Abstract
Background
Mpox infection is a zoonotic illness that resembles smallpox. Vaccination is widely regarded as a vital effective method of preventing mpox, however, there is lack of consensus of effectiveness of a single dose of mpox vaccine in the current 2022–2023 outbreak. We pooled data from real‐world studies to evaluate the efficacy of the JYNNEOS vaccination given as a single dosage.
Method
We carried out a thorough literature search in PubMed, Web of Science, and Scopus up until August 2023. We estimated the pooled vaccine effectiveness (VE) for mpox using inverse variance method in a random‐effects meta‐analysis. We expressed the results as VE, 95% confidence interval (95% CI), and 95% prediction interval (95% PI) using R v4.3.0. We assessed influence, heterogeneity contribution, and influence of studies using several tests and conducted sensitivity analysis accordingly. We used Doi plot and Luis Furuya‐Kanamori (LFK) index to evaluate publication bias.
Results
With a total sample size of 35,326 individuals, we involved 11 studies in the meta‐analysis. The VE of a single dose of JYNNEOS vaccine was 78.23% (95% CI: 62.79%–87.27%) by pooling data of 24,784 individuals over seven studies. The findings were heterogenous with a 95% PI of −32.14% to 96.41% depicting the expected range of VE in similar settings. Notably, VE increased to 83.02% (74.62%–88.64%) with a prediction interval of (44.67%–94.79) after sensitivity analysis by leaving out outliers. The results were robust in light of several sensitivity analyses. An asymmetric Doi plot with LFK index of −2.25 showed potential publication bias. Pooled prevalence of mpox infection among vaccinated individuals (breakthrough infection) in six studies was 2.19% (0.37%–5.32%).
Conclusion
The present findings provide compelling evidence that a single dose of JUNNEOS vaccine can protect recipients from mpox infection. With a 78.23% estimated efficacy rate, the vaccine is thought to be a useful tool in preventing further spread of mpox. However, more research and ongoing surveillance are required to fully understand the reasons behind breakthrough infections and to improve immunization strategies for better protection against mpox.
Keywords: effectiveness, JYNNEOS, Mpox: vaccine, MPXV
1. INTRODUCTION
Monkey pox (mpox) disease is caused by mpox virus (MPXV) that belongs to the Orthopoxvirus genus and is closely related to the variola virus causing smallpox. 1 Although mpox is endemic in Central and West African countries, sporadic outbreaks have been reported outside of Africa, including Asian and European countries. 2 , 3 , 4 , 5 The symptoms of mpox range from mild to severe including fever, headache, muscle aches, and a characteristic rash that progresses from maculopapular to vesicles and pustules. 6
The global health community has recently experienced an increase in mpox outbreaks, with the most recent one occurring in 2022, affecting approximately 113 countries worldwide. 7 In July 2022, The World Health Organization announced the current 2022 outbreak as a public health emergency of international concern. 8 The global confirmed cases have exceeded 89,000 cases as by August 2023. 7 In response to this threat to the public health, a number of infection control and prevention strategies have been implemented, including contact surveillance, case isolation, and vaccination campaigns using JYNNEOS vaccine. 9
JYNNEOS, also called modified vaccinia Ankara–Bavaria Nordic (MVA‐BN) vaccine, is a live attenuated vaccine that provides protection against both smallpox and mpox. 10 The JYNNEOS vaccine contains MVA virus strains expressing specific antigens from both viruses. 11 In 2019, JYNNEOS vaccine received approval from the Food and Drug Administration for use in high‐risk adults aged 18 years and older as a two‐dose subcutaneous (SC) regimen. 12 However, many countries have adopted a single‐dose course in the current outbreak due to shortage of vaccine supplies.
While JYNNEOS has demonstrated promising results in preclinical studies and clinical trials conducted before the current 2022 outbreak, 10 , 13 , 14 there is still debate about its effectiveness as a single‐dose vaccine during an actual outbreak scenario. Several published studies have reported varying results in terms of its effectiveness in real‐world settings. 15 , 16
Some studies have suggested that a single dose of JYNNEOS provides robust protection against mpox infection during this outbreak. 17 , 18 Sagy et al. 17 conducted a retrospective cohort study among high risk individuals who received a single SC dose of JYNNEOS vaccine. They found that only five of the 1037 vaccinated individuals developed symptomatic mpox, indicating a high level of VE. Contrarily, another study has reported suboptimal effectiveness of the JYNNEOS vaccine in outbreak settings. For instance, Deputy et al. 15 conducted a case‐control study in the United States and found that effectiveness of JYNNEOS single dose was only 35%, suggesting that additional doses or alternative vaccination strategies may be necessary for optimal protection.
These divergent results highlight the importance of conducting a comprehensive meta‐analysis to assess the overall effectiveness of a single dose of JYNNEOS vaccine during the 2022–2023 outbreak. We aim to conduct a comprehensive meta‐analysis to evaluate the effectiveness of a single dose of JYNNEOS vaccine during the current mpox outbreak. Additionally, this paper will discuss the implications of these findings for public health interventions and provide recommendations for future research and vaccination strategies.
2. METHODS
This review is registered with PROSPERO CRD42023455613 and reported in compliance with PRISMA criteria.
2.1. Search strategy
We used the following terms to search PubMed, Cochrane, Web Sciences, and SCOPUS from the beginning to August 15, 2023, with a restriction on articles written in English: “Monkeypox”, “Mpox”, “MPXV”, and “vaccin*”. (Table S1) After removing duplicates, two authors assessed the eligibility of the retrieved articles based on the title and abstract before moving on to the entire text. Any differences between the two authors were resolved by the third author.
2.2. Inclusion and exclusion criteria
We included studies fulfilling the following criteria:
-
1.
Study design: we included real‐world studies (observational studies).
-
2.
Population: individuals administered a single dose of JYNNEOS, regardless of age, sex, or ethnicity.
-
3.
Intervention: a single dose of the JYNNEOS vaccine.
-
4.
Outcomes: effectiveness of the vaccine in preventing disease. Studies must clearly state the vaccine dosage and administration details.
Exclusion criteria included animal studies, case reports, case series, commentaries, reviews, and editorials. Studies with incomplete outcome data or lacking essential information regarding vaccine administration or effectiveness measurement or non‐English studies were excluded. We also omitted trials that did not focus solely on a single dosage of JYNNEOS or that combined data with other vaccinations without obvious segregation.
2.3. Data extraction and quality assessment
We took the first author's name, the publication year, the nation, and the study population's characteristics (sample size, age, population inclusion criteria, percentage of men, sexual orientation, and administration method) from each study. Additionally, we extracted outcomes (VE, MPOX cases postvaccination/breakthrough infection) and breakthrough infection data (age, male sex, PLWH, and time from vaccination to symptoms). Using the New Castle Ottawa Scale (NOS), we determined if the included studies were of good, fair, or poor quality. 19 Quality of the involved articles was evaluated by two authors, and any discrepancies were settled by a third author.
2.4. Outcomes
Vaccine effectiveness (VE), defined as the reduction in the risk of mpox infection among vaccinated individuals versus unvaccinated individuals.
2.5. Data synthesis
By translating the vaccination efficacies into rate ratios, log‐transforming them, and then synthesizing them using the inverse variance approach, we were able to estimate the pooled VE for the MPOX virus. Due to the significant heterogeneity between studies, we utilized a random‐effects model. We back converted these numbers into VE with a 95% confidence interval (CI) to show them as forest plots. 20
The Q‐test, tau2 and I2 values, along with prediction interval (PI), were used to report heterogeneity. 21 Tau2 was calculated using the constrained maximum likelihood estimator, and the tau2 CI was reported using the Q‐profile method. When there was heterogeneity, the PI for the pooled measure was also shown. This provided epidemiologists and clinicians with more useful information because it not only provided the average effect size but also the range that could be anticipated in other studies with a similar design. 22 As a result, it provided the range that 95% of the values would fall inside. To investigate this heterogeneity based on potential moderating variables, a bubble plot was used. To identify heterogeneity contributors, overly influential studies, and outliers, we used graphical representations of heterogeneity, heterogeneity assessment by clustering algorithms, influence diagnostics, and Baujat plots. Sensitivity analyses were then conducted based on that information. We also carried out leave‐one‐out meta‐analyses to evaluate the reliability of the findings.
We were unable to utilize the funnel plot for publication bias or small‐study effects because there were fewer than 10 studies. Doi plot and Luis Furuya‐Kanamori (LFK) index were utilized as an alternative because they were validated for at least five studies. 23 The quantitative interpretation of a Doi graphic is the LFK index. An LFK score of less than −1 was indicative of potential publication bias since we anticipated research indicating vaccinations to be more efficacious would be reported more frequently. The meta, metafor, and metasens packages in R v4.3.0 were used to conduct the analyses. It was deemed significant if p < 0.05.
3. RESULTS
3.1. Study selection and characteristics
After conducting literature searches, we uncovered 4737 studies, and we ultimately decided to include 11 studies collecting data from 35236 individuals in the current systematic review and meta‐analysis. In Figure 1, the PRSIMA flow diagram is displayed. In the USA, four studies were carried out. There were seven cohort studies, three case‐control studies, and one cross‐sectional study. The bulk of the participants who were included were men with sexual orientation as GBMSM. Tables 1 and 2, respectively, displayed the participant baseline characteristics, the breakthrough infection data.
Figure 1.
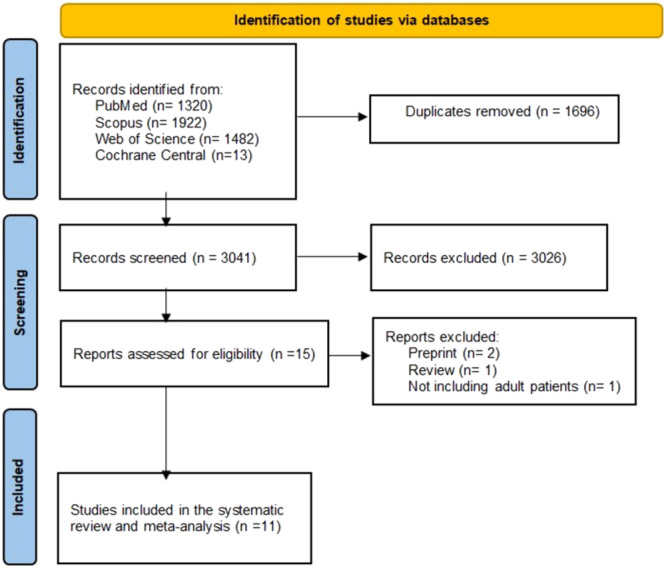
PRISMA figure.
Table 1.
Characteristics of included studies.
| ID | Group | Country | Sample size (N) | Age, mean (SD) | Male sex, N (%) | Sexual orientation, N (%) | Administration route | Inclusion criteria |
|---|---|---|---|---|---|---|---|---|
| Agunbiade 2023 34 | Vaccinated | UK | 10068 | NA | 10068 (100) | GBMSM | Subcutaneous | Individuals who received a first dose of modified vaccinia Ankara immunization at sexual health clinics part of Chelsea and Westminster Hospital NHS Foundation Trust, in London (UK). |
| Bertran 2023 16 | Total cohort | UK | 363 | 18–42 years | 363 (100) | GBMSM | Most doses were subcutaneous, others were intradermal | Individuals who were at‐risk gay, bisexual, and other men who have sex with men (GBMSM). This category included those with specific risk factors such as having multiple sexual partners, engaging in group sex, and attending sex on premises venues. Additionally, eligibility for HIV pre‐exposure prophylaxis (PrEP) was used as a criterion to identify eligible groups. |
| Dalton 2023 30 | Cases | US | 309 | 18–29 years: 78 (25.2) 30–39 years: 144 (46.6) 40–49 years: 87 (28.2) | Male 290 (94.2) Transgender female 6 (1.9) Transgender male 1 (0.3) Another gender identity 11 (3.6) | Male 290 (94.2 Transgender female 6 (1.9) Transgender male 1 (0.3) Another gender identity 11 (3.6) | Subcutaneous: 38 (65.5) Intradermal: 18 (31.0) Other/Missing: 2 (3.4) | 18–49 years MSM or transgender sexually active persons. |
| Control | 608 | 18–29 years: 184 (30.3) 30–39 years: 292 (48.0) 40–49 years: 132 (21.7) | Male 544 (89.5) Transgender female 13 (2.1) Transgender male 7 (1.2) Another gender identity 44 (7.2) | Male 544 (89.5) Transgender female 13 (2.1) Transgender male 7 (1.2) Another gender identity 44 (7.2) | Subcutaneous: 159 (67.1) Intradermal: 76 (32.1) Other/Missing: 2 (0.8) | 18–49 years MSM or transgender sexually active persons. | ||
| Deputy 2023 15 | Cases | US | 2266 | 18–35 years: 1252 (55.3) 36–49 years: 707 (31.2) 50–64 years: 273 (12.0) ≥65 years: 34 (1.5) | Legal sex Male: 2059 (90.9) Female: 204 (9.0) Other: 1 (<0.1) Unknown: 2 (0.1) | Male 2022 (89.2) Female 199 (8.8) Transgender male 2 (0.1) Transgender female 23 (1.0) Other 19 (0.8) Chose not to disclose 1 (<0.1) | For full vaccinations: Subcutaneous 6/25 (24.0) Intradermal 5/25 (20.0) Heterologous 8/25 (32.0) Other or missing 6/25 (24.0) For Partially vaccinated: Subcutaneous 106/146 (72.6) Intradermal 27/146 (18.5) Other or missing 13/146 (8.9) | Persons of any gender identity and age with either an initial mpox diagnosis code or a positive orthopoxvirus or MPXV laboratory test result during the study period. |
| Control | 8649 | 18–35 years: 4221 (48.8) 36–49 years: 2434 (28.1) 50–64 years: 1619 (18.7) ≥65 years: 375 (4.3) | Legal sex Male: 7821 (90.4) Female: 815 (9.4) Other: 5 (0.1) For control Unknown: 8 (0.1) | Male 7717 (89.2) Female 790 (9.1) Transgender male d 4 (0.1) Transgender female 75 (0.9) Other 19 62 (0.7) Chose not to disclose 1 (<0.1) | For full vaccinations: Subcutaneous 63/335 (18.8) For control Intradermal 42/335 (12.5) Heterologous 150/335 (44.8) For control Other or missing 80/335 (23.9) For control For Partially vaccinated: Subcutaneous 704/1000 (70.4) Intradermal 186/1000 (18.6) Other or missing 110/1000 (11.0) | Those with an incident clinical HIV diagnosis or a new positive HIV antibody or antigen–antibody test, or a new or refill order for HIV PrEP who also had an in‐person clinic visit during the study period. | ||
| Hazra 2022 | Vaccinated | US | 90 | >19 years | Cisgender man: 82 (91.1) | Gay: 87 Bisexual: 5 Queer: 6 Straight: 2 Declined: 1 Unspecified:13 | NA | Patients who tested positive for monkeypox at least 1 day after receiving the first dose of the MVA‐BN from June 28, 2022, through September 9, 2022, were included. |
| Merad 2022 27 | Vaccinated | France | 108 | 36 (11.27) | 97 (90) | MSM | NA | Adults (≥18 years old) who received one dose of MVA‐BN after exposure to mpox. |
| Morales 2023 18 | Vaccinated | Spain | 230 | 0–19 years: 4 (1.7) 20–39 years: 134 (58.2) 40–59 years: 83 (36) 60–93 years: 9 (3.9) | 194 (84.3) | MSM | Subcutaneous | Close contacts having contact with body fluids or lesions, contaminated fomites or clinical samples from a case since the time of appearance of the first symptoms. |
| Unvaccinated | 254 | 0–19 years: 12 (4.7) 20–39 years: 135 (53.1) 40–59 years: 76 (29.9) 60–93 years: 31 (12.2) | 183 (72) | MSM | Subcutaneous | |||
| Payne 2022 32 | Vaccinated | US | 1224 | (18–49)* | 1224 (100) | GBMSM | Subcutaneous and Intradermal | Confirmed and probable mpox cases among men aged 18–49 years with illness onset during July 31–October 1, 2022. |
| Unvaccinated | 8320 | (18–49)* | 8320 (100) | GBMSM | Confirmed and probable mpox cases among men aged 18–49 years with illness onset during July 31–October 1, 2022. | |||
| Rosenberg 2022 31 | Cases | US | 252 | 18–29 years: 94 (37.3) 30–39 years: 90 (35.7) 40–49 years: 37 (14.7) ≥50 years: 31 (12.3) | NA | GBMSM | NA | NA |
| Control | 255 | 18–29 years: 111 (43.5) 30–39 years: 75 (29.4) 40–49 years: 33 (12.9) ≥50 years: 36 (14.1) | NA | GBMSM | NA | NA | ||
| Sagy 2023 17 | Vaccinated | Israel | 1037 | 34.1 (4.9) | 1,037 (100) | NA | Subcutaneous | Males aged 18–42 years who were (a) dispensed HIV‐PrEP at least for 1 month since January 1, 2022, or (b) diagnosed with HIV and also were diagnosed with one or more STIs since January 1, 2022. |
| Unvaccinated | 1017 | 33.5 (5.8) | 1017 (100) | NA | Subcutaneous | Males aged 18–42 years who were (a) dispensed HIV‐PrEP at least for 1 month since January 1, 2022, or (b) diagnosed with HIV and also were diagnosed with one or more STIs since January 1, 2022. | ||
| Thy 2023 35 | Vaccinated | France | 276 | NA | NA | 84% MSM | Subcutaneous | Participants who had received smallpox vaccine after high risk exposure to mpox. |
Abbreviations: GBMSM, gay, bisexual, and other men who have sex with men; MSM, men who have sex with men; NA, not available; UK, United Kingdom; US, Unites States.
Data present as range.
Table 2.
Characteristics of mpox cases occurring postvaccination.
| ID | Age, mean (SD) | Male, N (%) | PLWH | STI, N (%) | Time from vaccination to symptoms in days, mean (SD) |
|---|---|---|---|---|---|
| Agunbiade 2022 34 | 37 (7.4) | 15 (100) | 3 (20) | Chlamydia trachomatis: 5 (33) | 5.3 (4.44) |
| Neisseria gonorrhoeae: 1 (7) | |||||
| Hazra 2022 | 34.33 (9.04) | 82 (91.1)* | 34 (37.8) | 34 (37.7) | 1–7 day: 37 cases |
| 8–14 day: 32 cases | |||||
| 15–28 day: 13 cases | |||||
| >28 days: 8 cases | |||||
| Merad 2022 27 | 32.66 (6.78) | 11 (100) | 3 (27.2) | 3 (27.2) | 4 (0.757) |
| Morales 2023 18 | 34 (6.11) | 7 (87.5) | 4 (50) | 4 (50) | NA |
| Sagy 2023 17 | NA | 5 (100) | NA | NA | NA |
| Thy 2023 35 | NA | NA | 4 (33.3) | 4 (33.3) | 1–5 days: 10 cases |
| 22 days: 1 case | |||||
| 25 days: 1 case |
Abbreviations: NA, not available; PLWH, people living with HIV; STI, sexually transmitted infection.
Cisgender males.
3.2. Quality of included studies
This meta‐analysis's included studies were all judged to be of good quality. Tables 3.1, 3.2, 3.3 (A–C) displayed the NOS.
Table 3.1.
New castle Ottawa Scale for quality assessment of cohort studies.
| Study ID | Agunbiade et al. (2022) 34 | Hazra et al. (2022) | Merad et al. (2022) 27 | Morales et al. (2023) 18 | Payne et al. (2022) 32 | Sagy et al. (2023) 17 | Thy et al. (2023) 35 |
|---|---|---|---|---|---|---|---|
| Representativeness of the exposed cohort | |||||||
| Selection of the non exposed cohort | ★ | ★ | ★ | ★ | ★ | ★ | ★ |
| Ascertainment of exposure | ★ | ★ | ★ | ★ | ★ | ★ | ★ |
| Demonstration that outcome of interest was not present at start of study | ★ | ★ | ★ | ★ | ★ | ★ | ★ |
| Comparability of cohorts on the basis of the design or analysis | ★★ | ★ | ★ | ★★ | ★★ | ||
| Assessment of outcome | ★ | ★ | ★ | ★ | ★ | ★ | ★ |
| Was follow‐up long enough for outcomes to occur | ★ | ★ | ★ | ★ | ★ | ★ | ★ |
| Adequacy of follow up of cohorts | ★ | ★ | ★ | ★ | ★ | ★ | ★ |
Table 3.2.
New castle Ottawa Scale for quality assessment of case‐control studies.
| Study ID | Dalton et al. (2023) 30 | Deputy et al. (2023) 15 | Rosenberg et al. (2022) 31 |
|---|---|---|---|
| Is the case definition adequate? | ★ | ★ | ★ |
| Representativeness of the cases | ★ | ★ | ★ |
| Selection of controls | ★ | ★ | ★ |
| Definition of controls | ★ | ★ | ★ |
| Comparability of cases and controls on the basis of the design or analysis | ★★ | ★★ | ★★ |
| Ascertainment of exposure | ★ | ★ | ★ |
| Same method of ascertainment for cases and controls | ★ | ★ | ★ |
| Nonresponse rate | ★ | ★ | ★ |
Table 3.3.
New castle Ottawa Scale for quality assessment of cross‐sectional studies.
| Study ID | Bertran et al. (2023) 16 |
|---|---|
| Representativeness of the exposed cohort | ★ |
| Sample size | |
| Ascertainment of exposure | ★ |
| Non respondants | |
| Comparability of cohorts on the basis of the design or analysis | |
| Assessment of outcome | ★ |
| Statistical test | ★ |
3.3. Outcomes
The primary outcome measure was VE. The combined VE against the Mpox virus was 78.23% (95% CI [62.79%, 87.27%]). The studies showed significant heterogeneity (I2 = 94.4%, 95% CI [90.8%, 96.6%]), (tau2 = 0.4174, 95% CI [0.1288, 1.9603]), and (Cochran's Q = 106.91, p < 0.001). As a result, the results were combined using a random‐effects model. (Figure 2A). The 95% PI of the vaccine's effectiveness against mpox infection ranged from −32.14% to 96.41%. The combined prevalence of mpox infection in people who had received vaccinations (breakthrough infection) was 2.19% (95% CI [0.37%, 5.32%]) (Figure 2B).
Figure 2.

(A) Forest plot showing pooled vaccine efficacy for mpox virus infection. (B) Forest plot showing pooled prevalence of mpox infection in vaccinated individuals.
By visually showing I2 against the pooled estimate (Figure S1) and by clustering (Figures S2–S4), the heterogeneity has also been further evaluated. Deputy et al. 15 showed significant heterogeneity contribution. (Figure 3) Influence diagnostics were used to show the influence that each study had. The same study was once more identified as having too much influence. (Figure 4) Deputy et al. 15 and Payne et al. 24 were recognized as outliers using the Baujat plot (Figure S5).
Figure 3.
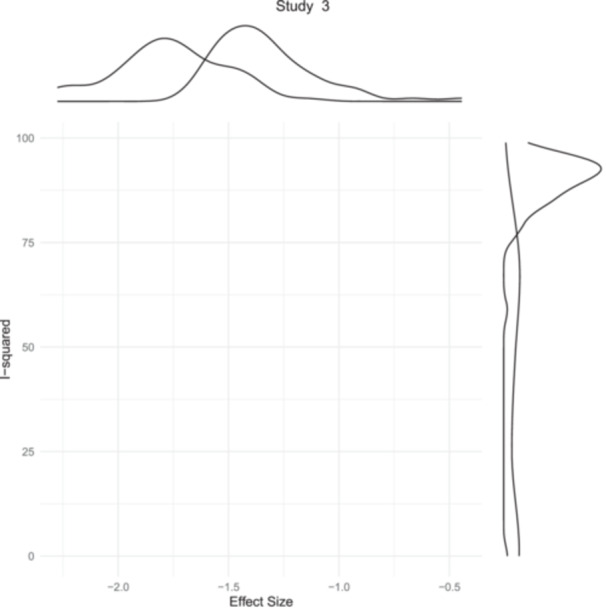
Graphical depiction of exaggerated contribution to between‐study heterogeneity by a single study.
Figure 4.
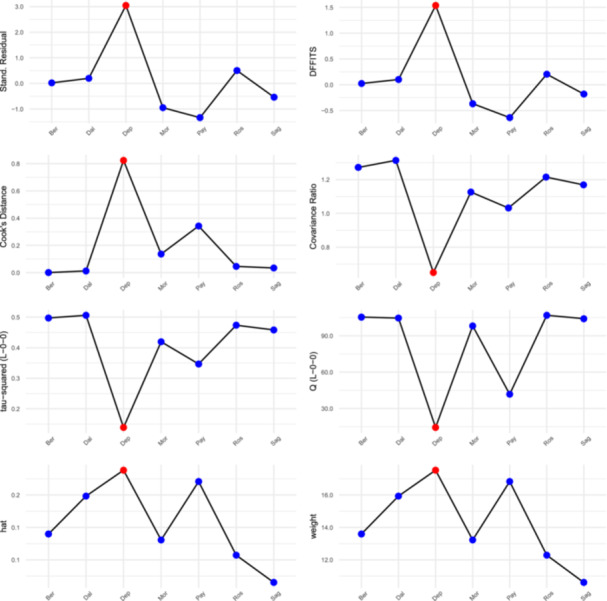
Influence diagnostics flagging a study for exerting overt influence.
A bubble plot was created to show how sex distribution and sample size influence the effectiveness of the mpox vaccination. Both the parameters (sex distribution: β = 0.76, p = 0.70) and (sample size: β = 0, p = 0.55) were not significant (Figures S6 and S7). Gross asymmetry could be shown in the Doi plot, with the left limb being overloaded with studies (Figure S8). This was supported by the LFK index, which had a value of −2.25. The projected direction of potential publication bias or small‐study effects was indicated by the negative LFK score and the negative inclination for rate ratios.
We conducted a sensitivity analysis by leaving out any outliers found on the Baujat plot. The effectiveness of the vaccine increased from 78.23% (95% CI [62.79%–87.27%]) to 83.02% (95% CI [74.62%–88.64%]), with PI ranging from 44.67% to 94.79% (Figure S9). Even when one study was excluded, the findings remain largely consistent (Figure S10).
4. DISCUSSION
This meta‐analysis and systemic review highlighted the effectiveness of a single in 24,784 patients. Our meta‐analysis included eleven studies that reported the effectiveness of a single dose of JYNNEOS vaccine for mpox prevention vaccine. These studies were conducted in various regions and populations, providing a diverse set of data for our analysis. The combined data from these studies allowed us to obtain a more accurate estimate of the vaccine's effectiveness. The overall VE of 78.23% (95% CI [62.79%, 87.27%]) suggests that the vaccine provides significant protection against mpox infection. The estimated prevalence of breakthrough mpox infections was 2.19% (0.37%−5.32%). It is important to note that the included studies were heterogeneous, which may be attributed to study design differences, population characteristics, and follow‐up duration. However, we conducted sensitivity analyses and they remained consistent indicating the robustness of our findings.
A prior meta‐analysis by Xu et al reported a combined efficacy of the JYNNEOS vaccination to be 89% (95% CI [0.86–0.91]) in Xu et al. 25 Authors included only five studies that evaluated the JYNNEOS vaccine's effectiveness, one of which was a preprint. 26 It's possible that the few studies included and the small sample size resulted in overestimated VE and the discrepancy with our findings. During the current outbreak, they also failed to record the frequency of breakthrough infections following immunization. 25
According to Morales et al., 18 one dosage of the JYNNEOS vaccine had a VE of 88.8% (95% CI [76.0–94.7]) for avoiding the disease in people who were in close contact to mpox patients. Taking into account the individuals' immunological health or sexual behavior had no effect on this high efficacy. 18 These results were not in agreement with those of Merad et al. 27 reporting that failure of postexposure prophylaxis was linked to sexual interaction, immunosuppression, and early immunization after exposure. 27 This high estimate can be the result of selection bias or other methodological flaws. There might have been certain close contacts who were unknown to the index case and therefore couldn't be identified. 18
The estimated JYNNEOS VE against symptomatic mpox after a single dose was 78% (95% CI [71%–85%]) in another trial conducted in England, which is comparable with our findings. Because clinical records were unavailable, they had to rely on self‐reported questionnaires to determine the vaccination status of each case. Low response rates (33%) were observed. VE would be underestimated if cases who had received vaccinations were more likely to react. 16
In the USA, Deputy et al. 15 showed that VE was 35.8% (95% CI [22.1–47.1]) for partial vaccination and 66.0% (95% CI [47.4–78.1]) for full vaccination after adjusting for age, race or ethnic group, and state of immunity. Additionally, they discovered that immunocompetent people had higher VEs than immunocompromised people. The VE discovered by Deputy et al. in 2023 (66%) in completely immunized is significantly less than earlier findings in the literature. 15 This might be as a result of the baseline HIV status differential between patients and controls. Control individuals solely had a new HIV diagnosis, while case patients had either an existing or new HIV diagnosis. Patients who had their vaccinations at non‐Epic locations outside of their home state may also be mistakenly labeled as unvaccinated. Both of the aforementioned elements may understate the VE. 15 Only the VE in completely immunized men reduced to 58.7% when VE in individuals between the ages of 18 and 49 was taken into account. This may be because older males (those 50 years of age or older) have already received a smallpox vaccination and are therefore less prone to contract the disease than younger people. 15 Since both MPXV and the smallpox virus are members of the Orthopoxvirus family, MPXV benefits from cross‐immunity with smallpox. According to about epidemiological African data, it was estimated that the incidence of mpox increased 20‐fold after the smallpox vaccine was discontinued, indicating that vaccination against smallpox could prevent mpox with an estimated VE ranging from 80% to 85%. 28 People who had received a smallpox vaccination in the past also fared better during an mpox outbreak in the USA in 2003. 29
The adjusted VE against mpox was 75.2% for partial vaccination and 85.9% for full vaccination in a case‐control study conducted by Dalton et al. 30 Depending on the level of immunity, different participants have different adjusted VE estimations. In immunocompromised patients, the VE was 51.0% for partial vaccination and 70.2% for full vaccination, while in those who were immunocompetent; it was 72.1% for partial vaccination and 87.8% for full vaccination. In sexual health and HIV clinics, controls were voluntarily recruited, which could have biased the results. Additionally, recall bias was applied to survey data. Both of these elements could have caused an inaccurate VE assessment. Additionally, they didn't employ clinical standards to categorize immunocompromised subjects, which may have resulted in an inaccurate prediction of VE based on immune status. 30
According to Rosenberg et al., 31 the VE of the two dosage regimen exceeded 88%. These results were in line with those of Payne et al., 32 who discovered that the two dose regimen was superior to the one dose regimen. The higher VE with two vaccination doses in the previous studies 15 , 30 , 31 , 32 emphasizes the significance of administering the full schedule of vaccination, particularly to high‐risk individuals such those with risky sexual behavior and immunocompromised patients. In a cohort study, males undergoing HIV PrEP or PLWH who had been diagnosed with one or more STIs experienced an 86% decrease in the incidence of MPOX illness after receiving a single SC dose of the MVA vaccine. Only 21 people with MPOX were included in the cohort, which led to erroneous estimates. Confounding by calendar time may result from failing to determine comparable time zero for the groups who received the vaccine and those who did not. 17 It's possible that the VE was overestimated as a result of all these factors. According to Brousseau et al.'s sensitivity study, 33 VE varied from 50% to 75%.
The effectiveness of a single dose of immunization varied greatly among studies, 15 , 16 , 17 , 18 , 30 ranging from 35.8% to 88.8%. This variation may have resulted from failure to account for confounders such as different exposure risk depending on vaccination status, as those who have received vaccinations may choose to limit their number of partners, 15 or calendar time, 16 , 32 as longer intervals between vaccination and mpox infection assessment may have given the immune system more time to mount a stronger immune response.
The proportion of mpox disease that developed despite vaccination varied considerably between studies. The prevalence of breakthrough infections was estimated to be 2.19% (95% CI [0.37%−5.32%]). In UK, 15 out of 10,068 people (0.15%) experienced a breakthrough infection following a single dose of JYNNEOS vaccine. 34 Infection rates following a single dose of immunization were determined to be 3.5% by Morales et al. 18 In France, Merad et al. and Thy et al. estimated the incidence of mpox infection after vaccination to be 10% and 4% respectively. 27 , 35 There was a considerable difference in the outcomes of the included studies. This can be attributable to different estimating techniques, selection standards, and baseline traits between people who received vaccinations and those who did not. Numerous factors, including the number of partners and sexual attitude after immunization, could not be evaluated. Further, there is lack of knowledge of JYNNEOS vaccine's long‐term protection and potential side effects,. The majority of studies that have been published have concentrated on immediate results and defense against mpox infection. Thus, it is imperative that future studies by evaluating the length of immunity provided by a single dose of JYNNEOS and potential side effects related to its administration.
It is important to consider that mpox breakthrough infections post immunization can be influenced by a number of factors, including previous immunity levels and the impact of existing comorbidities. Individual behaviors, such as multiple sexual partners and HIV status, increase the likelihood of breakthrough infections. 36 Furthermore, the time of immunization following exposure, as well as previous smallpox vaccination history, can influence vaccine efficacy. 36 These findings should assist enhance immunization policy by addressing risk factors, optimizing vaccine timing, and implementing targeted immunization techniques, particularly in high‐risk communities, to improve vaccine‐induced protection against mpox.
4.1. Strengthens and limitations
To our knowledge, this is the most comprehensive review evaluating mpox VE in the 2022 outbreak with robust statistical methodology. We included only peer‐reviewed studies and excluded preprints. 26 , 33 , 37 Despite the fact that it offers insightful information about mpox VE, there are some limitations to our study that should be acknowledged. Initially, the included studies' varied in study designs and levels of quality could introduce bias into our analysis. Secondly, publication bias cannot be completely disregarded because studies with contradictory or negative findings may be less likely to be published. The difference in VE according to immune status was not mentioned in every study that was considered. As immunocompromised people are more likely to contract mpox due to their impaired immune systems and risky sexual conduct.
5. CONCLUSION
Our finding concluded that a single dose of the current mpox vaccine has an overall VE of 78.23%. This result suggests that administering at least one dose of the vaccine can help prevent serious infections and the spread of the disease, particularly in immunocompromised individuals and those who engage in hazardous sexual conduct. However, more research is needed to understand the factors that influence VE variations across populations and geographical regions. These findings help in understanding the potential impact of mpox vaccination programmes and inform public health strategies for reducing mpox cases.
AUTHOR CONTRIBUTIONS
Amira Mohamed Taha: Conceptualization; writing—review and editing; writing—original draft. Abdelrahman Mohamed Mahmoud: Writing—original draft; writing—review and editing. Khaled Abouelmagd: Writing—original draft; writing—review and editing. Sara Adel Abdelkader Saed: Methodology; writing—review and editing; writing—original draft. Basma Badrawy Khalefa: Writing—original draft; writing—review and editing. Sangam Shah: Writing—review and editing; writing—original draft. Prakasini Satapathy: Writing—review and editing; writing–original draft. Muhammad Aaqib Shamim: Writing—original draft; writing—review and editing. Sanjit Sah: Software; Writing—review and editing. Hashem Abu Serhan: Writing—review and editing. Suzanne Donovan: Writing—review and editing; supervision. Ranjit Sah: Supervision; writing—review and editing; writing—original draft. Joshuan J. Barboza: Supervision; writing—original draft; writing—review and editing.
CONFLICT OF INTEREST STATEMENT
Authors declares no conflict of interest.
ETHICS STATEMENT
Not applied.
TRANSPARENCY STATEMENT
The lead author Ranjit Sah affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Supporting information
Supporting information.
Supporting information.
ACKNOWLEDGMENTS
Open Access funding provided by Qatar National Library.
Taha AM, Mahmoud AM, Abouelmagd K, et al. Effectiveness of a single dose of JYNNEOS vaccine in real world: a systematic review and meta‐analysis. Health Sci Rep. 2024;7:e70069. 10.1002/hsr2.70069
DATA AVAILABILITY STATEMENT
All authors have read and approved the final version of the manuscript Amira Mohamed Taha had full access to all of the data in this study and takes complete responsibility for the integrity of the data and the accuracy of the data analysis.
REFERENCES
- 1. Shchelkunov SN, Marennikova SS, Moyer RW. Orthopoxviruses Pathogenic for Humans. Springer Science & Business Media; 2006. [Google Scholar]
- 2. Yong SEF, Ng OT, Ho ZJM, et al. Imported monkeypox, Singapore. Emerg Infect Dis. 2020;26(8):1826‐1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Centers for Disease Control and Prevention . Past U.S. cases and outbreaks.2022.
- 4. Vaughan A, Aarons E, Astbury J, et al. Two cases of monkeypox imported to the United Kingdom, September 2018. Euro Surveill. 2018;23(38):1800509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Erez N, Achdout H, Milrot E, et al. Diagnosis of imported monkeypox, Israel, 2018. Emerg Infect Dis. 2019;25(5):980‐983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mitjà O, Ogoina D, Titanji BK, et al. Monkeypox. Lancet. 2023;401(10370):60‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Centers for Disease Control and Prevention . Mpox outbreak global map. 2022. Available from: https://www.cdc.gov/poxvirus/mpox/response/2022/world-map.html
- 8. World Health Organization . WHO Director‐General declares the ongoing monkeypox outbreak a Public Health Emergency of International Concern. 2022. Available from: https://www.who.int/europe/news/item/23-07-2022-who-director-general-declares-the-ongoing-monkeypox-outbreak-a-public-health-event-of-international-concern
- 9. Centers for Disease Control and Prevention . Infection control. 2022. Available from: https://www.cdc.gov/poxvirus/mpox/clinicians/infection-control.html
- 10. Frey SE, Newman FK, Kennedy JS, et al. Clinical and immunologic responses to multiple doses of IMVAMUNE®(Modified Vaccinia Ankara) followed by dryvax® challenge. Vaccine. 2007;25(51):8562‐8573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stickl H, Hochstein‐Mintzel V, Mayr A, Huber H, Schäfer H, Holzner A. MVA‐Stufenimpfung gegen Pocken. DMW ‐ Deutsche Medizinische Wochenschrift. 1974;99(47):2386‐2392. [DOI] [PubMed] [Google Scholar]
- 12. Food USDA . JYNNEOS package insert. Available from: https://wwwfdagov/media/131078/download
- 13. Earl PL, Americo JL, Wyatt LS, et al. Immunogenicity of a highly attenuated MVA smallpox vaccine and protection against monkeypox. Nature. 2004;428(6979):182‐185. [DOI] [PubMed] [Google Scholar]
- 14. Pittman PR, Hahn M, Lee HS, et al. Phase 3 efficacy trial of modified vaccinia Ankara as a vaccine against smallpox. N Engl J Med. 2019;381(20):1897‐1908. [DOI] [PubMed] [Google Scholar]
- 15. Deputy NP, Deckert J, Chard AN, et al. Vaccine effectiveness of JYNNEOS against Mpox disease in the United States. N Engl J Med. 2023;388:2434‐2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bertran M, Andrews N, Davison C, et al. Effectiveness of one dose of MVA–BN smallpox vaccine against mpox in England using the case‐coverage method: an observational study. Lancet Infect Dis. 2023;23:828‐835. [DOI] [PubMed] [Google Scholar]
- 17. Wolff Sagy Y, Zucker R, Hammerman A, et al. Real‐world effectiveness of a single dose of mpox vaccine in males. Nature Med. 2023;29(3):748‐752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Montero Morales L, Barbas del Buey JF, Alonso García M, et al. Post‐exposure vaccine effectiveness and contact management in the mpox outbreak, Madrid, Spain, May to August 2022. Eur Surveill. 2023;28(24):2200883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wells G Newcastle‐Ottawa Scale. http://wwwlrica/programs/ceu/oxfordhtm. 2007.
- 20. Wu N, Joyal‐Desmarais K, Ribeiro PAB, et al. Long‐term effectiveness of COVID‐19 vaccines against infections, hospitalisations, and mortality in adults: findings from a rapid living systematic evidence synthesis and meta‐analysis up to December, 2022. Lancet Respir Med. 2023;11(5):439‐452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. IntHout J, Ioannidis JPA, Rovers MM, Goeman JJ. Plea for routinely presenting prediction intervals in meta‐analysis. BMJ Open. 2016;6(7):e010247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shamim MA. Real‐life implications of prevalence meta‐analyses? Doi plots and prediction intervals are the answer. Lancet Microbe. 2023;4:e490. [DOI] [PubMed] [Google Scholar]
- 23. Furuya‐Kanamori L, Barendregt JJ, Doi S. A new improved graphical and quantitative method for detecting bias in meta‐analysis. Int J Evid Based healthc. 2018;16(4):195‐203. [DOI] [PubMed] [Google Scholar]
- 24. Payne AB, Ray LC, Cole MM, et al. Reduced risk for mpox after receipt of 1 or 2 doses of JYNNEOS vaccine compared with risk among unvaccinated persons − 43 U.S. jurisdictions, July 31‐October 1, 2022. MMWR Morb Mortal Wkly Rep. 2022;71(49):1560‐1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Xu M, Liu C, Du Z, et al. Real‐world effectiveness of mpox (monkeypox) vaccines: a systematic review. J Travel Med. 2023;30(5);taad048. 10.1093/jtm/taad048 [DOI] [PubMed] [Google Scholar]
- 26. Arbel R, Sagy YW, Zucker R, et al. Effectiveness of a single‐dose modified Vaccinia Ankara in Human Monkeypox: an observational study. Res Sq. 2022. 10.21203/rs.3.rs-1976861/v2 [DOI] [Google Scholar]
- 27. Merad Y, Gaymard A, Cotte L, et al. Outcomes of post‐exposure vaccination by modified vaccinia Ankara to prevent mpox (formerly monkeypox): a retrospective observational study in Lyon, France, June to August 2022. Euro Surveill. 2022;27(50):2200882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rimoin AW, Mulembakani PM, Johnston SC, et al. Major increase in human monkeypox incidence 30 years after smallpox vaccination campaigns cease in the Democratic Republic of Congo. Proc Nat Acad Sci. 2010;107(37):16262‐16267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hammarlund E, Lewis MW, Carter SV, et al. Multiple diagnostic techniques identify previously vaccinated individuals with protective immunity against monkeypox. Nat Med. 2005;11(9):1005‐1011. [DOI] [PubMed] [Google Scholar]
- 30. Dalton AF. Estimated effectiveness of JYNNEOS vaccine in preventing mpox: a multijurisdictional case‐control study—United States, August 19, 2022–March 31, 2023. Morb Mortal Wkly Rep. 2023;72(20);553‐558. https://www.cdc.gov/mmwr/volumes/72/wr/mm7220a3.htm [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rosenberg ES, Dorabawila V, Hart‐Malloy R, et al. Effectiveness of JYNNEOS vaccine against diagnosed mpox Infection—New York, 2022. Morb Mortal Wkly Rep. 2023;72(20):559‐563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Payne AB, Ray LC, Cole MM, et al. Reduced risk for mpox after receipt of 1 or 2 doses of JYNNEOS vaccine compared with risk among unvaccinated persons—43 US jurisdictions, July 31–October 1, 2022. Morb Mortal Wkly Rep. 2022;71(49):1560‐1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Brousseau N, Carazo S, Febriani Y, et al. Single‐dose effectiveness of mpox vaccine in Quebec, Canada: test‐negative design with and without adjustment for self‐reported exposure risk. Clin Infect Dis. 2024;78(2):461‐469. 10.1093/cid/ciad584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Agunbiade S, Burton F, Muirhead J, Whitlock GG, Girometti N. Clinical characteristics of mpox infection in individuals who received a first dose of modified vaccinia Ankara immunisation. Sex Transm Infect. 2023;99(3):198‐199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Thy M, Peiffer‐Smadja N, Mailhe M, et al. Breakthrough infections after postexposure vaccination against mpox. N Engl J Med. 2022;387(26):2477‐2479. [DOI] [PubMed] [Google Scholar]
- 36. Jiang W, Hu Y, Yang X, et al. Breakthrough infection and reinfection in patients with mpox. Rev Med Virol. 2024;34(2):e2522. [DOI] [PubMed] [Google Scholar]
- 37. Fontan‐Vela M, Hernando V, Olmedo C, et al. Effectiveness of modified vaccinia ankara‐bavaria nordic vaccination in a population at high risk of mpox: a spanish cohort study. Clin Infect Dis. 2024;78(2):476‐483. 10.1093/cid/ciad645 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Supporting information.
Data Availability Statement
All authors have read and approved the final version of the manuscript Amira Mohamed Taha had full access to all of the data in this study and takes complete responsibility for the integrity of the data and the accuracy of the data analysis.


