vertebra, using routine CT scans which has been proven comparable to DXA.
Results
Median overall survival (OS) of the Duesseldorf cohort after surgery was 527 days, one- and five-year survival probabilities were 62 and 18%. Patients with BMD above 156.5 HU had significantly improved OS (1435 days vs. 459 days; p = 0.002). The prognostic value for BMD was confirmed using Cox-regression analysis, as well as an external validation cohort. In subgroup analysis the prognostic effect of BMD was only present in female patients, suggesting sex specific differences.
Conclusion
BMD is a valuable, easily accessible and independent prognostic marker in patients receiving liver surgery for BTC.
Introduction
The assessment of the patients´ body composition for prognostic and predictive purposes has become increasingly important in medicine [1]. Measuring body composition allows the estimation of body tissues, organs and their distribution in living individuals. The role of changes in body composition has been analyzed in manifold malignancies and sarcopenia, the most common body composition abnormality, has been identified as a prognostic and predictive factor in several types of cancer, including gastrointestinal tumors [2, 3].
Cholangiocellular carcinoma (CCA) is the second most common liver tumor with an increasing incidence over the last years. In combination with gallbladder cancer it can be referred to as biliary tract cancer (BTC). Treatment options for all entities remain limited and tumor resection is the only potentially curative therapy [4]. However, many patients already have advanced disease at the time of diagnosis and the decision whether a patient will benefit from extensive surgery is often difficult to make in routine practice. To date, few objective markers exist beyond the clinical judgment of the treating physician to stratify patients according to their presumed clinical course after surgery. We previously established two markers for sarcopenia, L3SMI and L3PMI, as prognostic markers for survival in BTC patients [5, 6]. Bone mineral density (BMD) represents another surrogate for the patients´ body composition and was suggested as a potential marker for estimating the patients´ prognosis in many diseases [7]. In clinical routine, the BMD is assessed using dual-energy X-ray absorptiometry (DXA). However, DXA is not used for staging examinations in BTC and therefore not available for clinical decision making in most patients. Nevertheless, it was recently demonstrated that BMD can be analyzed by routine CT imaging, which is available in almost all cancer patients [8, 9]. In the present study we examined the role of BMD, determined from pretherapeutic CT scans performed as part of clinical staging, as a potentially novel tool to estimate whether a patient may benefit from extended surgery for BTC.
Material and methods
Selection of study patients
In total, 113 patients who underwent surgery for BTC at the Department of General, Visceral and Pediatric Surgery at the University Hospital Düsseldorf between 2011 and 2021 were screened for the study (Duesseldorf cohort). Detailed patient characteristics have recently been published and are further shown in Table 1 [5]. In total, 37 patients were excluded from the analysis due to different reasons like inoperability or poor CT scan quality (Fig. 1). For validation of our findings, we used a cohort of 34 patients treated at the University Hospital of Cologne (Cologne cohort, supplementary Table 1). The study was approved by the ethics committee of the medical faculty, Heinrich Heine university Düsseldorf (2021-1334_1).
Table 1.
Study cohort Düsseldorf.
| Parameter | Study cohort |
|---|---|
| BTC patients | n = 76 |
| sex (%) | |
| male | 48.7 (37) |
| female | 51.3 (39) |
| Age (years, median and range) | 68.5 (41–89) |
| BMI (kg/m2, median and range) | 26.08 (15.96–44.26) |
| Tumor localization (%) | |
| iCCA | 68.4 (52) |
| Klatskin | 11.8 (9) |
| dCCA | 3.9 (3) |
| gallbladder | 15.8 (12) |
| Staging (%) | |
| UICC I | 26.3 (20) |
| UICC II | 28.9 (22) |
| UICC III | 23.7 (18) |
| UICC IV | 18.4 (14) |
| ECOG PS (n, %) | |
| 0 | 59 (77.6) |
| 1 | 9 (11.8) |
| 2 | 4 (5.3) |
| unknown | 4 (5.3) |
| Chemotherapy (n, %) | |
| neoadjuvant | 4 (5.3) |
| adjuvant | 21 (27.6) |
| Surgical procedure (n, %) | |
| Hemihepatectomy | 14 (18.42) |
| Extended hemihepatectomy | 9 (11.84) |
| Segment resection | 35 (46.05) |
| Whipple / PPPD | 4 (5.26) |
| CHE | 1 (1.32) |
| Whipple + segment resection | 3 (3.95) |
| Hemihepatectomy + segment resection | 2 (2.63) |
| Bile duct resection | 1 (1.32) |
| Other | 7 (9.21) |
| Overall survival (days, median and range) | 527 (2–3087) |
| Recurrence free survival at 12 months (%) | 34.2% (26) |
| Bone mineral density L1 (HU, median and range) | 130 (51.65–253) |
BTC biliary tract cancer, iCCA intrahepatic cholangiocellular adenocarcinoma, dCCA distal cholangiocellular adenocarcinoma, UICC Union for International Cancer Control, ECOG PS Eastern Cooperative Oncology Group Performance Status.
Fig. 1. Patient selection of the Duesseldorf cohort.
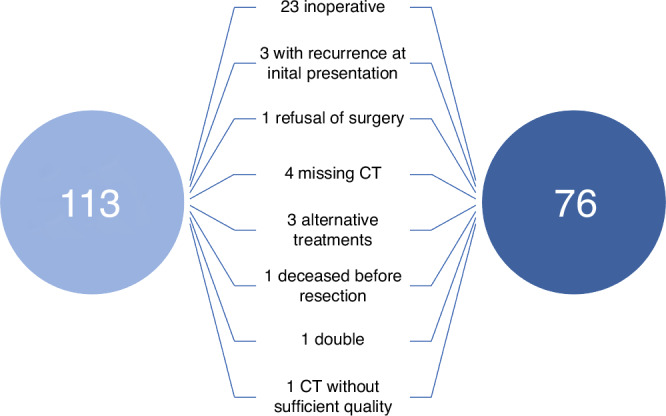
37 patients had to be excluded from the initial cohort due to reasons indicated.
Analysis of bone mineral density
The local PACS (IntelliSpace PACS, Philips, Amsterdam, The Netherlands) was used for BMD analysis of routine CT scans obtained during preoperative tumor staging. The trabecular BMD of the first lumbar vertebra was measured in Hounsfield units (HU) within a manually placed region of interest (ROI), as described recently (Supplementary Fig. 1) [10]. Variations due to the venous plexus in the posterior part or to inhomogeneous trabecular structure in the middle of the vertebral body were excluded by placing the ROI in the anterior part of the upper third of the vertebral body.
Statistical analysis
Statistical analysis was performed using SPSS 27 (SPSS, Chicago, IL, USA) as described in detail before, unless otherwise stated [11]. Correlation analysis was performed using Spearman correlation. Shapiro-Wilk test was used to assess normal distribution. Non-parametric data was compared by Mann–Whitney-U- or Kruskal-Wallis-Test. Box plots indicate medians, quartiles and ranges. The impact of different parameters on overall survival was investigated by Kaplan-Meier curve analysis. Log-rank test was used to indicate statistical differences between various groups. As described before, we used the optimal cut-off finder to assess the optimal cut-off values for bone density using method “significance of correlation with survival variable” that fits Cox proportional hazard model to the dichotomized variable and the survival variable [12]. For the analysis of the progostic value of different variables regarding overall survival we used univariate and multivariate Cox-regression analysis. 95% confidence interval (CI 95%) and hazard ratio (HR) are displayed. P values less than 0.05 were considered statistically significant. We used the Kaplan Meier method to estimate the one-year and 5-year survival probability using the survival package in R (v.4.2.2).
Results
Baseline characteristics of patients
A total of n = 76 patients receiving liver surgery for BTC between 2011 and 2021 were included into the training cohort (Duesseldorf cohort), as recently described [5]. We observed 51 events (deaths) among 76 patients. The median age was 68.5 years; 51.3% of patients were female. Intrahepatic cholangiocarcinoma (iCCA) was the most common localization of BTC (68.4%), while 11.8% of patients presented with Klatskin tumors, 15.8% of patients with gallbladder carcinoma and 3.9% with distal CCA (dCCA). Most patients were in good performance status (ECOG 0, 77.6%). 5.3% of patients had received neoadjuvant chemotherapy, 27.6% adjuvant chemotherapy. Median overall survival (OS) was 527 days, with a recurrence-free survival at 12 months of 34.2%. The one- and five-year survival probabilities were 62% (95% CI: 52–75%) and 18% (95% CI: 10–33%). For the external validation cohort, 34 patients were enrolled at the University Hospital Cologne (Cologne cohort). Table 1 and supplementary table 1 provide a detailed summary of clinical patient characteristics.
Bone mineral density is dependent on age but independent of sex or tumor stage
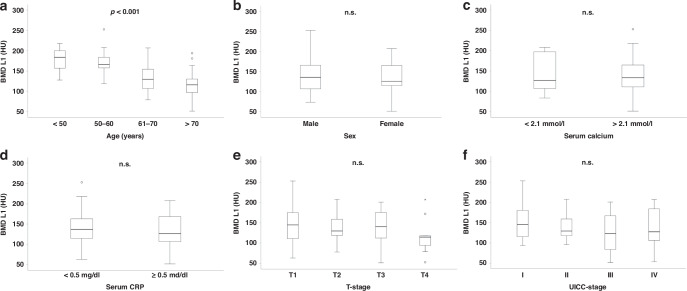
BMD was determined from preoperative CT scans within the trabecular space of the first lumbar vertebra. As expected, in the Duesseldorf cohort BMD was significantly reduced in older patients compared to younger ones (p < 0.001; Fig. 2a), which was also confirmed by Spearman correlation analysis (−0.571; p < 0.001). In contrast, neither the patients´ sex, nor serum calcium concentration or CRP had an influence on the individual BMD value (Fig. 2b–d). Similarly, the BMD was independent of the patients´ tumor stage (Fig. 2e, f), highlighting that BMD represents a stable parameter in patients with biliary tract cancer.
Fig. 2. BMD dependance on various clinical or laboratory parameters.
BMD was significantly decreased in older patients (a), but sex, serum calcium concentration, serum CRP, T- or UICC-stage had no influence on BMD (b–f). Box plots show medians, quartiles, and ranges; p ≤ 0.05 was considered statistically significant. Circles represent outliers, stars represent far outliers.
BMD is a prognostic marker for overall survival in patients undergoing surgery for BTC
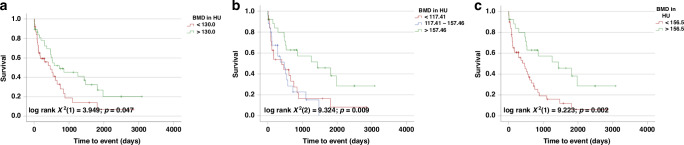
Based on previous studies suggesting a prognostic function of BMD in patients receiving systemic treatments for BTC, we evaluated the prognostic value of BMD in patients receiving curative intended liver surgery for BTC in the Duesseldorf cohort [13]. As there is no established cut-off value for BMD in CCA patients, we subdivided our cohort into two groups with BMD levels above or below the median (130 HU). Of note, in Kaplan-Meier curve analyses, patients with BMD above 130 HU had a significantly longer OS compared to patients with a BMD below this threshold (724 (95% CI: 0–1462) vs. 459 (95% CI: 119–799) days; p = 0.047; Fig. 3a). As the median level of BMD might not represent the ideal cut-off value for the differentiation between survivors and non-survivors, we next used the 33rd and 66th percentile as cut-off. Strikingly, patients with BMD above 157.46 HU—representing the 66th percentile–had a significantly improved survival compared to patients below that value (>66th: 1435 days (95% CI: 342–2528) vs. 33rd-66th: 473 days (95% CI: 193–753) vs. <33rd: 403 days (95% CI: 0–828); log rank X2(2) = 9.324; p = 0.009; Fig. 3b). Furthermore, patients under the 66th percentile or under the 33rd percentile had a comparably worse survival, indicating that the value of BMD for OS might not be linear and only relevant above a certain level (Fig. 3b). Finally, we used the optimal cut-off finder to establish an ideal cut-off value for BMD discriminating between survivors and non-survivors in our cohort [12]. The use of this optimal cut-off value (156.5 HU) – which turned out as almost identical to the value for the 66th percentile—further increased the prognostic potential of BMD (1435 days (95% CI: 342–2528) vs. 459 days (95% CI: 212–706); log rank X2(1) = 9.223; p = 0.002; Fig. 3c).
Fig. 3. Prediction of survival after resection of BTC using BMD.
Patients with BMD at level L1 above the median of 130 HU (a), the 66th percentile (157.46 HU) (b) or the optimal cut-off (156.5 HU) (c) had significantly improved OS.
To further substantiate the prognostic power of BMD, we next performed univariate Cox-regression analyses of routinely measured tumor markers for hepatobiliary malignancies (CEA, AFP, Ca19-9) and standard laboratory markers (hemoglobin, leukocyte count, thrombocyte count, sodium, potassium, calcium, AST, bilirubin, GGT, AP, CRP, INR and aPTT), as well as routine clinical and pathological parameters (age, gender, height, weight, BMI). In this analysis, serum levels of calcium, AST, age and BMD were predictive factors for long-term survival (Table 2). Subsequently, we included parameters with a significant result in the univariate analysis into a multivariate Cox-regression analysis, adding CEA and CA19-9 as relevant tumor markers for BTC. Strikingly, this multivariate analysis revealed BMD as the only independent marker predicting long-term survival after BTC tumor resection (HR 0.983 (95% CI: 0.967–0.999); p = 0.038; Table 2), supporting its use as a prognostic marker in patients receiving surgery for BTC.
Table 2.
Univariate and multivariate Cox-regression analysis for the prediction of postoperative overall survival.
| Parameter | Univariate cox regression | Multivariate cox regression | ||
|---|---|---|---|---|
| p value | Hazard ratio (95% CI) | p value | Hazard ratio (95% CI) | |
| Sex | 0.266 | 0.730 (0.419–1.271) | ||
| Age | 0.027 | 1.034 (1.004–1.064) | 0.320 | 0.968 (0.907–1.032) |
| Height cm | 0.855 | 0.997 (0.971–1.025) | ||
| Weight kg | 0.213 | 0.990 (0.976–1.006) | ||
| BMI | 0.208 | 0.963 (0.909–1.021) | ||
| Sodium | 0.684 | 0.986 (0.922–1.054) | ||
| Potassium | 0.149 | 0.647 (0.358–1.169) | ||
| Creatinin | 0.407 | 0.787 (0.446–1.388) | ||
| GFR ml/min | 0.484 | 0.996 (0.985–1.007) | ||
| Urea | 0.710 | 1.004 (0.981–1.028) | ||
| Uric acid | 0.920 | 1.002 (0.960–1.046) | ||
| Bilirubin | 0.191 | 1.116 (0.947–1.316) | ||
| γGT | 0.297 | 1.000 (1.000–1.001) | ||
| AP | 0.195 | 1.001 (1.000–1.002) | ||
| Albumin | 0.569 | 0.803 (0.377–1.710) | ||
| CRP | 0.001 | 1.112 (1.043–1.186) | 0.155 | 1.101 (0.964–1.257) |
| TSH | 0.885 | 0.986 (0.811–1.198) | ||
| Calcium | 0.005 | 0.162 (0.046–0.572) | 0.827 | 0.758 (0.064–9.029) |
| CEA | 0.112 | 1.014 (0.997–1.031) | 0.124 | 1.015 (0.996–1.034) |
| AFP | 0.217 | 1.017 (0.990–1.044) | ||
| CA19-9 | 0.069 | 1.000 (1.000–1.000) | 0.722 | 1.000 (1.000–1.000) |
| INR | 0.230 | 3.833 (0.427–34.423) | ||
| aPTT | 0.160 | 1.032 (0.988–1.078) | ||
| Bone mineral density L1 | 0.031 | 0.992 (0.985–0.999) | 0.038 | 0.983 (0.967–0.999) |
BMI body-mass-index, GFR glomerular filtration rate, AST aspartate-aminotransferase, γGT γ-glutamyltransferase; AP alkaline phosphatase, CRP C-reactive protein, TSH thyroid-stimulating hormone, CEA carcinoembryonic antigen, AFP α-fetoprotein, CA19-9 carbohydrate antigen 19-9, INR International normalized ratio, aPTT activated partial thromboplastin time.
Validation of the prognostic value of BMD in an external cohort
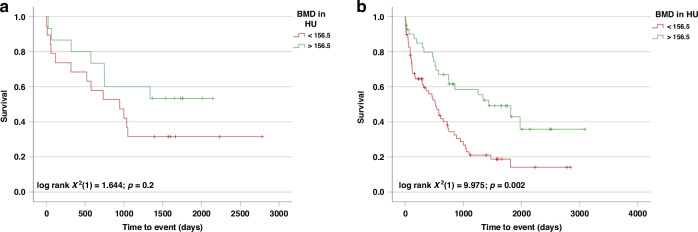
For external validation, a cohort of 34 (20/34 events = documented deaths) patients receiving curative intended liver surgery for BTC at the University Hospital Cologne was analyzed. Statistical significance was closely missed. This could be related to the small sample size of our validation cohort, but also other factors could impact this finding. Yet, patients with BMD values above the previously identified optimal cut off of 156.5 HU demonstrated a longer survival than those with lower BMD values (log rank X2(1) = 1.644; p = 0.2; Fig. 4a). To increase patient numbers, we combined both cohorts into a total cohort (combined cohort). In this analysis, patients with BMD values above 156.5 HU had a significantly improved OS (1435 days (95% CI: 699–2171) vs. 520 days (95% CI: 345–695); log rank X2(1) = 9.975, p = 0.002; Fig. 4b), confirming our initial finding that BMD is relevant for estimating the prognosis of patients receiving surgery for BTC.
Fig. 4. BMD as a prognostic marker in the validation (Cologne) and combined cohort.
Using the established optimal cut-off value for BMD from our Duesseldorf cohort (156.5 HU), a strong trend towards improved survival was found in the external validation cohort (Cologne cohort) (a). Combining both cohorts (combined cohort), BMD above 156.5 HU indicated statistically significant improved OS after resection of BTC (b).
BMD of the first lumbar vertebra is a sex specific predictor for overall survival in patients undergoing surgery for BTC
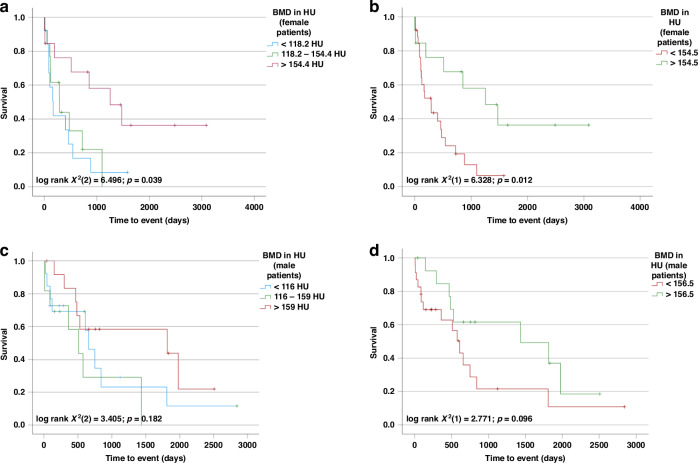
We recently demonstrated that the BMD is a predictor of mortality in female (but not in male) patients with BTC undergoing systemic chemotherapy, suggesting that the prognostic role of markers of body composition might be sex specific [13]. To test whether this observation might also hold true in patients receiving surgery, we performed Kaplan–Meier analysis separately in male and female patients of the Duesseldorf cohort. Using sex-specific tertiles only female (but not male) patients with BMD above the 66th percentile had a longer OS compared to patients with a BMD below this threshold (female: 1254 days (95% CI: 417–2091) vs. 290 days (95% CI: 26–554) vs. 172 days (95% CI: 62–282); log-rank X2(2) = 6.492; p = 0.039; male: 1814 days (95% CI: 0–4674) vs. 511 days (95% CI: 148–874) vs. 657 days (95% CI: 468–846); log-rank X2(2) = 3.405; p = 0.182; Fig. 5a, c). Interestingly, again a certain BMD threshold seems to be relevant for having an impact on OS as in female patients both curves of patients under the 33rd percentile but also between 33rd and 66th percentile seem to run in parallel. To further investigate potential differences between males and females, we used the optimal cut-off finder and determined sex-specific optimal cut-off values for BMD at L1. Within females a BMD value of 154.5 HU which is nearly identical with the sex-specific 66th percentile (154.4 HU), best discriminated between survivors and non survivors (1254 days (95% CI: 417–2091) days vs. 289 days (95% CI: 92–486); log-rank X2(1) = 6.328; p = 0.012; Fig. 5b). In contrast, male patients with BMD values above or below 156.5 HU (proposed by the cut-off finder as optimal value) displayed similar survival times (1435 days (95% CI: 0–3015) days vs. 608 days (95%CI: 438–778); log-rank X2(1) = 2.771; p = 0.096; Fig. 5d). Next, we performed univariate Cox regression analysis separately in male and female patients. We confirmed the prognostic value of BMD in L1 for female patients but could not detect an impact in male patients (female: HR 0.989 (95% CI: 0.979–1.000); p = 0.046; male: HR 0.996 (95% CI: 0.987–1.005); p = 0.391) (Supplementary Table 2).
Fig. 5. BMD is a prognostic marker in female, but not male patients after resection of BTC.
Using the sex-specific tertiles (33rd and 66th percentile) in the Duesseldorf cohort, only female patients with high BMD had improved OS (a, c). Even after establishing sex-specific optimal cut-off values (female: 154.5 HU; male: 156.5 HU), only female patients with BMD above the cut off had improved OS (b), while in male patients no statistical significance was observed (d).
Discussion
BTC is a rare but aggressive type of cancer. Despite various multimodal treatment options, including chemotherapy and surgery, survival rates of patients with BTC have not substantially improved in recent years [4]. With 5-year survival rates of only 7–20%, BTC is one of the most aggressive and treatment-resistant cancers, having the second-worst prognosis after pancreatic cancer. There is urgent need for improved clinical management of these patients, e.g. by identifying reliable and easily available markers for treatment selection [4]. Dual X-ray absorptiometry measurement (DXA) is the most reliable method for measuring BMD and is useful for assessing fracture risk and monitoring therapy for osteopenia [14–16]. However, it is not necessary for staging examinations in tumor patients and therefore not routinely performed. In contrast, CT scans are routinely performed before surgery in tumor patients. Since in most cases they can also be used to quantify BMD by determining radiodensity, the broad availability of CT scans eliminates the need for additional DXA for evaluating the patients´ body composition. Of note, recent studies have confirmed the accuracy of routine CT scans in assessing bone mineral density [8, 9], leading us to use this technique for our study. Surgical therapy cannot only alleviate symptoms, but also lead to long-term survival [17]. According to international guidelines, tumor resection is always recommended when complete resection (R0) is considered possible [17]. Yet just ~25% of BTC patients are deemed resectable at time of diagnosis [4]. In these patients, 5-year survival rates of 25–40% are achieved, depending on the stage of disease and patient selection [18]. The challenging decision of whether to perform surgery or recommend a more conservative therapy is often only based on the patient’s performance status and the technical feasibility of surgery, rather than on specific and objective indicators. Prognostic biomarkers are needed for the clinical management of a patient and used as decision aids to plan therapeutic approaches and should be reported according to the REMARK guidelines [19]. Biomarkers that can be measured before surgery may help to better identify patients who might benefit from additional attention in matters of prehabilitation before tumor surgery as part of a personalized treatment plan [20]. However, current tumor markers including the well-established CEA and CA19-9 often fail to identify the best candidates for surgery, although having generally proven useful in diagnosis, resection evaluation or treatment monitoring [4, 21]. Here, we demonstrate in two independent cohorts of patients with BTC receiving curative-intend surgical tumor resection that BMD as a prognostic marker provides valuable information on the patients’ postoperative outcome, as patients with impaired BMD had a significantly worse overall survival compared to other patients. Of note, our results are in line with previous findings in pancreatic cancer or colorectal cancer [22, 23]. In our analyses, BMD levels below a calculated optimal cut-off value (156.5 HU) identified a high-risk subgroup of BTC patients showing a substantially impaired long-term prognosis with a median OS of only 459 days. The prognostic relevance of BMD was further corroborated by uni- and multivariate Cox-regression analyses including various clinical and pathophysiological confounders. BMD is a measure of the amount of mineral, primarily calcium, in bones. Reduction of BMD is an increasingly important phenomenon, especially in an aging society, and the medical consequences are profound. It has already been shown that low BMD is associated with various conditions and diseases like cardiovascular diseases, stroke or chronic lung disease [24–26]. In oncology, an impaired BMD was recently identified as an independent marker for a poor prognosis in patients with digestive tract cancer [7] or in patients receiving palliative chemotherapy for BTC [13]. Our data on the prognostic role of BMD in patients receiving surgery for BTC therefore nicely integrate in the available body of literature and further support the broad use of such markers in the preoperative stratification of cancer patients. Nevertheless, it is unlikely that a patient who is a surgical candidate based on their performance status and imaging results would be denied surgery due to a single marker such the preoperative BMD. We therefore propose that preoperative measurements of BMD may be useful in identifying a group of high-risk BTC patients who should be closely monitored, especially in terms of clinical measurements related to cachexia or organ dysfunction. This could lead to more aggressive perioperative treatment for high-risk BTC patients or strategies to alleviate these findings in a prehabilitation setting. While clinical trials are currently investigating more aggressive adjuvant chemotherapy regimens, biomarkers such as BMD may help to identify patients who would particularly benefit from perioperative treatment or prehabilitation in terms of improved survival and/or quality of life.
We provide evidence that the prognostic value of BMD measurements is more prominent in women than in men, suggesting a sex-specific role of BMD in stratifying patients for surgery or other treatment modalities. These finding are striking since they reproduce our previous data from BTC-patients receiving systemic chemotherapy [13]. Moreover, they are in line with previous data providing evidence for muscle mass—another marker of the patients´ body composition - as a sex-specific prognostic factor in patients receiving surgery for BTC, where sarcopenia is only relevant in male but not in female patients [5]. Sarcopenia has also been demonstrated as relevant prognostic factor after liver transplantation only in male, but not in female patients [27]. The differences in BMD between men and women can be attributed to specific hormonal changes. BMD is considered an indicator of lifetime exposure to endogenous estradiol, a female hormone [28]. In women, the levels of reproductive hormones decline to prepubertal levels during midlife, whereas in men, they remain relatively stable until advanced age [29, 30]. Moreover, a substantial decrease in gonadal hormone levels is required to significantly affect BMD [31]. Thus, changes in estradiol levels strongly impact BMD in women [32], which may explain the observed effects. Testosterone, the primary male hormone, has a particularly strong influence on muscle mass [33]. Additionally, estradiol in men is primarily produced by the conversion of testosterone, and the levels of gonadal hormones are closely linked [34, 35]. The sex-specific changes in gonadal hormone levels may account for the differential changes in BMD and muscle mass between men and women. These findings also shed light on why BMD might be more relevant for women, whereas muscle mass is more relevant for men.
Our study has some limitations, which currently prevent applying its results to patients in clinical routine. First, our study included only 110 patients, representing a rather small cohort of patients when analyzing complex endpoints, such as OS. Second, our study represents a retrospective analysis conducted at two centers only, thus center-specific bias cannot be excluded. Furthermore, our validation cohort only consists of 34 patients due to the rarity of BTC. Moreover, due to the rarity of BTC, patients were included over a very long period of time, during which the methodology of liver surgery and postoperative treatment has changed. In addition, patients with differing demographics, risk factors, tumor localization, molecular structures and treatment approaches were included. Therefore, it cannot be ruled out that these effects may have impacted the results of the study. However, and most importantly, our study only looked at surgical resection as a treatment approach for BTC and did not include alternative treatments such as chemotherapy or loco-regional therapies. Thus, we cannot determine if patients with an initial BMD below our cut-off value would have had better outcomes with different treatments. Therefore, further research with larger patient numbers and multiple treatment modalities is needed to fully understand the relationship between BMD measurements and BTC surgery outcomes and potentially influence specific prehabilitation measurements before BTC surgery.
Supplementary information
Author contributions
MSJ, SHL, GF, CL, and CR designed the study; CL, LH, PM, GA and NGH established and provided methodology; CL, NGH, PM and GA assessed CT scan quality; LH, MSJ, TL, CL, GF, PM, NGH, PSP, AQ, AK, GA and WTK selected study patients and generated patient data; LH, CL, NGH and MSJ performed the measurements; LH, SHL and MSJ performed statistical analyses; LH, LW, CL, GF and MSJ generated figures and tables; CR, TL, GA, WTK and AQ validated the data and supervised the project; MSJ, GF, SHL, CL and CR wrote the manuscript, all authors revised the manuscript provided intellectual input and agreed to the final version of the manuscript.
Funding
MSJ was funded by Mildred Scheel-Stipendienprogramm für Krebsforschung, 2021 (57584079). Tom Luedde was funded by the European Research Council (ERC) Consolidator Grant PhaseControl (771083). Georg Fluegen was funded by the Deutsche Forschungsgemeinschaft (DFG, FL 865/2-1). Open Access funding enabled and organized by Projekt DEAL.
Data availability
Data included in this analysis represent highly sensitive medical data. It is directly against German (and European) law to publish such data in a way that would allow individual patients to be identified (e.g., by providing different clinical values of one distinct patient). Data are available upon request from the Department of Gastroenterology, Hepatology and Infectious Diseases at the University Hospital Düsseldorf for researchers who meet the criteria for access to confidential data: Wissenschaft.Gastro@med.uni-duesseldorf.de.
Competing interests
The authors declare no competing interests.
Ethics approval
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the ethics committee of the medical faculty of Heinrich Heine University Düsseldorf (2021-1334_1). Patient consent was waived owing to the retrospective data selection. This was approved by the ethics committee of the medical faculty of Heinrich Heine University Düsseldorf (2021-1334_1).
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Lisa Heinrichs, Georg Fluegen, Sven H. Loosen, Christina Loberg.
These authors jointly supervised this work: Christoph Roderburg, Markus S. Jördens.
Supplementary information
The online version contains supplementary material available at 10.1038/s44276-024-00094-2.
References
- 1.Schmeusser, BN, Ali, AA, Fintelmann, FJ, Garcia, JM, Williams, GR, Master, VA, et al. Imaging techniques to determine degree of sarcopenia and systemic inflammation in advanced renal cell carcinoma. Curr Urol Rep. 2023. 10.1007/s11934-023-01157-6. [DOI] [PMC free article] [PubMed]
- 2.Brown LR, Laird BJA, Wigmore SJ, Skipworth RJE. Understanding cancer cachexia and its implications in upper gastrointestinal cancers. Curr Treat Options Oncol. 2022;23:1732–47. 10.1007/s11864-022-01028-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bates DDB, Pickhardt PJ. CT-derived body composition assessment as a prognostic tool in oncologic patients: from opportunistic research to artificial intelligence-based clinical implementation. AJR Am J Roentgenol. 2022;219:671–80. 10.2214/AJR.22.27749. [DOI] [PubMed] [Google Scholar]
- 4.Banales JM, Marin JJG, Lamarca A, Rodrigues PM, Khan SA, Roberts LR, et al. Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nat Rev Gastroenterol Hepatol. 2020;17:557–88. 10.1038/s41575-020-0310-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jordens, MS, Heinrichs, L, Loosen, SH, Wittig, L, Keitel, V, Scholer D, et al. Sarcopenia predicts cancer mortality in male but not in female patients undergoing surgery for cholangiocellular carcinoma. Cancers (Basel). 2021; 13. 10.3390/cancers13215359. [DOI] [PMC free article] [PubMed]
- 6.Jordens, MS, Wittig, L, Heinrichs, L, Keitel, V, Schulze-Hagen, M, Antoch G, et al. Sarcopenia and myosteatosis as prognostic markers in patients with advanced cholangiocarcinoma undergoing palliative treatment. J Clin Med. 2021; 10. 10.3390/jcm10194340. [DOI] [PMC free article] [PubMed]
- 7.Watanabe J, Saitsu A, Miki A, Kotani K, Sata N. Prognostic value of preoperative low bone mineral density in patients with digestive cancers: a systematic review and meta-analysis. Arch Osteoporos. 2022;17:33 10.1007/s11657-022-01060-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schreiber JJ, Anderson PA, Rosas HG, Buchholz AL, Au AG. Hounsfield units for assessing bone mineral density and strength: a tool for osteoporosis management. J Bone Joint Surg Am. 2011;93:1057–63. 10.2106/JBJS.J.00160. [DOI] [PubMed] [Google Scholar]
- 9.Pickhardt PJ, Pooler BD, Lauder T, del Rio AM, Bruce RJ, Binkley N. Opportunistic screening for osteoporosis using abdominal computed tomography scans obtained for other indications. Ann Intern Med. 2013;158:588–95. 10.7326/0003-4819-158-8-201304160-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schulze-Hagen, MF, Roderburg, C, Wirtz, TH, Jordens, MS, Bundgens, L, Abu Jhaisha, S, et al. Decreased bone mineral density is a predictor of poor survival in critically Ill patients. J Clin Med. 2021; 10. 10.3390/jcm10163741. [DOI] [PMC free article] [PubMed]
- 11.Loosen, SH, van den Bosch, V, Gorgulho, J, Schulze-Hagen, M, Kandler, J, Jordens, MS, et al. Progressive sarcopenia correlates with poor response and outcome to immune checkpoint inhibitor therapy. J Clin Med. 2021; 10. 10.3390/jcm10071361. [DOI] [PMC free article] [PubMed]
- 12.Budczies J, Klauschen F, Sinn BV, Gyorffy B, Schmitt WD, Darb-Esfahani S, et al. Cutoff Finder: a comprehensive and straightforward Web application enabling rapid biomarker cutoff optimization. PLoS One. 2012;7:e51862 10.1371/journal.pone.0051862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jordens, MS, Wittig, L, Loberg, C, Heinrichs, L, Keitel, V, Schulze-Hagen, M, et al. Bone mineral density is a predictor of mortality in female patients with cholangiocellular carcinoma undergoing palliative treatment. Biomedicines. 2022; 10. 10.3390/biomedicines10071660. [DOI] [PMC free article] [PubMed]
- 14.Lupsa BC, Insogna K. Bone health and osteoporosis. Endocrinol Metab Clin North Am. 2015;44:517–30. 10.1016/j.ecl.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 15.Lane NE. Epidemiology, etiology, and diagnosis of osteoporosis. Am J Obstet Gynecol. 2006;194:S3–11. 10.1016/j.ajog.2005.08.047. [DOI] [PubMed] [Google Scholar]
- 16.Leib ES, Lewiecki EM, Binkley N, Hamdy RC, International Society for Clinical, D. Official positions of the international society for clinical densitometry. J Clin Densitom. 2004;7:1–6. 10.1385/jcd:7:1:1. [DOI] [PubMed] [Google Scholar]
- 17.Weber SM, Ribero D, O’Reilly EM, Kokudo N, Miyazaki M, Pawlik TM. Intrahepatic cholangiocarcinoma: expert consensus statement. HPB (Oxford). 2015;17:669–80. 10.1111/hpb.12441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mazzaferro V, Gorgen A, Roayaie S, Droz Dit Busset M, Sapisochin G. Liver resection and transplantation for intrahepatic cholangiocarcinoma. J Hepatol. 2020;72:364–77. 10.1016/j.jhep.2019.11.020. [DOI] [PubMed] [Google Scholar]
- 19.McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM, et al. Reporting recommendations for tumor marker prognostic studies. J Clin Oncol. 2005;23:9067–72. 10.1200/JCO.2004.01.0454. [DOI] [PubMed] [Google Scholar]
- 20.Loosen SH, Breuer A, Tacke F, Kather JN, Gorgulho J, Alizai PH, et al. Circulating levels of soluble urokinase plasminogen activator receptor predict outcome after resection of biliary tract cancer. JHEP Rep. 2020;2:100080 10.1016/j.jhepr.2020.100080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qin XL, Wang ZR, Shi JS, Lu M, Wang L, He QR. Utility of serum CA19-9 in diagnosis of cholangiocarcinoma: in comparison with CEA. World J Gastroenterol. 2004;10:427–32. 10.3748/wjg.v10.i3.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Motomura T, Uchiyama H, Iguchi T, Ninomiya M, Yoshida R, Honboh T, et al. Impact of osteopenia on oncologic outcomes after curative resection for pancreatic cancer. In Vivo. 2020;34:3551–7. 10.21873/invivo.12198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ikuta S, Aihara T, Nakajima T, Kasai M, Yamanaka N. Computed tomography-measured bone mineral density as a surrogate marker of survival after resection of colorectal liver metastases. Ann Transl Med. 2021;9:21 10.21037/atm-20-3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qu X, Huang X, Jin F, Wang H, Hao Y, Tang T, et al. Bone mineral density and all-cause, cardiovascular and stroke mortality: a meta-analysis of prospective cohort studies. Int J Cardiol. 2013;166:385–93. 10.1016/j.ijcard.2011.10.114. [DOI] [PubMed] [Google Scholar]
- 25.Campos-Obando N, Castano-Betancourt MC, Oei L, Franco OH, Stricker BH, Brusselle GG, et al. Bone mineral density and chronic lung disease mortality: the rotterdam study. J Clin Endocrinol Metab. 2014;99:1834–42. 10.1210/jc.2013-3819. [DOI] [PubMed] [Google Scholar]
- 26.Trivedi DP, Khaw KT. Bone mineral density at the hip predicts mortality in elderly men. Osteoporos Int. 2001;12:259–65. 10.1007/s001980170114. [DOI] [PubMed] [Google Scholar]
- 27.Kuo SZ, Ahmad M, Dunn MA, Montano-Loza AJ, Carey EJ, Lin S, et al. Sarcopenia predicts post-transplant mortality in acutely ill men undergoing urgent evaluation and liver transplantation. Transplantation. 2019;103:2312–7. 10.1097/TP.0000000000002741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cauley JA, Gutai JP, Sandler RB, LaPorte RE, Kuller LH, Sashin D. The relationship of endogenous estrogen to bone density and bone area in normal postmenopausal women. Am J Epidemiol. 1986;124:752–61. 10.1093/oxfordjournals.aje.a114451. [DOI] [PubMed] [Google Scholar]
- 29.Finkelstein JS, Brockwell SE, Mehta V, Greendale GA, Sowers MR, Ettinger B, et al. Bone mineral density changes during the menopause transition in a multiethnic cohort of women. J Clin Endocrinol Metab. 2008;93:861–8. 10.1210/jc.2007-1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR, Baltimore Longitudinal Study of, A. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. baltimore longitudinal study of aging. J Clin Endocrinol Metab. 2001;86:724–31. 10.1210/jcem.86.2.7219. [DOI] [PubMed] [Google Scholar]
- 31.Finkelstein JS, Lee H, Leder BZ, Burnett-Bowie SA, Goldstein DW, Hahn CW, et al. Gonadal steroid-dependent effects on bone turnover and bone mineral density in men. J Clin Invest. 2016;126:1114–25. 10.1172/JCI84137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Montalvo RN, Counts BR, Carson JA. Understanding sex differences in the regulation of cancer-induced muscle wasting. Curr Opin Support Palliat Care. 2018;12:394–403. 10.1097/SPC.0000000000000380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Szulc P, Duboeuf F, Marchand F, Delmas PD. Hormonal and lifestyle determinants of appendicular skeletal muscle mass in men: the MINOS study. Am J Clin Nutr. 2004;80:496–503. 10.1093/ajcn/80.2.496. [DOI] [PubMed] [Google Scholar]
- 34.Longcope C, Kato T, Horton R. Conversion of blood androgens to estrogens in normal adult men and women. J Clin Invest. 1969;48:2191–201. 10.1172/JCI106185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khosla S, Melton LJ 3rd, Atkinson EJ, O’Fallon WM, Klee GG, Riggs BL. Relationship of serum sex steroid levels and bone turnover markers with bone mineral density in men and women: a key role for bioavailable estrogen. J Clin Endocrinol Metab. 1998;83:2266–74. 10.1210/jcem.83.7.4924. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data included in this analysis represent highly sensitive medical data. It is directly against German (and European) law to publish such data in a way that would allow individual patients to be identified (e.g., by providing different clinical values of one distinct patient). Data are available upon request from the Department of Gastroenterology, Hepatology and Infectious Diseases at the University Hospital Düsseldorf for researchers who meet the criteria for access to confidential data: Wissenschaft.Gastro@med.uni-duesseldorf.de.