Abstract
To address the role of CXCR4 in the cell-surface attachment of the feline immunodeficency virus (FIV), a soluble fusion protein, gp95-Fc, consisting of the surface glycoprotein (SU, gp95) of either a primary (PPR) or cell line-adapted (34TF10) FIV strain was fused in frame with the Fc domain of human immunoglobulin G1. The recombinant SU-immunoadhesins were used as probes to investigate the cellular binding of FIV SU. In agreement with the host cell range properties of both viruses, binding of 34TF10 gp95-Fc was observed for all cell lines tested, whereas PPR gp95-Fc bound only to primary feline T cells. 34TF10 gp95-Fc also bound to Jurkat and HeLa cells, consistent with the ability of FIV to use human CXCR4 as a fusion receptor. As expected, 34TF10 gp95-Fc binding to Jurkat cells was blocked by addition of stromal cell-derived factor 1α (SDF-1α), as was binding to the 3201 feline lymphoma cell line. However, SDF-1α, RANTES, macrophage inflammatory protein 1β, and heparin all failed to inhibit the binding of either gp95-Fc to primary T cells, suggesting that a non-CXCR4 receptor is involved in the binding of FIV SU. In this regard, an unidentified 40-kDa protein species from the surface of primary T cells but not Jurkat and 3201 cells specifically coprecipitated with both gp95-Fc. Yet another type of binding of 34TF10 gp95-Fc to adherent kidney cells was noted. SDF-1α failed to block the binding of 34TF10 gp95-Fc to either HeLa, Crandel feline leukemia, or G355-5 cells. However, binding was severely impaired in the presence of soluble heparin, as well as after enzymatic removal of surface heparans or on cells deficient in heparan expression. These overall findings suggest that in addition to CXCR4, a non-CXCR4 receptor and cell-surface heparans also play an important role in FIV gp95 cell surface interactions on specific target cells.
The initial stage in infection with human immunodeficiency virus (HIV) is the interaction of the viral envelope (Env) with CD4 and a specific chemokine receptor on the surface of target cells which promote conformational changes that allow fusion of the viral envelope with the plasma membrane (reviewed in references 7, 15, and 81). The major coreceptors used by HIV type 1 (HIV-1) are CXCR4 and CCR5, two members of the superfamily of seven-transmembrane domain G-protein-coupled receptors. Syncytium-inducing or T-cell-tropic (T-tropic) strains of HIV-1 and laboratory-adapted viruses use predominantly CXCR4 (8, 31), whereas non-syncytium-inducing or macrophagetropic strains of HIV-1 use predominantly CCR5 (2, 14, 17, 21, 22). Additional members of the seven-transmembrane superfamily are also able to support infection with HIV-1, HIV-2, and simian immunodeficiency virus (SIV) (18, 24, 25, 30, 42, 48, 55, 64, 71).
The binding of HIV Env with the receptor complex follows a two-step model in which the binding determinants in the interaction of HIV Env with CD4 and the chemokine receptor are contained within gp120, the surface subunit of Env (7, 15, 81). In the first step, gp120 binds CD4, which triggers conformational changes in gp120 that expose or create the coreceptor binding site(s). In the second step, the CD4-gp120 complex interacts with the chemokine receptor, which promotes additional conformational changes that lead to the fusion of the viral and cellular membranes. Although CD4 is required for an efficient interaction between gp120 and the chemokine receptor, some HIV and SIV strains that have gained independence from CD4 can interact directly with their coreceptors on CD4-negative cells (9, 23, 26, 28, 40, 41, 44, 46, 50, 52, 56, 66, 67). While naturally occurring, CD4-independent isolates were reported for HIV-2 and SIV, CD4-independent HIV-1 isolates were observed only after long-term culture of the virus in CD4-positive cells or after adaptation of the virus to replicate in CD4-negative cells (23, 41, 50, 52). Determinants for CD4 independence are dispersed throughout Env in gp120 but also in gp41, the transmembrane subunit (23, 41, 50, 52). These mutations are believed to increase and stabilize the exposure of the coreceptor binding site on gp120.
Feline immunodeficiency virus (FIV) is the etiologic agent of feline AIDS in the domestic cat (62). One of the most interesting recent findings regarding the similarity between FIV and HIV is that FIV uses the chemokine receptor CXCR4 for efficient infection of target cells, similarly to T-tropic strains of HIV-1 (80). CXCR4 was first shown to be used by FIV isolates adapted for propagation in Crandel feline kidney (CrFK) cells (80); recently, the use of CXCR4 has been extended to primary T-tropic isolates of FIV (27, 68). Primary isolates of FIV (PI FIV) have a cellular tropism restricted to mitogen-activated peripheral blood mononuclear cells (PBMCs), thymocytes, macrophages, and interleukin-2 (IL-2)-dependent T-cell lines, and infection of these primary cells can be efficiently inhibited by stromal cell-derived factor 1α (SDF-1α) or the bicyclam AMD3100, two CXCR4 ligands (27, 68). However, PI FIV failed to productively infect CrFK and other nonlymphoid cells but can be adapted to propagate in these cells after several in vitro passages (53, 79). Adapted viruses have retained their parental cellular tropism but have gained the ability to infect CrFK cells as well as other nonlymphoid cells (53, 79). Determinants for the adapted phenotype have been mapped to Env in both the surface and transmembrane subunits (53, 79). The envelope of adapted viruses has also been shown to interact directly with CXCR4 (43), similar to what has been observed for CD4-independent CXCR4-using (X4) HIV isolates (40). It is yet to be determined if CXCR4 acts as a primary receptor or a coreceptor for FIV. However, it is possible that, by analogy with CD4-independent HIV isolates, CrFK-adapted FIV isolates may have gained independence from a CD4-like factor which is required by primary isolates.
To better address the role of CXCR4 and other receptors or coreceptors in the life cycle of FIV, we constructed chimeric fusion proteins between the surface glycoproteins (SU) from primary and CrFK-adapted isolates of FIV and the Fc domain of human immunoglobulin G1 (IgG1). The SU-immunoadhesins were used as tools to investigate the role of CXCR4 and other cell surface component(s) in the binding of FIV SU.
MATERIALS AND METHODS
Cell lines, reagents, and virus.
The Jurkat, CrFK, HeLa, G355-5, CHO-K1, and CHO-derived pgsA-745 cell lines were obtained from the American Type Culture Collection (Rockville, Md.). The feline lymphoma cell line 3201 was obtained from W. Hardy. The 104-C1 and MCH5-4 feline primary T-cell lines were isolated by limiting-dilution cloning from feline PBMCs (54). The feline primary T-cell lines were maintained in RPMI 1640 supplemented with 10% fetal bovine serum (Gemini Bioproducts, Calabasas, Calif.), 2 mM l-glutamine (Sigma, St. Louis, Mo.), 1 mM sodium pyruvate (Sigma), 10 mM HEPES buffer (Sigma), minimal essential medium-vitamins (Sigma), nonessential amino acids (Sigma), β-mercaptoethanol (Gibco-BRL, Gaithersburg, Md.), 2.5 μg of concanavalin A (Sigma) per ml, 50 U of human recombinant IL-2 (a gift from Hoffmann-La Roche) per ml, and 50 μg of gentamicin (Gemini Bioproducts) per ml. The Jurkat and 3201 cell lines were maintained in the same medium minus concanavalin A and IL-2. The fibroblastoid and glial cell lines were cultured in Dulbecco modified Eagle medium (DMEM) with the same supplements as described above for RPMI 1640 medium, without concanavalin A and IL-2. G-DMEM consists of DMEM prepared without glutamine, and to which was added 1 mM sodium pyruvate, 10 mM HEPES buffer, minimal essential medium-vitamins, 100 μM nonessential amino acids, β-mercaptoethanol, 500 μM glutamic acid (Sigma), 500 μM l-asparagine, 30 μM each adenosine, guanosine, cytidine, and uridine (Sigma), 10 μM thymidine (Sigma), and 5% immunoglobulin-depleted and dialyzed fetal bovine serum (Gibco-BRL). The FIV strains used in this study were PPR (63) and 34TF10 (76). The synthetic human chemokines, (SDF-1α), RANTES (regulated upon activation normal T-cell expressed and secreted), and macrophage inflammatory protein 1β (MIP-1β) were a kind gift from Gryphon Pharmaceuticals. The heparan sulfate antibody (clone F58-10E4; Seikagaku America) and the CXCR4 antibody (sc9046; Santa Cruz Biotechnology, Santa Cruz, Calif.) were a kind gift from P. Gallay. The CXCR4 antibodies sc6190 and clone 44717.111 were purchased from Santa Cruz Biotechnology and R&D Systems, respectively; 4G10 was a kind gift from C. Broder.
Inhibition of FIV infection.
104-C1 cells (105) were incubated with SDF-1α, MIP-1β, or RANTES at 0.3 and 1.0 μg/ml for 30 min at room temperature. Cells were then infected at a multiplicity of infection of 0.01 for 1 h at 37°C. After being washed three times in Hanks' balanced salt solution (Sigma) to remove unbound virus, cells were cultured in medium alone or medium containing the indicated concentration of chemokine. Virus production was monitored by a reverse transcriptase (RT) activity assay at 7 days after the initiation of infection. Briefly, cell-free supernatants (50 μl) were incubated for 15 min at room temperature with 10 μl of lysis buffer (0.75 M KCl, 20 mM dithiothreitol, 0.5% Triton X-100). The lysate was added to 40 μl of an enzyme cocktail containing 100 mM Tris-HCl (pH 8.1), 10 mM MgCl2, 2.5 μg of poly(rA)-poly(dT)12–18 (Pharmacia, Piscataway, N.J.), and 2.5 μCi of [3H]dTTP (Dupont NEN, Boston, Mass.) and was incubated for 90 min at 37°C. RT activity was measured as previously described (16). Percent inhibition was calculated by the formula 100 − [(t − c)/(m − c) × 100], where t represents the signal for the test sample, c represents the background signal of mock-infected cells, and m represents the signal obtained for cells infected in the absence of chemokine.
Plasmid construction.
SU of the envelopes of FIV-PPR and FIV-34TF10, extending from the leader sequence to the beginning of the transmembrane region, were subcloned by PCR amplification (see Fig. 2). The sense primers, including NotI sites (underlined), had the sequences 5′-AGAAATATTTATAATAATATTGCGGCCGCAACAATAATTATGGCAG-3′ and 5′-TAGAAATATTTATATTAATTTGCGGCCGCAACAATAAGAATGGCAG-3′ for PPR and 34TF10, respectively. The antisense primer, including BamHI sites (underlined), had the sequences 5′-GTATTTATAGGTCATAGGATCCGCTGGTACTACTATATA-3′ and 5′-AGGTTTATATTCCATGGGATCCGCTGGGACCAGTAAGTA-3′ for PPR and 34TF10, respectively. The amplified DNA product was digested with NotI and BamHI, gel purified, and ligated in frame with the Fc domain of human IgG1 (kindly provided by B. Seed [4]) in pCR3 (Invitrogen, Carlsbad, Calif.). For amplification of the expression of Fc and gp95-Fc protein, the glutamate synthetase (GS) amplification system was used (6). This system uses a vector that has the gene of interest and a GS gene which allows selection and amplification in a glutamine-free environment in the presence of methionine sulfoximine, an inhibitor of the GS encoded by the vector and the CHO cells. For this purpose the Fc and gp95-Fc fragments were subcloned under the control of the cytomegalovirus promoter in pRSC, a mammalian expression vector with two transcriptional units (kindly provided by T. C. Tsang, Arizona Cancer Center, Tucson) (78). The GS gene was cloned by PCR amplification from a CHO cDNA library. The sense (5′-CCCACACCAAGCTTCTCGCCGCTC-3′) and antisense (5′-GATGAACTAGGAAAGCTTCAAGATCACTCAAAG-3′) primers, including HindIII sites (underlined), were designed from the nucleotide sequence of the GS gene (38). The PCR product was digested with HindIII and cloned under the control of the Rous sarcoma virus promoter in pRSC.
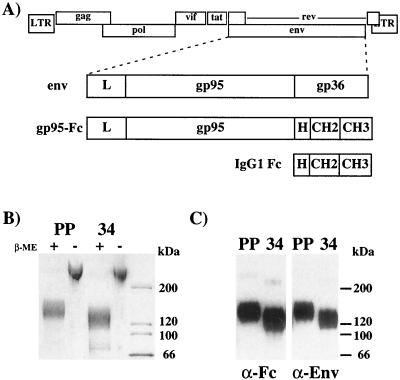
FIG. 2.
Construction of an FIV SU-immunoadhesin. (A) The FIV genome is represented at the top. The FIV Env region contains a leader (L) sequence followed by the surface (gp95) and transmembrane (gp36) subunits. FIV gp95, including the leader sequence, was cloned in frame to the hinge (H) region of human IgG Fc. The recombinant immunoadhesin proteins were stably transfected in CHO cells and batch purified from cell supernatants by affinity chromatography over protein A. (B) Samples (500 ng) of PPR (lanes PP) or 34TF10 (lanes 34) gp95-Fc were subjected to SDS-PAGE in the presence or absence of β-mercaptoethanol (β-ME) and stained by Coomassie blue. (C) Samples of PPR (lanes PP) and 34TF10 (lanes 34) gp95-Fc were subjected to SDS-PAGE under reducing conditions and immunoblotted with an antibody specific for human IgG1 Fc (α-Fc) or FIV Env (α-Env).
Expression, amplification, and purification of gp95-Fc fusion protein.
For production of stable cell lines expressing Fc and gp95-Fc, 106 CHO-K1 cells were transfected with 10 μg of pRSC-GS-Fc and pRSC-GS-gp95-Fc. At 36 h posttransfection, cells were trypsinized, plated in 96 wells of a microtiter plate, and grown in G-DMEM containing 25 μM methionine sulfoximine. After 14 to 21 days, clones surviving the selection were transferred to T25 flasks and grown to confluence. Supernatants were then harvested and incubated with 15 μl of protein A-Trisacryl at 4°C for 12 h. After three washes in phosphate-buffered saline (PBS), the beads were eluted in 25 μl of sodium dodecyl sulfate (SDS) sample buffer and loaded on an SDS–8 to 16% polyacrylamide gel, and the presence of Fc and gp95-Fc proteins was analyzed by Western blotting with antibodies to FIV Env and human IgG1 Fc. Blots were revealed by an enhanced chemiluminescence procedure (Pierce). Recombinant Fc and gp95-Fc proteins were batch purified by affinity chromatography over protein A as specified by the manufacturer (Pierce). The concentration of the purified Fc and gp95-Fc recombinant proteins was determined by using a bicinchoninic acid protein assay (Pierce).
Cytofluorimetric analysis.
Binding of gp95-Fc fusion proteins to the surface of Jurkat, 104-C1, 3201, HeLa, CrFK, G355-5, U87, and CHO cells was analyzed by flow cytometry. Briefly, 105 cells were incubated for 1 h at 4°C with 2.5 μg of gp95-Fc or Fc in PBS–0.1% bovine serum albumin. After two washes in ice-cold PBS, cells were labeled with a 1:100 dilution of fluorescein-conjugated goat anti-human IgG1 Fc antibody (Cappel, Durham, N.C.) for 1 h at 4°C. The cells were washed twice and then analyzed by flow cytometry on a FACScan using CellQuest software (BDIS, San Jose, Calif.). Specificity of SU binding was confirmed by a neutralization assay with a serum from an FIV-infected cat. For inhibition studies, chemokines or heparin were first added to the cells at the indicated concentration for 1 h at 4°C. The cells were then incubated with gp95-Fc recombinant proteins for 1 h, washed, further incubated with the anti-human IgG1 Fc antibody, and prepared for analysis as indicated above. Percent inhibition was calculated by the formula 100 − [(t − c)/(m − c) × 100], where t represents the signal for the test sample, c represents the background signal in the absence of gp95-Fc, and m represents the signal obtained for gp95-Fc in the absence of inhibitor.
Heparinase and chondroitinase digestions.
The enzymatic removal of cell surface heparans was performed essentially as described by Mondor et al. (57). Briefly, 106 cells were incubated in the absence or presence of either heparinase I (Sigma) or chrondroitinase ABC (Sigma) at a concentration of 10 U/ml at 37°C for 1 h. Cells were then washed three times in PBS, resuspended in PBS–0.1% bovine serum albumin, and prepared for fluorescence-activated cell sorting (FACS) analysis with the indicated antibodies or immunoadhesins as described above.
Cell-surface biotinylation and immunoprecipitation.
Cell-surface biotinylation was performed as previously described (3). Briefly, cells were washed twice in PBS and resuspended at 2.5 × 107 cells/ml in PBS (pH 8.0). The cell suspension was mixed with 0.5 mg/ml sulfo-N-hydroxysuccinimide-LC-biotin (Pierce) and incubated at room temperature for 30 min. The cells were then washed three times in ice-cold PBS to remove unreacted biotin. Cells were subsequently lysed in a 1% CHAPS {3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate Sigma} lysis buffer (20 mM Tris-HCl [pH 7.5], 150 mM NaCl, 10 μg of aprotinin/ml for 30 min at 4°C. Cell debris were removed by centifugation, and supernatants were precleared with 25 μl of protein A-Trisacryl for 2 h at 4°C. Precleared supernatants were next incubated with 2.5 μg of Fc and SU-Fc proteins and incubated overnight at 4°C. Complexes were immunoprecipated by addition 10 μl of protein A-Trisacryl and incubation overnight at 4°C. After three washes in lysis buffer, the pelleted beads were resuspended in SDS sample buffer containing β-mercaptoethanol, boiled, resolved on an SDS–8 to 16% polyacrylamide gel (Novex), and immunoblotted with either neutravidin (Pierce) or antibodies specific for human CXCR4. Blots were revealed by using an enhanced chemiluminescence procedure (Pierce).
RESULTS
FIV-PPR uses CXCR4 as a coreceptor for entry in primary T cells.
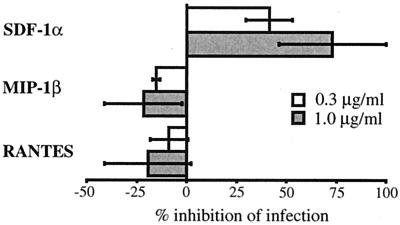
The two infectious molecular clones of FIV used in this study were previously reported (54, 63). FIV-PPR was obtained from a PI FIV (63), whereas FIV-34TF10 was cloned after adaptation of a PI FIV to propagate in CrFK cells (76). Both strains could be differentiated by their in vitro growth properties. While 34TF10 has a broad cell range tropism, FIV-PPR could propagate only in PBMCs, as well as IL-2-dependent primary feline T cell lines such as 104-C1 and MCH5-4 (54). Other feline cell lines, such as the lymphoma cell line 3201, the fibroblastoid cell line CrFK, and the glial cell line G355-5, are refractive to productive infection by FIV-PPR (54, 63). All of these cell lines are CXCR4 positive (see Fig. 8), indicating that there is a lack of correlation between CXCR4 expression and susceptibility to FIV-PPR infection. The 3201 cells could be distinguished from the other feline cell lines in that they expressed the highest level of CXCR4 (see Fig. 8). They also failed to be productively infected by FIV-PPR. However, long-term passage of FIV-PPR on these cells resulted in the production of a 3201-adapted FIV with an expanded host cell tropism that included productive infection of CrFK and G355-5 cells (53, 54). Adaptation of FIV-PPR in CrFK cells also resulted in the same expanded host cell tropism (A. de Parseval and J. H. Elder, unpublished data). These observations suggest that wild-type FIV-PPR might use a chemokine receptor different from CXCR4 and that adaptation in 3201 or CrFK cells resulted in a switch to CXCR4 use. We therefore tried to inhibit FIV-PPR infection of 104-C1 cells with SDF-1α, MIP-1β, and RANTES. Figure 1 shows that SDF-1α efficiently inhibited FIV-PPR infection of 104-C1 cells in a dose-dependent manner, whereas the β chemokines slightly increased the level of replication. These results suggest that CXCR4 is involved as a (co)receptor for FIV-PPR in 104-C1 cells, consistent with previous reports regarding the preferential use of CXCR4 by PI FIV (27, 68).
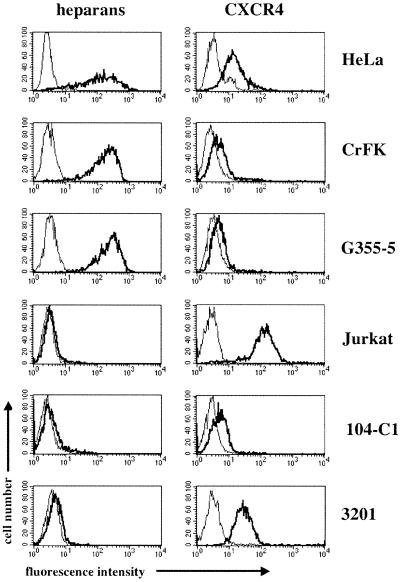
FIG. 8.
Comparative cell surface expression of heparans and CXCR4. Expression of heparans and CXCR4 (bold lines) was assessed by flow cytometry on HeLa, CrFK, G355-5, Jurkat, 104-C1, and 3201 cells. Background controls include isotype-matched antibodies (thin lines).
FIG. 1.
Inhibition of FIV-PPR infection of 104-C1 cells by SDF- 1α. 104-C1 cells were preincubated with either SDF-1α, MIP-1β, or RANTES at the indicated concentrations and infected with FIV-PPR at a multiplicity of infection of 0.01. Virus production was monitored 7 days after the initiation of infection by RT assay as described in Materials and Methods. Results are means and standard deviations for triplicate determinations.
Recombinant SU-immunoadhesins.
To analyze the interactions between FIV SU and specific cell surface receptor(s), a recombinant FIV SU-immunoadhesin was generated. Similar immunoadhesins have been previously reported for the SU glycoprotein of avian leukosis viruses (10), dengue virus (13), human herpesvirus 7 (73), and simian foamy virus (39). The FIV SU-immunoadhesins (designated gp95-Fc) consisted of amino acid residues 1 to 603 of FIV-PPR and FIV-34TF10 Env fused in frame to the hinge region of the Fc domain of IgG1 (Fig. 2A). The site corresponding to the disulfide bridge in Fc was preserved in order to maintain the homodimeric form of the recombinant proteins. The recombinant immunoadhesins were stably transfected in CHO cells, expressed at high levels in cell supernatants by using the GS gene amplification system (see Materials and Methods), and batch purified by affinity chromatography with protein A. By SDS-PAGE, gp95-Fc migrated at approximately 130 to 140 kDa under reducing conditions, and well above the 200-kDa marker in the absence of β-mercaptoethanol (Fig. 2B), consistent with the formation of disulfide-linked homodimers as reported for other SU-immunoadhesins (10, 13, 39, 73). Both gp95 immunoadhesins were specifically recognized by Western blotting with antibodies to FIV Env and human IgG1 Fc (Fig. 2C).
Binding of FIV gp95-Fc to feline cells correlates with susceptibility to infection.
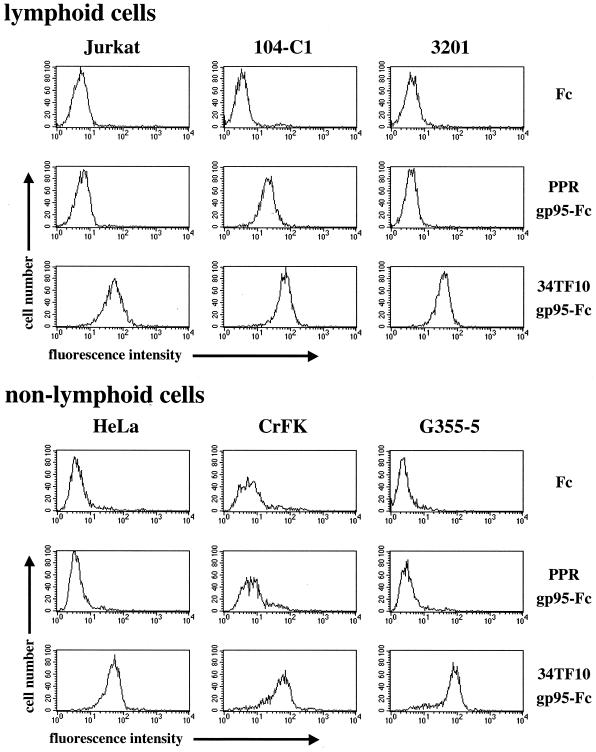
The recombinant immunoadhesins were used as probes to investigate the cellular binding of FIV SU by FACS analysis. Analyses were first performed on total PBMCs, before and after stimulation with concanavalin A and IL-2 (data not shown). As expected, a heterogeneous population of the unstimulated cells bound both SU proteins poorly. After 24 h on IL-2, the population of cells binding the two SU proteins became more homogeneous and the level of binding increased steadily and was nearly identical between both SU, consistent with the outgrowth of the T-cell population (data not shown). These studies demonstrated that the envelope proteins could specifically bind to distinct primary cell populations. However, it was clear that the heterogeneity of this population would preclude any rigorous characterization of receptor binding by either 34TF10 or PPR gp95-Fc. We thus turned to several cell lines, including primary cell lines that had been selected from outgrowth of PBMCs, for further studies (Fig. 3). Binding of Fc, which was used in our experiments as a negative control, was no greater than the background level (secondary antibody alone [data not shown]), indicating that the cellular binding of gp95-Fc was attributable solely to Env determinants. Binding of PPR gp95-Fc was observed only for the 104-C1 cells. This binding pattern is in good agreement with the host cell range properties of FIV-PPR, suggesting that the block in the replication observed with the 3201, CrFK, and G355-5 cells occurs at the level of cellular binding. Binding of 34TF10 gp95-Fc was observed for all cell lines tested, which reflected the in vitro cellular tropism of FIV-34TF10. Additionally, 34TF10 gp95-Fc also bound to the human Jurkat and HeLa cell lines, consistent with the ability of FIV-34TF10 to use human CXCR4 as a fusion receptor (65, 80).
FIG. 3.
Flow cytometry analysis of gp95-Fc binding to lymphoid and nonlymphoid cells. Cells were incubated with either Fc or gp95-Fc for 1 h at 4°C and washed, and cell-surface binding was detected by using fluorescein isothiocyanate-conjugated goat anti-human IgG1 Fc antibody. Fluorescence was analyzed by flow cytometry on 2.5 × 103 gated events.
A non-CXCR4 receptor is involved in the binding of FIV gp95-Fc to primary T cells.
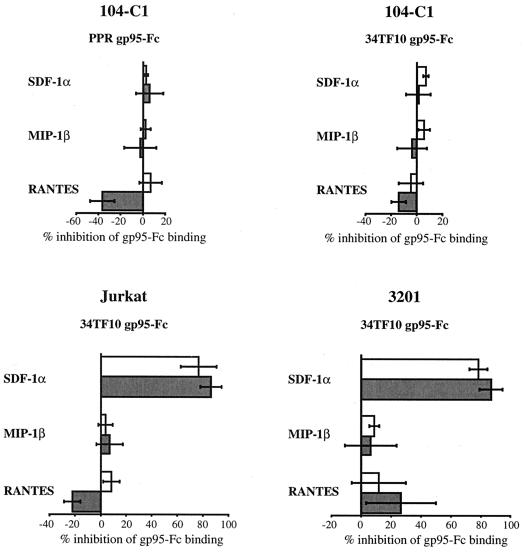
We next addressed the question of whether the α chemokine SDF-1α, the natural ligand of CXCR4, could inhibit the binding of gp95-Fc to 104-C1 cells. As shown in Fig. 4, SDF-1α failed to inhibit the binding of either PPR or 34TF10 gp95-Fc to 104-C1 cells, even at a concentration as high as 10 μg/ml (data not shown). Similar results were also obtained with MCH5-4 cells, another feline primary T-cell line (data not shown). Two β chemokines, MIP-1β and RANTES, were also unable to block the binding of gp95-Fc to 104-C1 cells (Fig. 4). RANTES actually increased the binding of both SU-immunoadhesins, a phenomenon previously observed with T-tropic HIV-1 virions (19, 35, 49). These results indicate that binding of FIV SU to primary feline T cells is not exclusively through CXCR4.
FIG. 4.
Inhibition of gp95-Fc binding to lymphoid cells. 104-C1, Jurkat, and 3201 cells were pretreated with the indicated chemokines at 0.3 or 1.0 μg/ml for 1 h at 4°C before incubation with gp95-Fc for another hour at 4°C. After washing, gp95-Fc binding was monitored by FACS analysis as described in the legend to Fig. 2. Results are means and standard deviations for triplicate determinations.
Binding of 34TF10 gp95-Fc to Jurkat and 3201 cells is CXCR4 dependent.
We next examined whether 34TF10 gp95-Fc used CXCR4 as a binding receptor on Jurkat and 3201 cells. Cells were preincubated with SDF-1α, and gp95-Fc binding was monitored by FACS analysis. Figure 4 shows that SDF-1α was able to inhibit 34TF10 gp95-Fc binding in a dose-dependent manner on both Jurkat and 3201 cells. An average of 85% inhibition in gp95-Fc binding could be observed on both cell lines with 1 μg of SDF-1α per ml. These results strongly indicate that 34TF10 gp95-Fc interacts directly with CXCR4 on the surface of Jurkat and 3201 cells. Preincubation with either MIP-1β or RANTES had no effect on 34TF10 gp95-Fc binding to Jurkat cells, whereas a slight inhibition of binding was observed with RANTES on 3201 cells (Fig. 4). Whether this inhibition was significant or not remains to be determined, since higher concentrations of RANTES increased the binding (data not shown).
Cell-surface heparans rather than CXCR4 contribute to the binding of 34TF10 gp95-Fc to adherent cells.
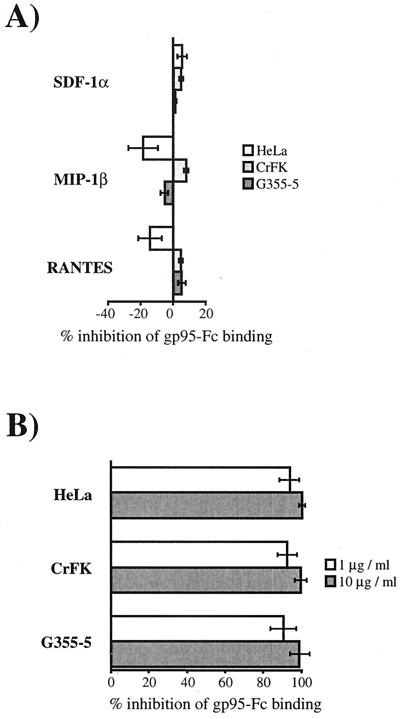
We next analyzed the binding of 34TF10 gp95-Fc to nonlymphoid cells. As with the lymphoid cells, we addressed the issue of whether SDF-1α could inhibit the binding of gp95-Fc to either the HeLa, CrFK, and G355-5 cell lines. Unexpectedly and contrary to what we observed with the Jurkat and 3201 cells, SDF-1α was unable to inhibit 34TF10 gp95-Fc binding to any of the three cell lines (Fig. 5A), even at concentrations as high as 10 μg/ml (data not shown). The β chemokines were also unable to inhibit gp95-Fc binding (Fig. 5A). Furthermore, gp95-Fc binding was also observed for the CXCR4-negative U87 cell line (data not shown), suggesting that CXCR4 is not required for the cellular binding of 34TF10 gp95-Fc. It is well established that heparan sulfate proteoglycans (HSPGs) serve as facilitators in the cellular attachment of HIV which is both cell type and HIV strain dependent (45, 57, 59, 61, 70, 72). Enzymatic removal of heparan sulfate chains by heparinase reduces HIV attachment and infectivity. These studies have also shown that soluble heparans such as heparin or dextran sulfate inhibited replication of X4 HIV strains by specific binding to the V3 loop of gp120. A similar inhibition mediated by heparin and dextran sulfate was also reported for FIV (77). To elucidate whether HSPGs could also be involved in 34TF10 gp95-Fc binding, we first performed a competition study with heparin. Heparin and 34TF10 gp95-Fc were coincubated with the adherent cell lines, and inhibition of gp95-Fc binding was monitored by FACS analysis. As shown on Fig. 5B, heparin strongly inhibited gp95-Fc binding.
FIG. 5.
Inhibition of 34TF10 gp95-Fc binding to adherent cells. Cells were preincubated with the indicated chemokines (1 μg/ml) at 4°C for 1 h before incubation with gp95-Fc (A) or coincubated with gp95-Fc and heparin at 1 and 10 μg/ml at 4°C for 1 h (B). After washing, gp95-Fc binding was monitored by FACS analysis. Results are means and standard deviations for triplicate determinations.
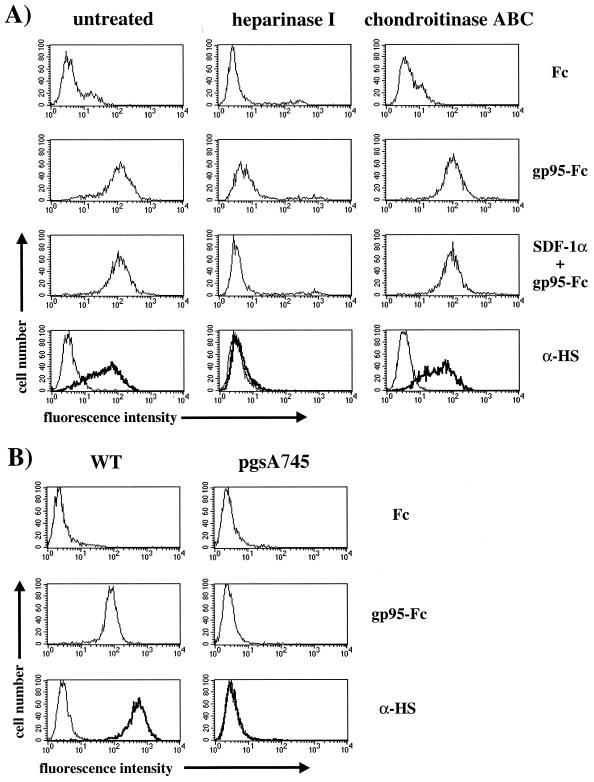
To further investigate the role of cell-surface heparans in mediating gp95-Fc binding, we treated HeLa (Fig. 6A) and CrFK (data not shown) cells with either heparinase I or chondroitinase ABC at a concentration of 10 U/ml for 1 h at 37°C and monitored gp95-Fc binding by FACS analysis. Heparinase and chondroitinase remove specifically the heparan and chondroitin sulfate chains, respectively, from the core of the proteoglycan. The specific enzymatic removal of heparan sulfate chains was assessed with an antibody specific for heparan sulfate chains (Fig. 6A). As expected, heparinase treatment abrogated 34TF10 gp95-Fc binding by approximately 90% compared to the untreated or chondroitinase-treated cells (Fig. 6A). These results strongly suggest that cell-surface heparans mediate binding of 34TF10 gp95-Fc to adherent cells. The residual gp95-Fc binding observed on heparinase-treated cells was CXCR4 dependent, since the residual binding was further inhibited by SDF-1α (Fig. 6A), consistent with the observation that CrFK-adapted viruses used CXCR4 as a fusion and entry receptor in either CrFK or HeLa cells (65, 80). To demonstrate further that HSPGs mediated binding of 34TF10 gp95-Fc, we used pgsA-745, a CHO cell line deficient in the biosynthesis of glycosaminoglycans including heparan sulfate chains (29). 34TF10 gp95-Fc efficiently bound to the CXCR4-negative wild-type CHO-K1 cells but failed to bind to pgsA-745 cells (Fig. 6B), suggesting that heparan sulfate chains are necessary for efficient binding of 34TF10 gp95-Fc to the cell membrane.
FIG. 6.
Cell-surface heparans mediate binding of 34TF10 gp95-Fc to adherent cells. (A) Cells were incubated with the glycosaminoglycan-specific enzymes heparinase I and chondroitinase ABC at 10 U/ml for 1 h at 37°C. Cells were washed and preincubated in the absence or presence of SDF-1α (1 μg/ml) at 4°C for 1 h prior to incubation with gp95-Fc. After washing, gp95-Fc binding was monitored by FACS analysis. Efficient removal of cell-surface heparans was monitored by using an antibody specific for heparan sulfate chains (α-HS; bold lines). Background controls include Fc for the detection of gp95-Fc binding and an isotype-matched antibody (thin lines) for the expression of cell-surface heparans. (B) Fc and 34TF10 gp95-Fc binding as well as the levels of expression of cell-surface heparans were assessed by flow cytometry on CHO wild-type (WT) cells and on a mutant cell line, pgsA745, deficient in the biosynthesis of cell-surface heparans.
Heparin inhibits 34TF10 gp95-Fc–CXCR4 interactions on Jurkat and 3201 cells.
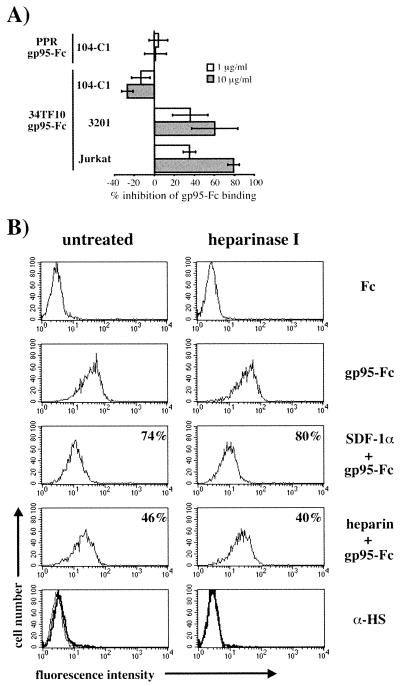
Since HSPGs played an important role in mediating 34TF10 gp95-Fc binding to adherent cells, we also sought to determine their potential role in the binding of FIV gp95-Fc to the lymphoid cells used in this study. Heparin had no effect on the binding of either PPR or 34TF10 gp95-Fc to primary 104-C1 T cells (Fig. 7A), whereas a dose-dependent inhibition in the binding of 34TF10 gp95-Fc was observed for the Jurkat and 3201 cells (Fig. 7A), the two cell lines for which we demonstrated a direct interaction between 34TF10 gp95-Fc and CXCR4 (Fig. 4). To address the issue of whether heparin inhibited an interaction between gp95 and CXCR4 rather than an interaction between gp95 and HSPGs, Jurkat (Fig. 7B) and 3201 (data not shown) cells were treated in the absence or presence of heparinase I (10 U/ml). As shown in Fig. 7B, heparinase treatment had no effect on the inhibition mediated by heparin. Coincubation of heparin and 34TF10 gp95-Fc resulted in 46 and 40% inhibition of gp95-Fc binding for cells treated in the absence or presence of heparinase, respectively. Inhibition mediated by SDF-1α was also unchanged (Fig. 7B). These results indicate that heparin interferes in the interactions between gp95 and CXCR4, consistent with a recent study in which heparin was shown to interfere with the gp120-CXCR4 association by binding to V3 as well as the coreceptor binding site (58). FACS analysis of the cellular expression of HSPGs and CXCR4 showed that Jurkat and 3201 cells expressed very low level of HSPGs and relatively high levels of CXCR4, whereas for HeLa and CrFK cells the opposite was observed (Fig. 8). Therefore, the preferential binding of 34TF10 gp95-Fc to either CXCR4 or HSPGs on a given cell line could be determined by the relative expression level of both markers.
FIG. 7.
Heparin interferes with gp95-Fc binding to CXCR4. (A) Cell were coincubated with 34TF10 gp95-Fc and heparin at 1 and 10 μg/ml for 1 h at 4°C. After washing, gp95-Fc binding was detected as described for Fig. 2. Results are means and standard deviations for triplicate determinations. (B) Jurkat cells were treated in the absence or presence of heparinase I at 10 U/ml. 34TF10 gp95-Fc binding was monitored by FACS analysis as described for Fig. 2. For inhibition studies, cells were preincubated with SDF-1α (1 μg/ml) at 4°C for 1 h before incubation with gp95-Fc or coincubated with gp95-Fc and heparin (1 μg/ml). After washing, gp95-Fc binding was monitored by FACS analysis. Percent inhibition of gp95-Fc binding is indicated in the top right corner of each histogram. Efficient removal of heparan sulfate chains by heparinase was monitored by FACS using an anti-heparan sulfate antibody (α-HS, bold lines; isotype-matched antibody, thin lines).
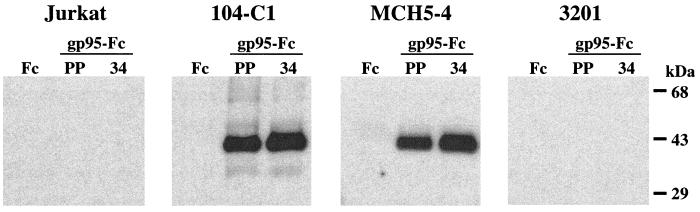
PPR and 34TF10 gp95-Fc coprecipitate with a 40-kDa protein species from the surface of feline primary T cells but not from Jurkat or 3201 cells.
Our overall results suggest that neither CXCR4 nor HSPGs interact with PPR or 34TF10 gp95-Fc on the surface of primary feline T cells, whereas only 34TF10 gp95-Fc interacts with CXCR4 on the surface of Jurkat or 3201 cells and with HSPGs on adherent cells. Successful coprecipitation of immunoadhesins with their specific ligands has been previously reported (1, 10, 33, 34, 74, 82). We therefore supplemented our flow cytometry results with immunoprecipitation assays. Primary feline T cells (104-C1 and MCH5-4), Jurkat cells, and 3201 cells were cell surface biotinylated and used in immunoprecipitation studies with either Fc or gp95-Fc, and coprecipitated complexes were immunoblotted with neutravidin. Figure 9 shows that PPR and 34TF10 gp95-Fc, but not Fc, coprecipitated with a 40-kDa protein species from lysates of primary T cells but not from CXCR4-rich Jurkat or 3201 cells, suggesting that this band is not CXCR4. Coprecipitated complexes were also immunoblotted with antibodies to CXCR4. However, we were unable to detect CXCR4 from Jurkat and 3201 cell lysates coprecipitated with 34TF10 gp95-Fc, even after using three different anti-CXCR4 antibodies (data not shown). Difficulties in solubilizing CXCR4 have previously been reported (12), which may explain the failure to detect CXCR4 by this approach.
FIG. 9.
gp95-Fc interacts with a 40-kDa protein species from the surface of feline primary T cells but not from Jurkat and 3201 cells. Cells were cell surface biotinylated and lysed, and precleared lysate supernatants were incubated with either Fc (lane 1), PPR gp95-Fc (PP; lane 2), or 34TF10 gp95-Fc (34; lane 3). Complexes were immunoprecipitated with protein A, resolved by SDS-PAGE, and immunoblotted with neutravidin. Bound neutravidin was revealed by an enhanced chemiluminescence procedure.
DISCUSSION
We report here the cellular binding properties of the SU protein of two divergent infectious molecular clones of FIV by using a recombinant soluble Env protein in which the transmembrane domain was replaced by the Fc domain of human IgG1. These gp95 immunoadhesins were used in FACS analyses to address the role of CXCR4 and other receptors in the binding of FIV SU to different cell lines. Although we observed a common mechanism of binding of FIV SU to primary feline T cells, binding of FIV SU to IL-2-independent T cells and adherent cells was both cell type and SU dependent.
The pattern of gp95-Fc binding to feline cells was consistent with the host cell range properties of both FIV-PPR and FIV-34TF10 (Fig. 3). 34TF10 gp95-Fc binding was observed for all feline cell lines tested, whereas PPR gp95-Fc binding was observed only for primary T-cell lines. This suggests that the inability of FIV-PPR to efficiently replicate in 3201, CrFK, and G355-5 cells is dictated by the first step in the virus life cycle, cellular attachment. This has profound implications for the mechanism by which the virus binds to and enters the target cell. FIV has been shown to use CXCR4 as a receptor for entry. However, certain cells, which are CXCR4 positive, are refractory to productive infection by primary FIV isolates. Here we demonstrate that the limiting step in the efficient replication of FIV-PPR in 3201, CrFK, and G355-5 cells was at a preentry rather than a postentry level, suggesting that a receptor different from CXCR4 is involved in the binding of FIV-PPR SU. This hypothesis was further confirmed by inhibition studies with SDF-1α. While SDF1-α was unable to efficiently block the binding of PPR gp95-Fc to 104-C1 (Fig. 4) and MCH5-4 (data not shown) cells, a dose-dependent inhibition of FIV-PPR replication in 104-C1 cells was observed (Fig. 1). These data demonstrate that FIV-PPR uses CXCR4 as an entry receptor in 104-C1 cells, consistent with previous reports showing that PI FIV use CXCR4. Most importantly, these data strongly suggest that a non-CXCR4 receptor is involved in the binding of PPR gp95 to primary T cells. Immunoprecipitation studies were used to complement our FACS analyses and a 40-kDa protein species distinct from CXCR4 was specifically coprecipitated from the surface of primary T cells but not from the nonpermissive Jurkat and 3201 cells (Fig. 9). Whether this protein is a primary binding receptor for FIV-PPR on primary T cells remains to be determined. Similar results were observed for 34TF10 gp95-Fc on primary T cells, which suggests that different isolates of FIV use a common mechanism of binding to primary T cells. However, FIV-34TF10 has a broader cellular tropism that was reflected by the ability of 34TF10 gp95-Fc to efficiently bind all cell lines tested (Fig. 3). Two mechanisms for the cellular binding of 34TF10 gp95-Fc could be inferred from our results. Our data clearly show that 34TF10 gp95-Fc uses CXCR4 as a primary binding receptor on Jurkat and 3201 IL-2-independent T cells (Fig. 4), whereas HSPGs contribute to the binding of 34TF10 gp95-Fc to adherent cells (Fig. 6). Binding of 34TF10 gp95-Fc to CXCR4 on adherent cells was observed only after enzymatic removal of heparan sulfate chains (Fig. 6A), suggesting that FIV (in this case FIV-34TF10) might be funneled to CXCR4 after initial binding to HSPGs. The low level of binding of 34TF10 gp95-Fc to heparinase-treated cells was unexpected but could be correlated to the low level of expression of CXCR4 on these cells (Fig. 8). The ability of 34TF10 gp95-Fc to bind either HSPGs or CXCR4 could be directly correlated to the level of expression of both markers on the cell surface (Fig. 8). Jurkat and 3201 cells expressed very low levels of heparans but high levels of CXCR4, and 34TF10 gp95-Fc was shown to bind exclusively to CXCR4 on these cells. On the other hand, 34TF10 gp95-Fc binding was predominantly if not exclusively through HSPGs on HeLa and CrFK cells, which expressed high levels of heparans but low levels of CXCR4 (Fig. 8).
Soluble heparans such as heparin and dextran sulfate interact with the V3 loop of T-tropic HIV-1 gp120 without interfering with the binding of gp120 to CD4 (5, 11, 36, 37, 69, 70). This interaction is dependent on the net charge of the V3 loop and is thought to be mediated via electrostatic interactions between the acidic sulfate groups of the heparan sulfate chains and the basic residues contained within the V3 loop. Recently, soluble heparans have also been shown to interact with the conserved coreceptor binding site on gp120 (58). The FIV strains used in our study have a V3 loop with net charges of +4 and +8 for PPR and 34TF10, respectively (Fig. 10). This main difference could explain the specific interaction of 34TF10 gp95-Fc with HSPGs on adherent cells. Our results also corroborate those reported by Moulard et al. (58), since heparin could specifically interfere with the interactions between gp95-Fc and CXCR4 on Jurkat and 3201 cells (Fig. 7).
FIG. 10.
Amino acid sequences and overall charge of FIV V3 loop. Residues differing between the clones are indicated. Amino acids (positions 407 and 409) that are important for CrFK tropism are boxed.
PI FIV adapted to propagate in CrFK cells have been shown to have a more basic V3 loop, involving in particular the mutation of either one or two glutamate residues (407 and 409) by lysine residues in the V3 loop (60, 75, 76, 79). Adaptation of FIV-PPR for propagation in CrFK cells also resulted in an increase in the net charge of V3 due to a change of glutamate to lysine at position 407 (Fig. 10) (de Perseval and Elder, unpublished). However, adaptation of FIV-PPR in 3201 cells resulted in several point mutations dispersed throughout the entire Env region, but none resulted in an increase in the net charge of V3 (Fig. 10) (53). Interestingly, 3201 cells expressed very low levels of HSPGs whereas CrFK cells express high levels of HSPGs (Fig. 8). Furthermore, X4 HIV isolates passaged in T-cell lines that expressed relatively high levels of HSPGs such as H9 and MT2 cells tend to have more basic V3 loops than those passaged in primary activated T cells which express small amounts of HSPGs (32, 45, 47, 59, 61). Therefore, the increase in the net charge of V3 associated with the CrFK phenotype could result from the adaptation of the virus to HSPGs. FIV-PPR adapted on 3201 cells has the ability to infect cells that were previously refractory for the parental virus (53). Infection of these cells was shown to be mediated through CXCR4 (53). It would therefore be interesting to analyze gp95-CXCR4 and gp95-HSPG interactions with gp95 immunoadhesins obtained from FIV-PPR adapted to either CrFK or 3201 cells.
The inability of SDF-1α to inhibit 34TF10 gp95-Fc binding to HeLa or CrFK cells (Fig. 5A) was unexpected, since FIV gp95 from another CrFK-adapted strain, FIV-F14, has been reported by Hosie and coworkers to compete with SDF-1α for binding to CXCR4 on CXCR4-transfected U87 cells (43). Here we found that 34TF10 gp95-Fc bound efficiently to the CXCR4-negative parental U87 cells through HSPGs (data not shown). FIV-14 and FIV-34TF10 have very similar amino acid sequences, since they were obtained from the same FIV primary isolate which was adapted for propagation in CrFK cells (60, 76). However, FIV-F14 as a V3 loop with a net charge of +6, compared to +8 for FIV-34TF10 (Fig. 10), and Hosie et al. (43) used a monomeric form of gp95 whereas our gp95 immunoadhesin is in a dimeric form (Fig. 2B). Recent modelings of oligomeric gp120 suggest that electrostatic properties are stronger for oligomeric gp120 than monomeric gp120, and are influenced by the net charge of V3 (51, 58). These reports confirmed an earlier study by Roderiquez et al. (70) in which monomeric gp120 from an X4 HIV isolate failed to interact with cell-surface heparans, whereas oligomeric gp120 from the same isolate bound specifically. Therefore, the decrease in both the electrostatic properties and the net charge of FIV-F14 gp95 V3 loop compared to 34TF10 gp95-Fc could explain the inability of F14 gp95 to interact with cell-surface heparans and its specific interaction with CXCR4.
The distinct host cell range properties and the preferential use of CXCR4 by primary as well as CrFK-adapted FIV isolates, combined with our current findings, support the possibility that a primary receptor could be required by FIV, similar to CD4 for HIV, a hypothesis already suggested by others (68, 81). CXCR4 expression in vitro does not correlate with susceptibility to infection with PI FIV (54). Several CXCR4-positive cell lines are refractory to productive infection by PI FIV. These observations are supported by our results, which suggest that PPR gp95-Fc failed to bind directly and specifically to CXCR4 (Fig. 4). However, CXCR4 is implicated, since replication of FIV-PPR (Fig. 1) and other PI FIV (27, 68) is inhibited by SDF-1α or AMD3100. This suggests that cellular attachment of PI FIV might follow a two-step model similar to the case for HIV. In a first step, gp95 would bind to a CD4-like factor that would induce conformational rearrangements in gp95; in a second step, gp95 would interact with CXCR4. This hypothesis would explain the inability of PPR gp95-Fc to bind directly to CXCR4 (Fig. 4). The fact that 34TF10 gp95-Fc interacted directly with CXCR4 on Jurkat and 3201 cells (Fig. 4) could be a result of its adaptation to propagate in CrFK cells similar to HIV isolates adapted to grow in CD4-negative cells. Consistent with the current opinion on HIV cellular binding and entry events (20), the differences we observed in the cellular binding of PPR and 34TF10 gp95-Fc suggest that binding affinity may be influenced by (i) the conformation of the SU in question, (ii) the lack or presence of a CD4-like factor that would induce conformational changes in SU to allow binding to CXCR4, (iii) the conformational heterogeneity of CXCR4, or (iv) some combination of the above.
Our study further addresses the issue of a candidate primary cell-surface receptor for FIV. gp95-Fc but not Fc specifically coprecipitated a 40-kDa protein from the surface of primary feline T cells but not from either Jurkat or 3201 cells (Fig. 9). Interestingly, SDF-1α was unable to inhibit the binding of 34TF10 gp95-Fc to primary feline T cells (Fig. 4), and we showed that 34TF10 gp95-Fc also coprecipitated this 40-kDa protein from primary T cells (Fig. 9). These findings suggest a preferential high-affinity binding of 34TF10 gp95-Fc for this protein rather than to CXCR4 as is the case for CD4-independent HIV variants which have retained the property of high-affinity binding to CD4 (20). Jurkat and 3201 cells do not expressed this gp95-Fc binding protein, or, in the case of the Jurkat cells, the human counterpart might not be specific for FIV. Adaptation of FIV-34TF10 to propagate in CrFK cells might have allowed the virus to gain independence from that 40-kDa protein. This would explain the ability of 34TF10 gp95-Fc to bind directly to CXCR4 on Jurkat and 3201 cells (Fig. 4). We were unable to show a specific coprecipitation of 34TF10 gp95-Fc with CXCR4 from Jurkat and 3201 cell lysates (data not shown). However, difficulties in solubilizing CXCR4 have been reported (12). The use of different detergent combinations might help us to successfully coprecipitate 34TF10 gp95-Fc with CXCR4. To definitively address the question of whether this gp95-Fc binding protein is a putative primary binding receptor for FIV, we are currently purifying the protein and attempting to isolate the gene encoding this protein by expression cloning.
ACKNOWLEDGMENTS
We thank Laure Moutouh-de Parseval and Philippe Gallay for careful reading of the manuscript and valuable comments. The contribution of certain cell lines, antibodies, and vectors by Chris Grant, Christopher Broder, Philippe Gallay, Brian Seed, and Tom Tsang is also gratefully acknowledged. We thank Hoffmann-La Roche and Gryphon Pharmaceuticals for the gift of IL-2 and chemokines.
This work was supported by a National Institutes of Health grant RO1AI25825.
REFERENCES
- 1.Adkins H B, Brojatsch J, Naughton J, Rolls M M, Pesola J M, Young J A T. Identification of a cellular receptor for subgroup E avian leukosis virus. Proc Natl Acad Sci USA. 1997;94:11617–11622. doi: 10.1073/pnas.94.21.11617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alkhatib G, Combadiere C, Broder C C, Feng Y, Kennedy P E, Murphy P M, Berger E A. CC CKR5: a RANTES, MIP-1alpha, MIP-1beta receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–1958. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- 3.Altin J G, Pagler E B. A one-step procedure for biotinylation and chemical cross-linking of lymphocyte surface and intracellular membrane-associated molecules. Anal Biochem. 1995;224:382–389. doi: 10.1006/abio.1995.1054. [DOI] [PubMed] [Google Scholar]
- 4.Aruffo A, Stamenkovic I, Melnick M, Underhill C B, Seed B. CD44 is the principal cell surface receptor for hyaluronate. Cell. 1990;61:1303–1313. doi: 10.1016/0092-8674(90)90694-a. [DOI] [PubMed] [Google Scholar]
- 5.Batinic D, Robey F A. The V3 region of the envelope glycoprotein of human immunodeficiency virus type 1 binds sulfated polysaccharides and CD4-derived synthetic peptides. J Biol Chem. 1992;267:6664–6671. [PubMed] [Google Scholar]
- 6.Bebbington C R, Renner G, Thomson S, King D, Abrams D, Yarranton G T. High-level expression of a recombinant antibody from myeloma cells using a glutamate synthetase gene as an amplifiable selectable marker. Bio Technology. 1992;10:169–175. doi: 10.1038/nbt0292-169. [DOI] [PubMed] [Google Scholar]
- 7.Berger E A, Murphy P M, Farber J M. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annu Rev Immunol. 1999;17:657–700. doi: 10.1146/annurev.immunol.17.1.657. [DOI] [PubMed] [Google Scholar]
- 8.Berson J F, Long D, Doranz B J, Rucker J, Jirik F R, Doms R W. A seven-transmembrane domain receptor involved in fusion and entry of T-cell-tropic human immunodeficiency virus type 1 strains. J Virol. 1996;70:6288–6295. doi: 10.1128/jvi.70.9.6288-6295.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borsetti A, Parolin C, Ridolfi B, Sernicola L, Geraci A, Ensoli B, Titti F. CD4-independent infection of two CD4−/CCR5−/CXCR4+ pre-T-cell lines by human and simian immunodeficiency viruses. J Virol. 2000;74:6689–6694. doi: 10.1128/jvi.74.14.6689-6694.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brojatsch J, Naughton J, Rolls M M, Zingler K, Young J A T. CAR1, a TNFR-related protein, is a cellular receptor for cytopathic avian leukosis-sarcoma viruses and mediates apoptosis. Cell. 1996;87:845–855. doi: 10.1016/s0092-8674(00)81992-3. [DOI] [PubMed] [Google Scholar]
- 11.Callahan L N, Phelan M, Mallinson M, Norcross M A. Dextran sulfate blocks antibody binding to the principal neutralizing domain of human immunodeficiency virus type 1 without interfering with gp120-CD4 interactions. J Virol. 1991;65:1543–1550. doi: 10.1128/jvi.65.3.1543-1550.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chabot D J, Chen H, Dimitrov D S, Broder C C. N-linked glycosylation of CXCR4 masks coreceptor function for CCR5-dependent human immunodeficiency virus type 1 isolates. J Virol. 2000;74:4404–4413. doi: 10.1128/jvi.74.9.4404-4413.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen Y, Maguire T, Marks R M. Demonstration of binding of dengue virus envelope protein to target cells. J Virol. 1996;70:8765–8772. doi: 10.1128/jvi.70.12.8765-8772.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choe H, Farzan M, Sun Y, Sullivan N, Rollins B, Ponath P D, Wu L, Mackay C R, LaRosa G, Newman W, Gerard N, Gerard C, Sodroski J. The beta-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell. 1996;85:1135–1148. doi: 10.1016/s0092-8674(00)81313-6. [DOI] [PubMed] [Google Scholar]
- 15.Clapham P R, Reeves J D, Simmons G, Dejucq N, Hibbitts S, McKnight A. HIV coreceptors, cell tropism and inhibition by chemokine receptor ligands. Mol Membr Biol. 1999;16:49–55. doi: 10.1080/096876899294751. [DOI] [PubMed] [Google Scholar]
- 16.de Parseval A, Lerner D L, Borrow P, Willett B J, Elder J H. Blocking of feline immunodeficiency virus infection by a monoclonal antibody to CD9 is via inhibition of virus release rather than interference with receptor binding. J Virol. 1997;71:5742–5749. doi: 10.1128/jvi.71.8.5742-5749.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Di Marzio P, Marmon S, Sutton R E, Hill C M, Davis C B, Peiper S C, Schall T J, Littman D R, Landau N R. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 18.Deng H K, Unutmaz D, KewalRamani V N, Littman D R. Expression cloning of new receptors used by simian and human immunodeficiency viruses. Nature. 1997;388:296–300. doi: 10.1038/40894. [DOI] [PubMed] [Google Scholar]
- 19.Dolei A, Biolchini A, Serra C, Curreli S, Gomes E, Dianzani F. Increased replication of T-cell-tropic HIV strains and CXC-chemokine receptor-4 induction in T cells treated with macrophage inflammatory protein (MIP)-1alpha, MIP-1beta and RANTES beta-chemokines. AIDS. 1998;12:183–190. doi: 10.1097/00002030-199802000-00008. [DOI] [PubMed] [Google Scholar]
- 20.Doms R W. Beyond receptor expression: the influence of receptor conformation, density, and affinity in HIV-1 infection. Virology. 2000;276:229–237. doi: 10.1006/viro.2000.0612. [DOI] [PubMed] [Google Scholar]
- 21.Doranz B J, Rucker J, Yi Y, Smyth R J, Samson M, Peiper S C, Parmentier M, Collman R G, Doms R W. A dual-tropic primary HIV-1 isolate that uses fusin and the beta-chemokine receptors CKR-5, CKR-3, and CKR-2b as fusion cofactors. Cell. 1996;85:1149–1158. doi: 10.1016/s0092-8674(00)81314-8. [DOI] [PubMed] [Google Scholar]
- 22.Dragic T, Litwin V, Allaway G P, Martin S R, Huang Y, Nagashima K A, Cayanan C, Maddon P J, Koup R A, Moore J P, Paxton W A. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature. 1996;381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 23.Dumonceaux J, Nisole S, Chanel C, Quivet L, Amara A, Baleux F, Briand P, Hazan U. Spontaneous mutations in the env gene of the human immunodeficiency virus type 1 NDK isolate are associated with a CD4-independent entry phenotype. J Virol. 1998;72:512–519. doi: 10.1128/jvi.72.1.512-519.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Edinger A L, Hoffman T L, Sharron M, Lee B, O'Dowd B, Doms R W. Use of GPR1, GPR15, and STRL33 as coreceptors by diverse human immunodeficiency virus type 1 and simian immunodeficiency virus envelope proteins. Virology. 1998;249:367–378. doi: 10.1006/viro.1998.9306. [DOI] [PubMed] [Google Scholar]
- 25.Edinger A L, Hoffman T L, Sharron M, Lee B, Yi Y, Choe W, Kolson D L, Mitrovic B, Zhou Y, Faulds D, Collman R G, Hesselgesser J, Horuk R, Doms R W. An orphan seven-transmembrane domain receptor expressed widely in the brain functions as a coreceptor for human immunodeficiency virus type 1 and simian immunodeficiency virus. J Virol. 1998;72:7934–7940. doi: 10.1128/jvi.72.10.7934-7940.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Edinger A L, Mankowski J L, Doranz B J, Margulies B J, Lee B, Rucker J, Sharron M, Hoffman T L, Berson J F, Zink M C, Hirsch V M, Clements J E, Doms R W. CD4-independent, CCR5-dependent infection of brain capillary endothelial cells by a neurovirulent simian immunodeficiency virus strain. Proc Natl Acad Sci USA. 1997;94:14742–14747. doi: 10.1073/pnas.94.26.14742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Egberink H F, De Clercq E, Van Vliet A L, Balzarini J, Bridger G J, Henson G, Horzinek M C, Schols D. Bicyclams, selective antagonists of the human chemokine receptor CXCR4, potently inhibit feline immunodeficiency virus replication. J Virol. 1999;73:6346–6352. doi: 10.1128/jvi.73.8.6346-6352.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Endres M J, Clapham P R, Marsh M, Ahuja M, Turner J D, McKnight A, Thomas J F, Stoebenau-Haggarty B, Choe S, Vance P J, Wells T N, Power C A, Sutterwala S S, Doms R W, Landau N R, Hoxie J A. CD4-independent infection by HIV-2 is mediated by fusin/CXCR4. Cell. 1996;87:745–756. doi: 10.1016/s0092-8674(00)81393-8. [DOI] [PubMed] [Google Scholar]
- 29.Esko J D, Stewart T E, Taylor W H. Animal cell mutants defective in glycosaminoglycan biosynthesis. Proc Natl Acad Sci USA. 1985;82:3197–3201. doi: 10.1073/pnas.82.10.3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Farzan M, Choe H, Martin K, Marcon L, Hofmann W, Karlsson G, Sun Y, Barrett P, Marchand N, Sullivan N, Gerard N, Gerard C, Sodroski J. Two orphan seven-transmembrane segment receptors which are expressed in CD4-positive cells support simian immunodeficiency virus infection. J Exp Med. 1997;186:405–411. doi: 10.1084/jem.186.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feng Y, Broder C C, Kennedy P E, Berger E A. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272:872–877. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 32.Fouchier R A, Groenink M, Kootstra N A, Tersmette M, Huisman H G, Miedema F, Schuitemaker H. Phenotype-associated sequence variation in the third variable domain of the human immunodeficiency virus type 1 gp120 molecule. J Virol. 1992;66:3183–3187. doi: 10.1128/jvi.66.5.3183-3187.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Golovkina T V, Dzuris J, van den Hoogen B, Jaffe A B, Wright P C, Cofer S M, Ross S R. A novel membrane protein is a mouse mammary tumor virus receptor. J Virol. 1998;72:3066–3071. doi: 10.1128/jvi.72.4.3066-3071.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goodwin R G, Alderson M R, Smith C A, Armitage R J, VandenBos T, Jerzy R, Tough T W, Schoenborn M A, Davis-Smith T, Hennen K, et al. Molecular and biological characterization of a ligand for CD27 defines a new family of cytokines with homology to tumor necrosis factor. Cell. 1993;73:447–456. doi: 10.1016/0092-8674(93)90133-b. [DOI] [PubMed] [Google Scholar]
- 35.Gordon C J, Muesing M A, Proudfoot A E, Power C A, Moore J P, Trkola A. Enhancement of human immunodeficiency virus type 1 infection by the CC-chemokine RANTES is independent of the mechanism of virus-cell fusion. J Virol. 1999;73:684–694. doi: 10.1128/jvi.73.1.684-694.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harrop H A, Coombe D R, Rider C C. Heparin specifically inhibits binding of V3 loop antibodies to HIV-1 gp120, an effect potentiated by CD4 binding. AIDs. 1994;8:183–192. doi: 10.1097/00002030-199402000-00005. [DOI] [PubMed] [Google Scholar]
- 37.Harrop H A, Rider C C. Heparin and its derivatives bind to HIV-1 recombinant envelope glycoproteins, rather than to recombinant HIV-1 receptor, CD4. Glycobiology. 1998;8:131–137. doi: 10.1093/glycob/8.2.131. [DOI] [PubMed] [Google Scholar]
- 38.Hayward B E, Hussain A, Wilson R H, Lyons A, Woodcock V, McIntosh B, Harris T J R. The cloning and nucleotide sequence of cDNA for an amplified glutamine synthetase gene from the Chinese hamster. Nucleic Acids Res. 1996;14:999–1008. doi: 10.1093/nar/14.2.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Herchenroder O, Moosmayer D, Bock M, Pietschmann T, Rethwilm A, Bieniasz P D, McClure M O, Weis R, Schneider J. Specific binding of recombinant foamy virus envelope protein to host cells correlates with susceptibility to infection. Virology. 1999;255:228–236. doi: 10.1006/viro.1998.9570. [DOI] [PubMed] [Google Scholar]
- 40.Hesselgesser J, Halks-Miller M, DelVecchio V, Peiper S C, Hoxie J, Kolson D L, Taub D, Horuk R. CD4-independent association between HIV-1 gp120 and CXCR4: functional chemokine receptors are expressed in human neurons. Curr Biol. 1997;7:112–121. doi: 10.1016/s0960-9822(06)00055-8. [DOI] [PubMed] [Google Scholar]
- 41.Hoffman T L, LaBranche C C, Zhang W, Canziani G, Robinson J, Chaiken I, Hoxie J A, Doms R W. Stable exposure of the coreceptor-binding site in a CD4-independent HIV-1 envelope protein. Proc Natl Acad Sci USA. 1999;96:6359–6364. doi: 10.1073/pnas.96.11.6359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Horuk R, Hesselgesser J, Zhou Y, Faulds D, Halks-Miller M, Harvey S, Taub D, Samson M, Parmentier M, Rucker J, Doranz B J, Doms R W. The CC chemokine I-309 inhibits CCR8-dependent infection by diverse HIV-1 strains. J Biol Chem. 1998;273:386–391. doi: 10.1074/jbc.273.1.386. [DOI] [PubMed] [Google Scholar]
- 43.Hosie M J, Broere N, Hesselgesser J, Turner J D, Hoxie J A, Neil J C, Willett B J. Modulation of feline immunodeficiency virus infection by stromal cell-derived factor. J Virol. 1998;72:2097–2104. doi: 10.1128/jvi.72.3.2097-2104.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hoxie J A, LaBranche C C, Endres M J, Turner J D, Berson J F, Doms R W, Matthews T J. CD4-independent utilization of the CXCR4 chemokine receptor by HIV-1 and HIV-2. J Reprod Immunol. 1998;41:197–211. doi: 10.1016/s0165-0378(98)00059-x. [DOI] [PubMed] [Google Scholar]
- 45.Ibrahim J, Griffin P, Coombe D R, Rider C C, James W. Cell-surface heparan sulfate facilitates human immunodeficiency virus Type 1 entry into some cell lines but not primary lymphocytes. Virus Res. 1999;60:159–169. doi: 10.1016/s0168-1702(99)00018-0. [DOI] [PubMed] [Google Scholar]
- 46.Iyengar S, Schwartz D H, Clements J E, Hildreth J E. CD4-independent, CCR5-dependent simian immunodeficiency virus infection and chemotaxis of human cells. J Virol. 2000;74:6720–6724. doi: 10.1128/jvi.74.15.6720-6724.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jiang S. HIV-1–co-receptors binding. Nat Med. 1997;3:367–368. doi: 10.1038/nm0497-367. [DOI] [PubMed] [Google Scholar]
- 48.Kanbe K, Shimizu N, Soda Y, Takagishi K, Hoshino H. A CXC chemokine receptor, CXCR5/BLR1, is a novel and specific coreceptor for human immunodeficiency virus type 2. Virology. 1999;265:264–273. doi: 10.1006/viro.1999.0036. [DOI] [PubMed] [Google Scholar]
- 49.Kinter A, Catanzaro A, Monaco J, Ruiz M, Justement J, Moir S, Arthos J, Oliva A, Ehler L, Mizell S, Jackson R, Ostrowski M, Hoxie J, Offord R, Fauci A S. CC-chemokines enhance the replication of T-tropic strains of HIV-1 in CD4(+) T cells: role of signal transduction. Proc Natl Acad Sci USA. 1998;95:11880–11885. doi: 10.1073/pnas.95.20.11880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kolchinsky P, Mirzabekov T, Farzan M, Kiprilov E, Cayabyab M, Mooney L J, Choe H, Sodroski J. Adaptation of a CCR5-using, primary human immunodeficiency virus type 1 isolate for CD4-independent replication. J Virol. 1999;73:8120–8126. doi: 10.1128/jvi.73.10.8120-8126.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kwong P D, Wyatt R, Sattentau Q J, Sodroski J, Hendrickson W A. Oligomeric modeling and electrostatic analysis of the gp120 envelope glycoprotein of human immunodeficiency virus. J Virol. 2000;74:1961–1972. doi: 10.1128/jvi.74.4.1961-1972.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.LaBranche C C, Hoffman T L, Romano J, Haggarty B S, Edwards T G, Matthews T J, Doms R W, Hoxie J A. Determinants of CD4 independence for a human immunodeficiency virus type 1 variant map outside regions required for coreceptor specificity. J Virol. 1999;73:10310–10319. doi: 10.1128/jvi.73.12.10310-10319.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lerner D L, Elder J H. Expanded host cell tropism and cytopathic properties of feline immunodeficiency virus strain PPR subsequent to passage through interleukin-2-independent T cells. J Virol. 2000;74:1854–1863. doi: 10.1128/jvi.74.4.1854-1863.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lerner D L, Grant C K, de Parseval A, Elder J H. FIV infection of IL-2-dependent and -independent feline lymphocyte lines: host cells range distinctions and specific cytokine upregulation. Vet Immunol Immunopathol. 1998;65:277–297. doi: 10.1016/S0165-2427(98)00162-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liao F, Alkhatib G, Peden K W, Sharma G, Berger E A, Farber J M. STRL33, a novel chemokine receptor-like protein, functions as a fusion cofactor for both macrophage-tropic and T cell line-tropic HIV-1. J Exp Med. 1997;185:2015–2023. doi: 10.1084/jem.185.11.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Martin K A, Wyatt R, Farzan M, Choe H, Marcon L, Desjardins E, Robinson J, Sodroski J, Gerard C, Gerard N P. CD4-independent binding of SIV gp120 to rhesus CCR5. Science. 1997;278:1470–1473. doi: 10.1126/science.278.5342.1470. [DOI] [PubMed] [Google Scholar]
- 57.Mondor I, Ugolini S, Sattentau Q J. Human immunodeficiency virus type 1 attachment to HeLa CD4 cells is CD4 independent and gp120 dependent and requires cell surface heparans. J Virol. 1998;72:3623–3634. doi: 10.1128/jvi.72.5.3623-3634.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moulard M, Lortat-Jacob H, Mondor I, Roca G, Wyatt R, Sodroski J, Zhao L, Olson W, Kwong P D, Sattentau Q J. Selective interactions of polyanions with basic surfaces on human immunodeficiency virus type 1 gp120. J Virol. 2000;74:1948–1960. doi: 10.1128/jvi.74.4.1948-1960.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ohshiro Y, Murakami T, Matsuda K, Nishioka K, Yoshida K, Yamamoto N. Role of cell surface glycosaminoglycans of human T cells in human immunodeficiency virus type-1 (HIV-1) infection. Microbiol Immunol. 1996;40:827–835. doi: 10.1111/j.1348-0421.1996.tb01148.x. [DOI] [PubMed] [Google Scholar]
- 60.Olmsted R A, Hirsch V M, Purcell R H, Johnson P R. Nucleotide sequence analysis of feline immunodeficiency virus: genome organization and relationship to other lentiviruses. Proc Natl Acad Sci USA. 1989;86:8088–8092. doi: 10.1073/pnas.86.20.8088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Patel M, Yanagishita M, Roderiquez G, Bou-Habib D C, Oravecz T, Hascall V C, Norcross M A. Cell-surface heparan sulfate proteoglycan mediates HIV-1 infection of T-cell lines. AIDS Res Hum Retroviruses. 1993;9:167–174. doi: 10.1089/aid.1993.9.167. [DOI] [PubMed] [Google Scholar]
- 62.Pedersen N C, Ho E W, Brown M L, Yamamoto J K. Isolation of a T-lymphotropic virus from domestic cats with an immunodeficiency-like syndrome. Science. 1987;235:790–793. doi: 10.1126/science.3643650. [DOI] [PubMed] [Google Scholar]
- 63.Phillips T R, Talbott R, Lamont C, Muir S, Lovelace K, Elder J H. Comparison of two host cell range variants of feline immunodeficiency virus. J Virol. 1990;64:4605–4613. doi: 10.1128/jvi.64.10.4605-4613.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pleskoff O, Treboute C, Alizon M. The cytomegalovirus-encoded chemokine receptor US28 can enhance cell-cell fusion mediated by different viral proteins. J Virol. 1998;72:6389–6397. doi: 10.1128/jvi.72.8.6389-6397.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Poeschla E M, Looney D J. CXCR4 is required by a nonprimate lentivirus: heterologous expression of feline immunodeficiency virus in human, rodent, and feline cells. J Virol. 1998;72:6858–6866. doi: 10.1128/jvi.72.8.6858-6866.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Potempa S, Picard L, Reeves J D, Wilkinson D, Weiss R A, Talbot S J. CD4-independent infection by human immunodeficiency virus type 2 strain ROD/B: the role of the N-terminal domain of CXCR-4 in fusion and entry. J Virol. 1997;71:4419–4424. doi: 10.1128/jvi.71.6.4419-4424.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Reeves J D, McKnight A, Potempa S, Simmons G, Gray P W, Power C A, Wells T, Weiss R A, Talbot S J. CD4-independent infection by HIV-2 (ROD/B): use of the 7-transmembrane receptors CXCR-4, CCR-3, and V28 for entry. Virology. 1997;231:130–134. doi: 10.1006/viro.1997.8508. [DOI] [PubMed] [Google Scholar]
- 68.Richardson J, Pancino G, Merat R, Leste-Lasserre T, Moraillon A, Schneider-Mergener J, Alizon M, Sonigo P, Heveker N. Shared usage of the chemokine receptor CXCR4 by primary and laboratory-adapted strains of feline immunodeficiency virus. J Virol. 1999;73:3661–3671. doi: 10.1128/jvi.73.5.3661-3671.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rider C C, Coombe D R, Harrop H A, Hounsell E F, Bauer C, Feeney J, Mulloy B, Mahmood N, Hay A, Parish C R. Anti-HIV-1 activity of chemically modified heparins: correlation between binding to the V3 loop of gp120 and inhibition of cellular HIV-1 infection in vitro. Biochemistry. 1994;33:6974–6980. doi: 10.1021/bi00188a029. [DOI] [PubMed] [Google Scholar]
- 70.Roderiquez G, Oravecz T, Yanagishita M, Bou-Habib D C, Mostowski H, Norcross M A. Mediation of human immunodeficiency virus type 1 binding by interaction of cell surface heparan sulfate proteoglycans with the V3 region of envelope gp120-gp41. J Virol. 1995;69:2233–2239. doi: 10.1128/jvi.69.4.2233-2239.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rucker J, Edinger A L, Sharron M, Samson M, Lee B, Berson J F, Yi Y, Margulies B, Collman R G, Doranz B J, Parmentier M, Doms R W. Utilization of chemokine receptors, orphan receptors, and herpesvirus-encoded receptors by diverse human and simian immunodeficiency viruses. J Virol. 1997;71:8999–9007. doi: 10.1128/jvi.71.12.8999-9007.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Saphire A C, Bobardt M D, Gallay P A. Host cyclophilin A mediates HIV-1 attachment to target cells via heparans. EMBO J. 1999;18:6771–6785. doi: 10.1093/emboj/18.23.6771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Secchiero P, Sun D, De Vico A L, Crowley R W, Reitz M S, Zauli G, Lusso P, Gallo R C. Role of the extracellular domain of human herpes virus 7 glycoprotein B in virus binding to cell surface heparan sulfate proteoglycans. J Virol. 1997;71:4571–4580. doi: 10.1128/jvi.71.6.4571-4580.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shao H, Lou L, Pandey A, Pasquale E B, Dixit V M. cDNA cloning and characterization of a ligand for the Cek5 receptor protein-tyrosine kinase. J Biol Chem. 1994;269:26606–26609. [PubMed] [Google Scholar]
- 75.Siebelink K H, Karlas J A, Rimmelzwaan G F, Osterhaus A D, Bosch M L. A determinant of feline immunodeficiency virus involved in Crandell feline kidney cell tropism. Vet Immunol Immunopathol. 1995;46:61–69. doi: 10.1016/0165-2427(94)07006-s. [DOI] [PubMed] [Google Scholar]
- 76.Talbott R L, Sparger E E, Lovelace K M, Fitch W M, Pedersen N C, Luciw P A, Elder J H. Nucleotide sequence and genomic organization of feline immunodeficiency virus. Proc Natl Acad Sci USA. 1989;86:5743–5747. doi: 10.1073/pnas.86.15.5743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tanabe-Tochikura A, Tochikura T S, Blakeslee J R, Jr, Olsen R G, Mathes L E. Anti-human immunodeficiency virus (HIV) agents are also potent and selective inhibitors of feline immunodeficiency virus (FIV)-induced cytopathic effect: development of a new method for screening of anti-FIV substances in vitro. Antiviral Res. 1992;19:161–172. doi: 10.1016/0166-3542(92)90075-g. [DOI] [PubMed] [Google Scholar]
- 78.Tsang T C, Harris D T, Akporiaye E T, Chu R S, Brailey J, Liu F, Vasanwala F H, Schluter S F, Hersh E M. Mammalian expression vector with two multiple cloning sites for expression of two foreign genes. BioTechniques. 1997;22:68. doi: 10.2144/97221bm13. [DOI] [PubMed] [Google Scholar]
- 79.Verschoor E J, Boven L A, Blaak H, van Vliet A L, Horzinek M C, de Ronde A. A single mutation within the V3 envelope neutralization domain of feline immunodeficiency virus determines its tropism for CRFK cells. J Virol. 1995;69:4752–4757. doi: 10.1128/jvi.69.8.4752-4757.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Willett B J, Picard L, Hosie M J, Turner J D, Adema K, Clapham P R. Shared usage of the chemokine receptor CXCR4 by the feline and human immunodeficiency viruses. J Virol. 1997;71:6407–6415. doi: 10.1128/jvi.71.9.6407-6415.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wyatt R, Sodroski J. The HIV-1 envelope glycoproteins: fusogens, antigens, and immunogens. Science. 1998;280:1884–1888. doi: 10.1126/science.280.5371.1884. [DOI] [PubMed] [Google Scholar]
- 82.Yao Z, Fanslow W C, Seldin M F, Rousseau A M, Painter S L, Comeau M R, Cohen J I, Spriggs M K. Herpesvirus Saimiri encodes a new cytokine, IL-17, which binds to a novel cytokine receptor. Immunity. 1995;3:811–821. doi: 10.1016/1074-7613(95)90070-5. [DOI] [PubMed] [Google Scholar]