Abstract
Murine leukemia virus (MuLV) M813 was originally isolated from the Southeast Asian rodent Mus cervicolor. As with the ecotropic MuLVs derived from Mus musculus, its host range is limited to rodent cells. Earlier studies have mapped its receptor to chromosome 2, but it has not been established whether M813 shares a common receptor with any other MuLVs. In this study, we have performed interference assays with M813 and viruses from four interference groups of MuLV. The infection efficiency of M813 was not compromised in cells expressing any one of the other MuLVs, demonstrating that M813 must use a distinct receptor for cell entry. The entire M813 env coding region was molecularly cloned. Sequence analysis revealed high similarity with other MuLVs but with a unique receptor-binding domain. Substitution of M813 env sequences in Moloney MuLV resulted in a replication-competent virus with a host range and interference profile similar to those of the biological clone M813. M813 thus defines a novel receptor interference group of type C MuLVs.
Viral subgroups of the murine C-type viruses have been defined on the basis of host range, generally corresponding to distinct interference groups defined by receptor usage (40). Five distinct interference groups have been established for the well-studied isolates of Mus musculus, and the cellular receptors for these viruses have all been identified (Table 1). Three families of receptors have been characterized for these viruses, all of which share multiple transmembrane-spanning topology and are implicated in transporter activity: (i) cationic amino acid transport for the cationic amino acid transporter (CAT) family (20, 48), (ii) inorganic phosphate transport for the Pit family (18, 37), and (iii) a presumed phosphate transport activity for the newly identified Sgy1 family (44). Whereas some viruses, such as 10A1 murine leukemia virus (10A1 MuLV), recognize several members of the same transporter family (e.g., Pit1 and Pit2) as well as homologous receptors of different species (e.g., mouse, rat, and human) (34), other viruses recognize only one or at most two members of a receptor family and are unable to recognize homologous receptors of other species (e.g., ecotropic MuLV recognizes only the rodent CAT1 and, with less affinity, the CAT3 receptor, but not the CAT2 receptor or CAT homologues of other species) (19, 30). Interestingly, viruses of different interference groups may use the same receptor but show different affinities to homologues in other species (e.g., the xenotropic MuLV does not recognize all murine homologues of its human cellular receptor, all of which, however, are recognized by polytropic MuLVs [5, 28, 44, 50]). Determining the domains in the viral Env protein complex and the receptor molecule which dictate this specificity has been a subject of intense research over the last few years (11).
TABLE 1.
Classification of type C MuLVs based on receptor usage or homology
| Species and interference group | Prototype | Receptor | Chromosome locationa
|
Reference(s) | |
|---|---|---|---|---|---|
| Mouse | Human | ||||
| M. musculus | |||||
| Ecotropic | Mo-MuLV | mCAT1 | 5 | 13b | 20, 48 |
| Xenotropic | NZB-MuLVc | Syg1 | 1d | 1 | 5, 44, 50 |
| Polytropic | Mo-MCFV | Syg1 | 1 | 1 | 5, 44, 50 |
| Amphotropic | 4070A | Pit2 | 8 | 8 | 18 |
| 10A1 | 10A1 | Pit1, Pit2 | 2, 8 | 2, 8 | 34, 37 |
| M. dunni, Multitropic | MDEV | Unknown | ND | ND | 49 |
| M. cervicolor | |||||
| CI | CERV CI | Unknown | ND | ND | 7 |
| CII | M813 | Unknown | 2 | ND | 7, 29 |
| M. caroli CI | CARO CI | Unknown | ND | ND | 25 |
Chromosome location of receptor gene. ND, not determined.
Homologous gene in human, the product of which cannot be used by ecotropic viruses.
NZB, New Zealand Black xenotropic MuLV.
The gene allele of laboratory mouse strains encodes a protein that is not recognized by xenotropic MuLVs.
In addition to being isolated from M. musculus, type C retroviruses have also been isolated from Mus caroli (25), Mus cervicolor (7), and, more recently, Mus dunni (9). With the exception of the latter, the interference groups of these viruses have not been well defined. Earlier studies showed that two classes of type C retroviruses could be isolated from M. cervicolor. Type CI has a classical xenotropic host range (i.e., does not infect cells of inbred mouse strains but infects cells of wild mice and other mammalian species) and, based on immunological and nucleic acid hybridization criteria, is closely related to the virus isolated from M. caroli (7) and antigenically related to type C viruses from woolly monkeys (e.g., simian sarcoma-associated virus) and gibbon apes (gibbon ape leukemia virus [GALV]). Based on close sequence similarity to GALV, the M. dunni endogenous virus (MDEV) isolate is also related to this group (49), although it uses a virus receptor distinct from that of GALV.
The host range of the CII type isolates of M. cervicolor is limited to M. musculus. Based on antigenic determinants and RNA homology, these type CII viruses appear to be closely related to MuLVs isolated from M. musculus (7). Despite similarities to the ecotropic MuLVs, genomic mapping studies showed that CII isolates use a distinct cellular receptor (29, 39). Whereas the cellular receptor for the CII isolate M813 is located on mouse chromosome 2, the CAT1 gene encoding the ecotropic MuLV receptor is located on chromosome 5. The mapping of the gene encoding the Pit1 receptor to chromosome 2 led to the speculation that M813 may use the same receptor as MuLV 10A1 (1). As we have recently shown that viruses that use this family of phosphate transporters for cell entry induce a severe spongiform encephalomyelopathy in mice (35, 36), we were interested in establishing the interference group of M813.
The studies presented here show that the CII isolate M813 uses none of the known MuLV retroviral receptors for cell entry. Sequence analysis of a molecular clone of the env gene confirmed its homology with other MuLVs but showed considerable sequence diversity in the regions known to be important for receptor recognition. Replacement of the env gene of Moloney MuLV (Mo-MuLV) with that of M813 results in a replication-competent virus with a host range and receptor interference analogous to those of the original M813. Thus, M813 defines a novel receptor-interference group of the type C MuLVs.
(This work was done as part of the doctoral thesis of S. Hein in the Department of Biology at the University of Hamburg.)
MATERIALS AND METHODS
Virus-producing cell lines and cell culture.
SC1 mouse fibroblasts were used as virus producers in all experiments. SC1 cells expressing M813 were obtained by infection of SC1 cells with supernatant of either M813(NIH) or M813/M813 cell lines, kindly provided by U. Rapp (39). No differences in virus titers or host interference between the virus expressed by these two independent sources were observed, so all experiments described here were performed with virus derived from M813(NIH). Molecular clones of ecotropic (Mo-MuLV, clone mov3 [15]), amphotropic (Mo-AmphoV, clone AMS [33]), polytropic (Moloney mink cell focus-forming virus [Mo-MCFV], clone 16I [10]), and 10A1 (10A1 and Mo-10A1V, clone RR1 [38]) MuLVs were transfected into SC1 fibroblasts to obtain virus producers of these different interference groups. All cells were grown in Dulbecco's modified Eagle's medium with 10% fetal bovine serum.
Virus assays.
Marker rescue assays were employed to verify the host range of M813 and to determine the virus interference groups. For these assays, either MPEV or SFEV retroviral vector was used, both of which are based on the murine embryonal stem cell virus (14) with either LTRs from the myeloproliferative sarcoma virus (23) or the spleen focus-forming virus SFFVp (6), respectively. For the various assays, one of the following marker genes was incorporated in the retroviral backbone: (i) the neomycin resistance gene (neo) derived from the Tn5 transposon, which confers resistance to G418 in eukaryotes; (ii) the hph gene of Escherichia coli, conferring resistance to hygromycin; or (iii) the gene encoding the enhanced mutant of the green fluorescence protein (EGFP) from Aequorea victoria. SC1 fibroblasts expressing these retroviral vectors were established by DNA transfer by electroporation, followed either by selection in G418 or hygromycin or by cell sorting. The resulting cell lines were infected with supernatant from virus-producing cells and cultured for a minimum of 10 days to allow complete virus spread. Medium was replaced 24 h prior to harvesting of virus stocks from confluent monolayers and passed through a Millex-GP 0.22-μm-pore-size filter (Millipore).
Titers of virus stocks of MPEVneo and MPEVhyg pseudotypes were determined by end-point dilution. Target SC1 cells were plated at 104 cells per 1.7-cm-diameter well of a 24-well plate on day 1. On day 2, medium was replaced with medium containing virus stock serial dilutions (1:5) and 6 to 8 μg of Polybrene per ml. After 12 h, medium was again replaced to remove Polybrene. On day 3 to 4, G418 (400 μg/ml; specific activity, 0.6) or hygromycin (400 μg/ml; specific activity, 0.34) was added. After 10 to 14 days, drug-resistant colonies were counted, and data were expressed as either G418-resistant transfer units (GTU) or hygromycin transfer units per ml. All virus dilutions were analyzed in triplicate.
To assay the expression of mouse CAT1 (mCAT1) in human TE671 cells, the SFEV vector SF11α (16) carrying the EGFP cDNA was pseudotyped with either Mo-MuLV, 10A1V or M813 by coexpression in SC1 cells. TE671 and SC1 (2 × 105) cells were infected with 1 ml of virus-containing supernatant in the presence of 6 μg of Polybrene/ml and after 24 h were analyzed by flow cytometry on a FACScalibur (Becton Dickinson).
Construction of human cell lines expressing the ecotropic CAT1 receptor.
The murine CAT1 cDNA was amplified from the plasmid pJET (kindly provided by L. Albritton) by PCR using primers incorporating a NotI site upstream of the ATG (at position 199) and a HindIII site downstream of stop codon at position 2065 (2). The resulting fragment was cloned in the EcoRV site of pBluescript SK (Stratagene), excised with NotI and HindIII, and then cloned into the SFEV vector SF1-ires-Neo using the same sites. The resulting plasmid (SFEV-mCAT1-neo; R664) and MPEVneo, as a control, were transfected independently into Phoenix-ampho packaging cells together with a plasmid encoding the vesicular stomatitis virus G protein (47). After 48 h, supernatant was collected and used to infect cells of the human fibroblast line TE671. Cultures were selected for neomycin resistance in the presence of 800 to 1,000 μg of G418/ml (specific activity of 0.6). DNAs from the resulting cell lines, TE671neo and TE671neo-mCAT1, were subjected to Southern blot analysis to confirm the presence and intactness of the vector (data not shown).
Molecular cloning of env gene and sequence analysis.
Virus particles collected from the supernatant of confluent monolayers of SC1-M813(NIH) cells were filtered through Millex-HA 0.45-μm-pore-size filters (Millipore) and pelleted over a 4-ml 20% sucrose cushion in SW28 ultracentrifuge tubes at 26,000 rpm for 2 h at 4°C in a Beckman LS-65 ultracentrifuge. Virus particles were resuspended in 500 μl of TNE+S buffer (50 mM Tris, pH 7.4; 150 mM NaCl; 50 mM EDTA; 2% sarcosyl), and viral poly(A)+ RNA was extracted using the Micro-Fast Track kit (Invitrogen). cDNA was synthesized using oligo(dT) primers. A fragment encompassing the 3′ pol and 5′ env sequences was amplified by PCR with Taq polymerase using the primers 5′-GGTCAGGTAGAAAGAATGAA-3′ (cs252) and 5′-CACAGCCAACATTCTTG-3′ (cs245) (corresponding to nucleotides [nt] 5259 and 5278 and nt 6226 to 6245 of the Mo-MuLV genome [GenBank accession no. J02255] or nt 1 to 20 and nt 1383 to 1364 of the M813 sequence compiled from this study [GenBank accession no. AF327437]). An identical fragment was also isolated from genomic DNA prepared from SC1-M813(NIH) cl5 cells.
To obtain sequences encoding the TM subunit of the Env protein, a 3′-RACE (rapid amplification of 3′ cDNA ends) reaction was performed with genomic RNA isolated from SC1-M813(NIH) cl5 and the 3′-RACE kit from Boehringer Mannheim. Two independent reactions were performed, using as a 5′ primer either 5′-CCACATGGCAGATTCATCGC-3′ (cs314) or 5′-GGCATATCTAGCCCTCAACT-3′ (cs324), corresponding to nt 521 to 40 and nt 1334 to 1353, respectively, of the compiled M813 sequence. Both RACE products overlapped with the PCR products from the viral RNA and had identical 3′ ends.
Sequence identity between different mammalian type C virus Env proteins was determined using the Clustal V method with a PAM 250 residue weight. The multiple sequence alignment was created with Clustal W (1.81). Sequences used for comparison are found in the GenBank database with the following accession numbers: ecotropic MuLVs Mo-MuLV (J02255), Friend MuLV cl FB29 (NC 001362), and C3H/He-MuLV (M19005); polytropic MuLVs cl MX33 (M17327) and cl MX27 (M17326); xenotropic MuLVs cl NZB-9-1 (K02730) and cl bxv-CWD (M59793); amphotropic MuLVs cl 4070A (M33469) and 10A1 (M33470); multitropic MuLV cl MDEV (AF053745); feline leukemia virus A (FeLV-A) (M12500); GALV (U20589); and koala retrovirus (AF151794).
Construction of a Mo-MuLV/M813 env recombinant.
To generate a Mo-MuLV recombinant containing M813 env sequences, a primer was designed to insert a ScaI restriction site immediately upstream of the sequence encoding the proposed cleavage site of the mature M813 gp85env (5′-GTCAGTACTCAGGGAGGGAGTCC-3′; cs265, corresponding to nt 624 to 637 of the M813 sequences.) A ScaI site (underlined) is found in Mo-MuLV at this position. The second primer (5′-CGTTCCCGCCGGCGTCTGGGGTTGCTTGGT-3′; cs284, corresponding to nt 1278 to 1293 in M813) was designed to introduce an SgrA1 site (underlined) at the 5′ end of the proline-rich region of the SU, a site found in Mo-MuLV at this location. The 5′ half of M813 env was amplified using these primers with Pfx polymerase and, after digestion with ScaI and SgrA1, used to replace the corresponding fragment in a plasmid encoding the entire Mo-MuLV (pUC-MoMuLV; R686). The resulting plasmid (Mo-M813; R704) was transfected into SC1 cells, and supernatant was monitored for virus spread by reverse transcriptase activity.
Nucleotide sequence accession number.
The nucleotide sequences of the 3′ M813 viral genome, including partial sequences of pol, the complete env gene, and the U3 and R region of the long terminal repeat (LTR), have been deposited in GenBank under the accession no. AF327437.
RESULTS
M813 MuLV efficiently infects cells of mouse but not rat or human origin.
Using a reverse transcriptase assay, it has been previously shown that type CII viruses from M. cervicolor infect cells derived from M. musculus (e.g., SC1 and NIH 3T3 cells) but not cells derived from M. cervicolor or from other diverse species, including rat and human (7). To verify that the clone M813 belongs to this same virus type, we used a marker rescue assay to determine the virus titers on mouse, rat, and human cells. For these studies, SC1 cells expressing the MPEVneo retroviral vector were infected with either M813 or as controls, Mo-MuLV or Mo-10A1V. After complete virus spread (10 to 14 days), the supernatant of these cells was used as a source of MPEVneo pseudotypes with different Env proteins. The transfer efficiency of these pseudotypes was determined on two or three different cell types from all three species, and the number of GTU per milliliter was calculated.
As shown in Table 2, M813 was able to efficiently infect cells only of mouse origin. This is in contrast to Mo-10A1V and Mo-MuLV, which could infect both mouse and rat cells and, in the case of Mo-10A1V, human cells. A few G418-resistant clones were observed after M813-pseudotype infections of rat cells, but this was at a frequency 4 orders of magnitude lower than that obtained with M813-infected murine cells or when either Mo-10A1V or Mo-MuLV was used to infect the same cultures. This assay confirmed that M813 has the same host range as the previously described CII MuLVs. Furthermore, this host range is distinct from that of the other MuLVs isolated from M. musculus or M. dunni, all of which can infect rat and, with the exception of the ecotropic MuLVs, most human cells (26, 31, 41).
TABLE 2.
M813 has a restricted host range
| Species | Cell line | Titer (GTU/ml)a of MPEVneo pseudotyped with:
|
||
|---|---|---|---|---|
| M813 | Mo-MuLV | 10A1 | ||
| Mouse | SC1 | 3.5 × 104 | 1.6 × 105 | 2.6 × 105 |
| NIH 3T3 | 8.0 × 104 | 2.6 × 105 | 3.0 × 105 | |
| Rat | RAT1 | 37 | 8.6 × 104 | 5.6 × 104 |
| NRK | 30 | 1.2 × 105 | 1.8 × 105 | |
| REF52 | 130 | ND | 1.7 × 105 | |
| Human | HeLa | 0 | 0 | 8.3 × 105 |
| HT1080 | 0 | ND | 4.3 × 104 | |
| TE671 | 0 | ND | 1.4 × 105 | |
Average of at least two independent experiments.
ND, not determined.
M813 MuLV shows only slight or no interference with other MuLVs.
Despite its different host range, it remains possible that M813 uses the same receptor for cell entry as other MuLVs. To test this, we performed interference assays using a neoR rescue assay. In the first set of experiments, MPEVneo(M813) pseudotype titers were determined on SC1 cells that either were uninfected or expressed virus of one of the four known interference groups, i.e., Mo-MuLV (ecotropic), Mo-AmphoV (amphotropic); 10A1V (10A1), and Mo-MCFV (polytropic). Xenotropic MuLV was not tested, as these viruses do not infect all M. musculus cells and use the same receptor as polytropic viruses (24). As a control for infectivity of the cell lines, MPEVneo(10A1) pseudotype infections were carried out in parallel. As shown in Table 3, expression of M813 in SC1 cells inhibits infection of M813 by more than 4 orders of magnitude, but no or only slight interference (at most fourfold) was observed in SC1 cells expressing any one of the other MuLVs.
TABLE 3.
M813 infection is not blocked by MuLVs of the different interference groups
| Target cells | Titer (GTU/ml)a of MPEVneo pseudotyped with:
|
|
|---|---|---|
| M813 | 10A1 | |
| SC1 | 3.4 × 104 | 7.6 × 104 |
| SC1 + M813 | 1.5 | 7.4 × 104 |
| SC1 + Mo-MuLV | 3.5 × 104 | 7.6 × 104 |
| SC1 + Mo-AmphoV | 5.7 × 104 | 8.8 × 104 |
| SC1 + 10A1V | 8.8 × 103 | 2 |
| SC1 + Mo-MCFV | 4.3 × 104 | 2.0 × 105 |
Average of two or more independent experiments.
In the reciprocal experiment, where the infection frequencies of all four MuLVs were compared between uninfected and M813-infected SC1 cells, no interference in infection frequency was observed, with the exception of the Mo-MuLV infection, where a reproducible 50-fold decrease was seen with M813-infected cells (Table 4).
TABLE 4.
M813 expression slightly interferes with ecotropic MuLV infection but not with other MuLVs
| Pseudotypes of MPEVneo | Titer on target cells (GTU/ml)a
|
|
|---|---|---|
| SC1 | SC1-M813 | |
| M813 | 5.4 × 104 | 0 |
| Mo-MuLV | 1.8 × 105 | 5.1 × 103 |
| Mo-AmphoV | 1.2 × 105 | 1.2 × 105 |
| 10A1V | 7.8 × 104 | 8.2 × 104 |
| Mo-MCFV | 2.6 × 103 | 3.0 × 103 |
Average of a minimum of two independent experiments.
Taken together, the data yield two conclusions. First of all, as the infection efficiency of M813 is not compromised on cells expressing any of the four MuLVs assayed, it must use a unique receptor for cell entry. However, as ecotropic MuLV infection is slightly inhibited in cells expressing M813, we cannot completely rule out the possibility that M813 can also use the mCAT1 protein as a receptor, albeit with a low transfer frequency.
Human TE671 cells expressing the mCAT1 protein are susceptible to Mo-MuLV infections but not M813 infections.
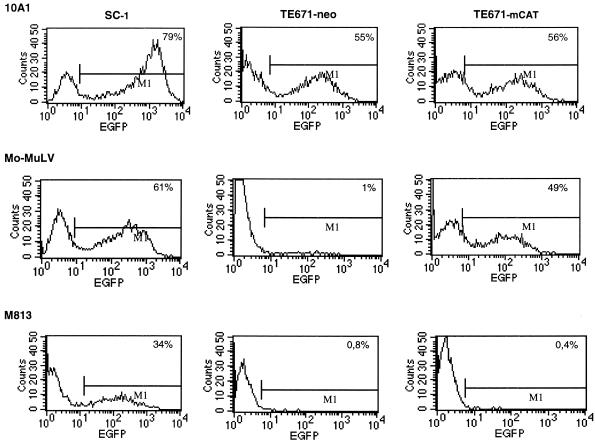
To address the question of whether M813 can use mCAT1 as a receptor, a cDNA encoding mCAT1 was introduced into the human fibroblast line TE671 by the retroviral vector MPEVneo. After selection for G418 resistance, the resulting cell line, TE671neo-mCAT1, as well as the control cell line TE671neo, were assayed for expression of the mCAT1 gene by infection with Mo-MuLV. For this assay, we used an SFEV vector containing the EGFP cDNA, which was pseudotyped with either Mo-MuLV or, as a control, 10A1V. Forty-eight hours after infection, target cells were monitored for EGFP expression. As shown in Fig. 1, TE671 cells carrying the mCAT1 construct were susceptible to Mo-MuLV infections, in contrast to control TE671neo cells. The infection efficiency of Mo-MuLV was reduced by approximately 25% (average of two independent experiments) on TE671neo-CAT1 cells, compared to SC1 cells; however, a comparable decrease was also observed with 10A1V. We therefore conclude that the TE671neo-CAT1 cells express sufficient levels of CAT1 to allow infection by Mo-MuLV.
FIG. 1.
Susceptibility to Mo-MuLV infection conferred to human TE671 cells by expression of mCAT1 was confirmed by EGFP transfer. Target cells were incubated for 48 h with supernatant containing the SFEV-EGFP vector pseudotyped with either 10A1, Mo-MuLV, or M813 produced from SC1 fibroblasts. Infected cells were then monitored for expression of EGFP by flow cytometry.
We then infected the same set of cells with SFEV-EGFP(M813) pseudotypes. No transfer of EGFP could be detected, despite relatively efficient transfer to SC1 cells. However, as M813 decreased Mo-MuLV titers only 50-fold, it could be argued that the use of mCAT1 by M813 is very inefficient and thus not detectable in this assay. We therefore tested M813 infectivity of TE671neo-CAT1 by pseudotyping with MPEVhyg vectors. As shown in Table 5, no hygromycin-resistant clones were observed, despite titers up to 104 on SC1 cells. Thus, mCAT1 is either never used by M813 or used 0.001-fold less efficiently than a second cellular receptor for cell entry.
TABLE 5.
Expression of mCAT1 in TE671 cells does not render the cells susceptible to M813 infection
| Target cells | Titer (HTU/ml)a of MPEVhyg pseudotyped with:
|
|
|---|---|---|
| Mo-MuLV | M813 | |
| SC1 | 1 × 103 | 2 × 104 |
| TE671neo | 0 | 0 |
| TE671neo-mCAT1 | 2.2 × 103 | 0 |
Results of one of two independent experiments with similar results are shown. HTU, hygromycin transfer units.
Molecular cloning of the M813 env gene.
To gain insight into the relationship of M813 MuLV with other MuLVs, as well as to define the molecular basis for its unique receptor usage, the env gene of M813 was molecularly cloned. Viral RNA was isolated from virus particles and subjected to cDNA synthesis. Primers designed to recognize conserved sequences in the pol and env genes of MuLVs were used to amplify the intervening sequences (see Materials and Methods). 3′-RACE was successfully employed to obtain 3′ env sequences and LTR U3 and R regions. Compiled sequence data of the overlapping clones have been submitted to the public data banks.
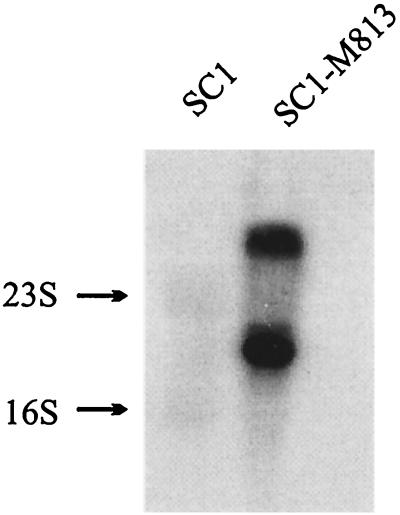
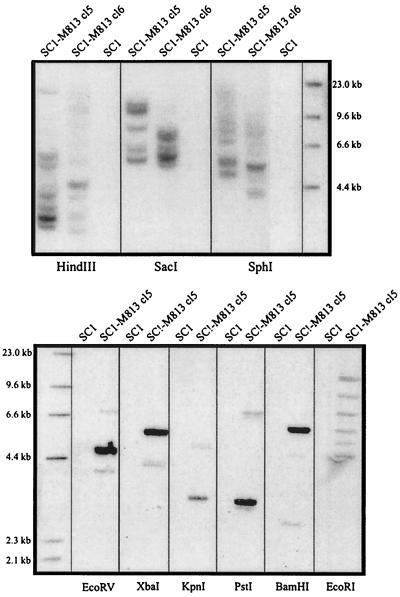
To confirm that the isolated env sequences originated from M813 and not from an endogenous virus in M. musculus, Northern and Southern analyses were performed. RNA and DNA isolated from either uninfected SC1 cells or SC1-M813 producer cells were hybridized to an 840-bp probe encompassing the 5′ terminus of the M813 env gene. As shown in Fig. 2, the probe hybridizes with two transcripts of about 8.5 and 3.2 kb, corresponding to the expected size of unspliced and spliced MuLV transcripts, thus providing evidence that these sequences are contained within a full-length virus. Furthermore, as would be expected if the sequences are derived from M813, the env probe hybridized under stringent conditions with DNA isolated from SC1 cells infected with M813 but not with DNA from uninfected SC1 cells (Fig. 3). When DNAs were digested with EcoRV, XbaI, PstI, and BamHI, a single strong band was detected, in addition to a much fainter second band. In contrast, digests with EcoRI, HindIII, SacI, and SphI resulted in multiple bands. As all cell lines analyzed were derived from single cell clones, we can conclude that the multiple bands represent unique integration sites and thus, multiple copies (6 to 12) of the provirus are found in each cell line. The first set of restriction enzymes must have recognition sites flanking the env probe and within the proviral genome and thus confirm that the majority of the proviral copies have a conserved restriction digest pattern. The faint bands observed in these blots most likely represent one or two rearranged proviral copies found in the genome. The high proviral copy number most likely reflects a relatively high titer of the M813(NIH) cell supernatant used to infect the SC1 cells, thereby resulting in multiple integrations before infection interference had occurred.
FIG. 2.
Northern blot analysis confirms that the env gene is expressed within a full-length retrovirus expressed in the SC1-M813 virus producer. Total RNA from SC1 and SC1-M813 producer was isolated, separated by agarose gel electrophoresis, and transferred to a nylon membrane. The blot was hybridized under standard conditions with a probe extending from nt 519 to 1358, corresponding to the first 840 bp of the env coding region.
FIG. 3.
Southern blot analysis of SC1-M813 and SC1 cells confirms that M813 is not an endogenous M. musculus MuLV. DNA isolated from M813-infected and uninfected SC1 cells was digested with the indicated restriction enzymes and separated by agarose gel electrophoresis. After transfer to a nylon membrane, DNA was hybridized with an M813 env probe (nt 519 to 1358).
Replacement of the env gene of Mo-MuLV with that of M813 MuLV results in a replication-competent virus with an M813 interference profile.
To functionally show that the isolated env sequences are derived from M813, the cloned sequences were replaced with those of Mo-MuLV to generate a replication-competent virus. Using the strategy described in Materials and Methods, a Mo-M813V recombinant was constructed in which the sequences encoding the first 225 amino-terminal residues of the mature gp70env protein are derived from the M813 env clone. This includes the proposed receptor recognition domain (including variable regions A, C, and B), as well as the proline-rich domain of the SU. Plasmid DNA was transfected into SC1 cells containing the MPEVneo construct and then monitored for reverse transcriptase activity and marker rescue. After 10 days, the supernatant of SC1–Mo-M813 cells was positive for both reverse transcriptase activity (data not shown) and its ability to transfer G418 resistance to SC1 cells (Table 6), conclusively showing that the cloned env sequences encoded an Env protein that was able to bind to a cellular receptor and mediate cell penetration.
TABLE 6.
Mo-M813V has the same interference pattern as wild-type M813
| Target cells | Titer of MPEVneo (GTU/ml)a pseudotyped with:
|
||
|---|---|---|---|
| Mo-M813V | Mo-MuLV | Mo-10A1V | |
| SC1 | 1.0 × 105 | 5.2 × 105 | 8.3 × 104 |
| SC1 + Mo-M813V | 0 | 7.0 × 105 | 7.8 × 104 |
| SC1 + Mo-MuLV | 1.0 × 105 | ND | ND |
| SC1 + Mo-AmphoV | 1.5 × 105 | ND | ND |
| SC1 + Mo-10A1V | 0.8 × 105 | ND | ND |
| SC1 + M813 | 0 | ND | ND |
Results of one experiment (plated in triplicate); a second experiment gave similar results but with lower titers. ND, not determined.
Interference assays were performed to determine if Mo-M813V exhibited the same receptor usage as the original M813. As expected if Mo-M813 uses the same receptor, a complete block to infection was observed on SC1 cells expressing M813, but no interference was observed in cells expressing Mo-MuLV, Mo-10A1V, and Mo-AmphoV (Table 6). Furthermore, SC1 cells expression Mo-M813V were completely permissive for infection with Mo-MuLV and Mo-10A1V (Table 6). In conclusion, the env sequence we have cloned and characterized must represent a functional equivalent of the M813 env gene. The fact that no inhibition of Mo-MuLV infection was observed supports the conclusion that M813 does not use CAT1 as a receptor.
Sequence analysis of the M813 env gene confirms its close but distinct relationship to M. musculus MuLVs.
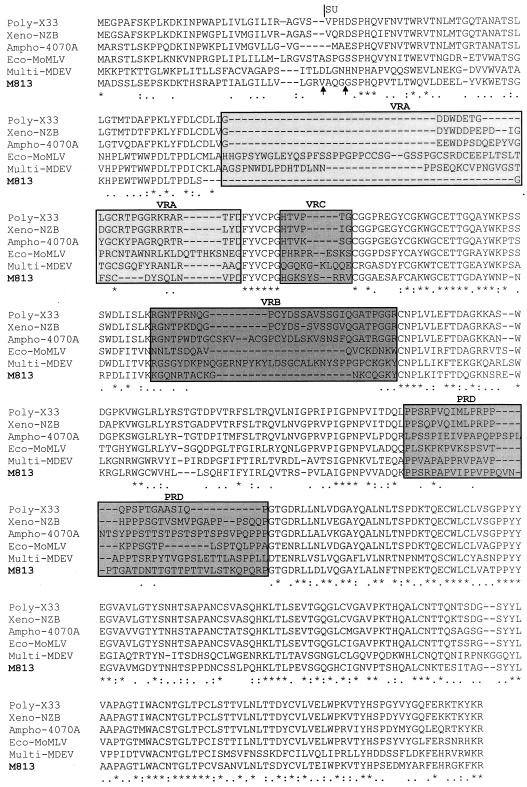
The amino acid sequence of M813 Env was compared to that of several other MuLVs (see Materials and Methods for list of viruses used for comparison). The M813 Env protein shares an overall sequence identity of 51 to 58% with other MuLV Envs, the highest identity being with the Env protein of the C3H ecotropic virus. Sequence alignments with other mammalian C-type viruses showed relatively high identity with FeLV-A (50.4%) but less identity with the M. dunni multitropic MDEV-MuLV isolate, GALV, or the koala retrovirus (42.5, 39, and 42.4%, respectively), consistent with the hypothesis that FeLV-A originated from MuLV (8).
A multiple-sequence alignment of the SU domain of Env from ecotropic, xenotropic, polytropic, amphotropic, multitropic, and M813 MuLVs is shown in Fig. 4. The alignment clearly shows the conserved scaffold regions surrounding the variable regions, corresponding to the variable receptor specificity domains, identified in other MuLVs (4, 13). As would be expected if M813 uses a distinct receptor, no sequence identity is observed in the variable regions. In particular, the variable region A (VRA) domain, shown to be essential for receptor binding for other MuLVs (3, 4, 27), is quite distinct in M813, comprised of only 12 amino acids. Indeed, a highly conserved cysteine upstream of VRA, which has been shown to form a disulfide bond with a conserved cysteine in the VRA (13), is absent in M813.
FIG. 4.
Multiple-sequence alignment (Clustal W) of the SU Env of several MuLVs. Two potential cleavage sites of the signal peptide in the M813 sequence are indicated by arrows (46). The variable regions and the proline-rich domain (PRD) are boxed and shaded. Identical amino acid residues are denoted with asterisks, whereas residues with highly similar or fairly similar physiochemical properties (e.g., polar, hydrophobic, or charged) are denoted with colons or dots, respectively.
DISCUSSION
Interest in the type C MuLVs stems from various disciplines. MuLVs have proven to be outstanding tools for studying oncogenesis, for establishing principles of viral persistence in host organisms, for tracking evolutionary relationships among mice and viruses, and finally as gene transfer vectors in both the laboratory and the clinical setting. MuLVs are generally classified into different host range or interference groups that reflect sequence homology in the env gene. These sequences encode the SU protein, which is incorporated into the virus envelope and mediates cell-specific binding to a transmembrane receptor. Receptor usage has several implications for both the pathogenicity of the virus (36, 38) and its usefulness as a vector for gene transfer (31, 32, 47). In addition, these unique env sequences have been used to facilitate phylogenetic analysis of mouse strains and viruses (17, 22, 43, 45). Although the best-studied MuLVs have been isolated from M. musculus, MuLVs have also been isolated from several other strains. In the present study, we have defined a novel receptor group of the MuLVs by characterizing the M813 MuLV isolate from M. cervicolor.
Due to its high homology with MuLVs from M. musculus and its limited host range, M813 was originally classified as a type CII MuLV with an ecotropic (i.e., M. musculus) host range (7, 29). In this study, we have presented data that support the unique receptor usage of M813. This was initially demonstrated by interference assays—M813 infection was not hampered in cells expressing any one of the tested MuLVs. Although there was slight interference of ecotropic Mo-MuLV infections in cells expressing M813, Mo-MuLV expression did not block M813 infection. Such a nonreciprocal interference pattern is suggestive of at least two receptors, one of which would be shared by both virus groups. However, expression of the ecotropic receptor mCAT1 cDNA in human cells did not impart susceptibility to M813 infection. Although we cannot completely rule out the possibility that this interference is due to expression and utilization of the mCAT3 receptor, we find this unlikely, as ecotropic MuLV infection using CAT3 is inefficient (30) and CAT3 expression is limited to the brain and thus probably not present in the SC1 fibroblasts used in this study. We find it more likely that the slight interference was directly due not to M813 expression but to other sequences present in the original M813-expressing cell line. In support of this, we found that expression of molecularly cloned M813 env sequences did not show any detectable interference with Mo-MuLV infections.
To characterize the sequences that imparted the unique receptor usage to M813, we molecularly cloned the entire env sequences. Due to relatively high homology with other known MuLVs, we were able to amplify the env sequences using oligonucleotide primers in conserved regions of the pol and env genes. Importantly, incorporation of the M813 env sequences into the Mo-MuLV genome imparted an altered host range that was restricted by M813 but not by the other tested MuLVs, verifying that these sequence were of M813 origin.
As would be expected for a virus that recognizes a different receptor, only 37% sequence identity at most was found with other MuLVs within the amino-terminal domain of the SU protein, known to be important for receptor binding (4, 13), whereas sequences encoding the carboxyl end of the SU and the entire TM were more highly conserved (76%). Sequence similarity is not always an accurate prediction of receptor usage, however, as demonstrated for 10A1 and GALV, which use the same receptor but show only 24% identity in the receptor-binding and proline-rich domains. Nevertheless, the distinctive structure of the VRA domain in M813 is striking evidence that it has a unique binding domain.
No sequence homology was detected between M813 5′ env sequences and M. musculus sequences by hybridization. This not only verifies that our molecular clone is not derived from an endogenous M. musculus provirus but also has implications with regard to its evolution. The absence of M813 5′ env sequences in the M. musculus genome is in striking contrast to the wide distribution of both polytropic and xenotropic MuLVs in different mouse species but is analogous to ecotropic MuLVs, whose distribution is limited (22). This suggests that both ecotropic MuLVs from M. musculus and M813 from M. cervicolor may have more recent origins. In agreement with the high divergence in the amino-terminal region, M813 may have arisen by replacement of a region in the env gene of an endogenous xenotropic MuLV by the analogous regions of an unknown virus (not fixed in the germ line) or with cellular sequences that bind to a novel receptor, as has been suggested for ecotropic MuLVs (42). Further studies, such as screening other mouse species for homologous M813 env sequences, as well as sequence comparison of other M813 structural gene with the different MuLVs are necessary for establishing definitive phylogenetic relationships.
Interestingly, the original characterization of type CII viruses (such as M813) showed infectivity for M. musculus- but not M. cervicolor-derived cells (7). This implies that the host must have developed a mechanism of resistance after the virus was introduced into the genome. As the provirus appears to be in an active state in the M. cervicolor genome (induction of virus release was not necessary to isolate the original CII variant), the resistance mechanism most likely involves blocking virus binding to the cell surface receptors. This could be accomplished by ectopic expression of env sequences, as has been described for the Fv4 resistance locus, or accumulation of point mutations in the cellular receptor that prevent virus binding without disruption of its normal function, as predicted for the xenotropic receptor in laboratory mouse strains and shown for the ecotropic receptor in M. dunni (12, 21, 24). In this light, it will be of interest to determine the number of M813 env-related sequences that are present in the M. cervicolor genome.
In conclusion, we have identified a unique MuLV interference group defined by the type CII M813 MuLV isolated from M. cervicolor tissue. We predict that this virus may have evolved through “domain swapping” of either cellular or viral sequences with more ancient endogenous MuLVs, yielding a retrovirus with altered receptor usage. Characterization of this novel receptor will help us better understand the mechanisms by which retroviruses gain entry into the cell.
ACKNOWLEDGMENTS
We are indebted to Ulf Rapp for providing the M813 producer cell lines, to Marc Sitbon for help with the multiple-sequence alignments, and to Wolfram Ostertag for many fruitful discussions. We also thank Dusty Miller, Alan Rein, and Klaus Harbers for making available molecular clones of the viruses used in these studies. We gratefully acknowledge the expert technical assistance of Stephanie Peters.
This work was supported by a grant of the Deutsche Forschungsgemeinschaft (Sto 225-5-1/2). The Heinrich-Pette-Institut is financed by the Freie und Hansestadt Hamburg and the Bundesministerium für Gesundheit.
REFERENCES
- 1.Adamson M, Silver J, Kozak C. The mouse homolog of the gibbon ape leukemia virus receptor: genetic mapping and a possible receptor function in rodents. Virology. 1991;183:778–781. doi: 10.1016/0042-6822(91)91010-e. [DOI] [PubMed] [Google Scholar]
- 2.Albritton L, Tseng L, Scadden D, Cunningham J. A putative murine ecotropic retrovirus receptor gene encodes a multiple membrane-spanning protein and confers susceptibility to virus infection. Cell. 1989;57:659–666. doi: 10.1016/0092-8674(89)90134-7. [DOI] [PubMed] [Google Scholar]
- 3.Battini J-L, Danos O, Heard J. Receptor-binding domain of murine leukemia virus envelope glycoproteins. J Virol. 1995;69:713–719. doi: 10.1128/jvi.69.2.713-719.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Battini J-L, Heard J, Danos O. Receptor choice determinants in the envelope glycoprotein of amphotropic, xenotropic, and polytropic murine leukemia viruses. J Virol. 1992;66:1468–1475. doi: 10.1128/jvi.66.3.1468-1475.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Battini J-L, Rasko J, Miller A. A human cell-surface receptor for xenotropic and polytropic murine leukemia viruses: possible role in G protein-coupled signal transduction. Proc Natl Acad Sci USA. 1999;96:1385–1390. doi: 10.1073/pnas.96.4.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baum C, Hegewisch-Becker S, Eckert H-G, Stocking C, Ostertag W. Novel retroviral vectors for efficient expression of the multidrug resistance (mdr-1) gene in early hematopoietic cells. J Virol. 1995;69:7541–7547. doi: 10.1128/jvi.69.12.7541-7547.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benveniste R, Callahan R, Sherr C, Chapman V, Todaro G. Two distinct endogenous type C viruses isolated from the Asian rodent Mus cervicolor: conservation of viral gene sequences in related rodent species. J Virol. 1977;21:849–862. doi: 10.1128/jvi.21.3.849-862.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benveniste R, Todaro G. Evolution of C-type viral genes: inheritance of exogenously acquired viral genes. Nature. 1974;252:456–459. doi: 10.1038/252456a0. [DOI] [PubMed] [Google Scholar]
- 9.Bonham L, Wolgamot G, Miller A. Molecular cloning of Mus dunni endogenous virus: an unusual retrovirus in a new murine viral interference group with a wide host range. J Virol. 1997;71:4663–4670. doi: 10.1128/jvi.71.6.4663-4670.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bosselman R, van Straaten F, Van Beveren C, Verma I, Vogt M. Analysis of the env gene of a molecularly cloned and biologically active Moloney mink cell focus-forming proviral DNA. J Virol. 1982;44:19–31. doi: 10.1128/jvi.44.1.19-31.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cosset F-L, Russell S. Targeting retrovirus entry. Gene Ther. 1996;3:946–956. [PubMed] [Google Scholar]
- 12.Eiden M, Farell K, Warsowe J, Mahan L, Wilson C. Characterization of a naturally occurring ecotropic receptor that does not facilitate entry of all ecotropic murine retroviruses. J Virol. 1993;67:4056–4061. doi: 10.1128/jvi.67.7.4056-4061.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fass D, Davey R, Hamson C, Kim P, Cunningham J, Burger J. Structure of a murine leukemia virus receptor-binding glycoprotein at 2.0 Angstrom resolution. Science. 1997;277:1662–1666. doi: 10.1126/science.277.5332.1662. [DOI] [PubMed] [Google Scholar]
- 14.Grez M, Akgün E, Hilberg F, Ostertag W. Embryonic stem cell virus, a recombinant murine retrovirus with expression in embryonic stem cells. Proc Natl Acad Sci USA. 1990;87:9202. doi: 10.1073/pnas.87.23.9202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harbers K, Schnieke A, Stuhlmann H, Jahner D, Jaenisch R. DNA methylation and gene expression: endogenous retroviral genome becomes infectious after molecular cloning. Proc Natl Acad Sci USA. 1981;78:7609–7613. doi: 10.1073/pnas.78.12.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hildinger M, Abel K, Ostertag W, Baum C. Design of 5′ untranslated sequences in retroviral vectors developed for medical use. J Virol. 1999;73:4083–4089. doi: 10.1128/jvi.73.5.4083-4089.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jenkins N, Copeland N, Taylor B, Lee B. Organization, distribution, and stability of endogenous ecotropic murine leukemia virus DNA sequences in chromosomes of Mus musculus. J Virol. 1982;43:26–36. doi: 10.1128/jvi.43.1.26-36.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kavanaugh M, Miller D, Zhang W, Law W, Kozak S, Kabat D, Miller A. Cell-surface receptors for gibbon ape leukemia virus and amphotropic murine retrovirus are inducible sodium-dependent phosphate symporters. Proc Natl Acad Sci USA. 1994;91:7071–7075. doi: 10.1073/pnas.91.15.7071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kavanaugh M, Wang H, Zhang Z, Thang W, Wu Y-N, Dechant E, North R, Kabat D. Control of cationic amino acid transport and retroviral receptor functions in a membrane protein family. J Biol Chem. 1994;269:15445–15450. [PubMed] [Google Scholar]
- 20.Kim J, Closs E, Albritton L, Cunningham J. Transport of cationic amino acids by the mouse ecotropic retrovirus receptor. Nature. 1991;352:725–728. doi: 10.1038/352725a0. [DOI] [PubMed] [Google Scholar]
- 21.Kozak C. Susceptibility of wild mouse cells to exogenous infection with xenotropic leukemia viruses: control by a single dominant locus on chromosome 1. J Virol. 1985;55:690–695. doi: 10.1128/jvi.55.3.690-695.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kozak C, O'Neil R. Diverse wild mouse origins of xenotropic, mink cell focus-forming, and two types of ecotropic proviral genes. J Virol. 1987;61:3082–3088. doi: 10.1128/jvi.61.10.3082-3088.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laker C, Meyer J, Schopen A, Friel J, Ostertag W, Stocking C. Retroviruses permissive for expression in embryonic cells are subject to host cis-mediated extinction. J Virol. 1998;72:339–348. doi: 10.1128/jvi.72.1.339-348.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levy J. Xenotropism: the elusive viral receptor finally uncovered. Proc Natl Acad Sci USA. 1999;96:802–804. doi: 10.1073/pnas.96.3.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lieber M, Sherr C, Todaro G, Benveniste R, Callahan R, Coon H. Isolation from the Asian mouse Mus caroli of endogenous type C virus related to infectious primate type C viruses. Proc Natl Acad Sci USA. 1975;72:2315–2319. doi: 10.1073/pnas.72.6.2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Loiler S, DiFronzo N, Holland C. Gene transfer to human cells using retrovirus vectors produced by a new polytropic packaging cell line. J Virol. 1997;71:4825–4828. doi: 10.1128/jvi.71.6.4825-4828.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.MacKrell A, Soong N, Curtis C, Anderson W. Identification of a subdomain in the Moloney murine leukemia virus envelope protein involved in receptor binding. J Virol. 1996;70:1768–1764. doi: 10.1128/jvi.70.3.1768-1774.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marin M, Tailor C, Nouri A, Kozak S, Kabat D. Polymorphisms of the cell surface receptor control mouse susceptibilities to xenotropic and polytropic leukemia viruses. J Virol. 1999;73:9362–9368. doi: 10.1128/jvi.73.11.9362-9368.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marshall T, Rapp U. Genes controlling receptors for ecotropic and xenotropic type C virus in Mus cervicolor and Mus musculus. J Virol. 1979;29:501–506. doi: 10.1128/jvi.29.2.501-506.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Masuda M, Kakushima N, Wilt S, Ruscetti S, Hoffman P, Iwamoto A, Masuda M. Analysis of receptor usage by ecotropic murine retroviruses, using green fluorescent protein-tagged cationic amino acid transporters. J Virol. 1999;73:8623–8629. doi: 10.1128/jvi.73.10.8623-8629.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller A, Chen F. Retrovirus packaging cells based on 10A1 murine leukemia virus for production of vectors that use multiple receptors for cell entry. J Virol. 1996;70:5564–5571. doi: 10.1128/jvi.70.8.5564-5571.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller A, Garcia J, von Suhr N, Lynch C, Wilson C, Eiden M. Construction and properties of retrovirus packaging cell based on gibbon ape leukemia virus. J Virol. 1991;65:2220–2224. doi: 10.1128/jvi.65.5.2220-2224.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller A, Law M, Verma I. Generation of helper-free amphotropic retroviruses that transduce a dominant-acting, methotrexate-resistant dihydrofolate reductase gene. Mol Cell Biol. 1985;5:431–437. doi: 10.1128/mcb.5.3.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller D, Miller A. A family of retroviruses that utilize related phosphate transporters for cell entry. J Virol. 1994;68:8270–8276. doi: 10.1128/jvi.68.12.8270-8276.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Münk C, Löhler J, Prassolov V, Just U, Stockschläder M, Stocking C. Amphotropic murine leukemia viruses induce spongiform encephalomyelopathy. Proc Natl Acad Sci USA. 1997;94:5837–5842. doi: 10.1073/pnas.94.11.5837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Münk C, Thomsen S, Stocking C, Löhler J. Murine leukemia virus recombinants that use phosphate transporters for cell entry induce similar spongiform encephalomyelopathies in newborn mice. Virology. 1998;252:318–323. doi: 10.1006/viro.1998.9476. [DOI] [PubMed] [Google Scholar]
- 37.Olah Z, Lehel C, Anderson W, Eiden M, Wilson C. The cellular receptor for gibbon ape leukemia virus is a novel high affinity sodium-dependent phosphate transporter. J Biol Chem. 1994;269:25426–25431. [PubMed] [Google Scholar]
- 38.Ott D, Keller J, Sill K, Rein A. Phenotypes of murine leukemia virus-induced tumors: influence of 3′ viral coding sequences. J Virol. 1992;66:6107–6116. doi: 10.1128/jvi.66.10.6107-6116.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rapp U, Marshall T. Cell surface receptors for endogenous mouse type C viral glycoprotein and epidermal growth factor: tissue distribution in vivo and possible participation in specific cell-cell interaction. J Supramol Struct. 1980;14:343–352. doi: 10.1002/jss.400140308. [DOI] [PubMed] [Google Scholar]
- 40.Rein A, Schultz A. Different recombinant murine leukemia viruses use different cell surface receptors. Virology. 1984;136:144–152. doi: 10.1016/0042-6822(84)90255-1. [DOI] [PubMed] [Google Scholar]
- 41.Sorge J, Wright D, Erdman V, Cutting A. Amphotropic retrovirus vector system for human cell gene transfer. Mol Cell Biol. 1984;4:1730–1737. doi: 10.1128/mcb.4.9.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stoye J, Coffin J. The four classes of endogenous murine leukemia viruses: structural relationships and potential for recombination. J Virol. 1987;61:2659–2669. doi: 10.1128/jvi.61.9.2659-2669.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stoye J, Coffin J. Polymorphism of murine endogenous proviruses revealed by using virus class-specific oligonucleotide probes. J Virol. 1988;62:168–175. doi: 10.1128/jvi.62.1.168-175.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tailor C, Nouri A, Lee C, Kozak C, Kabat D. Cloning and characterization of a cell surface receptor for xenotropic and polytropic murine leukemia viruses. Proc Natl Acad Sci USA. 1999;96:927–932. doi: 10.1073/pnas.96.3.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tomonga K, Coffin J. Structures of endogenous nonecotropic murine leukemia virus (MLV) long terminal repeats in wild mice: implications for evolution of MLVs. J Virol. 1999;73:4327–4340. doi: 10.1128/jvi.73.5.4327-4340.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986;14:4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.von Laer D, Thomsen S, Vogt B, Donath M, Kruppa J, Rein A, Ostertag W, Stocking C. Entry of amphotropic and 10A1 pseudotyped murine retroviruses is restricted in hematopoietic stem cell lines. J Virol. 1989;72:1424–1430. doi: 10.1128/jvi.72.2.1424-1430.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang H, Kavanaugh M, North R, Kabat D. Cell-surface receptor for ecotropic murine retroviruses is a basic amino-acid transporter. Nature. 1991;352:729–731. doi: 10.1038/352729a0. [DOI] [PubMed] [Google Scholar]
- 49.Wolgamot G, Bonhan L, Miller A. Sequence analysis of Mus dunni endogenous virus reveals a hybrid VL30/gibbon ape leukemia virus-like structure and a distinct envelope. J Virol. 1998;72:7459–7466. doi: 10.1128/jvi.72.9.7459-7466.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang Y-L, Guo L, Xu S, Holland C, Kitamura T, Hunter K, Cunningham J. Receptors for polytropic and xenotropic mouse leukaemia viruses encoded by a single gene at Rmc1. Nat Genet. 1999;21:216–219. doi: 10.1038/6005. [DOI] [PubMed] [Google Scholar]