Abstract
Measles virus (MV) interacts with cellular receptors on the surface of peripheral blood lymphocytes (PBL) which mediate virus binding and uptake. Simultaneously, the direct contact of the viral glycoproteins with the cell surface induces a negative signal blocking progression to the S phase of the cell cycle, resulting in a pronounced proliferation inhibition. We selected a monoclonal antibody (MAb 5C6) directed to the surface of highly MV-susceptible B cells (B95a), which inhibits binding to and infection of cells with MV wild-type and vaccine strains. By screening a retroviral cDNA library from human splenocytes (ViraPort; Stratagene) with this antibody, we cloned and identified the recognized molecule as signaling lymphocytic activation molecule (SLAM; CD150), which is identical to the MV receptor recently found by H. Tatsuo et al. (Nature 406:893–897, 2000). After infection of cells, and after surface contact with MV envelope proteins, SLAM is downregulated from the cell surface of activated PBL and cell lines. Although anti-SLAM and/or anti-CD46 antibodies block virus binding, they do not interfere with the contact-mediated proliferation inhibition. In addition, the cell-type-specific expression of SLAM does not correlate with the sensitivity of cells for proliferation inhibition. The data indicate that proliferation inhibition induced by MV contact is independent of the presence or absence of the virus-binding receptors SLAM and CD46.
Measles virus (MV) is among the most widespread human pathogens, causing approximately 1 million deaths worldwide each year mainly due to its immunosuppressive potential (for reviews, see references 4, 11, and 37). During and weeks after acute measles, delayed-type hypersensitivity skin test responses to recall antigens are suppressed, and there is an increased susceptibility to opportunistic infections, which aggravates the course of the disease. One aspect of the viral immunosuppression is the proliferation inhibition in response to mitogens, T-cell receptor cross-linking, or recall antigens of peripheral blood mononuclear cells (PBMC) isolated from patients (ex vivo) and in vitro. Recently, we found that direct contact of the MV glycoproteins hemagglutinin (H) and fusion protein (F) with the cell surface of lymphocytes or lymphoid cell lines induces a dominant negative signal in the contacted cells, leading to this proliferative inhibition (33, 44). It is likely that this negative signal is transduced by a receptor present on the surface of lymphoid cells.
Using MV vaccine strains such as Edmonston (Edm), CD46 was identified as a cellular receptor for MV (8, 30). However, MV wild-type isolates do not or only with low affinity interact with CD46 (3, 16, 23). It has been demonstrated that MV can efficiently be isolated from patients using B-cell lines, such as B95a (19), which lack a complete CD46 (15, 28), indicating the presence of another receptor. To identify this receptor, we selected a monoclonal antibody (MAb) directed to the surface of B95a cells which inhibits MV binding and infection and identified the recognized molecule as SLAM (CD150). Thus, it is identical to the MV receptor recently found by Tatsuo et al. by different means (40).
SLAM is a glycoprotein belonging to the CD2 subset of the immunoglobulin (Ig) superfamily and is expressed on the surface of a proportion of primary B cells and Epstein-Barr virus (EBV)-transformed B cells, activated T cells, memory T cells, T-cell clones, and immature thymocytes (39). It is rapidly induced on naive lymphocytes after activation, and cross-linking antibodies to SLAM stimulate B-and T-cell proliferation (2, 7, 32). Since SLAM is a signal-transducing molecule, the antiproliferative effect exerted by MV contact to the cell surface of lymphocytes could possibly be mediated by SLAM. We therefore assessed the involvement of SLAM in this process. We investigated the virus-mediated SLAM modulation and the effect of SLAM engagement on the viral contact-mediated proliferation inhibition of lymphocytes.
MATERIALS AND METHODS
Antibodies, cells, and viruses.
To raise MAbs to enriched surface proteins of B95a cells, we biotinylated 107 cells with sulfo-d-biotin-N-hydroxysuccinimide ester (sulfo-NHS-LC-biotin; Pierce) and prepared a lysate with radioimmunoprecipitation assay buffer (150 mM NaCl, 10 mM Tris, 0.1% sodium dodecyl sulfate [SDS], 1% sodium desoxy cholate, 1% Triton X-100). The biotinylated proteins were precipitated using 50 μl of streptavidin-agarose (Pierce) and washed four times with phosphate-buffered saline (PBS). BALB/c mice were immunized intraperitoneally three times with the beads in 500 μl of PBS before spleen cells were fused with SP2/0 myeloma cells.
The MAbs 5C6 (anti-SLAM), L77, K4, and K29 (directed against the MV H, protein [MV-H] [21]), 13/42 (anti-CD46 CCP1 [5, 34]), and 10/88 (anti-CD46 CCP3/4 [3]) were produced using RPMI 1640 medium containing 10% fetal calf serum (FCS) and purified over protein G-Sepharose in our laboratory. The fluorescein isothiocyanate (FITC)-conjugated rabbit anti-mouse Ig and rabbit anti-human IgG antibodies were purchased from DAKO, anti-SLAM MAb clone IPO-3 was purchased from Kamiya Biomedicals, and anti-CD69 MAb was purchased from PharMingen. MAb K4 was biotinylated using sulfo-NHS-biotin according to the protocol provided by the company.
The EBV-positive marmoset (Saguinus oedipus) B-cell line B95a (19), the EBV-negative human lymphoblastoid B-cell line BJAB (25), Jurkat cells, and primary human peripheral blood lymphocytes (PBL) were cultured in RPMI 1640 medium containing 10% FCS. PBL of human blood donors (Department of Transfusion Medicine, University of Würzburg) were isolated by centrifugation of blood cells (buffy coat) on a Percoll (Pharmacia) cushion (density, 1.123 g/ml) for 20 min at 1,000 × g and removal of monocytes by adherence. Vero, HeLa, CHO, and CD46-transfected CHO (CHO-CD46) cells (CHO-5.3; a gift of B. Loveland, Heidelberg, Australia) (22) were cultured in minimal essential medium containing 10% FCS.
The MV vaccine strains Edm and Edmonston Zagreb (EdmZag) were propagated on Vero cells. Wild-type MV strains WTFb and Wü5679 (same as TC5679 in reference 35) were isolated from patients with acute measles (Erlangen, Germany, 1990, and Würzburg, Germany, 1996, respectively [35]) and propagated on BJAB cells, since these cells, in contrast to B95a cells, do not contain EBV and express CD46 and SLAM and therefore do not exert a selective pressure for one of the receptors. The BJAB cells cultivated in our laboratory express considerably more SLAM than those described by Tatsuo et al. (40). For virus production, cells were infected with a multiplicity of infection (MOI) of 0.01, and virus was harvested when maximum giant cell formation was observed by one cycle of freezing-thawing and cleared by centrifugation. Supernatants were stored at −80°C. All viruses were titrated using B95a cells.
Expression cloning using a retroviral cDNA library.
The cDNA gene bank containing cDNA of a pool of five normal human spleen tissue specimens cloned unidirectional into the ViraPort pFB-XR vector (Stratagene), a replication-defective Moloney MLV-based vector, was purchased from Stratagene. The retrovirus was produced by transiently transfecting tissue culture cells with the pFB-XR plasmid cDNA library together with two additional vectors from which the Gag-Pol and vesicular stomatitis virus G (VSV-G) proteins are expressed (pVPack-GP and pVPack-VSV-G). Virus produced using the plasmid libraries is pseudotyped with the VSV-G envelope protein and is thus capable of infecting most dividing cell types, including CHO cells. After infection of cells with the retroviral supernatants, the cDNAs inserted between two long terminal repeats integrate in the host genome. CHO cells (106) were transduced with an MOI of approximately 1; 48 h postinfection, cells expressing the 5C6 epitope were selected by fluorescence-activated cell sorting (FACS) using a FACS Vantage (Becton Dickinson). Sorted cells were expanded and resorted four times. The cDNA insert was then recovered from genomic DNA of sorted cells by PCR using the vector-specific primers (5′ retro primer 5′-GGCTGCCGACCCCGGGGGTGG-3′ and 3′ pFB primer 5′-CGAACCCCAGAGTCCCGCTCA-3′; provided by Stratagene). Amplified cDNA was sequenced using an Abi Prism 310 genetic analyzer (Perkin-Elmer).
Virus-cell binding assay.
Cells (5 × 104) in 100 μl of PBS were incubated at 37°C for 1 h with viruses at a given MOI (titrated using B95a cells), washed with FACS buffer (PBS containing 0.4% bovine serum albumin and 0.02% sodium azide), and stained with MAb L77 against MV-H and FITC-conjugated goat anti-mouse antibodies. Bound virus was determined by analysis with a FACscan (Becton Dickinson). In case of the virus binding inhibition assay, cells were preincubated with increasing concentrations of MAbs to SLAM and/or CD46. Bound virus was then detected with biotinylated MAb K4 to MV-H and streptavidin-FITC. The concentrations of SLAM and CD46 antibodies alone were as indicated in the figure legends. In case of both antibodies together, SLAM and CD46 antibodies were used added in half amounts to sum up to the indicated concentrations.
SLAM downregulation assays.
To measure the infection-induced receptor modulation, cells were infected with an MOI of 1 for 2 to 3 days. Cells were fixed with 3.7% paraformaldehyde and stained with antibodies to SLAM (MAb 5C6) or to MV-H (MAb L77) as control of infection. For contact-mediated downregulation, persistently MV-infected BJAB cells (BJAB-pEdm or BJAB-pWTF) were mixed 1:1 with uninfected cells for 1 to 4 h at 37°C. Cells were chilled on ice and fixed with 3.7% paraformaldehyde. As control, cells were chilled on ice, fixed, and then mixed. The surface expression of SLAM was detected with MAb 5C6 and FITC-conjugated second antibody by flow cytometry using a FACscan (Becton Dickinson).
Proliferation assay.
The assay was done as described elsewhere (33). Briefly, responder cells (105/well/100 μl; PBL, B95a, BJAB, or Jurkat) were incubated with antibodies to SLAM and/or CD46 (2, 10, or 50 μg/ml) for 45 min at room temperature before mixing with UV-inactivated viruses. PBL were activated with 2.5 μg of phytohemagglutinin (PHA; Sigma) per ml at the start of the test or 24 h before, with no difference in results. Inhibition of proliferation was induced by adding MV strain Edm or WTFb in various amounts, which were inactivated by UV irradiation (1.5 J/cm2) to prevent infection of cells. After incubation of the cells at 37°C for 48 h, the cells were incubated for 16 h with [3H]thymidine (0.5 μCi/ml). The cells were harvested, and [3H]thymidine incorporation in DNA was measured using a scintillation counter (1205 Betaplate; LKB). All assays were performed as triplicates.
RESULTS
Selection of a receptor blocking antibody.
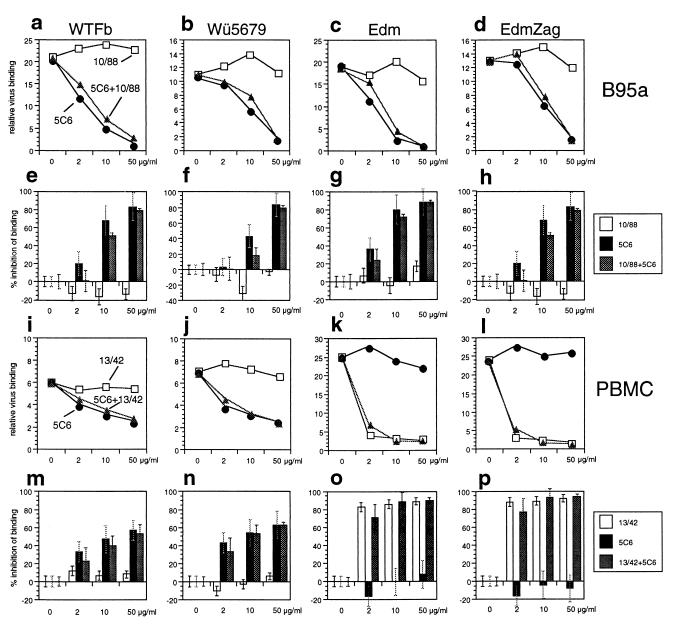
We raised MAbs to enriched surface proteins of B95a cells and selected a MAb (5C6) which inhibits syncytium formation and infection of these cells with MV. The binding of both wild-type MV (WTFb and Wü5679), and vaccine strains (Edm and EdmZag) to the surface of B95a cells was nearly completely inhibited by MAb 5C6 (Fig. 1a to h). An antibody directed to the truncated form of CD46 present on B95a cells (MAb 10/88) had no effect on virus binding, and both antibodies together inhibited virus binding like MAb 5C6 alone. In case of human PBMC, which express CD46 and the molecule recognized by MAb 5C6, MAb 5C6 blocked the binding of wild-type MV (WTFb and Wü5679) (Fig. 1i, j, m, and n) but not the binding of strains interacting with high affinity with CD46 (Edm and EdmZag) (Fig. 1k, l, o, and p). In contrast, binding of the vaccine strains to PBMC was inhibited by MAb 13/42 directed to the first domain of CD46. Both antibodies together had no detectable additive effects. These results suggest that MAb 5C6 recognizes the only receptor present on B95a cells for MV and a receptor for wild-type MV on human PBMC.
FIG. 1.
Inhibition of MV binding to B95a cells and human PBMC. B95a cells (a to h) or human PBMC (i to p) were preincubated for 1 h with increasing amounts of MAb 5C6 (black circles and columns), MAb 10/88 recognizing the truncated CD46 on B95a cells, or 13/42 recognizing the first domain of CD46 on PBMC (white squares and columns) alone, or with 5C6 and anti-CD46 antibodies in combination (grey triangles and columns). Two MV wild-type strains, WTFb (a, e, i, and m) and Wü5679 (b, f, j, and n), and two vaccine strains, Edm (c, g, k, and o) and EdmZag (d, h, l, and p), were incubated with cells at an MOI of 2.5, and bound virus was detected by flow cytometry. Results of three experiments are presented as mean values of relative binding of virus over background (a to d, and i to l) and as percentage inhibition of virus binding with standard deviations (e to h and m to p).
Analysis of cell surface proteins binding to wild-type MV.
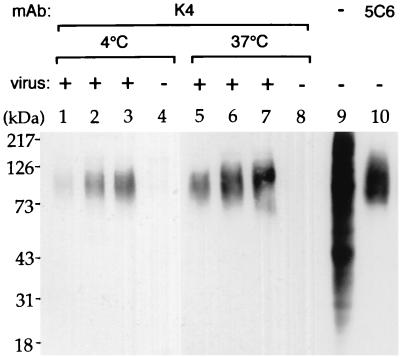
Surface proteins of B95a cells binding to MV strain WTFb were assessed by a coprecipitation assay, a method independent of antibody 5C6. The cell surface of B95a cells was biotinylated, the cells were incubated with MV strain WTFb at 4 or 37°C overnight, and virus-receptor complexes were precipitated using a MAb to MV-H (K4). The immunoprecipitated proteins were separated by SDS-polyacrylamide gel electrophoresis (PAGE) and blotted, and bands were visualized with streptavidin-peroxidase (Fig. 2). Bound proteins were detected using increasing doses of virus (Fig. 2, lanes 1 to 3 and 5 to 7). Virus binding at 37°C (Fig. 2, lanes 5 to 7) was more efficient than that at 4°C (Fig. 2, lanes 1 to 3). For comparison, an immunoprecipitation using MAb 5C6 is shown (Fig. 2, lane 10). The diffuse band of biotinylated surface protein bound to MV appeared to have the same molecular weight as the band precipitated with MAb 5C6.
FIG. 2.
Virus-receptor coprecipitation assay. The surface of B95a cells was biotinylated and incubated with MV wild-type strain WTFb overnight at 4°C (lanes 1 to 3) and 37°C (lanes 5 to 7), and complexes were precipitated with MAb K4 to MV-H. The immunoprecipitated proteins were separated by SDS-PAGE on a 10% gel and blotted on a polyrinylidene fluoride membrane, and bands were visualized with streptavidin-peroxidase. Bound proteins were detected using increasing doses of virus (85, 190, and 380 μg in lanes 1 to 3 and 5 to 7, respectively). As controls, cells were incubated with protein extracts not containing virus (lanes 4 and 8), the complete extract of biotinylated proteins was separated (lane 9), and proteins were immunoprecipitated using MAb 5C6 (lane 10).
Expression cloning and identification of the protein recognized by MAb 5C6.
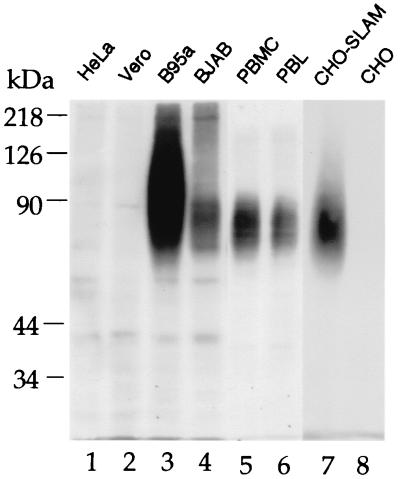
Using MAb 5C6, we screened a retroviral cDNA library from human splenocytes transduced in CHO cells by selecting positive cells by FACS. Selected cells were expanded and reselected four times. After recloning of the sorted 5C6-positive cells, we obtained a stably transduced cell line, since the retrovirus integrates in the cellular genome. Using retrovirus-specific primers complementary to the boundaries between which the cDNA inserts were cloned, we could amplify a 1.7-kb band present in the DNA of the FACS-sorted transduced CHO cells. This amplified 1.7-kb DNA fragment was sequenced and identified as encoding the signaling lymphocytic activation molecule (SLAM, CD150), which was recently identified also by Tatsuo et al. as an MV receptor (40). The sequence was identical to the published sequence for the transmembrane form of human SLAM (7). We analyzed by Western blotting the proteins recognized by MAb 5C6. HeLa, Vero, and CHO cells did not provide specific signals, whereas MAb 5C6 recognized proteins in lysates of B95a, BJAB cells, human activated PBMC and PBL, and the FACS-sorted SLAM-transduced CHO (CHO-SLAM) cells (Fig. 3). SLAM was most strongly expressed by B95a cells. The band appeared as a broad smear since SLAM is highly glycosylated. In addition, SLAM can interact with itself to form homodimers and trimers (24), as seen in BJAB cells (Fig. 3, lane 4). Similar bands were detected with a commercial anti-SLAM antibody (IPO-3 [not shown]). Thus, MAb 5C6 recognizes SLAM of monkey and human lymphoid cells and the selected transduced CHO cells.
FIG. 3.
Proteins recognized by MAb 5C6 under nonreducing conditions on a Western blot. Cell extracts (100 μg of protein each) from HeLa, Vero, B95a, and BJAB cells, PHA-activated human PBMC and PBL, and selected transduced (CHO-SLAM) and parental CHO cells (lanes 1 to 8) were separated by SDS-PAGE on an 8% gel and blotted (semidry) on a polyrinylidene difluoride membrane. The blot was incubated with MAb 5C6 and peroxidase-conjugated secondary antibody, and bands were visualized using an enhanced chemiluminescence system (Amersham).
MV susceptibility of CHO-SLAM cells.
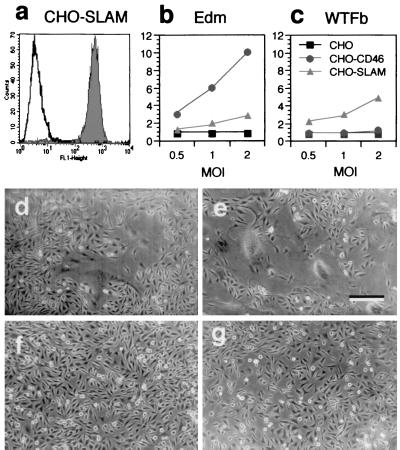
The FACS-sorted transduced CHO cells express high amounts of SLAM and were designated CHO-SLAM cells (Fig. 4a). CHO-SLAM cells gained the capacity to bind and replicate both wild-type and vaccine MV. The MV vaccine-like strain Edm bound with high affinity to CHO-CD46 cells and with lower affinity to CHO-SLAM cells (Fig. 4b), whereas wild-type strain WTFb bound only to CHO-SLAM cells (Fig. 4c). After infection with Edm or WTFb, the CHO-SLAM cells formed large syncytia (Fig. 4d and e), whereas the parental CHO cells did not support syncytium formation (Fig. 4f and g). These data demonstrate that SLAM mediates viral binding, uptake, and syncytium formation and is a cellular receptor for wild-type and vaccine-like MV.
FIG. 4.
MV binding to and susceptibility of CHO-SLAM and CHO-CD46 cells. FACS-sorted CHO-SLAM cells express high amounts of SLAM at their cell surface (a). CHO-SLAM, CHO-CD46, and parental CHO cells were incubated with increasing amounts (MOI = 0.5, 1, and 2) of Edm (b) and WTFb (c), and bound virus was detected by flow cytometry. CHO-SLAM (d and e) and CHO (f and g) cells were incubated with MV Edm (d and f) or WTFb (e and g) for 48 h (MOI = 1), and syncytium formation observed in the microscope. Syncytia were present only in SLAM-transduced cells. The bar in panel e represents 50 μm.
SLAM downregulation after infection or contact with MV-infected cells.
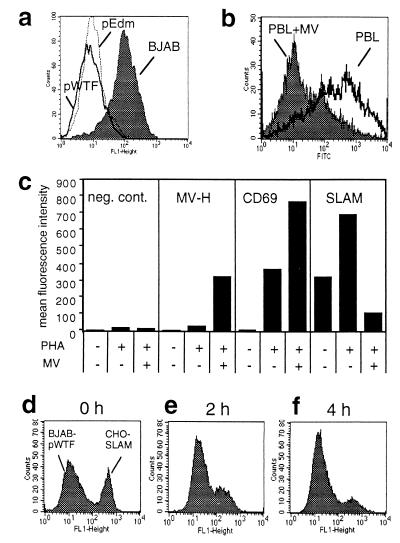
Since SLAM is a costimulatory molecule, the virus-SLAM interaction could possibly interfere with the stimulation of lymphocytes and contribute to immunosuppression during acute measles. This could happen either by blocking a costimulatory signal or by inducing a negative signal via SLAM. The first aspect, blocking costimulatory signaling, could be achieved by binding of virus or virus-infected cells blocking the binding of natural ligands or by the induction of receptor modulation. We therefore investigated whether SLAM is downregulated from the surface of cells after infection or contact with MV-infected cells. SLAM was strongly downregulated from the surface of BJAB cells persistently infected with WTFb or Edm (Fig. 5a). Infection of primary human PBL led to the specific downregulation of SLAM from the cell surface, whereas the activation marker CD69 was simultaneously upregulated (Fig. 5b and c). Infection of cell lines such as BJAB and B95a with WTFb or Edm also induced the receptor modulation (not shown).
FIG. 5.
Downregulation of SLAM after infection or contact of cells with MV glycoproteins. SLAM expression on MV WTFb or Edm persistently infected BJAB cells (a) and 72-h WTFb-infected human PBL (b) was detected by flow cytometry. (c) Mean fluorescence intensities of signals detected on nonactivated, PHA-activated, and PHA-activated, MV-infected PBL using isotype control antibody, anti-MV-H antibody (L77), anti-CD69 MAb, and anti-SLAM MAb 5C6. For measuring contact-mediated receptor modulation, CHO-SLAM cells were incubated in a ratio of 1:1 with BJAB-pWTF cells for 0 h (d), 2 h (e), and 4 h (f) at 37°C, and SLAM expression was detected by flow cytometry.
Since during acute measles only a small proportion of PBL are infected, SLAM downregulation following infection may not have a major effect on the immune response. However, as described for CD46 (20, 36), a rapid receptor modulation may also be induced by contact of MV glycoproteins with SLAM on uninfected cells. We measured contact-mediated downregulation by mixing persistently infected BJAB cells with uninfected SLAM-positive cells. SLAM was rapidly downregulated from the surface of CHO-SLAM cells (Fig. 5d to f). Similar results were obtained with primary human PBL, B95a, or BJAB cells (not shown).
Role of SLAM in contact-mediated proliferation inhibition of lymphocytes.
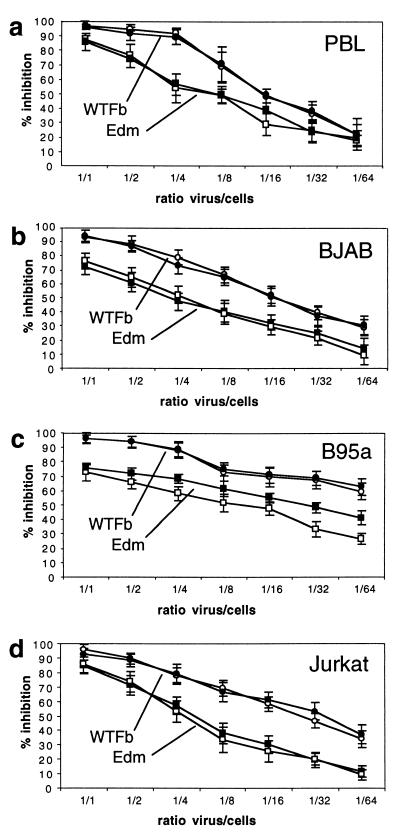
The direct contact of MV-infected cells or virus with target cells leads not only to receptor modulation but also to a proliferative inhibition of these contacted cells (responder cells) (33). To test whether SLAM is involved in the induction of this negative signal, we assessed the influence of MV receptor antibodies in proliferation inhibition tests. We showed above that the anti-SLAM antibody 5C6 (50 μg/ml) efficiently blocks high-affinity binding of MV to SLAM. However, also high concentrations of MAb 5C6 had no significant influence on the proliferative inhibition of the tested cells induced by either wild-type or vaccine MV (Fig. 6). Using human PBL or BJAB cells as responder cells which express both virus receptors, CD46 and SLAM, antibodies to both receptors together (Fig. 6a and b) and to SLAM or CD46 alone (not shown) had no significant effect on the induction of proliferative inhibition by MV WTFb and Edm. In the case of B95a cells expressing only SLAM as receptor, 5C6 had also no effect on proliferation inhibition induced by WTFb and a slight enhancing effect on the proliferation inhibition induced by Edm (Fig. 6c). Using CD46-positive, SLAM-negative Jurkat cells, both viruses induced the proliferative inhibition also in the absence of SLAM, and anti-CD46 antibodies had no effect on proliferation inhibition (Fig. 6d). Antibodies were used in concentrations of 2, 10, and 50 μg/ml without effects (the data with 50 μg/ml are shown in Fig. 6). As controls, the cells were treated with antibodies in the absence of virus. Under these conditions, anti-SLAM antibodies led to a slight (up to 8%) stimulation of proliferation, and anti-CD46 had no effect on proliferation. Furthermore, we have described earlier that the monocytic U937 cells are susceptible to proliferative inhibition by MV contact (33), although these cells lack SLAM. The absence of SLAM in Jurkat and U937 cells was confirmed by reverse transcription-PCR and flow cytometry (not shown). These data indicate that the proliferative inhibition induced by MV contact is independent of the presence or absence of the MV-binding receptors SLAM and CD46.
FIG. 6.
Proliferative inhibition of primary human PBL and cell lines after contact with UV-inactivated MV in the absence and presence of receptor antibodies. As responder cells, PBL (a), BJAB (b), B95a (c), and Jurkat (d) were preincubated with (black symbols) or without (open symbols) receptor-specific antibodies prior to incubation with UV-inactivated MV WTFb (circles) or Edm (squares) in ratios of 1 PFU/1 responder cell to 1 PFU/64 responder cells. PHA-activated human PBL and BJAB cells were incubated with anti-SLAM (5C6) and anti-CD46 (13/42) MAbs, B95a cells were incubated with anti-SLAM MAb alone, and Jurkat cells were incubated with anti-CD46 MAb alone. 3H incorporation was determined after 72 h, and results are expressed as percentage inhibition of proliferation in comparison to proliferation rates in the absence of virus.
DISCUSSION
CD46 was identified as an MV receptor with high affinity for MV vaccine-like strains Edm and Hallé (8, 30) and has low or no affinity to a number of wild-type isolates (3, 16, 23, 27). Using an antibody which blocks virus binding and infection of B95a cells, we selected transduced CHO cells expressing the molecule and identified it as the costimulatory molecule SLAM. The binding of wild-type and vaccine MV to B95a cells was inhibited by MAb 5C6 in a dose-dependent manner and nearly completely at 50 μg/ml. This suggests that SLAM is the only accessible binding receptor for MV on these cells. When CD46 is present in addition to SLAM on cells such as human PBL, the binding of MV with high affinity to CD46 was not influenced by anti-SLAM antibodies. Vice versa, anti-CD46 antibodies did not disturb the binding of wild-type viruses to SLAM. This indicates that both receptors can interact independently with MV. Since B95a cells have been used successfully to isolate a number of MV strains from patients and are susceptible for infection with MV laboratory strains, and since SLAM-expressing CHO cells are infected by all MV strains tested, SLAM is presumably a receptor for all MV strains. Our results fully confirm the data published recently by Tatsuo et al. (40).
We were interested in whether the virus-SLAM interaction (in the absence of infection) can induce contact-mediated proliferative inhibition of cells. Sensitive responder cells for contact-mediated proliferation inhibition in vitro are primary PBL and cell lines of hematopoietic origin such as B95a, BJAB, Jurkat, Molt4, U937, and HL60 (33, 44). In both contacted (9, 38) and infected (29) cells, progression to the S phase of the cell cycle is prevented by a dominant negative signal. The fact that several of these cell lines are SLAM negative (or SLAM and CD46 negative, like murine lymphoid cells) in conjunction with our data using SLAM-blocking antibodies indicate that SLAM is not involved in the induction of this negative signal. In addition, the published findings and data presented here demonstrate that this is also the case for CD46. Thus, both MV-binding receptors SLAM and CD46 are not required, and cell surface structures mediating the virus contact-mediated inhibition of proliferation have not been identified. On the viral side, it is known that a complex of the MV H and F proteins is required for this interaction and that activation of the F protein by cleavage is essential for contact-mediated proliferation inhibition (33, 44).
MV-induced immunosuppression is a multifactorial process to which the infection of certain subpopulations of regulatory cells, the direct contact between MV envelope proteins with cell surface receptors (31, 33, 44), and soluble factors such as interleukin (IL-12) (18) may contribute. Measles patients suffer from pronounced lymphopenia and an unresponsiveness of lymphocytes to mitogens, CD3-cross-linking agents, and secondary and recall antigens (for a review, see reference 4). Since SLAM is a signaling transmembrane molecule present on the surface of memory and activated T and B lymphocytes (2, 7, 32, 39), its engagement in virus binding and subsequent downregulation may well influence the immune response in vivo. Our finding that the contact-mediated proliferative inhibition of lymphocytes is not mediated by SLAM does not exclude involvement of SLAM in other virally caused disturbances of the immune response.
It is interesting that SLAM is not expressed by monocytes/macrophages (for a review, see reference 39), although these cells are primary target cells for MV (10, 14), which besides other cytokines produce IL-12. This interleukin is critical for the development of the cell-mediated immune response as inducer of gamma interferon (IFN-γ) from T and NK cells, required for the development of Th1 responses, and is required as a comitogen for T and NK cells (41). IL-12 has been found to be diminished after infection of monocytes with MV or cross-linking of CD46, and this may play a role for suppression of cell-mediated immunity (18). In the light of receptor usage by various MV strains, this raises the question as to how wild-type MV isolates, which do not interact with CD46, may infect SLAM-negative monocytes and reduce IL-12 levels. On these cells, MV might use either CD46, although with a low binding affinity not detected by standard methods, another unknown receptor, or another uptake mechanism.
Interaction of activated human lymphocytes with MV simultaneously induced both proliferative inhibition and downregulation of SLAM. In B lymphocytes, ligation of SLAM induces the rapid dephosphorylation of both Src homology 2-containing inositol phosphatase and SLAM, as well as the association of Lyn and Fgr kinases with this phosphatase, and enhances CD95-mediated apoptosis (26). MV infection was found earlier to induce apoptosis in stimulated PBMC and T-cell cultures (17) and in SCID mice engrafted with human thymus/liver implants (1, 42). It remains to be elucidated whether MV-induced apoptosis is related to SLAM signaling.
During acute measles, there is an initial Th1 response with release of IFN-γ and IL-2, followed by a prolonged presence of IL-4 in the plasma of patients when the rash subsides (12, 13). In vitro, stimulation of PBMC from patients with T-cell mitogens such as PHA or anti-CD3 antibodies elicits little IFN-γ and IL-2 but large amounts of IL-4 (43). Interestingly, engagement of SLAM induces IFN-γ in T cells and redirects the Th2 phenotype of helper T lymphocytes to a Th1/Th0 phenotype (6). Vice versa, absence of SLAM may bias the immune response in the opposite direction to a Th2 type. One mechanism leading to absence of SLAM or blocking SLAM signaling in lymphocytes is the virus-induced downregulation of SLAM. Thus, MV-induced downregulation of SLAM may contribute to the prolonged Th2 response in measles patients at times postinfection (after the rash) when sufficient MV-infected cells and glycoproteins are produced to contact a significant number of lymphocytes.
ACKNOWLEDGMENTS
We thank Franziska Dimpfel for technical assistance and Sonja Rotzoll for assistance with the fluorescence-activated cell sorter.
We thank the Deutsche Forschungsgemeinschaft for financial support.
REFERENCES
- 1.Auwaerter P G, Kaneshima H, McCune J M, Wiegand G, Griffin D E. Measles virus infection of thymic epithelium in the SCID-hu mouse leads to thymocyte apoptosis. J Virol. 1996;70:3734–3740. doi: 10.1128/jvi.70.6.3734-3740.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aversa G, Chang C-C J, Carballido J M, Cocks B G, de Vries J E. Engagement of the signalling lymphocytic activation molecule (SLAM) on activated T cells results in IL-2-independent, cyclosporin A-sensitive T cell proliferation and IFN-gamma production. J Immunol. 1997;158:4036–4044. [PubMed] [Google Scholar]
- 3.Bartz R, Firsching R, Rima B, ter Meulen V, Schneider-Schaulies J. Differential receptor usage by measles virus strains. J Gen Virol. 1998;79:1015–1025. doi: 10.1099/0022-1317-79-5-1015. [DOI] [PubMed] [Google Scholar]
- 4.Borrow P, Oldstone M B A. Measles virus-mononuclear cell interactions. In: Billeter M A, ter Meulen V, editors. Measles virus. Vol. 191. Berlin, Germany: Springer-Verlag; 1995. pp. 85–100. [DOI] [PubMed] [Google Scholar]
- 5.Buchholz C J, Koller D, Devaux P, Mumenthaler C, Schneider-Schaulies J, Braun W, Gerlier D, Cattaneo R. Mapping of the primary binding site of measles virus to its receptor CD46. J Biol Chem. 1997;272:22072–22079. doi: 10.1074/jbc.272.35.22072. [DOI] [PubMed] [Google Scholar]
- 6.Carballido J, Aversa G, Kaltoft K, Cocks B G, Punnonen J, Yssel H, Thestrup-Pedersen K, de Vries J E. Reversal of human allergic T helper 2 responses by engagement of signalling lymphocyte activation molecule. J Immunol. 1997;159:4316–4321. [PubMed] [Google Scholar]
- 7.Cocks B G, Chang C-C J, Carballido J M, Yssel H, de Vries J E, Aversa G. A novel receptor involved in T-cell activation. Nature. 1995;376:260–263. doi: 10.1038/376260a0. [DOI] [PubMed] [Google Scholar]
- 8.Dörig R E, Marcil A, Chopra A, Richardson C D. The human CD46 molecule is a receptor for measles virus (Edmonston strain) Cell. 1993;75:295–305. doi: 10.1016/0092-8674(93)80071-l. [DOI] [PubMed] [Google Scholar]
- 9.Engelking O, Fedorov L M, Lilischkis R, ter Meulen V, Schneider-Schaulies S. Measles virus-induced immunosuppression in vitro is associated with deregulation of G1 cell cycle control proteins. J Gen Virol. 1999;80:1599–1608. doi: 10.1099/0022-1317-80-7-1599. [DOI] [PubMed] [Google Scholar]
- 10.Esolen L M, Ward B J, Moench T R, Griffin D E. Infection of monocytes during measles. J Infect Dis. 1993;168:47–52. doi: 10.1093/infdis/168.1.47. [DOI] [PubMed] [Google Scholar]
- 11.Griffin D E, Bellini W J. Measles virus. In: Fields B N, Knipe D M, Howley P M, et al., editors. Fields virology. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 1267–1312. [Google Scholar]
- 12.Griffin D E, Ward B J, Jauregui E, Johnson R T, Vaisberg A. Immune activation during measles: interferon-gamma and neopterin in plasma and cerebrospinal fluid in complicated and uncomplicated disease. J Infect Dis. 1990;161:449–453. doi: 10.1093/infdis/161.3.449. [DOI] [PubMed] [Google Scholar]
- 13.Griffin D E, Ward J B. Differential CD4 T cell activation in measles. J Infect Dis. 1993;168:275–281. doi: 10.1093/infdis/168.2.275. [DOI] [PubMed] [Google Scholar]
- 14.Helin E, Salmi A A, Vanharanta R, Vainionpää R. Measles virus replication in cells of myelomonocytic lineage is dependent on cellular differentiation stage. Virology. 1999;253:35–42. doi: 10.1006/viro.1998.9460. [DOI] [PubMed] [Google Scholar]
- 15.Hsu E C, Dorig R E, Sarangi F, Marcil A, Iorio C, Richardson C D. Artificial mutations and natural variations in the CD46 molecules from human and monkey cells define regions important for measles virus binding. J Virol. 1997;71:6144–6154. doi: 10.1128/jvi.71.8.6144-6154.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsu E C, Sarangi F, Iorio C, Sidhu M S, Udem S A, Dillehay D L, Xu W, Rota P A, Bellini W J, Richardson C D. A single amino acid change in the hemagglutinin protein of measles virus determines its ability to bind CD46 and reveals another receptor on marmoset B cells. J Virol. 1998;72:2905–2916. doi: 10.1128/jvi.72.4.2905-2916.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ito M, Watanabe M, Ihara T, Kamiya H, Sakurai M. Measles virus induces apoptotic cell death in lymphocytes activated with phorbol 12-myristate 13-acetate (PMA) plus calcium ionophore. Clin Exp Immunol. 1997;108:266–271. doi: 10.1046/j.1365-2249.1997.d01-995.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karp C L, Wysocka M, Wahl L M, Ahearn J M, Cuomo P J, Sherry B, Trinchieri G, Griffin D E. Mechanism of suppression of cell-mediated immunity by measles virus. Science. 1996;273:228–231. doi: 10.1126/science.273.5272.228. . (Erratum, 275:1053, 1997.) [DOI] [PubMed] [Google Scholar]
- 19.Kobune F, Sakata H, Sugiura A. Marmoset lymphoblastoid cell as a sensitive host for isolation of measles virus. J Virol. 1990;64:700–705. doi: 10.1128/jvi.64.2.700-705.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krantic S, Gimenez C, Rabourdin-Combe C. Cell-to-cell contact via measles virus haemagglutinin-CD46 interaction triggers CD46 downregulation. J Gen Virol. 1995;76:2793–2800. doi: 10.1099/0022-1317-76-11-2793. [DOI] [PubMed] [Google Scholar]
- 21.Liebert U G, Flanagan S G, Löffler S, Baczko K, ter Meulen V, Rima B K. Antigenic determinants of measles virus hemagglutinin associated with neurovirulence. J Virol. 1994;68:1486–1493. doi: 10.1128/jvi.68.3.1486-1493.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loveland B E, Johnstone R W, Russell S M, Thorley B R, McKenzie I F C. Different membrane cofactor protein (CD46) isoforms protect transfected cells against antibody and complement mediated lysis. Transplant Immunol. 1993;1:101–108. doi: 10.1016/0966-3274(93)90002-p. [DOI] [PubMed] [Google Scholar]
- 23.Manchester M, Eto D S, Valsamakis A, Liton P B, Fernandez-Munoz R, Rota P A, Bellini W J, Forthal D N, Oldstone M B A. Clinical isolates of measles virus use CD46 as a cellular receptor. J Virol. 2000;74:3967–3974. doi: 10.1128/jvi.74.9.3967-3974.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mavaddat N, Mason D W, Atkinson P D, Evans E J, Gilbert R J C, Stuart D I, Fennelly J A, Barclay A N, Davis S J, Brown M H. Signaling lymphocytic activation molecule (CDw150) is homophilic but self-associates with very low affinity. J Biol Chem. 2000;275:28100–28109. doi: 10.1074/jbc.M004117200. [DOI] [PubMed] [Google Scholar]
- 25.Menezes J, Leibold W, Klein G, Clements G. Establishment and characterization of an Epstein-Barr virus (EBV)-negative lymphoblastoid B cell line (BJA-B) from an exceptional, EBV-genome-negative African Burkitt's lymphoma. Biomedicine. 1975;22:276–284. [PubMed] [Google Scholar]
- 26.Mikhalp S V, Shlapatska L M, Berdowa A G, Law C-L, Clark E A, Sidorenko S P. CDw150 associates with Src-homology 2-containing inositol phosphatase and modulates CD95-mediated apoptosis. J Immunol. 1999;162:5719–5727. [PubMed] [Google Scholar]
- 27.Murakami Y, Fukui A, Seya T, Ueda S, Nagasawa S. Effect of mutations at the residues R25, D27, P69, and N70 of B95a-MCP on receptor activities for the measles viruses Nagahata wild-type strain and CAM vaccine strain. Int J Mol Med. 1999;3:25–32. doi: 10.3892/ijmm.3.1.25. [DOI] [PubMed] [Google Scholar]
- 28.Murakami Y, Seya T, Kurita M, Fukui A, Ueda S, Nagasawa S. Molecular cloning of membrane cofactor protein (MCP; CD46) on B95a cell, an Epstein-Barr virus-transformed marmoset B cell line: B95a-MCP is susceptible to infection by the CAM, but not the Nagahata strain of the measles virus. Biochem J. 1998;330:1351–1359. doi: 10.1042/bj3301351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Naniche D, Reed S I, Oldstone M B A. Cell cycle arrest during measles virus infection: a G0-like block leads to suppression of retinoblastoma protein expression. J Virol. 1999;73:1894–1901. doi: 10.1128/jvi.73.3.1894-1901.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Naniche D, Varior-Krishnan G, Cervoni F, Wild T F, Rossi B, Rabourdin-Combe C, Gerlier D. Human membrane cofactor protein (CD46) acts as a cellular receptor for measles virus. J Virol. 1993;67:6025–6032. doi: 10.1128/jvi.67.10.6025-6032.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Niewiesk S, Eisenhuth I, Fooks A, Clegg J C, Schnorr J J, Schneider-Schaulies S, ter Meulen V. Measles virus-induced immune suppression in the cotton rat (Sigmodon hispidus) model depends on viral glycoproteins. J Virol. 1997;71:7214–7219. doi: 10.1128/jvi.71.10.7214-7219.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Punnonen J, Cocks B G, Carballido J M, Bennett B, Peterson D, Aversa G, de Vries J. Soluble and membrane-bound forms of signalling lymphocytic activation molecule (SLAM) induce proliferation and Ig synthesis by activated human B lymphocytes. J Exp Med. 1997;185:993–1004. doi: 10.1084/jem.185.6.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schlender J, Schnorr J J, Spielhoffer P, Cathomen T, Cattaneo R, Billeter M A, ter Meulen V, Schneider-Schaulies S. Interaction of measles virus glycoproteins with the surface of uninfected peripheral blood lymphocytes induces immunosuppression in vitro. Proc Natl Acad Sci USA. 1996;93:13194–13199. doi: 10.1073/pnas.93.23.13194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schneider-Schaulies J, Dunster L M, Schwartz-Albiez R, Krohne G, ter Meulen V. Physical association of moesin and CD46 as a receptor complex for measles virus. J Virol. 1995;69:2248–2256. doi: 10.1128/jvi.69.4.2248-2256.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schneider-Schaulies J, Martin M, Logan J S, Firsching R, ter Meulen V, Diamond L E. CD46-transgene expression in pig peripheral blood mononuclear cells does not alter their susceptibility to measles virus nor the capacity to downregulate endogenous and transgenic CD46. J Gen Virol. 2000;81:1431–1438. doi: 10.1099/0022-1317-81-6-1431. [DOI] [PubMed] [Google Scholar]
- 36.Schneider-Schaulies J, Schnorr J J, Schlender J, Dunster L M, Schneider-Schaulies S, ter Meulen V. Receptor (CD46) modulation and complement-mediated lysis of uninfected cells after contact with measles virus-infected cells. J Virol. 1996;70:255–263. doi: 10.1128/jvi.70.1.255-263.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schneider-Schaulies S, Niewiesk S, Schneider-Schaulies J, ter Meulen V. Measles virus induced immunosuppression: targets and effector mechanisms. Curr Mol Med. 2001;1:163–181. doi: 10.2174/1566524013363960. [DOI] [PubMed] [Google Scholar]
- 38.Schnorr J J, Seufert M, Schlender J, Borst J, Johnston I C, ter Meulen V, Schneider-Schaulies S. Cell cycle arrest rather than apoptosis is associated with measles virus contact-mediated immunosuppression in vitro. J Gen Virol. 1997;78:3217–3226. doi: 10.1099/0022-1317-78-12-3217. [DOI] [PubMed] [Google Scholar]
- 39.Tangye S G, Phillips J H, Lanier L L. The CD2-subset of the Ig superfamily of cell surface molecules: receptor-ligand pairs expressed by NK cells and other immune cells. Semin Immunol. 2000;12:149–157. doi: 10.1006/smim.2000.0217. [DOI] [PubMed] [Google Scholar]
- 40.Tatsuo H, Ono N, Yanagi Y. SLAM (CDw150) is a cellular receptor for measles virus. Nature. 2000;406:893–897. doi: 10.1038/35022579. [DOI] [PubMed] [Google Scholar]
- 41.Trinchieri G. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu Rev Immunol. 1995;13:251–276. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]
- 42.Valsamakis A, Auwaerter P G, Rima B K, Kaneshima H, Griffin D E. Altered virulence of vaccine strains of measles virus after prolonged replication in human tissue. J Virol. 1999;73:8791–8797. doi: 10.1128/jvi.73.10.8791-8797.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ward B J, Johnson R T, Vaisberg A, Jauregui E, Griffin D E. Cytokine production in vitro and the lymphoproliferative defect of natural measles virus infection. Clin Immunol Immunopathol. 1991;61:236–248. doi: 10.1016/s0090-1229(05)80027-3. [DOI] [PubMed] [Google Scholar]
- 44.Weidmann A, Maisner A, Garten W, Seufert M, ter Meulen V, Schneider-Schaulies S. Proteolytic cleavage of the fusion protein but not membrane fusion is required for measles virus-induced immunosuppression in vitro. J Virol. 2000;74:1985–1993. doi: 10.1128/jvi.74.4.1985-1993.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]