Abstract
Three different emulsions of myofibrillar protein (MP), soy protein isolate (SPI) and egg white protein isolate (EPI) were individually mixed with MP sol to form composite gels. N-ethylmaleimide (NEM) was used as a sulfhydryl group blocker to evaluate the effects of sulfhydryl and disulfide bonds on the properties of different protein–emulsion composite gels. The results show that the disulfide bond contents in the MP (SPI, EPI) emulsion composite gel decreased from the initial 2.4 ± 0.1, (2.3 ± 0.2, 1.8 ± 0.4) mol/kg to 0.6 ± 0.1, (0.5 ± 0.3, 0.7 ± 0.1) mol/kg with the NEM content increased. In addition, the microstructure showed that the interfacial protein membrane of the emulsion globules were broken in different degrees, indicating that the interaction between the emulsion and the gel matrix was weakened. Meanwhile, gel strength, water distribution and elastic modulus of the composite gels were reduced with NEM contents increased.
Keywords: Sulfhydryl blocker N-ethylmaleimide, Sulfhydryl group, Disulfide bond, Emulsion, Gel properties
Highlights
-
•
Myofibrillar protein (MP) composite gels were prepared with three different protein emulsion.
-
•
NEM caused the rupture of the emulsion interfacial protein membrane in the gel system.
-
•
The reduction of disulfide bonds destroyed the composite gel system after the three different protein emulsion added.
-
•
MP and EPI emulsion composite gels had more compact gel networks and complete emulsion granules.
1. Introduction
Proteins can be adsorbed at the oil and water interface to stabilize oil droplets in the system and are often used as emulsifiers for emulsions (Dickinson, Evison, Gramshaw, & Schwope, 1994). The emulsion consisting of protein and vegetable oil has been diffusely used to replace animal fat in meat products (Ansorena & Astiasaran, 2004), and the protein emulsion is usually added to the myofibrillar protein (MP) matrix to simulate the gel-based meat products. The non-covalent interaction (hydrogen bond, hydrophobic and electrostatic interaction) and covalent interaction (disulfide bond) are important factors determining the gels properties in the formation of heat-induced gel (Cao et al., 2018). Under heating conditions, the structure of the protein expands, exposing a large number of hydrophobic groups and forming disulfide bonds, which are important force to maintain the stability of the gel structure (Wang, Wang, Yang, Wu, & Sun, 2021). Wu et al. (2019) found that during the heat-induced gel formation process, the contents of total sulfhydryl and free sulfhydryl groups were reduced and converted into disulfide bonds, which greatly promoted protein cross-linking and enhanced the stability of the gel structure when the temperature exceeded 50 °C.
In the process of thermal denaturation and aggregation, new intramolecular or intermolecular disulfide bonds can be formed between unfolded polypeptide chains through thiol oxidation or sulfhydryl-disulfide bond exchange reactions (Yang et al., 2016). With the formation of disulfide bonds on amino acid side chains, cysteine residues on protein polypeptides are converted to cystine (Fritz, Swartz, & Greaser, 1989). Although the disulfide bond is covalent, it is easily reduced and decomposed into sulfhydryl groups, which can be reoxidized to form disulfide bonds, indicating that thiol bonds and disulfide bonds can be converted to each other under certain conditions. The disulfide bond can affect the interaction between proteins, and then affect the emulsifying properties of proteins (Kim & Kinsella, 1987). Soy protein isolate, myofibrillar protein, egg white protein isolate and whey protein are common proteins involved in the emulsification of protein and oil. Due to the different structures of different proteins, the emulsification properties are different, resulting in the formation of different emulsion properties. At present, there have been some studies on the role of disulfide bonds in protein emulsions. Kim and Kinsella (1986) added dithiothreitol to soybean protein isolate solution to study the effects of disulfide bonds on the surface hydrophobicity, viscosity and turbidity of protein molecules. The results showed that reducing the disulfide bond contents would affect the molecular properties of protein, and thus destroy the emulsification properties of protein. However, the research on the mechanism of disulfide bond in different protein emulsions is relatively lacking and needs to be further explored.
Disulfide bonds play a very important role in protein folding and conformational structure. Sano, Ohno, Otsukafuchino-fuchino, Matsumoto, and Tsuchiya (1994) studied the changes of the content of sulfhydryl groups during the heating process of actomyosin and found that the active sulfhydryl groups were oxidized into disulfide bond in the temperature range of 30 °C–50 °C. Niwa, Matsubara, Nakayama, and Hamada (1982) found that disulfide bonds were formed in the heat-induced gels formed by fish myosin when the heating temperature reached 40–45 °C. It can be seen that disulfide bond has a stabilizing effect on the higher structure of protein gel. Previous reports have shown that Dithiothreitol (DTT) or NEM, as a small organic reducing agent, can prevent the formation of disulfide bonds when added to myosin (Visessanguan, Ogawa, Nakai, & An, 2000). Although it does not change the heat transfer temperature of myosin, the initial temperature of myosin gel formation increases and thus decreases the gel strength, suggesting that disulfide bonds play an important role in the aggregation and formation of gel networks. Previously, we used NEM to study the effect of different proteins (MP, EPI, SPI) as emulsifier on the properties of stabilizing emulsions in the case of decreased disulfide bonds. The results indicated that under conditions of lower disulfide bond content, these proteins were unable to form enough interfacial protein films with good viscoelasticity, leading to poor stability of the protein emulsions (Wu et al., 2021). This provides an important theoretical basis for us to further explore the role of disulfide bonds in composite gels.
In this study, three different protein emulsions of myofibrillar protein (MP, animal meat protein), egg-white protein isolate (EPI, animal non-meat protein), and soybean protein isolate (SPI, vegetable protein) were added to MP sol and heated to form composite gels to simulate meat products. The roles of sulfhydryl and disulfide bond contents on the gel strength, rheology, microstructure and water distribution of different protein emulsion composite gels were explored. The purpose of this study was to deepen the understanding of the role of disulfide bonds in diversified protein emulsion complex gel systems, and to explore the universal applicability and importance of disulfide bonds in stabilizing protein gel structures. This study elucidates the role of disulfide bonds in the construction and stability of MP composite gels, and provides a theoretical basis for the research in related fields.
2. Materials and methods
2.1. Materials
Fresh pork tender loin muscles were obtained from a local supermarket (24–48 h post mortem, pH 5.6–5.9). Then, visible external fat and connective tissue were trimmed. The tissue was cut into cubes of approximately 1cm3. Finally, the samples were sealed in vacuum plastic bags and stored at −75 °C until use in less than two months.
Olive oil was acquired from Sinopharm Chemical Reagent Co., Ltd. (Beijing, China). Egg-white protein isolate (EPI) was supplied by Qianyu Co. (Zhejiang, China). Soy protein isolate (SPI) was obtained from Gushen Chemical Co. (Shandong, China). The rest of the reagents were analytical grade reagents supplied by Yuanye Biotechnology Co., Ltd. (Shanghai, China).
2.2. Preparation of myofibrillar protein
The extraction of myofibrillar protein is based on the previously described methods (Wu, Xiong, Chen, Tang, & Zhou, 2009). Partially thawed (taken out of the −80 °C freezer and placed at 4 °C for 4 h) muscle cubes were minced with a knife; minced muscle was suspended in 4 vol (w/v, based on muscle weight) of isolation buffer (0.1 M NaCl, 10 mM sodium phosphate, 2 mM MgCl2, 1 mM EGTA, pH 7.0) and homogenized with a tissue homogenizer at maximum speed setting for 90 s. The slurry was centrifuged at 2000g for 15 min and the supernatant was discarded. The above operation was repeated twice with the same phosphate buffer. Subsequently, the pellets were washed 2 more times using the same homogenization and centrifugation conditions as above except that the washing solution was 0.1 M NaCl and the protein suspension was adjusted to pH 6.2 prior to centrifugation. The purified MP pellets needed to be kept on crushed ice and utilized within 2 days of separation. The protein concentration was determined by the Biuret method using bovine serum albumin (Xiong, Aguilera, & Kinsella, 1991) as the standard.
2.3. Preparation of protein emulsion and Myofibrillar protein complex sols
Three different protein-emulsion systems were prepared by mixing 24 g of 1 % MP (1 % EPI or 1 % SPI) with 6 g olive oil at 12,000 rpm for 60 s by using an Ultra-Turrax homogenizer (IKA T18 Basic, Staufen, Germany). Subsequently, each protein emulsion group was individually added with 0, 1, 5, and 10 mM NEM, and then continuously stirred at 2000 rpm under 25 °C for 30 min. The conditions for the preparation of protein emulsions are listed in Table 1. The fresh emulsions were separately mixed with MP to produce composite gels with a concentration of 2 % (w/w) protein and 5 % (w/w) olive oil.
Table 1.
Preparation of protein emulsions.
| Treatment | High speed homogenization | Low-speed stirring | NEM (mM) |
|---|---|---|---|
| Pre-emulsion | Yes | No | 0 |
| control | Yes | Yes | 0 |
| 1 mM NEM | Yes | Yes | 1 |
| 5 mM NEM | Yes | Yes | 5 |
| 10 mM NEM | Yes | Yes | 10 |
Note: Low speed stirring is equivalent to secondary emulsification. That is, in addition to the pre-emulsification group, all four subsequent groups were subjected to secondary emulsification. “Yes” respresented the sample treated with the procedure; “No” represented not.
2.4. Preparation of the emulsion protein complex gels
Gómez-Guillén, BorderíAs, and Montero (1997) found that disulfide bonds are the most abundant in different non-meat protein and MP composite gels at 60 °C. Therefore, 60 °C was used as the heating temperature. The composite sols (10 g each) were divided into glass tubes (height 50 mm × inner diameter 16.5 mm), covered with aluminum foil, and heated in a water bath at 60 °C for 30 min, immediately removed after heating, put into ice water for 5 min, and kept in an environment of 4 °C for 12 h for subsequent experiments.
2.5. Thiol(—SH) and disulfide bond (S—S)content
The contents of thiol and disulfide bonds were determined as previously reported by Yongsawatdigul and Park (2003). The samples to be tested were centrifuged at 10000g for 15 min. With bovine serum albumin as the standard protein, the content of protein in supernatant was determined by the Lorry method. Subsequently, 1 mL of phosphate buffer (0.6 M NaCl, 20 mM sodium phosphate, 10 mM ethylene diamine tetraacetic acid, pH 7.0) and 400 μL of DTNB 10 mM (5,5′-dithiobis (2-nitrobenzoic acid), pH 7.0) were added to 100 μL samples. After the samples were mixed at 37 °C for 30 min and centrifuged at 20000g for 5 min. The absorbance was measured at 412 nm. Thiol content was calculated as follows:
| (1) |
where C0 is the concentration of -SH (mol/1000 g protein), A represents the absorbance at 412 nm, e is the absorbance coefficient (13,600 M−1·cm−1), D indicates the dilution factor of the sample.
Theoretically, the disulfide bond content was calculated using the following equation:
| (2) |
where CS is the concentration of S—S (mol/1000 g protein), CT represents the concentration of total —SH (mol/1000 g protein), and CR is the concentration of reactive —SH (mol/1000 g protein).
2.6. Gel strength and water holding capacity
The texture properties of the MP gel samples were analyzed using a texture analyzer (TMS-PRO, Food Technology Corporation, USA) fitted with a cylindrical probe. The test speed of the sample was 1.0 mm/s, the pre-test speed and post-test speed were 2.0 mm/s, and the compression ratio was 50 %. (Montejano, Hamann, & Lanier, 1985). The gels stored at 4 °C were restored to room temperature, the samples were placed in a 50 mL centrifuge tube, centrifuged at 10000g for 15 min, and then the supernatant was poured out and weighed. (Tang, Wang, Yang, & Li, 2009). The water holding capacity was calculated as:
| (3) |
where W0 is the weight of the centrifuge tube and gel after the supernatant is poured out, W represents the weight of the centrifuge tube, and W1 indicates the weight of the centrifuge tube and gel before centrifugation.
2.7. Dynamic rheological testing during gelation
The viscoelastic properties of MP-emulsion composite sols were determined by KINEXUS Pro, which was equipped with parallel plates (diameter = 40 mm), spaced 1 m apart. The heating temperature of sols was 20–90 °C, the heating rate was 2 °C/min, the heating frequency was 0.1 MHz, and the maximum strain was generated under the condition of 2 %. The storage modulus (G') was the required data to indicate the rheological properties of the gels.
2.8. Light microscopy
Light microscopy was determined as reported by the method of Wu, Xiong, and Chen (2011). The gels were cut into approximately 3 cm × 3 cm × 3 cm gel blocks, which were immobilized over the night in paraformaldehyde (4 % w/v) and then washed with distilled water to remove the fixative. The samples were dehydrated with a range of organic solvents commonly used in tissue preparation for optical microscopy: 50 % ethanol, 2 h; 70 % ethanol, 2 h; 90 % ethanol, 2 h; 100 % ethanol, 2 h. Then it was treated with wood-based ethanol (100 %) (1:1) for 1 h and xylene for 2 h; Then the paraffin was heated in an oven at 60 °C for 1.5 h, the paraffin was soaked at room temperature for 1 h, and finally embedded in the paraffin. Slices (8 μm thick) were cut with a microtome and then transferred to a glass slide coated with gelatin. The samples were dewaxed in xylene, then stained with Ehrlich hematoxylin, covered with a drop of neutral balsam, and finally viewed and photographed with a light microscope.
2.9. Water distribution measurement
Complex sols (10 g each) were transferred to an NMR sample tube measuring 20 mm (inside diameter) × 300 mm (length), stored at 4 °C for 12 h. The samples were heated at 60 °C for 30 min. After heating was completed, the samples were allowed to equilibrate at room temperature (25 °C ± 1 °C) for 30 min. Relaxation measurements were performed at room temperature (25 °C ± 1 °C) under the following conditions: Transverse relaxation, T2, was measured using the Carr–Purcell–Gill sequence. The T2 measurements were performed using a τ-value (time between 90° and 180° pulses) of 200 μs and a scan range of 0–10,000 ms, 18,000 echoes, and at least four repeats (Gravelle, Marangoni, & Barbut, 2016). The low-field nuclear magnetic resonance (LF–NMR) relaxation curve was fitted to a multi-exponential curve by using the program MultiExp Inv Analysis, and the relaxation time (T2) was obtained.
2.10. Statistical analysis
Each experiment was repeated at least three times, and the data were analyzed statistically using one-way ANOVA and the post-hoc Duncan's Multiple Range Test (P < 0.05) in SPSS ver. 22.0. All statistical analyses were performed using SigmaPlot ver 10.0.
3. Results and discussion
3.1. Influence of NEM on the contents of sulfhydryl group and disulfide bond
Changes in sulfhydryl group and disulfide bond contents influence the interaction of protein molecules and thermal induction gelation (Plancken, Loey, & Hendrickx, 2005; Soladoye, Juárez, Aalhus, Shand, & Estévez, 2015). The effects of NEM on the total sulfhydryl group, free sulfhydryl group, and disulfide bond in the composite gel consisting of different protein emulsion are illustrated in Fig. 1A, B and C. The contents of samples are significantly lower in the composite gels than in the control group (P < 0.05). In the protein matrix of composite gels, the oil droplets in the emulsion often exist in the form of granules (Vliet, 1988). The granules can participate in the interaction within the gel network (Gu, Campbell, & Euston, 2009). The lower stirring speed could cause the droplet size to be smaller and the size distribution narrower. The heated MP solution can form into composite gels, and the interaction between the emulsion granules and protein matrix is enhanced, leading to an increase in the sulfhydryl group and disulfide bond contents (Matsumura, Kang, Sakamoto, Motoki, & Mori, 1993). NEM binds to sulfhydryl groups and blocks the transformation of sulfhydryl groups into disulfide bonds. Thus, the sulfhydryl group and disulfide bond contents continuously decreased with an increase in NEM content. Diao, Guan, Zhao, Diao, and Kong (2016) showed that although disulfide bonds were not the main force affecting the formation of composite gels, disulfide bonds on the interfacial protein membrane of emulsion particles and disulfide bonds between the interfacial film and the continuous phase protein matrix were important for maintaining the stability of composite gels. The broken disulfide bonds lead to the rearrangement of the gel network structure during heating. They also influence the molecular interaction of proteins (Arntfield, Murray, & Ismond, 1991) and properties of the composite gel.
Fig. 1.
Changes in the total sulfhydryl groups (A), free sulfhydryl groups (B), and disulfide bonds (C) of different emulsion-filled protein gels by N-ethylmaleimide. (*a-i Different letters indicate significant differences between means (P < 0.05)).
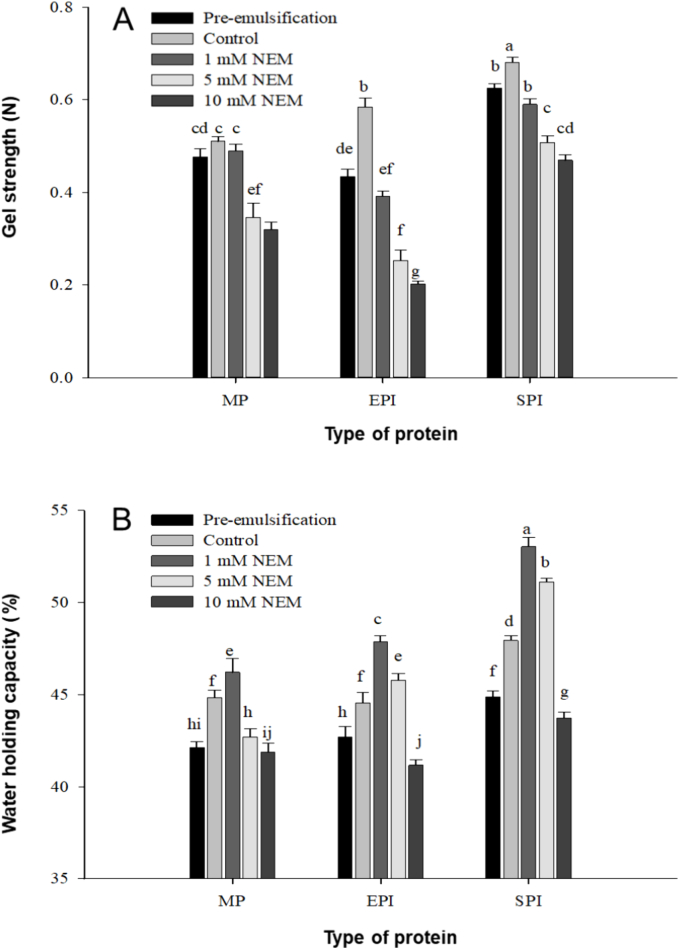
3.2. Influence of NEM on the gel strength and water holding capacity of composite gels
Gel strength is an important physical property of protein gel, which directly affects the appearance and taste of the products. Fig. 2A illustrates the influences of NEM on the gel strength of different protein emulsion MP composite gels. The gel strength of the control group is higher than that of the pre-emulsification group. The fat granules can enter into the gel network because of the network structure of protein gels. The strong interaction between fat granules and gel matrix can enhance the gel strength (Ring & Stainsby, 1982). The emulsion granules after heating possess a similar effect (Gu et al., 2009). The continuous low stirring renders the droplets small and even, easily filling the interspace within the protein network. It enhances the interaction within the protein matrix and improves gel strength (Matsumura et al., 1993; Xiong et al., 1991).
Fig. 2.
Changes in the strength (A) and water holding capacity (B) of different emulsion-filled protein gels by N-ethylmaleimide. (*a-j Different letters indicate significant differences between means (P < 0.05)).
After the addition of NEM, the gel strength of the MP, EPI and SPI emulsion composite gels was all significantly decreased (P < 0.05), with reductions of 28.18 %, 65.36 % and 31.13 %, especially when the addition amount of NEM reached 10 mM. The contents of disulfide bonds between proteins decreased, resulting in a decrease in the gel strength of the protein. Correlation analysis indicated that after NEM addition, gel strength exhibited a significant positive correlation with total sulfhydryl group, free sulfhydryl group, and disulfide bond contents (P < 0.05). Utsumi and Kinsella (1985) also reached the same conclusion in their study of soybean protein. The gel strength of SPI emulsion gel decreased as the amount of NEM increased. Shimada and Cheftel (1988) also found that at pH 7–10, NEM reduced the strength of SPI gel. However, Plancken et al. (2005) obtained opposite results. Their findings indicated that under a specific concentration, the sulfhydryl group content exhibited a significant negative correlation with gel strength. Hayakawa and Nakai (1985) examined the correlation between the gel strength of ovalbumin and total sulfhydryl group content and supported the conclusion (Plancken et al., 2005). Although the sulfhydryl groups and disulfide bonds could influence the strength of composite gels, the solidification of protein gel was not correlated with sulfhydryl group and disulfide bond contents (Ma & Harwalkar, 1987; Olakshmi & Nandi, 1979). The difference in gel strength may be attributed to the initial sulfhydryl group content and surface hydrophobicity in the gel (Voutsinas, Nakai, & Harwalkar, 1983). In addition, the increase in NEM content considerably influences the gel strength of EPI emulsion composite gels. More NEM residues are present in the EPI emulsion, largely affecting the sulfhydryl group and disulfide bond contents and gel strength of composite gels.
Water holding capacity is generally used as one of the indices of the quality of meat products. Higher water holding capacity indicates higher juiciness (Rosenvold & Andersen, 2003). Fig. 2B illustrates the influence of NEM on the water holding capacity of the composite gels. Under the same condition, the water holding capacity of SPI composite gel was superior to those of the two remaining groups (P < 0.05). When the MP sol was added to the emulsion, with the increase of temperature, the emulsion granules were connected to the protein network structure in the matrix and then formed a cage-like structure, and the formed composite gel was expected to generate a physical barrier protecting water and fat (Gravelle et al., 2016). Diao et al. (2016) reported that the gel with a good structure could fix more water and proteins. The disulfide bond content in the gel might be reduced after 1 mM NEM was added; however, the water holding capacity was expected to be improved. An appropriate reduction of the disulfide bond content is deduced to increase the flexibility of protein and emulsibility (Wagner & Gueguen, 1995), stabilize the filling of emulsion droplets in the gel network, and improve the water holding capacity. However, the continuous decrease in disulfide bond content damages the physical barrier of the composite gels when the addition amount of NEM is higher than 1 mM, and the water holding capacity of the three composite gels is reduced correspondingly.
3.3. Influence of NEM on the rheological properties of composite gels
Rheological properties are important for characterizing the interactions between oil droplets and matrix in different protein–emulsion composite gels. Elasticity modulus (G') is one of the important rheological indices of protein–emulsion composite gels. Fig. 3 illustrates the effect of NEM on the rheological properties of composite gels. The rheological properties depend on the property of the gel matrix, property of the emulsion granules, emulsion concentration, and interaction between the granules and protein (Tolstoguzov & Braudo, 1983). In the pre-emulsification group, the G' of the MP emulsion composite gel is relatively larger below 60 °C. At temperatures ranging from 35 °C to 45 °C, G' is modified by the formation of protein dynamic networks arising from the unfolding of the linkage between the head and the tail of myosin (Egelandsdal, Fretheim, & Samejima, 1986; Samejima, Ishioroshi, & Yasui, 1981). In MP emulsion complex gel, both the protein emulsion and the protein matrix are MP. The protein particles and matrix in the composite gel are continuously unfolded simultaneously after heating, resulting in an increase in G'. At 55 °C, the G' values of the three composite gels linearly increase with temperature. This behavior indicates that the system transforms from an oil-like dispersed phase to a solid-like gel (Kim, Renkema, & Vliet, 2001). At temperatures higher than 60 °C, the G' of SPI composite gels increased rapidly because high temperature allows the SPI emulsion droplets to fill the network of MP gels.
Fig. 3.
Changes in the storage modules of different emulsion-filled protein gels by N-ethylmaleimide (NEM). (A): Myofibrillar protein (MP), (B): Egg white protein isolate (EPI) and (C): Soybean protein isolate (SPI). ●: Pre-emulsification; ○: Control; ▼: 1 mM NEM; Δ: 5 mM NEM; ■: 10 mM NEM.
Under low-speed stirring emulsification, the G' value of the MP emulsion composite gels in the control group exhibited no significant change. The SPI emulsion composite gels also showed the same result (Fig. 3C). However, in the EPI emulsion composite gels, G' was increased. Fig. 4 illustrates that continuous emulsification exhibits no significant influence on the droplets of MP and SPI emulsions, in contrast to its influence on the droplets of EPI emulsion. Low-speed stirring emulsification increases the number of small droplets and reduces the size distribution, which is conducive to the interaction between the emulsion and protein network. When the addition of NEM is 1 mM, the G' value of MP and EPI emulsion composite gels exhibits no significant change. However, when the temperature of SPI emulsion composite gel exceeds 60 °C, G' decreases. The G' value shows different degrees of change with the increase of NEM addition, because the disulfide bond content influences the formation of viscoelastic gels and densification of the gel network (Phillips, Whitehead, & Kinsella, 1994). The decrease in the disulfide bond content leads to the breakdown of the gel network, resulting in a reduction in G'.
Fig. 4.
Changes in the optical microstructure of different emulsion-filled protein gels by N-ethylmaleimide (NEM). Column A: MP; Column B: EPI; and Column C: SPI. Row (1): Pre-emulsification; Row (2): Control; Row (3): 1 mM NEM; Row (4): 5 mM NEM; and Row (5): 10 mM NEM.
3.4. Influence of NEM on the microstructure of composite gels
Emulsion granules embedded into the protein network matrix were observed by optical microscopy. Fig. 4 illustrates the effects of NEM on the microstructure of composite gels. In the pre-emulsification group, spherical emulsion granules on the surface of the composite gels network are clearly observed. The MP is still a continuous phase and is the main component of the gel network. (Fan et al., 2017). The same occurrence can be observed in the control group. Fig. 2A and 3 illustrate that owing to the complete interfacial protein film of emulsion granules, the gel matrix exhibits a considerably strong interaction, which promotes crosslinking between granules and gels. When the protein on the interface membrane of emulsion particles interacts with the surrounding protein matrix through disulfide bonds, the physical properties of the protein gels strengthened by the emulsion particles are strengthened (Wu et al., 2011). Gravelle et al. (2016) reported that emulsion granules could be used as filling agents to enhance the protein gel and improve the rheological property of the gels.
After the addition of 1 mM NEM, some of the emulsion particles in the gels of MP and EPI showed irregular shape and some were slightly broken. However, in SPI composite gel, most of the interfacial protein membranes of emulsion particles were broken. Youssef and Barbut (2010) studied the beef emulsion added with rapeseed oil and found that a similar phenomenon occurred when the emulsion was unstable. In heat-induced gels, the protein film in contact with the gel matrix is cracked, meaning that the interaction between the emulsion particles and the gel matrix is weakened (Sala, Aken, Stuart, & Velde, 2007). With an increase in the amount of NEM, all emulsion granules in the gel showed cracks at varying degrees. Fig. 1C and 3 illustrate that the addition of NEM decreases the disulfide bond content, leading to cracks in the protein film and further influencing the characteristics of the gel. In particular, in the 10 mM NEM group of SPI composite gel, some emulsion particles did not have interface protein membrane residue. Wu et al. (2011) evaluated the effect of different concentrations of NEM (0, 1, 3, 5 and 10 mM) on interface protein membranes and also found similar phenomena. The results showed that with the increase of NEM addition, the damage degree of protein membrane was intensified, and some droplets atrophied when the NEM concentration was 10 mM.
3.5. Influence of NEM on the water distribution of composite gels
When the solid gel network is formed, the water is completely retained within the gel network. However, the water is still mobile at this time (Jr & Kauzmann, 1974). The effect of NEM on the migration rate of water molecules in the composite gels was investigated using the T2 relaxation time of LF-NMR (Zhang, Yang, Tang, Chen, & Yuan, 2015). By fitting, the NMR decay signal can be divided into exponential functions with one to three independent peak distributions (Sherman, 1973). The T2 relaxation time can be divided into T2b, T21, and T22. T2b (in the range of 1–10 ms) indicates the presence of water molecules in the protein (immobile water); T21 (in the range of 40–60 ms) is closely associated with macromolecules (weakly movable water); T22 (in the range of 300–800 ms) is the water in the composite gels (moderately movable water) (Shaarani, Nott, & Hall, 2006); T23 (in the range of 1000–1600 ms) may at times appear, representing free water in the gels: water that is released after heating. Fig. 5 illustrates the effects of NEM on the T2 relaxation time distribution of the three composite gels. NEM exhibits a nonlinear influence on T2, meaning that the change in relaxation time alone cannot sufficiently reflect the difference in the composite gels, which should be investigated based on the change in the peak area (Zhang et al., 2015). The T2 relaxation time and P2 peak area ratio are illustrated in Fig. 6.
Fig. 5.
Changes in the T2 relaxation times of different emulsion protein composite gels by N-ethylmaleimide. (A): Myofibrillar protein (MP) group, (B): Egg white protein isolate (EPI) group, and (C): Soybean protein isolate (SPI) group.
Fig. 6.
Changes in T2b, T21, T22, and T23 relaxation times (left) and peak areas (right) of different emulsion protein composite gels by N-ethylmaleimide (NEM) (Left:  : T2b; □: T21;
: T2b; □: T21;  : T22; and ■: T23; Right:
: T22; and ■: T23; Right: : P2b; □: P21;
: P2b; □: P21;  : P22; and ■: P23. ME: MP pre-emulsification group; MC: MP control group; M1: MP 1 mM NEM group; M5: MP 5 mM NEM group; and M10: MP 10 mM NEM group. EE: EPI pre-emulsification group; EC: EPI control group; E1: EPI 1 mM NEM group; E5: EPI 5 mM NEM group; and E10: EPI 10 mM NEM group. SE: SPI pre-emulsification group; SC: SPI control group; S1: SPI 1 mM NEM group; S5 SPI 5 mM NEM group; and S10: SPI 10 mM NEM group).
: P22; and ■: P23. ME: MP pre-emulsification group; MC: MP control group; M1: MP 1 mM NEM group; M5: MP 5 mM NEM group; and M10: MP 10 mM NEM group. EE: EPI pre-emulsification group; EC: EPI control group; E1: EPI 1 mM NEM group; E5: EPI 5 mM NEM group; and E10: EPI 10 mM NEM group. SE: SPI pre-emulsification group; SC: SPI control group; S1: SPI 1 mM NEM group; S5 SPI 5 mM NEM group; and S10: SPI 10 mM NEM group).
The variation in T2 distribution indicates the differences in the physical and chemical properties of gels (Raun et al., 1999; Salomonsen, Sejersen, Viereck, Ipsen, & Engelsen, 2007). Fig. 5A, 6A and D illustrate that 4 peaks appear in the composite gel in the control group. The corresponding relaxation times are 1.24–1.51 ms, 37.74–49.77 ms, 174.75–533.67 ms, and 811.13–1629.75 ms; the corresponding peak area ratios are 0.0054, 0.020, 0.65, and 0.33. The appearance of T23 indicates water release after gelation. The peak area of T22 is the largest, indicating that the moderately mobile water content is the highest. T2b and T21 in the control group disappear, while the T22 peak appears later and the peak area increases. The T23 peak appears late and the area is decreased, indicating the transfer of a small amount of water from T23 to T22. The results showed that the content of moderate movable water was higher in the control group. Although T2b and T21 are in the control group, the contents are low. Therefore, the water holding capacity of the control group was higher than that of the pre-emulsified group. After 1 mM NEM is added, T2b and T21 reappear. Compared with the pre-emulsified group, all four peaks appeared later in the 1 mM NEM group, with increased peak areas for three of them, while the T23 peak area decreased. This occurrence indicates a reduction in water mobility and a tighter binding with MP emulsion composite gels, which are consistent with the result for water holding capacity. With the addition of NEM, T2b and T21 disappear again, and the peak areas of T22 and T23 change correspondingly. The increase in the T23 peak area indicates that the free water is expelled from the gel during gelation, and the migration rate increases. It also demonstrates that the network of the MP composite gel is reduced, and the water holding capacity becomes worse.
As shown in Fig. 5B, 6B and E, T2b and T21 in EPI emulsion composite gel are hardly observed, and the peak area of T23 is smaller than 0.07, suggesting moderately movable water is the main water in the gel. After adding 1 mM NEM, no significant change in peak area is observed, compared with the control group. However, the relaxation time shifts into the rapid-speed direction, indicating that the water binding in the composite gels is tighter with a higher water holding capacity. With the addition of NEM, T22 peak appeared earlier in the 5 mM NEM group than in the 10 mM NEM group. This finding suggests that the water holding capacity is higher in the 5 mM NEM group than in the 10 mM NEM group. Although only a small amount of T23 was present in the 1 mM NEM group (peak area = 0.07), the T22 peak intensity was lower and wider than that in the 5 mM NEM group. This finding suggests limited water mobility within the 1 mM NEM group (Noronha, Duggan, Ziegler, O'Riordan, & O'Sullivan, 2008). Therefore, the water holding capacity of the 1 mM NEM group in EPI emulsion composite gel is the best, which is consistent with the results in Fig. 2B.
Fig. 5C, 6C and F illustrate that the SPI emulsion composite gel in the 1 mM NEM group has a higher water holding capacity. In summary, disulfide bond content influences the water distribution of the three protein-emulsion composite gels, but the specific effect varies according to the gel types. The water holding capacity of the three composite gels in the 1 mM NEM group is high, which is consistent with the results in Fig. 2B.
4. Conclusions
In conclusion, the composite gels formed by the heating of the different protein emulsions with MP were used as study subjects to evaluate the effects of disulfide bonds and sulfhydryl groups on the composite system. The results show that sulfhydryl groups and disulfide bonds influence the physicochemical properties of the composite gels by affecting the interaction between the protein film and gel network. With the increase of NEM concentration, the sulfhydryl and disulfide bonds of the MP, EPI and SPI emulsion composite gels significantly decreased. The sulfhydryl groups reduced by 60.15 %, 77.35 %, 67.85 %, while the disulfide bonds reduced by 75.58 %, 62.89 %, 79.19 %, respectively. The addition of NEM reduced the content of sulfhydryl bonds and disulfide bonds, resulting in protein film cracks on the surface of emulsion particles, thus destroying the structure of the composite gels, reducing the gel strength and water holding capacity, loosening the binding of water molecules, and reducing the elastic modulus. The above experimental results show that the effects of different NEM additions on the properties of MP, SPI and EPI emulsion composite gels are similar. Furthermore, the findings of this study demonstrate that the stabilizing role of disulfide bonds in different protein emulsion composite gels is universal. This study provides a theoretical basis for understanding the role of disulfide bonds in MP composite gels.
CRediT authorship contribution statement
Yuyu Xu: Writing – original draft, Data curation. Jingjing Yang: Software, Investigation, Formal analysis, Data curation. Mangang Wu: Writing – review & editing, Writing – original draft, Supervision, Funding acquisition, Conceptualization. Shumin Lei: Writing – original draft, Software, Methodology, Formal analysis, Data curation. Peipei Yin: Writing – review & editing, Validation, Software, Resources, Methodology, Investigation. Tianhao Zhu: Writing – review & editing, Validation, Methodology, Formal analysis, Data curation. Qingling Wang: Software, Methodology, Formal analysis, Data curation. Xinxin Zhao: Validation, Investigation. Duxin Jin: Writing – review & editing. Rui Liu: Writing – review & editing. Qingfeng Ge: Writing – review & editing. Hai Yu: Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This study was funded by the projects from National Natural Science Foundation of China (Grant No. 32272335, 32101859, 32302110), and supported by Yangzhou University Student Science and Technology Innovation Fund (XCX20231060), Postgraduate Research & Practice Innovation Program of Jiangsu Province (SJCX24_2367). Outstanding Youth Science Fund Project of Natural Science Foundation of Jiangsu Province (BK20230070), China.
Data availability
Data will be made available on request.
References
- Ansorena D., Astiasaran I. The use of linseed oil improves nutritional quality of the lipid fraction of dry-fermented sausages. Food Chemistry. 2004;87(1):69–74. doi: 10.1016/j.foodchem.2003.10.019. [DOI] [Google Scholar]
- Arntfield S.D., Murray E.D., Ismond M.A.H. Role of disulfide bonds in determining the rheological and microstructural properties of heat-induced protein networks from ovalbumin and vicilin. Journal of Agricultural and Food Chemistry. 1991;39(8):1378–1385. doi: 10.1021/jf00008a005. [DOI] [Google Scholar]
- Cao H., Fan D., Jiao X., Huang J., Zhao J., Yan B., Zhang H. Effects of microwave combined with conduction heating on surimi quality and morphology. Journal of Food Engineering. 2018;228:1–11. doi: 10.1016/j.jfoodeng.2018.01.021. [DOI] [Google Scholar]
- Diao X., Guan H., Zhao X., Diao X., Kong B. Physicochemical and structural properties of composite gels prepared with myofibrillar protein and lard diacylglycerols. Meat Science. 2016;121:333–341. doi: 10.1016/j.meatsci.2016.07.002. [DOI] [PubMed] [Google Scholar]
- Dickinson E., Evison J., Gramshaw J.W., Schwope D. Flavour release from a protein-stabilized water-in-oil-in-water emulsion. Food Hydrocolloids. 1994;8(1):63–67. doi: 10.1016/S0268-005X(09)80145-8. [DOI] [Google Scholar]
- Egelandsdal B., Fretheim K., Samejima K. Dynamic rheological measurements on heat-induced myosin gels: Effect of ionic strength, protein concentration and addition of adenosine triphosphate or pyrophosphate. Journal of the Science of Food and Agriculture. 1986;37(9):915–926. doi: 10.1002/jsfa.2740370914. [DOI] [Google Scholar]
- Fan M., Hu T., Zhao S., Xiong S., Xie J., Huang Q. Gel characteristics and microstructure of fish myofibrillar protein/cassava starch composites. Food Chemistry. 2017;218:221–230. doi: 10.1016/j.foodchem.2016.09.068. [DOI] [PubMed] [Google Scholar]
- Fritz J.D., Swartz D.R., Greaser M.L. Factors affecting polyacrylamide gel electrophoresis and electroblotting of high-molecular-weight myofibrillar proteins myofibrillar proteins. Analytical Biochemistry. 1989;180(2):205–210. doi: 10.1016/0003-2697(89)90116-4. [DOI] [PubMed] [Google Scholar]
- Gómez-Guillén M.C., BorderíAs A.J., Montero P. Chemical interactions of nonmuscle proteins in the network of sardine (Sardina pilchardus) muscle gels. LWT - Food Science and Technology. 1997;30(6):602–608. doi: 10.1006/fstl.1997.0239. [DOI] [Google Scholar]
- Gravelle A.J., Marangoni A.G., Barbut S. Insight into the mechanism of myofibrillar protein gel stability: Influencing texture and microstructure using a model hydrophilic filler. Food Hydrocolloids. 2016;60:415–424. doi: 10.1016/j.foodhyd.2016.04.014. [DOI] [Google Scholar]
- Gu X., Campbell L.J., Euston S.R. Effects of different oils on the properties of soy protein isolate emulsions and gels. Food Research International. 2009;42(8):925–932. doi: 10.1016/j.foodres.2009.04.015. [DOI] [Google Scholar]
- Hayakawa S., Nakai S. Contribution of hydrophobicity, net charge and sulfhydryl groups to thermal properties of ovalbumin. Canadian Institute of Food Science and Technology Journal. 1985;18(4):290–295. doi: 10.1016/S0315-5463(85)71960-8. [DOI] [Google Scholar]
- Jr K.I.D., Kauzmann W. Hydration of proteins and polypeptides. Advances in Protein Chemistry. 1974;28(1):239–345. doi: 10.1007/BF02435776. [DOI] [PubMed] [Google Scholar]
- Kim K.H., Renkema J.M.S., Vliet T.V. Rheological properties of soybean protein isolate gels containing emulsion droplets. Food Hydrocolloids. 2001;15(3):295–302. doi: 10.1016/S0268-005X(01)00028-5. [DOI] [Google Scholar]
- Kim S.H., Kinsella J.E. Effects of reduction with dithiothreitol on some molecular properties of soy glycinin. Journal of Agricultural and Food Chemistry. 1986;34(4):623–627. [Google Scholar]
- Kim S.H., Kinsella J.E. Surface active properties of food proteins: Effects of reduction of disulfide bonds on film properties and foam stability of glycinin. Journal of Food Science. 1987;52(1):128–131. doi: 10.1111/j.1365-2621.1987.tb13987.x. [DOI] [Google Scholar]
- Ma C.Y., Harwalkar V.R. Thermal coagulation of oat globulin. Cereal Chemistry. 1987;64(4):212–218. [Google Scholar]
- Matsumura Y., Kang I.J., Sakamoto H., Motoki M., Mori T. Filler effects of oil droplets on the viscoelastic properties of emulsion gels. Food Hydrocolloids. 1993;7(3):227–240. doi: 10.1016/S0268-005X(09)80174-4. [DOI] [Google Scholar]
- Montejano J.G., Hamann D.D., Lanier T.C. Comparison of two instrumental methods with sensory texture of protein gels. Journal of Texture Studies. 1985;16(4):403–424. doi: 10.1111/j.1745-4603.1985.tb00705.x. [DOI] [Google Scholar]
- Niwa E., Matsubara Y., Nakayama T., Hamada I. Participation of SS bonding in the appearance of setting. Nippon Suisan Gakkaishi. 1982;48(5):727. doi: 10.2331/suisan.48.727. [DOI] [Google Scholar]
- Noronha N., Duggan E., Ziegler G.R., O’Riordan E.D., O’Sullivan M. Investigation of imitation cheese matrix development using light microscopy and NMR relaxometry. International Dairy Journal. 2008;18(6):641–648. doi: 10.1016/j.idairyj.2007.12.004. [DOI] [Google Scholar]
- Olakshmi T.S., Nandi P.K. Studies on the effect of heat on the dissociation, denaturation, and aggregation of sesame alpha-globulin. Journal of Agricultural and Food Chemistry. 1979;27(4):818–821. doi: 10.1021/jf60224a057. [DOI] [PubMed] [Google Scholar]
- Phillips L.G., Whitehead D.M., Kinsella J.E. Structure-function properties of food proteins. Food Science and Technology International. 1994:251–265. [Google Scholar]
- Plancken I.V.D., Loey A.V., Hendrickx M.E.G. Changes in sulfhydryl content of egg white proteins due to heat and pressure treatment. Journal of Agricultural and Food Chemistry. 2005;53(14):5726–5733. doi: 10.1021/jf050289+. [DOI] [PubMed] [Google Scholar]
- Raun R.R., Wang X., Chen P.L., Fulcher R.G., Pesheck P., Chakrabarti S. Study of water in dough using nuclear magnetic resonance. Cereal Chemistry. 1999;76(2):231–235. doi: 10.1094/CCHEM.1999.76.2.231. [DOI] [Google Scholar]
- Ring S., Stainsby G. Filler reinforcement of gels. Progress in Food and Nutrition Science. 1982:17–19. [Google Scholar]
- Rosenvold K., Andersen H.J. Factors of significance for pork quality-a review. Meat Science. 2003;64(3):219–237. doi: 10.1016/S0309-1740(02)00186-9. [DOI] [PubMed] [Google Scholar]
- Sala G., Aken G.A.V., Stuart M.A.C., Velde F.V.D. Effect of droplet-matrix interactions on large deformation properties of emulsion-filled gels. Journal of Texture Studies. 2007;38(4):511–535. doi: 10.1111/j.1745-4603.2007.00110.x. [DOI] [Google Scholar]
- Salomonsen T., Sejersen T.M., Viereck N., Ipsen R., Engelsen S.B. Water mobility in acidified milk drinks studied by low-field 1H NMR. International Dairy Journal. 2007;17(4):294–301. doi: 10.1016/j.idairyj.2006.04.003. [DOI] [Google Scholar]
- Samejima K., Ishioroshi M., Yasui T. Relative roles of the head and tail portions of the molecule in heat-induced gelation of myosin. Journal of Food Science. 1981;46(5):1412–1418. doi: 10.1111/j.1365-2621.1981.tb04187.x. [DOI] [Google Scholar]
- Sano T., Ohno T., Otsukafuchino-fuchino H., Matsumoto J.J., Tsuchiya T. Carp natural actomyosin: Thermal denaturation mechanism. Journal of Food Science. 1994;59(5):1002–1008. doi: 10.1111/j.1365-2621.1994.tb08177.x. [DOI] [Google Scholar]
- Shaarani S.M., Nott K.P., Hall L.D. Combination of NMR and MRI quantitation of moisture and structure changes for convection cooking of fresh chicken meat. Meat Science. 2006;72(3):398–403. doi: 10.1016/j.meatsci.2005.07.017. [DOI] [PubMed] [Google Scholar]
- Sherman P. Structure and textural properties of foods. Texture Measurements of Foods. 1973:52–70. [Google Scholar]
- Shimada K., Cheftel J.C. Determination of sulfhydryl groups and disulfide bonds in heat-induced gels of soy protein isolate. Journal of Agricultural and Food Chemistry. 1988;36(1):147–153. doi: 10.1021/jf00079a038. [DOI] [Google Scholar]
- Soladoye O.P., Juárez M.L., Aalhus J.L., Shand P., Estévez M. Protein oxidation in processed meat: Mechanisms and potential implications on human health. Comprehensive Reviews in Food Science and Food Safety. 2015;14(2):106–122. doi: 10.1111/1541-4337.12127. [DOI] [PubMed] [Google Scholar]
- Tang C.H., Wang X.Y., Yang X.Q., Li L. Formation of soluble aggregates from insoluble commercial soy protein isolate by means of ultrasonic treatment and their gelling properties. Journal of Food Engineering. 2009;92(4):432–437. doi: 10.1016/j.jfoodeng.2008.12.017. [DOI] [Google Scholar]
- Tolstoguzov V.B., Braudo E.E. Fabricated foodstuffs as multicomponent gels. Journal of Texture Studies. 1983;14(3):183–212. doi: 10.1111/j.1745-4603.1983.tb00344.x. [DOI] [Google Scholar]
- Utsumi S., Kinsella J.E. Forces involved in soy protein gelation: Effects of various reagents on the formation, hardness and solubility of heat-induced gels made from 7S, 11S, and soy isolate. Journal of Food Science. 1985;50(5):1278–1282. doi: 10.1111/j.1365-2621.1985.tb10461.x. [DOI] [Google Scholar]
- Visessanguan W., Ogawa M., Nakai S., An H. Physicochemical changes and mechanism of heat-induced gelation of arrowtooth flounder myosin. Journal of Agricultural and Food Chemistry. 2000;48(4):1016–1023. doi: 10.1021/jf9900332. [DOI] [PubMed] [Google Scholar]
- Vliet T.V. Rheological properties of filled gels. Influence of filler matrix interaction. Colloid and Polymer Science. 1988;266(6):518–524. doi: 10.1007/bf01420762. [DOI] [Google Scholar]
- Voutsinas L.P., Nakai S., Harwalkar V.R. Relationships between protein hydrophobicity and thermal functional properties of food proteins. Canadian Institute of Food Science and Technology Journal. 1983;16(3):185–190. doi: 10.1016/S0315-5463(83)72205-4. [DOI] [Google Scholar]
- Wagner J.R., Gueguen J. Effects of dissociation, deamidation, and reducing treatment on structural and surface active properties of soy glycinin. Journal of Agricultural and Food Chemistry. 1995;43(8):1993–2000. doi: 10.1021/jf00056a007. [DOI] [Google Scholar]
- Wang X., Wang L., Yang K., Wu D., Sun W. Radio frequency heating improves water retention of pork myofibrillar protein gel: An analysis from water distribution and structure. Food Chemistry. 2021;350(1) doi: 10.1016/j.foodchem.2021.129265. [DOI] [PubMed] [Google Scholar]
- Wu M., Cao Y., Lei S., Liu Y., Wang J., Hu J.…Yu H. Protein structure and sulfhydryl group changes affected by protein gel properties: Process of thermal-induced gel formation of myofibrillar protein. International Journal of Food Properties. 2019;22(1):1834–1847. doi: 10.1080/10942912.2019.1656231. [DOI] [Google Scholar]
- Wu M., Li Z., Wei R., Luan Y., Hu J., Wang Q.…Yu H. Role of disulfide bonds and sulfhydryl blocked by N-ethylmaleimide on the properties of different protein-stabilized emulsions. Foods. 2021;10(12):3079. doi: 10.3390/foods10123079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M., Xiong Y.L., Chen J. Role of disulphide linkages between protein-coated lipid droplets and the protein matrix in the rheological properties of porcine myofibrillar protein–peanut oil emulsion composite gels. Meat Science. 2011;88(3):384–390. doi: 10.1016/j.meatsci.2011.01.014. [DOI] [PubMed] [Google Scholar]
- Wu M., Xiong Y.L., Chen J., Tang X., Zhou G. Rheological and microstructural properties of porcine myofibrillar protein–lipid emulsion composite gels. Journal of Food Science. 2009;74(4):E207–E217. doi: 10.1111/j.1750-3841.2009.01140.x. [DOI] [PubMed] [Google Scholar]
- Xiong Y.L., Aguilera J.M., Kinsella J.E. Emulsified milkfat effects on rheology of acid-induced milk gels. Journal of Food Science. 1991;56(4):920–925. doi: 10.1111/j.1365-2621.1991.tb14606.x. [DOI] [Google Scholar]
- Yang H., Zhang W., Li T., Zheng H., Khan M.A., Xu X.…Zhou G. Effect of protein structure on water and fat distribution during meat gelling. Food Chemistry. 2016;204:239–245. doi: 10.1016/j.foodchem.2016.01.053. [DOI] [PubMed] [Google Scholar]
- Yongsawatdigul J., Park J.W. Thermal denaturation and aggregation of threadfin bream actomyosin. Food Chemistry. 2003;83(3):409–416. doi: 10.1016/S0308-8146(03)00105-5. [DOI] [Google Scholar]
- Youssef M.K., Barbut S. Physicochemical effects of the lipid phase and protein level on meat emulsion stability, texture, and microstructure. Journal of Food Science. 2010;75(2):S108–S114. doi: 10.1111/j.1750-3841.2009.01475.x. [DOI] [PubMed] [Google Scholar]
- Zhang Z., Yang Y., Tang X., Chen Y., Yuan Y. Chemical forces and water holding capacity study of heat-induced myofibrillar protein gel as affected by high pressure. Food Chemistry. 2015;188(1):111–118. doi: 10.1016/j.foodchem.2015.04.129. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.