Abstract
Summary
The study found that in osteoporosis patients who had not previously received bisphosphonate treatment and were in a treatment cycle of over 12 months, both teriparatide and denosumab significantly increased bone mineral density compared to bisphosphonates. Additionally, teriparatide was also shown to significantly decrease the risk of fractures.
Objective
The systematic review and meta-analysis aimed to assess and compare the safety and efficacy of teriparatide vs. bisphosphonates and denosumab vs. bisphosphonates in patients with osteoporosis who had not previously received bisphosphonates.
Methods
We conducted a search of published literature from inception to May 31, 2023, including databases such as PubMed, Embase, Cochrane Library, CNKI, SinoMed, VIP, and WanFang. The study only included head-to-head randomized controlled trials (RCTs) that compared teriparatide and denosumab with bisphosphonates to treat patients with osteoporosis. Fixed-effect model and random-effect model were used due to clinical heterogeneity. Meta-analysis was performed via Stata 17.0.
Results
A total of 6680 patients were enrolled across 23 eligible trials. The results of the meta-analysis showed that teriparatide was superior to bisphosphonates in decreasing the risk of fracture (risk ratio (RR) = 0.61, 95% confidence interval (CI) (0.51, 0.74), P < 0.001). Denosumab showed no benefit compared to bisphosphonates in reducing the risk of fracture in treating osteoporosis (RR 0.99, 95% CI (0.62, 1.57), P = 0.96). Compared with bisphosphonates, teriparatide and denosumab could significantly improve femoral neck, total hip, and lumbar spine bone mineral density (BMD) (P < 0.05). Furthermore, teriparatide and denosumab did not increase the incidence of adverse events (teriparatide vs. bisphosphonates, RR 0.92, 95% CI (0.79, 1.08), P = 0.32; denosumab vs. bisphosphonates, RR 0.98, 95% CI (0.95, 1.02), P = 0.37).
Conclusions
Teriparatide is superior to bisphosphonates in decreasing the risk of fracture in patients with osteoporosis. In addition, teriparatide and denosumab were more efficacious than bisphosphonates in increasing the percentage change in BMD at the femoral neck, total hip, and lumbar spine.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11657-024-01447-7.
Keywords: Teriparatide, Denosumab, Bisphosphonates, Osteoporosis, Randomized controlled trials
Introduction
Population aging is a widespread global phenomenon, leading to an increasing prevalence of diseases related to the elderly, with osteoporosis being a prominent example. Osteoporosis is a systemic bone disease characterized by decreased bone tissue microarchitecture and low bone mineral density, making bones more fragile and prone to fractures [1]. In the USA alone, approximately 1.5 million individuals experience fragility fractures annually [2], while osteoporosis affects nearly 90 million patients in China, with about 15% of individuals over the age of 50 suffering from fractures [3]. Fractures due to osteoporosis can significantly impact patients’ quality of life and lead to substantial financial burdens [4, 5]. The annual cost of fragility fractures in the United States is projected to rise by over 20%, reaching $25.3 billion by 2025 [6].The economic strain associated with osteoporosis underscores the urgent need for effective prevention and treatment strategies.
The primary strategies for osteoporosis prevention and treatment encompass fundamental measures, medicinal intervention, and rehabilitation. Clinical practice typically employs a range of pharmaceuticals, with bisphosphonates (BPs) having been the first-line treatment for osteoporosis since the 1990s [7]. Bisphosphonates work mainly by hampering osteoclast function to inhibit bone resorption [8]. After a 5-year treatment period with oral BPs or 3 years with intravenous BPs, a discontinuation phase may become necessary [9]. Currently, teriparatide is mostly used for patients with severe and high-risk osteoporosis [10]. Teriparatide increases bone formation by boosting the number of active osteoblasts while decreasing osteoblast apoptosis [11, 12]. Denosumab, a fully human monoclonal antibody, binds to the nuclear factor κB ligand-receptor activator (RANKL), which plays a vital role in osteoclast development, differentiation, and survival. This binding offers a possible treatment option for patients with osteoporosis [13]. However, due to the high side effects and poor adherence to anti-osteoporosis drugs, continuous research is needed to improve osteoporosis treatment strategies.
Numerous relevant meta-analyses that compared the effectiveness of teriparatide and denosumab with bisphosphonates have previously been published [14–19]. In studies by Yuan et al., the effectiveness of teriparatide vs. bisphosphonates in the treatment of postmenopausal osteoporosis was analyzed through a direct comparison [14]. Similarly, Lyu et al. employed a head-to-head comparison strategy to examine the clinical impacts of denosumab and bisphosphonates on osteoporosis patients [15, 16]. Furthermore, there have been network meta-analyses that indirectly assessed the effects of bisphosphonates, teriparatide, and denosumab [17–19].
However, these studies had some limitations. For instance, many of them exclusively focused on postmenopausal osteoporosis, thereby excluding male osteoporosis, and lacked a follow-up duration of at least 12 months. Most of the trial groups in these studies had already been treated with bisphosphonates, which hindered the direct distinction between the therapeutic effects of the different anti-osteoporosis medications. Moreover, these studies did not provide a comprehensive evaluation of teriparatide, denosumab, and bisphosphonates. For these reasons, we performed a systematic review and meta-analysis, including a direct comparison of randomized controlled trials (RCTs). Our methodology took into account the latest and high-quality RCTs and employed a wealth of data to validate our conclusions. In contrast to prior meta-analyses, this study was defined by its advanced evidence and the inclusion of a larger male patient population suffering from osteoporosis. We implemented new criteria, whereby participants in the experimental group refrained from bisphosphonate treatment and observed an extended follow-up period. In this study, we explored the influence of the three different anti-osteoporosis drugs (teriparatide, denosumab, and bisphosphonates) on fracture risk reduction, as well as the enhancement of femoral neck, total hip, and lumbar spine bone mineral density in patients with osteoporosis. Our goal was to provide the most comprehensive analysis available to patients.
Materials and methods
The systematic review and meta-analysis were performed in line with the recommendations of the Cochrane Handbook and conducted in compliance with preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [20]. A formal protocol was enrolled on the International Prospective Register of Systematic Reviews (PROSPERO). Registration ID: CRD42023442508.
Data sources and searches
Two authors systematically scanned published literature, we searched in PubMed, Embase, Cochrane Library, CNKI, SinoMed, VIP, and WanFang databases from inception to May 31, 2023. The retrieval strategy included a full-text search of Medical Subject Headings (MeSH) terms as well as word variations that have been searched. The following search terms were used: “osteoporosis” AND “Teriparatide” OR “Denosumab” [MeSH terms] AND Randomized controlled trial. Detailed term variations and complete search strategy results derived from the database can be seen in Table S1. References of all included articles were limited to English-language and Chinese-language studies. Besides, it only included randomized controlled trials, not review reports and animal trials. We also manually searched other electronic sources to identify other potentially eligible trials.
Inclusion and exclusion criteria
Articles eligible for inclusion have satisfied the following criteria: (1) Population: Patients diagnosed with osteoporosis (T-score < − 2.5), no severe or long-term disabling conditions; (2) Intervention: Patients enrolled in the trial group received either teriparatide or denosumab; (3) Comparison: patients in the control group with bisphosphonates; (4) Outcomes: Percentage BMD change in femoral neck, total hip and lumbar spine, risk of fracture, and incidence of adverse events. Additionally, we included studies that were double-blind and open-label randomized controlled trials published in both English and Chinese languages. All included participants had a follow-up of at least 12 months and trials measuring at least one outcome of interest.
Exclusions from this study include (1) duplicate publication articles or studies from the same trials; (2) the type of articles was animal experiments, case report meta-analysis, and other non-RCTs; (3) the included population with cancer or glucocorticoid-induced patients; (4) osteoporotic patients with severe heart disease, severe liver disease, severe diabetes mellitus, severe renal impairment, etc. were excluded; (5) studied exclusion with participants who had previously combination or crossover treatment with bisphosphonates; (6) the follow-up time was less than 12 months; (7) articles exclusion with no outcome of interest and no original data from texts.
Data retrieval and risk of bias assessment
Two reviewers independently screened and extracted the data using a predefined extraction form. In the first step, authors screened the title and abstracts from the retrieval literature and excluded articles if they failed to meet the eligibility criteria. Secondly, the authors browsed the full text, selecting the articles that met the qualified criteria and excluding the irrelevant ones. Then, performing data extraction and quantitative analysis from the included studies. If there is insufficient relevant data, reach out to the primary author of the article. In case the data is unavailable, utilize GetData software for extracting essential information from the figures. Any discrepancy or uncertain error was determined by means of discussion or a third reviewer check. Collected data contains the following parameters: the first name of the author, year of publication, country, basic treatment of included trials, gender, sample size, average age and body mass index of patients, intervention measures between the experimental group and control group, follow-up time, and outcomes. The first outcome was the incidence of risk of fracture; to confirm the influence of various factors on the fracture, we performed a subgroup analysis of this outcome according to the drug type of the control group, sample size, and follow-up time. Other outcomes included percent changes in bone mineral density in the femoral neck, total hip, lumbar spines, and adverse events.
Then, two reviewers independently assessed the risk of bias according to the Cochrane risk-of-bias tool [21]. If there was a dispute between the two authors, then disagreement was confirmed by discussion or consultation with a third author. The risk assessment includes the following items: (1) random sequence generation; (2) allocation concealment; (3) blinding of participants and personnel; (4) blinding of outcome assessment; (5) incomplete outcome data; (6) selective reporting; and (7) other bias. Each bias item was deemed to be high risk, unclear risk, or low risk in accordance with the bias score. The trials were deemed to possess a substantial risk of bias due to the assessment of one bias item as high risk, while all other items exhibited low risk of bias and some remained unclear. Besides, we drafted a funnel plot, Begg’s test, and Egger’s test to examine publication bias and further performed a heterogeneity test to assess the sensitivity of our meta-analysis.
Data synthesis and statistical analysis
All data analyses were performed with Stata 17.0. Our determined outcomes were divided into two categories of data, one was continuous data (percent changes in femoral neck, total hip, lumbar spine BMD) and the other was dichotomous variables data (the risk of fracture and adverse events). Calculated using the weighted mean difference (WMD) with 95% confidence interval (CI) for continuous outcomes and the risk ratio (RR) with 95% CI for dichotomous outcomes. Possible heterogeneity between included studies, the I2 statistic is used to demonstrate the degree of heterogeneity: I2 > 50% reveals remarkable heterogeneity, outcomes data were pooled using a random-effect model; I2 ≤ 50% indicates slight heterogeneity, outcomes data were pooled using a fixed-effect model. To evaluate the deviation of included outcomes, publication bias was assessed by funnel plot, Begg’s test, and Egger’s test. All the tests were set at P < 0.05.
Results
Search results
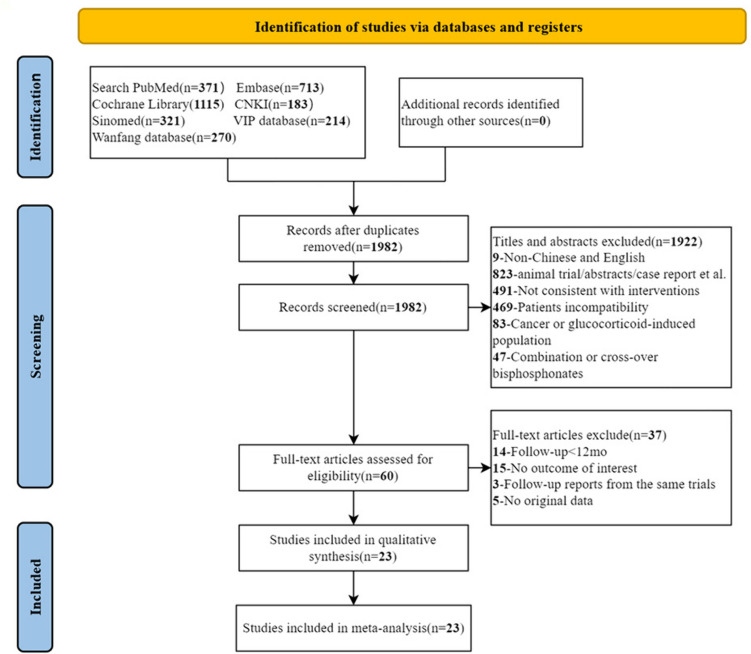
The process of literature screening met the PRISMA guideline flowchart (Fig. 1). An initial search for the effect of teriparatide or denosumab on osteoporosis identified 3187 articles: PubMed (371), Embase (713), Cochrane Library (1 115), CNKI (183), SinoMed (321), VIP database (214), and WanFang database (270). After the removal of duplicates and browsing the title and abstracts, 3127 articles were excluded, and 60 articles were possibly considered to meet the inclusion criteria. Finally, after screening the full texts, 37 studies were excluded based on the institutional inclusion//exclusion criteria, and 23 studies were selected for qualitative analysis and included in the meta-analysis.
Fig. 1.
The flow chart of selection in this systematic review and meta-analysis
Trial characteristics of patients
The major baseline characteristics of the trials are provided in Tables 1 and 2. Included trials were published from 2002 to 2023 and contained a total of 6680 patients; there were 3679 patients in the experimental group and 3001 patients in the control group. All patients (mean age ranged from 51 to 77 years) were given supplemental treatment such as daily oral calcium and vitamin D. The sample size of subjects ranged from 9 to 680, follow-up time of trials ranged from 12 to 30 months. Among the included 23 trials, subjects of 17 trials were female osteoporosis, 2 trials were male osteopetrosis, and 4 trials were female and male osteoporosis. Of these, 16 of 23 trials compared teriparatide with bisphosphonates, and 7 of 23 trials compared denosumab with bisphosphonates. The bisphosphonates included risedronate, alendronate, and zoledronic acid.
Table 1.
Basic characteristics of patients
| Author | Year | Country | Sex (Female/Male) | Basic treatment | Follow-up (month) | Outcomes |
|---|---|---|---|---|---|---|
| Anastasilakis | 2008 | Greece | F | Calcium (500 mg/day), vitamin D (400 IU/day) | 12 | 5 |
| Body | 2002 | USA | F | Calcium (1000 mg/day), vitamin D (400–1200 IU/day) | 12 | 12,345 |
| Chiba | 2022 | Japan | F | Alfacalcidol (1 µg/day), vitamin D) | 18 | 1235 |
| Cosman | 2011 | USA | F | Calcium (1000–1200 mg/day), vitamin D (400–800 IU/day) | 12 | 45 |
| Finkelstein | 2003 | USA | M | Calcium (1000–1200 mg/day), vitamin D (400 IU/day) | 30 | 123 |
| Finkelstein | 2010 | USA | F | Calcium (1000–1200 mg/day), vitamin D (400 IU/day) | 30 | 1235 |
| Hadji | 2012 | Germany | F | Calcium (1000 mg/day), vitamin D (800 IU/day) | 18 | 12,345 |
| Keaveny | 2007 | USA | F | Calcium (1000 mg/day), vitamin D (400–800 IU/day) | 18 | 13 |
| Keaveny | 2012 | USA | F | Calcium (1000 mg/day), vitamin D (400–800 IU/day) | 18 | 12 |
| Kendler | 2018 | Canada | F | Calcium (500–1000 mg/day), vitamin D (400–800 IU/day) | 24 | 45 |
| Li | 2022 | China | F | Calcium (500 mg/day), vitamin D (200 IU/day) | 12 | 345 |
| Malouf-Sierra | 2017 | UK | F/M | Calcium (500–1000 mg/day), vitamin D (800 IU/day) | 18 | 12,345 |
| McClung | 2005 | Brazil | F | Calcium (1000 mg/day), vitamin D (400–800 IU/day) | 18 | 45 |
| Panico | 2011 | Italy | F | Calcium (1 g/day), vitamin D (800 IU/day) | 18 | 1345 |
| Walker | 2013 | Columbia | M | Calcium (500 mg/day), vitamin D (400 IU/day) | 18 | 1345 |
| Ji | 2021 | China | F | Calcium carbonate, calcitriol | 12 | 2345 |
| Beck | 2008 | USA | F | Calcium (1000 mg/day), vitamin D (400 IU/day) | 24 | 1 |
| Brown | 2009 | Spain | F | Calcium (500 mg/day), vitamin D (400 or 800 IU/day) | 12 | 12,345 |
| Iseri | 2019 | Japan | F/M | Calcitriol (0.25 µg/day), calcium lactate (1.5 g/day) | 12 | 1345 |
| Lewiecki | 2007 | USA | F | Calcium (1 g/day), vitamin D (400 IU/day) | 24 | 45 |
| McClung | 2006 | USA | F | Calcium (1 g/day), vitamin D (400 IU/day) | 12 | 2345 |
| Nakamura | 2014 | Japan | F/M | Calcium (600 mg/day), vitamin D (400 IU/day) | 24 | 12,345 |
| Nakura | 2023 | Japan | F/M | Calcium (600 mg/day), vitamin D (400 IU/day) | 24 | 15 |
1, risk of fracture; 2, change in femoral neck BMD; 3, change in total hip BMD; 4, change in lumbar spine BMD; 5, adverse events
SC subcutaneous injection
Table 2.
General characteristics of patients interventions
| Author | Teriparatide OR denosumab | Bisphosphonates | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No | Age (year) | BMI (kg/m2) | Intervention | No | Age (year) | BMI (kg/m2) | Intervention | ||||
| Anastasilakis | 22 | 65.4 | 28.2 | SC teriparatide (20 µg/day) | 22 | 64.7 | 28.1 | Oral risedronate (35 mg/week) | |||
| Body | 73 | 65 | 23.9 | SC teriparatide (40 µg/day) | 73 | 66 | 24.4 | Oral alendronate (10 mg/day) | |||
| Chiba | 46 | 71.7 | 23.4 | SC teriparatide (20 µg/day) | 40 | 71.9 | 23.1 | Oral alendronate (35 mg/week) | |||
| Cosman | 138 | 63.8 | 25.3 | SC teriparatide (20 µg/day) | 137 | 66.1 | 25.3 | Intravenous zoledronic acid (5 mg/year) | |||
| Finkelstein | 25 | 57 | 25.3 | SC teriparatide (40 µg/day) | 28 | 58 | 25.7 | Oral alendronate (10 mg/day) | |||
| Finkelstein | 20 | 65 | 24.9 | SC teriparatide (40 µg/day) | 29 | 64 | 25.6 | Oral alendronate (10 mg/day) | |||
| Hadji | 360 | 70.5 | 26.3 | SC teriparatide (20 µg/day) | 350 | 71.6 | 26.4 | Oral risedronate (35 mg/week) | |||
| Keaveny | 28 | 64.5 | 26.3 | SC teriparatide (20 µg/day) | 25 | 62.5 | 26.6 | Oral alendronate (1 0 mg/day) | |||
| Keaveny | 27 | 64.2 | 25.6 | SC teriparatide (20 µg/day) | 21 | 62.2 | 26.5 | Oral alendronate (10 mg/day) | |||
| Kendler | 680 | 72.6 | 26.9 | SC teriparatide (20 µg/day) | 680 | 71.6 | 21.7 | Oral risedronate (35 mg/week) | |||
| Li | 391 | 64.2 | 23.1 | SC teriparatide (20 µg/day) | 196 | 63.6 | 23.2 | Oral alendronate (70 mg/week) | |||
| Malouf-Sierra | 86 | 77.2 | _ | SC teriparatide (20 µg/day) | 85 | 76.4 | _ | Oral risedronate (35 mg/week) | |||
| McClung | 102 | 65.3 | 25.7 | SC teriparatide (20 µg/day) | 101 | 66.6 | 25.3 | Oral alendronate (10 mg/day) | |||
| Panico | 42 | 65 | 24.5 | SC teriparatide (20 µg/day) | 39 | 60 | 22.8 | Oral alendronate (70 mg/week) | |||
| Walker | 9 | 51.6 | 25.2 | SC teriparatide (20 µg/day) | 10 | 54 | 24.8 | Oral risedronate (35 mg/week) | |||
| Ji | 84 | 66.8 | 23.7 | SC teriparatide (20 µg/day) | 120 | 68.1 | 23.5 | Intravenous zoledronic Acid (5 mg/year) | |||
| Beck | 39 | 63 | 28.32 | SC denosumab (60 mg/6 month) | 38 | 63 | 27.39 | Oral alendronate (70 mg/week) | |||
| Brown | 594 | 64.1 | _ | SC denosumab (60 mg/6 month) | 595 | 64.6 | _ | Oral alendronate (70 mg/week) | |||
| Iseri | 24 | 71.3 | 19.9 | SC denosumab (60 mg/6 month) | 24 | 71.5 | 20.7 | Intravenous alendronate (900 µg/4 week) | |||
| Lewiecki | 319 | 62.3 | _ | SC denosumab (60 mg/6 month) | 47 | 62.8 | _ | Oral alendronate (70 mg/week) | |||
| McClung | 47 | 63.1 | 15.7 | SC denosumab (60 mg/6 month) | 47 | 62.8 | 13.7 | Oral alendronate (70 mg/week) | |||
| Nakamura | 472 | 69.9 | 22.6 | SC denosumab (60 mg/6 month) | 242 | 70.2 | 22.3 | Oral risedronate (35 mg/week) | |||
| Nakura | 51 | 69.9 | 23.2 | SC denosumab (60 mg/6 month) | 52 | 68.3 | 22.5 | Oral risedronate (17.5 mg/week) | |||
Risk of bias assessment
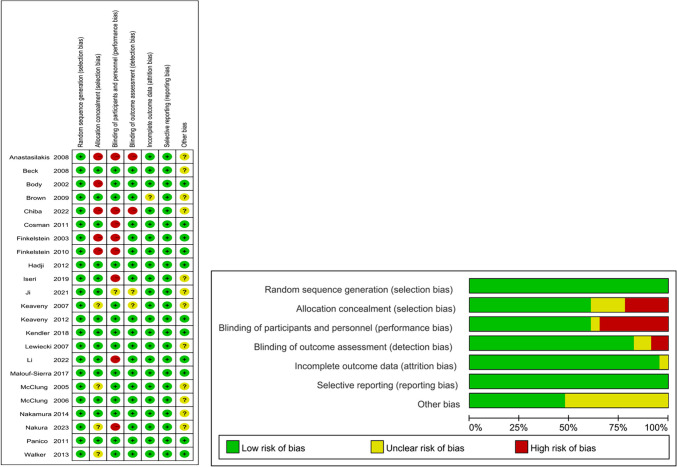
Assessment of the risk of bias was summarized in Fig. 2. Overall, five trials were well-arranged with a low risk of bias, nine trials in unclear, and nine trials in high risk of bias. Random sequence generation and selective reporting were reported and sorted as being at low risk of bias in all trials. Blinding of outcome assessment and incomplete outcome data were generated in over 75% of these trials. Fourteen trials reported appropriate allocation concealment and the assessment of blinding of participants was unclear or inadequately reported in eight trials.
Fig. 2.
Assessment of risk of bias. Green, low risk of bias; yellow, unclear risk of bias; red, high risk of bias
Results of meta-analysis
Risk of fractures
The analysis included 15 trials involving 6264 patients that assessed the risk of fracture incidence. This encompassed 10 trials with 3602 patients comparing teriparatide directly with bisphosphonates, as well as 5 trials with 2662 patients comparing denosumab with bisphosphonates [22–36].
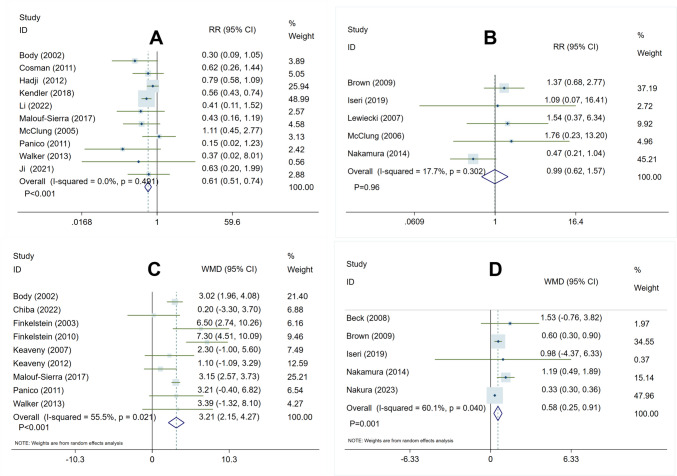
In the comparison of teriparatide vs. bisphosphonate treatment, the heterogeneity test yielded the following results: I2 = 0.0%, P = 0.49, indicating a lack of significant heterogeneity among the studies. This suggested the use of a fixed-effect model for analysis. The pooled analysis demonstrated that teriparatide treatment, in comparison to bisphosphonates, significantly reduced the risk of fracture occurrence (RR 0.61, 95% CI 0.51–0.74, P < 0.001) (Fig. 3A).
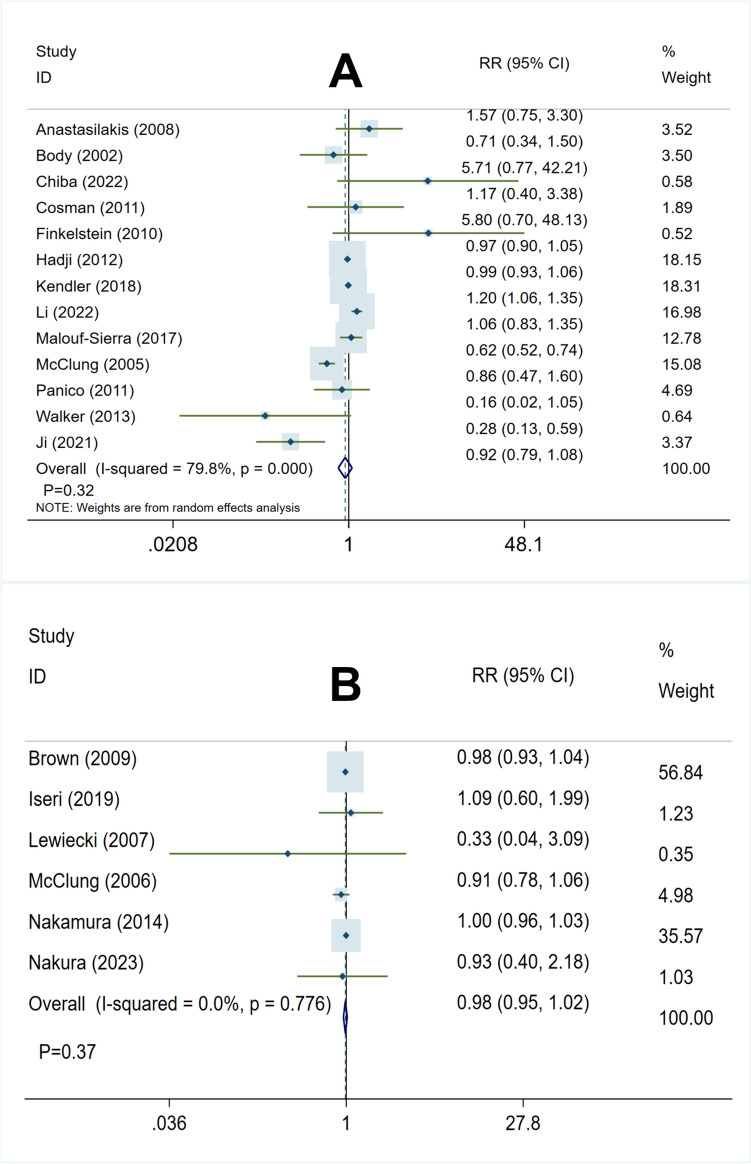
Fig. 3.
Forest plot of meta-analysis result in risk of fractures and percent changes at femoral neck BMD. A Teriparatide vs. bisphosphonates in risk of fractures. B denosumab vs. bisphosphonates in risk of fractures. C Teriparatide vs. bisphosphonates in percent changes at femoral neck BMD. D Denosumab vs. bisphosphonates in percent changes at femoral neck BMD
In the comparison of denosumab vs. bisphosphonate treatment, the heterogeneity test yielded the following results: I2 = 17.7%, P = 0.30, indicating no significant heterogeneity among the trials. This suggested the utilization of a fixed-effect model for analysis. The pooled analysis indicated that denosumab had a similar impact to bisphosphonates in reducing the occurrence of fracture risk (RR 0.99, 95% CI 0.62–1.57, P = 0.96) (Fig. 3B).
Percent changes at femoral neck BMD
In this analysis, percent changes at femoral neck BMD were assessed in 14 trials comprising 2706 patients with 9 trials involving 611 patients comparing teriparatide and bisphosphonates, and 5 trials involving 2095 patients comparing denosumab and bisphosphonates [22, 28, 30–33, 36–43].
When comparing teriparatide treatment with bisphosphonates, the heterogeneity test yielded the following results: I2 = 55.5%, P < 0.05, indicating a slight degree of heterogeneity among the trials. Subsequently, a sensitivity analysis was conducted by systematically removing one study at a time, which confirmed the stability of the included studies (Fig. S1) and recommended the use of a random-effect model for the analysis. The pooled analysis revealed that teriparatide treatment, in comparison to bisphosphonates, resulted in a further increase in percent changes in femoral neck BMD (WMD 3.21, 95% CI 2.15–4.27, P = 0.001) (Fig. 3C).
After conducting a heterogeneity test, it was found that there was slight heterogeneity among the included studies comparing denosumab with bisphosphonates, with I2 = 60.1% and P < 0.05. To ensure data accuracy, a sensitivity analysis was performed by systematically excluding one study at a time. The results indicated that the included studies demonstrated stability (Fig. S2), leading to the recommendation of using a random-effect model for the analysis. The pooled analysis revealed that denosumab was more effective than bisphosphonates in increasing the percent changes at femoral neck BMD (WMD 0.58, 95%CI 0.25–0.91, P = 0.001) (Fig. 3D).
Percent changes at total hip BMD
In this analysis, 11 trials involving 3175 subjects reported percent changes in total hip BMD. The teriparatide vs. bisphosphonates group comprised 8 RCTs with 1190 subjects, while the denosumab vs. bisphosphonates group included 3 RCTs with 1985 subjects [22, 24, 25, 32, 36–41, 43].
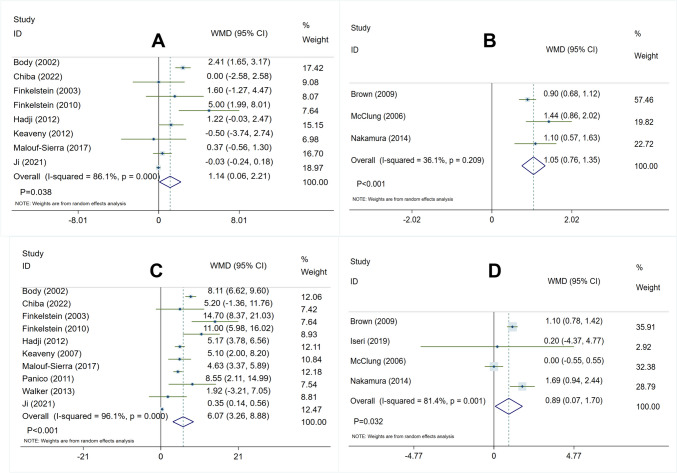
In the comparison between the teriparatide group and the bisphosphonates group, the heterogeneity test yielded as follows: I2 = 86.1%, P < 0.05, indicating potential high heterogeneity among the included trials. To ensure the accuracy of the study, a sensitivity analysis was conducted where each study was systematically excluded to enhance the stability of the results (Fig. S3). A random-effect model was utilized for analysis. The overall pooled analysis revealed that teriparatide was significantly more effective than bisphosphonates in increasing percent changes in total hip BMD (WMD 1.14, 95% CI 0.06–2.21, P = 0.038) (Fig. 4A).
Fig. 4.
Forest plot of meta-analysis result in percent changes at total hip and lumbar spine BMD. A Teriparatide vs. bisphosphonates in percent changes at total hip. B Denosumab vs. bisphosphonates in percent changes at total hip. C Teriparatide vs. bisphosphonates in percent changes at lumbar spine BMD. D Denosumab vs. bisphosphonates in percent changes at lumbar spine BMD
In the comparison between the denosumab group and the bisphosphonates group, the heterogeneity test revealed as follows: I2 = 36.1%, P = 0.21, indicating no significant heterogeneity among the trials. A fixed-effect model was employed for analysis. The overall pooled analysis showed that denosumab was significantly more effective than bisphosphonates in increasing percent changes in total hip BMD (WMD 1.05, 95% CI 0.76–1.35, P < 0.001) (Fig. 4B).
Percent changes at lumbar spine BMD
A total of 14 trials with 3332 subjects, comprising 10 randomized controlled trials involving 1301 subjects comparing teriparatide and bisphosphonates, and 4 RCTs involving 2031 subjects comparing denosumab and bisphosphonates, reported percent changes in total hip BMD [22, 24, 25, 28, 30–33, 35–40].
In the comparison between teriparatide and bisphosphonates treatment, the heterogeneity test showed as follows: I2 = 96.1%, P < 0.05, indicating potential heterogeneity among the randomized controlled trials. Therefore, a sensitivity analysis was performed by leaving out one study in turn, and results showed these RCTs have better stability (Fig. S4). Then, a random-effect model was utilized for analysis. The overall pooled analysis demonstrated that compared with bisphosphonates, teriparatide significantly improved percent changes at lumbar spine BMD (WMD 6.07, 95% CI 3.26–8.88, P < 0.001) (Fig. 4C).
In the comparison between denosumab and bisphosphonates treatment, the heterogeneity test showed as follows: I2 = 81.4%, P < 0.05, suggesting potential heterogeneity among the RCTs. A sensitivity analysis was conducted to verify the accuracy of the included studies by sequentially omitting one study at a time, resulting in better stability in the included trials (Fig. S5), therefore implying the use of a random-effects model for analysis. The overall pooled analysis demonstrated that denosumab treatment significantly increased percent changes in lumbar spine bone mineral density compared to bisphosphonates (WMD 0.89, 95% CI 0.07–1.70, P = 0.032) (Fig. 4D).
Incidence of adverse events
The analysis included a total of 19 trials with 6221 patients assessing the incidence of adverse events, comprising 13 RCTs with 4010 patients comparing teriparatide with bisphosphonates, and 6 RCTs with 2211 patients comparing denosumab with bisphosphonates [22–37, 39, 43, 44]. The adverse included nausea, pyrexia, back pain, arthralgia, myalgia, skin injury, and leg cramps.
In comparison between the teriparatide group and bisphosphonates group, the heterogeneity test showed as follows: I2 = 79.8%, P < 0.05, suggesting potential heterogeneity among studies, then we performed a sensitivity analysis to ensure study accuracy, leaving out one study in turn, and results found included studies had better stability (Fig. S6), therefore implying the use of a random-effects model for analysis. The overall pooled analysis has shown that teriparatide and bisphosphonates were similar in the incidence of adverse events (RR 0.92, 95% CI 0.79–1.08, P = 0.32) (Fig. 5A).
Fig. 5.
Forest plot of meta-analysis result in incidence of adverse. A Teriparatide vs. bisphosphonates in incidence of adverse. B Denosumab vs. bisphosphonates in incidence of adverse
Comparison of the denosumab and bisphosphonates groups revealed the following results from the heterogeneity test: I2 = 0.0%, P = 0.78. These findings indicate that no significant heterogeneity was present among the studies. Therefore, a fixed-effect model was utilized for the analysis. The overall pooled analysis has shown that denosumab and bisphosphonates were equal in the incidence of adverse events (RR 0.98, 95% CI 0.95–1.02, P = 0.37) (Fig. 5B).
Subgroup analysis
Performed subgroup analysis on decreasing the incidence of risk of fracture according to types of bisphosphonates in the control group, sample size, and follow-up time (Tables 3 and 4). For comparison of teriparatide and bisphosphonates, when the control group was treated with alendronate and risedronate, the experimental group could significantly decrease the incidence of risk of fracture. However, compared with zoledronic acid, teriparatide has no obvious advantage (RR 0.62, 95% CI 0.31–1.23, P = 0.17). Subgroup analysis of sample size and follow-up time was consistent with previous results. For comparison of denosumab and bisphosphonates, the findings of all subgroup analyses that reported denosumab and bisphosphonates were not significantly different regarding the incidence of risk of fracture.
Table 3.
Subgroup analysis for teriparatide vs. bisphosphonates for risk of fracture
| Subgroup | No. trials | P value | RR (95% CI) | I-squared |
|---|---|---|---|---|
| Drug of bisphosphonates | ||||
| Alendronate | 4 | 0.022 | 0.51 (0.28, 0.91) | 38.00% |
| Risedronate | 4 | 0.000 | 0.63 (0.51, 0.77) | 10.30% |
| Zoledronic acid | 2 | 0.173 | 0.62 (0.31, 1.23) | 0.00% |
| Sample size of participants | ||||
| No. (≤ 100 of participants) | 4 | 0.008 | 0.37 (0.18, 0.77) | 0.00% |
| No. (> 100 of participants) | 6 | 0.000 | 0.64 (0.53, 0.77) | 1.30% |
| Follow-up | ||||
| = 12 months | 4 | 0.011 | 0.50 (0.29, 0.85) | 0.00% |
| = 18 months | 5 | 0.027 | 0.73 (0.55, 0.96) | 10.90% |
| = 24 months | 1 | 0.000 | 0.56 (0.43, 0.74) | _ |
Table 4.
Subgroup analysis for denosumab vs. bisphosphonates for risk of fracture
| Subgroup | No. trials | P value | RR (95% CI) | I-squared |
|---|---|---|---|---|
| Drug of bisphosphonates | ||||
| Alendronate | 4 | 0.242 | 1.42 (0.79, 2.56) | 0.00% |
| Risedronate | 1 | 0.063 | 0.47 (0.21, 1.04) | _ |
| Sample size of participants | ||||
| No. (≤ 100 of participants) | 3 | 0.434 | 1.53 (0.53, 4.45) | 0.00% |
| No. (> 100 of participants) | 2 | 0.605 | 0.87 (0.52, 1.46) | 74.30% |
| Follow-up | ||||
| = 12 months | 3 | 0.313 | 1.39 (0.73, 2.66) | 0.00% |
| = 24 months | 2 | 0.229 | 0.66 (0.34, 1.30) | 52.00% |
Publication bias
To access the publication bias of the 10 trials in this meta-analysis, compared teriparatide and bisphosphonates on the risk of fracture, there was no significant publication bias according to the examination of the funnel plot (Fig. 6), and further, the Begg’s test yielded P = 0.28 (Fig. S7) and Egger’s test yielded P = 0.24 (Fig. S8).
Fig. 6.
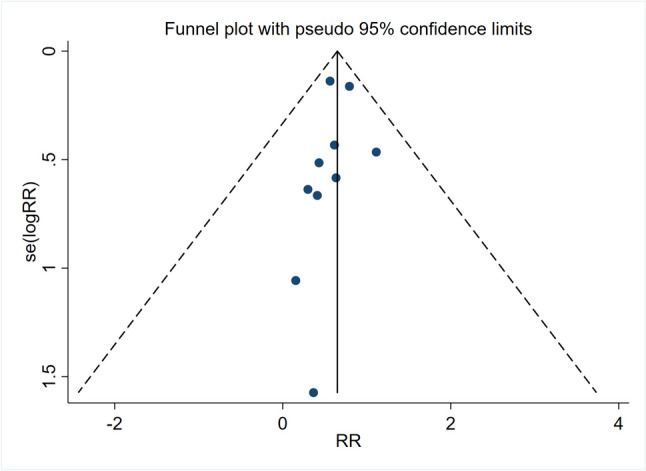
Funnel plot of risk of fracture
Discussion
In clinical practice, selecting the appropriate anti-osteoporosis drug treatment is vital for patients, given that long-term medication is necessary to delay disease progression and improve the quality of life. Effective anti-osteoporosis drug therapy increases bone density, improves bone quality, and significantly reduces the risk of fractures. However, due to limitations in the effectiveness of the original medication used, patients often need to switch to other medications. For example, romosozumab, zoledronic acid, teriparatide, denosumab, and elcatonin are often used clinically; however, patients often need to be switched to other medications due to the limited efficacy of the original medications used. Therefore, this work focuses on comparing the efficacy and safety of different anti-osteoporosis drugs in osteoporosis not previously treated with bisphosphonates.
After carefully examining 23 randomized controlled trials involving 6680 patients, our meta-analysis compared the effectiveness and safety of teriparatide and bisphosphonates, denosumab, and bisphosphonates in treating osteoporosis. The results indicated that teriparatide significantly reduced the risk of fractures compared to bisphosphonates. Furthermore, both teriparatide and denosumab surpass bisphosphonates in improving bone mineral density in the femoral neck, total hip, and lumbar spine. There are no notable differences found in the incidence of adverse events between teriparatide, denosumab, and bisphosphonates.
Though several relevant meta-analyses have been previously published, there were several points of differences between our meta-analysis and those of earlier studies [14–19]. Firstly, two prior meta-analyses were conducted solely on postmenopausal women [14, 16]. While the prevalence of osteoporosis is significantly higher in women, the likelihood of osteoporotic fractures in men is comparable to that in women. Hence, attention must also be directed at preventing and treating osteoporosis in men. Our meta-analysis includes both men and women suffering from osteoporosis, thus broadening the scope of the population studied and increasing the reliability of the results. Secondly, in a meta-analysis comparing denosumab and bisphosphonates, patients in the denosumab group had previously received bisphosphonate treatment [15]. However, the patients in our denosumab group had not received bisphosphonate treatment, leading to a significant reduction in the heterogeneity of the meta-analysis. Thirdly, several orthodox meta-analyses have compared the effectiveness and safety of anti-osteoporosis drugs in treating osteoporosis [17–19]. However, the comparison among teriparatide, denosumab, and bisphosphonates in those studies was indirect head-to-head comparisons. Moreover, in our study, the osteoporosis patients were under treatment for at least 12 months. We also excluded patients with osteoporosis induced by malignant diseases or those taking other hormone drugs, allowing us to effectively evaluate the drug treatment for patients suffering from age-induced osteoporosis. In summary, our meta-analysis is the most comprehensive comparison of the efficacy of teriparatide, denosumab, and bisphosphonates, including the latest and high-quality randomized controlled trials.
In this systematic review and meta-analysis, a total of 15 randomized controlled trials showed that teriparatide and denosumab are more effective than bisphosphonates in increasing the bone mineral density (BMD) in the femoral neck. Eleven RCTs demonstrated that teriparatide and denosumab are more effective than bisphosphonates in improving the BMD of the total hip, and 14 RCTs reported that teriparatide and denosumab are beneficial in enhancing lumbar spine BMD. This suggests that teriparatide and denosumab can be selected to augment the body’s bone mineral density and restore it to normal levels in patients with low bone mass. Fifteen RCTs included in this meta-analysis compared the three kinds of anti-osteoporosis drugs in reducing the risk of fractures, revealing that teriparatide outperformed bisphosphonates after 12, 18, and 24 months of treatment. This suggests that teriparatide could be preferentially considered in the clinical treatment of patients with extremely high fracture risk. Compared with bisphosphonates, denosumab did not yield any significant effect on fractures for the following reasons. Firstly, the lack of sequential or combination treatment with bisphosphonate may explain why the benefit of denosumab is not apparent. Consequently, it can be inferred that denosumab needs to be used for a long period before it can play a pivotal role in reducing the risk of fractures. In addition to this, it has also been shown in several studies that sequential treatment with bisphosphonates followed by denosumab helps to reduce fractures, and therefore, based on the findings of this study, it is suggested that sequential treatment with denosumab and bisphosphonates in patients with osteoporosis is more effective in achieving a reduction in fractures [45–47]. Secondly, the number of patients included in the RCTs of the denosumab group and the bisphosphonate group was small, leading to unclear effects. Hence, more research is needed to validate this hypothesis. The results from subgroup analyses showed that teriparatide surpassed alendronate and risedronate in reducing the risk of fractures, but there was no significant difference between teriparatide and zoledronic acid. This suggested that zoledronic acid may be very effective in reducing the risk of fractures among bisphosphonates. Therefore, using teriparatide and denosumab in clinical practice greatly benefits the treatment of patients suffering from osteoporosis. The safety of drugs for treating osteoporosis was also examined, with results indicating that all three types of drugs did not have significant differences in terms of side effects. Therefore, when it comes to selecting an anti-osteoporosis medication for treating osteoporosis, the priority should be to choose drugs that demonstrate significant effectiveness and minimal adverse effects.
Despite these findings, several limitations must be taken into account. Firstly, two different doses of teriparatide were used in this systematic review and meta-analysis—20 µg and 40 µg. Most of the studies used 20 µg of teriparatide. As such, no comparisons were conducted regarding the efficacy of varying doses of teriparatide and bisphosphonates in reducing fracture risks, enhancing bone mineral density, and decreasing the occurrence of adverse events. Secondly, the heterogeneity of the study could be ascribed to the type of bisphosphonates used, different follow-up durations, and adjuvant therapy. And there is an impact of RCT studies with small samples on the results, and more and larger sample sizes are needed for validation. Thirdly, the evaluation of drug efficacy in this meta-analysis only considered changes in bone mineral density and risk of fracture as observational indicators, ignoring other indicators such as bone metabolism markers. Fourthly, this study did not compare the serious adverse reactions caused by teriparatide, denosumab, and bisphosphonates. Finally, in subgroup analyses, there was no significant difference in fracture reduction between zoledronic acid and teriparatide. However, as only two RCTs were included for comparison, further studies are required to confirm whether teriparatide indeed surpasses zoledronic acid in terms of its fracture reduction efficacy.
Conclusions
The results of our research reveal that teriparatide and denosumab outperform bisphosphonates in increasing percentage changes in femoral neck, total hip, and lumbar spine BMD for patients suffering from osteoporosis. Additionally, teriparatide significantly reduces the risk of fractures compared to bisphosphonates. Existing data suggests no substantial difference in the incidence of adverse events among teriparatide, denosumab, and bisphosphonates. As such, both teriparatide and denosumab have proven to be effective and do not result in additional adverse events when treating osteoporosis patients. However, additional randomized controlled trials are still required for further confirmation.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contribution
Mingnian Li: writing—review and editing, writing—original draft, validation, methodology, investigation, formal analysis, data curation. Zhuoqi Ge: review and editing, visualization, supervision, investigation. Benqi Zhang: investigation, data curation. Li Sun: review and editing, validation, conceptualization. Tao Zou: data curation, review. Zhongyuan Wang: validation, supervision. Qi Chen: methodology, review and editing, visualization, validation, supervision, project administration.
Funding
This work was supported by Guizhou Province’s high-level innovative talent “thousands levels” training funds (GZSYQCC[2023]008).
Data availability
The datasets for this study can be found in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Declarations
Ethics approval
Not applicable.
Conflicts of interest
None.
Footnotes
The original online version of this article was revised: The original article, due to our oversight, had the following incorrect affiliation as follows:
Mingnian Li1,2 · Zhuoqi Ge2 · Benqi Zhang1 · Li Sun2 · Zhongyuan Wang2 · Tao Zou3 · Qi Chen1,2
1 Department of Pharmacy, Guizhou Provincial People’s Hospital, Guiyang, Guizhou, China.
2 College of Pharmacy, Guizhou Medical University, Guiyang, Guizhou, China.
3 Department of Pharmacy, Beijing Jishuitan Hospital Guizhou Hospital, Guiyang, Guizhou, China.
In this article the affiliation details for Author Mingnian Li were incorrectly given as 'Department of Pharmacy, Guizhou Provincial People’s Hospital, Guiyang, Guizhou, China' but should have been 'College of Pharmacy, Guizhou Medical University, Guiyang, Guizhou, China'.
The corrected affiliation details are as follows:
Mingnian Li1 · Zhuoqi Ge2 · Benqi Zhang1 · Li Sun2 · Zhongyuan Wang2 · Tao Zou3 · Qi Chen1,2
1 College of Pharmacy, Guizhou Medical University, Guiyang, Guizhou, China.
2 Department of Pharmacy, Guizhou Provincial People’s Hospital, Guiyang, Guizhou, China.
3 Department of Pharmacy, Beijing Jishuitan Hospital Guizhou Hospital, Guiyang, Guizhou, China.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
11/16/2024
The original online version of this article was revised: The original article, due to our oversight, had the following incorrect affiliation as follows:
Mingnian Li1,2 · Zhuoqi Ge2 · Benqi Zhang1 · Li Sun2 · Zhongyuan Wang2 · Tao Zou3 · Qi Chen1,2
1 Department of Pharmacy, Guizhou Provincial People’s Hospital, Guiyang, Guizhou, China.
2 College of Pharmacy, Guizhou Medical University, Guiyang, Guizhou, China.
3 Department of Pharmacy, Beijing Jishuitan Hospital Guizhou Hospital, Guiyang, Guizhou, China.
In this article the affiliation details for Author Mingnian Li were incorrectly given as 'Department of Pharmacy, Guizhou Provincial People’s Hospital, Guiyang, Guizhou, China' but should have been 'College of Pharmacy, Guizhou Medical University, Guiyang, Guizhou, China'.
The corrected affiliation details are as follows:
Mingnian Li1 · Zhuoqi Ge2 · Benqi Zhang1 · Li Sun2 · Zhongyuan Wang2 · Tao Zou3 · Qi Chen1,2
1 College of Pharmacy, Guizhou Medical University, Guiyang, Guizhou, China.
2 Department of Pharmacy, Guizhou Provincial People’s Hospital, Guiyang, Guizhou, China.
3 Department of Pharmacy, Beijing Jishuitan Hospital Guizhou Hospital, Guiyang, Guizhou, China.
Change history
11/21/2024
A Correction to this paper has been published: 10.1007/s11657-024-01478-0
References
- 1.Rachner TD, Khosla S, Hofbauer LC (2011) Osteoporosis: now and the future. Lancet 377(9773):1276–1287. 10.1016/s0140-6736(10)62349-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clynes MA, Harvey NC, Curtis EM, Fuggle NR, Dennison EM, Cooper C (2020) The epidemiology of osteoporosis. Br Med Bull 133(1):105–117. 10.1093/bmb/ldaa005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang Z (2023) Guideline for diagnosis and treatment of primary osteoporosis(in Chinese) (2022). Chin Gen Pract 26(14):1671–1691 [Google Scholar]
- 4.Adachi JD, Loannidis G, Berger C, Joseph L, Papaioannou A, Pickard L, Papadimitropoulos EA, Hopman W, Poliquin S, Prior JC et al (2001) The influence of osteoporotic fractures on health-related quality of life in community-dwelling men and women across Canada. Osteoporos Int 12(11):903–908. 10.1007/s001980170017 [DOI] [PubMed] [Google Scholar]
- 5.Tarride J, Adachi JD, Brown JP, Schemitsch E, Slatkovska L, Burke N (2021) Incremental costs of fragility fractures: a population-based matched -cohort study from Ontario, Canada. Osteoporos Int 32(9):1753–1761. 10.1007/s00198-021-05877-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A (2007) Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res 22(3):465–475. 10.1359/jbmr.061113 [DOI] [PubMed] [Google Scholar]
- 7.Khosla S, Bilezikian JP, Dempster DW, Lewiecki EM, Miller PD, Neer RM, Recker RR, Shane E, Shoback D, Potts JT (2012) Benefits and risks of bisphosphonate therapy for osteoporosis. J Clin Endocrinol Metab 97(7):2272–2282. 10.1210/jc.2012-1027 [DOI] [PubMed] [Google Scholar]
- 8.McClung MR (2021) Role of bone-forming agents in the management of osteoporosis. Aging Clin Exp Res 33(4):775–791. 10.1007/s40520-020-01708-8 [DOI] [PubMed] [Google Scholar]
- 9.Adler RA, El-Hajj Fuleihan G, Bauer DC, Camacho PM, Clarke BL, Clines GA, Compston JE, Drake MT, Edwards BJ, Favus MJ et al (2016) Managing osteoporosis in patients on long-term bisphosphonate treatment: report of a Task Force of the American Society for Bone and Mineral Research. J Bone Miner Res 31(1):16–35. 10.1002/jbmr.2708 [DOI] [PubMed] [Google Scholar]
- 10.Blick SK, Dhillon S, Keam SJ (2008) Teriparatide: a review of its use in osteoporosis. Drugs 68(18):2709–2737. 10.2165/0003495-200868180-00012 [DOI] [PubMed] [Google Scholar]
- 11.Girotra M, Rubin MR, Bilezikian JP (2006) The use of parathyroid hormone in the treatment of osteoporosis. Rev Endocr Metab Disord 7(1–2):113–121. 10.1007/s11154-006-9007-z [DOI] [PubMed] [Google Scholar]
- 12.Brixen KT, Christensen PM, Ejersted C, Langdahl BL (2004) Teriparatide (biosynthetic human parathyroid hormone 1–34): a new paradigm in the treatment of osteoporosis. Basic Clin Pharmacol Toxicol 94(6):260–270. 10.1111/j.1742-7843.2004.pto940602.x [DOI] [PubMed] [Google Scholar]
- 13.Dennison EM, Cooper C, Kanis JA, Bruyère O, Silverman S, McCloskey E, Abrahamsen B, Prieto-Alhambra D, Ferrari S (2019) Fracture risk following intermission of osteoporosis therapy. Osteopor Int 30(9):1733–1743. 10.1007/s00198-019-05002-w [DOI] [PubMed] [Google Scholar]
- 14.Yuan F, Peng W, Yang C, Zheng J (2019) Teriparatide versus bisphosphonates for treatment of postmenopausal osteoporosis: A meta-analysis. Int J Surg 66:1–11. 10.1016/j.ijsu.2019.03.004 [DOI] [PubMed] [Google Scholar]
- 15.Lyu H, Jundi B, Xu C, Tedeschi SK, Yoshida K, Zhao S, Nigwekar SU, Leder BZ, Solomon DH (2019) Comparison of denosumab and bisphosphonates in patients with osteoporosis: a meta-analysis of randomized controlled trials. J Clin Endocrinol Metab 104(5):1753–1765. 10.1210/jc.2018-02236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu J, Zhang Q, Yan G, Jin X (2018) Denosumab compared to bisphosphonates to treat postmenopausal osteoporosis: a meta-analysis. J Orthop Surg Res 13(1):194. 10.1186/s13018-018-0865-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ayers C, Kansagara D, Lazur B, Fu R, Kwon A, Harrod C (2023) Effectiveness and safety of treatments to prevent fractures in people with low bone mass or primary osteoporosis: a living systematic review and network meta-analysis for the American College of Physicians. Ann Intern Med 176(2):182–195. 10.7326/m22-0684 [DOI] [PubMed] [Google Scholar]
- 18.Simpson EL, Martyn-St James M, Hamilton J, Wong R, Gittoes N, Selby P, Davis S (2020) Clinical effectiveness of denosumab, raloxifene, romosozumab, and teriparatide for the prevention of osteoporotic fragility fractures: a systematic review and network meta-analysis. Bone 130:115081. 10.1016/j.bone.2019.115081 [DOI] [PubMed] [Google Scholar]
- 19.Wang WY, Chen LH, Ma WJ, You RX (2023) Drug efficacy and safety of denosumab, teriparatide, zoledronic acid, and ibandronic acid for the treatment of postmenopausal osteoporosis: a network meta-analysis of randomized controlled trials. European Review for Medical and Pharmacological Sciences 27(17):8253–8268. 10.26355/eurrev_202309_33586 [DOI] [PubMed] [Google Scholar]
- 20.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ (Clinical research ed) 339:b2700. 10.1136/bmj.b2700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Higgins JPT, Altman DG, Gtzsche PC, Jüni P, Sterne JAC: Higgins JP, Altman DG, Gotzsche PC et al., The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343 5928 [DOI] [PMC free article] [PubMed]
- 22.Body JJ, Gaich GA, Scheele WH, Kulkarni PM, Miller PD, Peretz A, Dore RK, Correa-Rotter R, Papaioannou A, Cumming DC et al (2002) A randomized double-blind trial to compare the efficacy of teriparatide [recombinant human parathyroid hormone (1–34)] with alendronate in postmenopausal women with osteoporosis. J Clin Endocrinol Metab 87(10):4528–4535. 10.1210/jc.2002-020334 [DOI] [PubMed] [Google Scholar]
- 23.Cosman F, Eriksen EF, Recknor C, Miller PD, Guañabens N, Kasperk C, Papanastasiou P, Readie A, Rao H, Gasser JA et al (2011) Effects of intravenous zoledronic acid plus subcutaneous teriparatide [rhPTH(1–34)] in postmenopausal osteoporosis. J Bone Miner Res 26(3):503–511. 10.1002/jbmr.238 [DOI] [PubMed] [Google Scholar]
- 24.Hadji P, Zanchetta JR, Russo L, Recknor CP, Saag KG, McKiernan FE, Silverman SL, Alam J, Burge RT, Krege JH et al (2012) The effect of teriparatide compared with risedronate on reduction of back pain in postmenopausal women with osteoporotic vertebral fractures. Osteoporos Int 23(8):2141–2150. 10.1007/s00198-011-1856-y [DOI] [PubMed] [Google Scholar]
- 25.Ji C, Zhang L, Yang H (2021) Comparative the efficacy of teriparatide and zoledronic acid in the treatment of postmenopausal osteoporotic vertebral fractures(in Chinese). Chin J Bone Joint Surg 14(12):1011–1015 [Google Scholar]
- 26.Kendler DL, Marin F, Zerbini CAF, Russo LA, Greenspan SL, Zikan V, Bagur A, Malouf-Sierra J, Lakatos P, Fahrleitner-Pammer A et al (2018) Effects of teriparatide and risedronate on new fractures in post-menopausal women with severe osteoporosis (VERO): a multicentre, double-blind, double-dummy, randomised controlled trial. Lancet (London, England) 391(10117):230–240. 10.1016/s0140-6736(17)32137-2 [DOI] [PubMed] [Google Scholar]
- 27.Li M, Zhang Z, Xue Q, Li Q, Jin X, Dong J, Cheng Q, You L, Lin H, Tang H et al (2022) Efficacy of generic teriparatide and alendronate in Chinese postmenopausal women with osteoporosis: a prospective study. Arch Osteoporos 17(1):103. 10.1007/s11657-022-01131-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Malouf-Sierra J, Tarantino U, García-Hernández PA, Corradini C, Overgaard S, Stepan JJ, Borris L, Lespessailles E, Frihagen F, Papavasiliou K et al (2017) Effect of teriparatide or risedronate in elderly patients with a recent pertrochanteric hip fracture: final results of a 78-week randomized clinical trial. J Bone Miner Res 32(5):1040–1051. 10.1002/jbmr.3067 [DOI] [PubMed] [Google Scholar]
- 29.McClung MR, San Martin J, Miller PD, Civitelli R, Bandeira F, Omizo M, Donley DW, Dalsky GP, Eriksen EF (2005) Opposite bone remodeling effects of teriparatide and alendronate in increasing bone mass. Arch Intern Med 165(15):1762–1768. 10.1001/archinte.165.15.1762 [DOI] [PubMed] [Google Scholar]
- 30.Panico A, Lupoli GA, Marciello F, Lupoli R, Cacciapuoti M, Martinelli A, Granieri L, Iacono D, Lupoli G (2011) Teriparatide vs. alendronate as a treatment for osteoporosis: changes in biochemical markers of bone turnover, BMD and quality of life. Med Sci Monit 17(8):442–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walker MD, Cusano NE, Sliney J Jr, Romano M, Zhang C, McMahon DJ, Bilezikian JP (2013) Combination therapy with risedronate and teriparatide in male osteoporosis. Endocrine 44(1):237–246. 10.1007/s12020-012-9819-4 [DOI] [PubMed] [Google Scholar]
- 32.Brown JP, Prince RL, Deal C, Recker RR, Kiel DP, De Gregorio LH, Hadji P, Hofbauer LC, Álvaro-Gracia JM, Wang H et al (2009) Comparison of the effect of denosumab and alendronate on BMD and biochemical markers of bone turnover in postmenopausal women with low bone mass: a randomized, blinded, phase 3 trial. J Bone Miner Res 24(1):153–161. 10.1359/jbmr.0809010 [DOI] [PubMed] [Google Scholar]
- 33.Iseri K, Watanabe M, Yoshikawa H, Mitsui H, Endo T, Yamamoto Y, Iyoda M, Ryu K, Inaba T, Shibata T (2019) Effects of denosumab and alendronate on bone health and vascular function in hemodialysis patients: a randomized, controlled trial. J Bone Miner Res 34(6):1014–1024. 10.1002/jbmr.3676 [DOI] [PubMed] [Google Scholar]
- 34.Lewiecki EM, Miller PD, McClung MR, Cohen SB, Bolognese MA, Liu Y, Wang A, Siddhanti S, Fitzpatrick LA (2007) Two-year treatment with denosumab (AMG 162) in a randomized phase 2 study of postmenopausal women with low BMD. J Bone Miner Res 22(12):1832–1841. 10.1359/jbmr.070809 [DOI] [PubMed] [Google Scholar]
- 35.McClung MR, Michael Lewiecki E, Cohen SB, Bolognese MA, Woodson GC, Moffett AH, Peacock M, Miller PD, Lederman SN, Chesnut CH et al (2006) Denosumab in postmenopausal women with low bone mineral density. N Engl J Med 354(8):821–831. 10.1056/NEJMoa044459 [DOI] [PubMed] [Google Scholar]
- 36.Nakamura T, Matsumoto T, Sugimoto T, Hosoi T, Miki T, Gorai I, Yoshikawa H, Tanaka Y, Tanaka S, Sone T et al (2014) Clinical trials express: fracture risk reduction with denosumab in Japanese postmenopausal women and men with osteoporosis: Denosumab Fracture Intervention Randomized Placebo Controlled Trial (DIRECT). J Clin Endocrinol Metab 99(7):2599–2607. 10.1210/jc.2013-4175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chiba K, Okazaki N, Kurogi A, Watanabe T, Mori A, Suzuki N, Adachi K, Era M, Yokota K, Inoue T et al (2022) Randomized controlled trial of daily teriparatide, weekly high-dose teriparatide, or bisphosphonate in patients with postmenopausal osteoporosis: the TERABIT study. Bone 160:116416. 10.1016/j.bone.2022.116416 [DOI] [PubMed] [Google Scholar]
- 38.Finkelstein JS, Hayes A, Hunzelman JL, Wyland JJ, Lee H, Neer RM (2003) The effects of parathyroid hormone, alendronate, or both in men with osteoporosis. N Engl J Med 349(13):1216–1226. 10.1056/NEJMoa035725 [DOI] [PubMed] [Google Scholar]
- 39.Finkelstein JS, Wyland JJ, Lee H, Neer RM (2010) Effects of teriparatide, alendronate, or both in women with postmenopausal osteoporosis. J Clin Endocrinol Metab 95(4):1838–1845. 10.1210/jc.2009-1703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Keaveny TM, Donley DW, Hoffmann PF, Mitlak BH, Glass EV, San Martin JA (2007) Effects of teriparatide and alendronate on vertebral strength as assessed by finite element modeling of QCT scans in women with osteoporosis. J Bone Miner Res 22(1):149–157. 10.1359/jbmr.061011 [DOI] [PubMed] [Google Scholar]
- 41.Keaveny TM, McClung MR, Wan X, Kopperdahl DL, Mitlak BH, Krohn K (2012) Femoral strength in osteoporotic women treated with teriparatide or alendronate. Bone 50(1):165–170. 10.1016/j.bone.2011.10.002 [DOI] [PubMed] [Google Scholar]
- 42.Beck TJ, Lewiecki EM, Miller PD, Felsenberg D, Liu Y, Ding B, Libanati C (2008) Effects of denosumab on the geometry of the proximal femur in postmenopausal women in comparison with alendronate. J Clin Densitom 11(3):351–359. 10.1016/j.jocd.2008.04.001 [DOI] [PubMed] [Google Scholar]
- 43.Nakura N, Hirakawa K, Takayanagi S, Mihara M (2023) Denosumab prevented periprosthetic bone resorption better than risedronate after total hip arthroplasty. J Bone Miner Metab 41(2):239–247. 10.1007/s00774-023-01405-2 [DOI] [PubMed] [Google Scholar]
- 44.Anastasilakis AD, Goulis DG, Polyzos SA, Gerou S, Koukoulis GN, Efstathiadou Z, Kita M, Avramidis A (2008) Head-to-head comparison of risedronate vs. teriparatide on bone turnover markers in women with postmenopausal osteoporosis: a randomised trial. Int J Clin Pract 62(6):919–924. 10.1111/j.1742-1241.2008.01768.x [DOI] [PubMed] [Google Scholar]
- 45.Miller PD, Pannacciulli N, Malouf-Sierra J, Singer A, Czerwiński E, Bone HG, Wang C, Huang S, Chines A, Lems W et al (2020) Efficacy and safety of denosumab vs. bisphosphonates in postmenopausal women previously treated with oral bisphosphonates. Osteoporos Int 31(1):181–191. 10.1007/s00198-019-05233-x [DOI] [PubMed] [Google Scholar]
- 46.Anastasilakis AD, Polyzos SA, Gkiomisi A, Saridakis ZG, Digkas D, Bisbinas I, Sakellariou GT, Papatheodorou A, Kokkoris P, Makras P (2015) Denosumab versus zoledronic acid in patients previously treated with zoledronic acid. Osteoporos Int 26(10):2521–2527. 10.1007/s00198-015-3174-2 [DOI] [PubMed] [Google Scholar]
- 47.Miller PD, Pannacciulli N, Brown JP, Czerwinski E, Nedergaard BS, Bolognese MA, Malouf J, Bone HG, Reginster JY, Singer A et al (2016) Denosumab or zoledronic acid in postmenopausal women with osteoporosis previously treated with oral bisphosphonates. J Clin Endocrinol Metab 101(8):3163–3170. 10.1210/jc.2016-1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets for this study can be found in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.