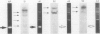Abstract
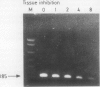
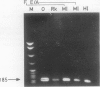
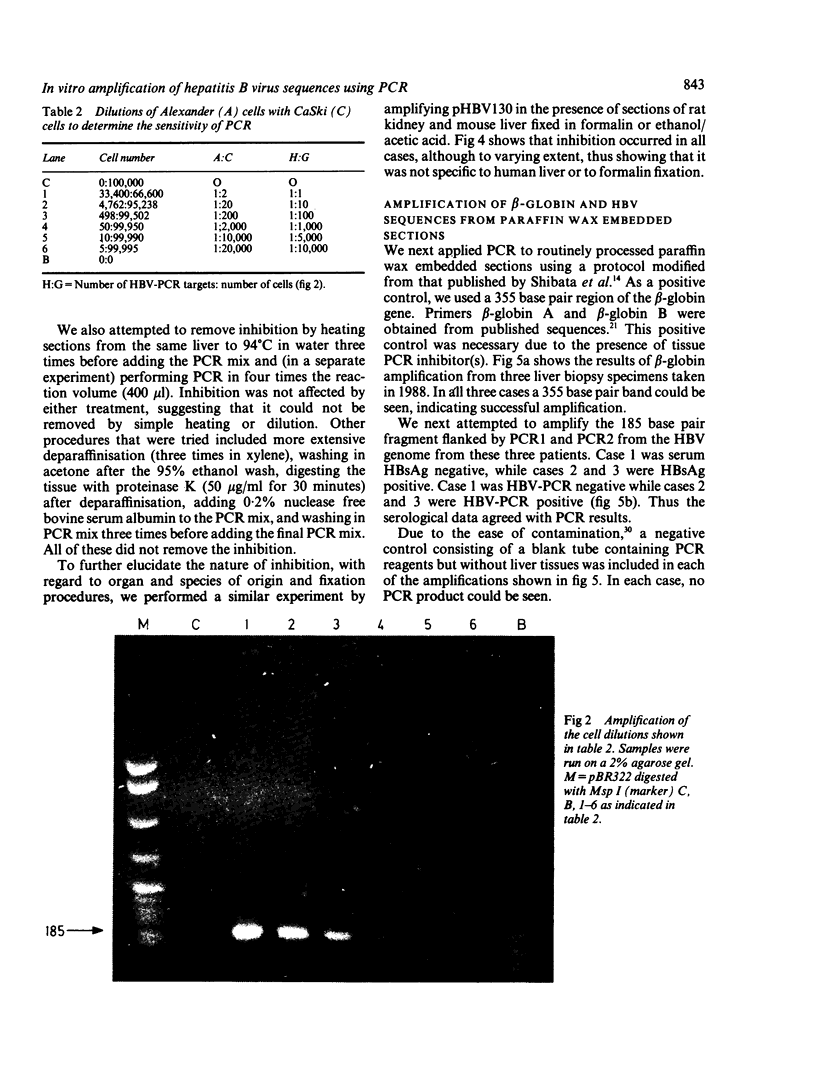
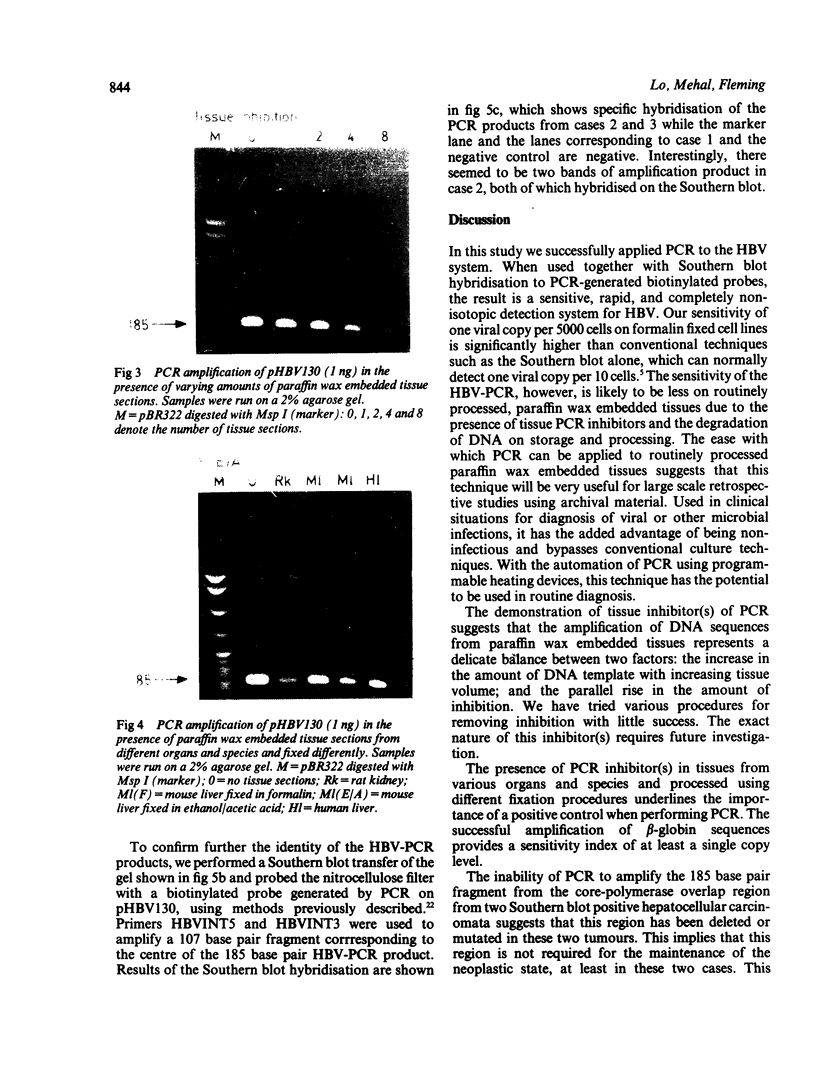
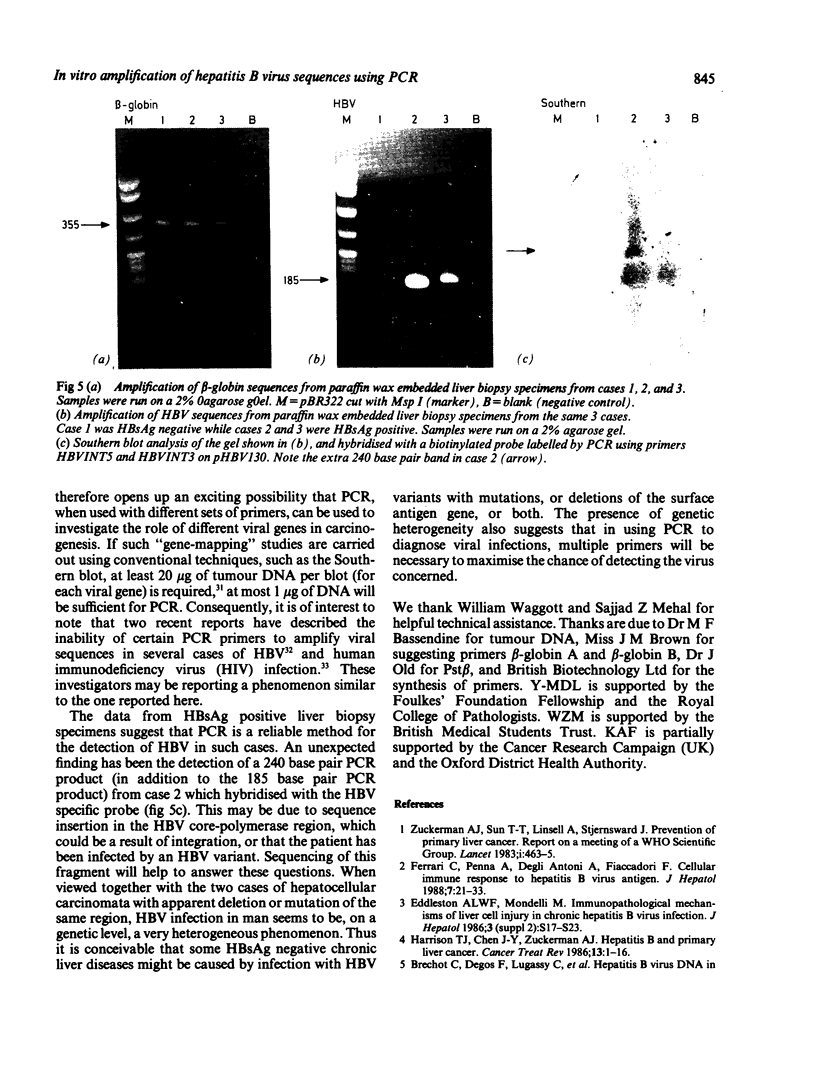
A 185 base pair fragment from the core-polymerase overlap region of the hepatitis B virus (HBV) genome was amplified using the polymerase chain reaction (PCR). The results were compared with those of Southern blotting on extracted DNA from eight hepatocellular carcinomata. The data agreed with those of Southern blotting in six cases (two positive, four negative) but in two other positive cases PCR failed to amplify HBV sequences. This suggests deletion or mutation, or both, of this viral region in these cases. PCR was also used to amplify HBV sequences from formalin fixed, paraffin wax embedded tissue. Tissue inhibition of PCR occurred which increased with the number of tissue sections. It was present in tissues from different organs and species and fixed by different procedures, thus highlighting the need for a positive control during amplification. Use of formalin fixed Alexander cells, however, showed a sensitivity of one viral copy per 5000 cells. Confirmation of the identity of the PCR products was carried out using PCR-generated biotinylated probes, and suggested the insertion of extra nucleotide sequences or infection with an HBV variant in one case.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander J. J., Bey E. M., Geddes E. W., Lecatsas G. Establishment of a continuously growing cell line from primary carcinoma of the liver. S Afr Med J. 1976 Dec 18;50(54):2124–2128. [PubMed] [Google Scholar]
- Brechot C., Pourcel C., Louise A., Rain B., Tiollais P. Presence of integrated hepatitis B virus DNA sequences in cellular DNA of human hepatocellular carcinoma. Nature. 1980 Jul 31;286(5772):533–535. doi: 10.1038/286533a0. [DOI] [PubMed] [Google Scholar]
- Bréchot C., Degos F., Lugassy C., Thiers V., Zafrani S., Franco D., Bismuth H., Trépo C., Benhamou J. P., Wands J. Hepatitis B virus DNA in patients with chronic liver disease and negative tests for hepatitis B surface antigen. N Engl J Med. 1985 Jan 31;312(5):270–276. doi: 10.1056/NEJM198501313120503. [DOI] [PubMed] [Google Scholar]
- Chan V. T., Fleming K. A., McGee J. O. Detection of sub-picogram quantities of specific DNA sequences on blot hybridization with biotinylated probes. Nucleic Acids Res. 1985 Nov 25;13(22):8083–8091. doi: 10.1093/nar/13.22.8083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. Y., Harrison T. J., Lee C. S., Chen D. S., Zuckerman A. J. Detection of hepatitis B virus DNA in hepatocellular carcinoma: analysis by hybridization with subgenomic DNA fragments. Hepatology. 1988 May-Jun;8(3):518–523. doi: 10.1002/hep.1840080315. [DOI] [PubMed] [Google Scholar]
- Eddleston A. L., Mondelli M. Immunopathological mechanisms of liver cell injury in chronic hepatitis B virus infection. J Hepatol. 1986;3 (Suppl 2):S17–S23. doi: 10.1016/s0168-8278(86)80096-4. [DOI] [PubMed] [Google Scholar]
- Edman J. C., Gray P., Valenzuela P., Rall L. B., Rutter W. J. Integration of hepatitis B virus sequences and their expression in a human hepatoma cell. Nature. 1980 Jul 31;286(5772):535–538. doi: 10.1038/286535a0. [DOI] [PubMed] [Google Scholar]
- Ferrari C., Penna A., DegliAntoni A., Fiaccadori F. Cellular immune response to hepatitis B virus antigens. An overview. J Hepatol. 1988 Aug;7(1):21–33. doi: 10.1016/s0168-8278(88)80503-8. [DOI] [PubMed] [Google Scholar]
- Figus A., Blum H. E., Vyas G. N., De Virgilis S., Cao A., Lippi M., Lai E., Balestrieri A. Hepatitis B viral nucleotide sequences in non-A, non-B or hepatitis B virus-related chronic liver disease. Hepatology. 1984 May-Jun;4(3):364–368. doi: 10.1002/hep.1840040303. [DOI] [PubMed] [Google Scholar]
- Fowler M. J., Monjardino J., Weller I. V., Bamber M., Karayiannis P., Zuckerman A. J., Thomas H. C. Failure to detect nucleic acid homology between some non-A, non-B viruses and hepatitis B virus DNA. J Med Virol. 1983;12(3):205–213. doi: 10.1002/jmv.1890120306. [DOI] [PubMed] [Google Scholar]
- Gough N. M., Murray K. Expression of the hepatitis B virus surface, core and E antigen genes by stable rat and mouse cell lines. J Mol Biol. 1982 Nov 25;162(1):43–67. doi: 10.1016/0022-2836(82)90161-9. [DOI] [PubMed] [Google Scholar]
- Harrison T. J., Chen J. Y., Zuckerman A. J. Hepatitis B and primary liver cancer. Cancer Treat Rev. 1986 Mar;13(1):1–16. doi: 10.1016/0305-7372(86)90011-3. [DOI] [PubMed] [Google Scholar]
- Impraim C. C., Saiki R. K., Erlich H. A., Teplitz R. L. Analysis of DNA extracted from formalin-fixed, paraffin-embedded tissues by enzymatic amplification and hybridization with sequence-specific oligonucleotides. Biochem Biophys Res Commun. 1987 Feb 13;142(3):710–716. doi: 10.1016/0006-291x(87)91472-0. [DOI] [PubMed] [Google Scholar]
- Lai-Goldman M., Lai E., Grody W. W. Detection of human immunodeficiency virus (HIV) infection in formalin-fixed, paraffin-embedded tissues by DNA amplification. Nucleic Acids Res. 1988 Aug 25;16(16):8191–8191. doi: 10.1093/nar/16.16.8191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laure F., Courgnaud V., Rouzioux C., Blanche S., Veber F., Burgard M., Jacomet C., Griscelli C., Brechot C. Detection of HIV1 DNA in infants and children by means of the polymerase chain reaction. Lancet. 1988 Sep 3;2(8610):538–541. doi: 10.1016/s0140-6736(88)92659-1. [DOI] [PubMed] [Google Scholar]
- Lawn R. M., Efstratiadis A., O'Connell C., Maniatis T. The nucleotide sequence of the human beta-globin gene. Cell. 1980 Oct;21(3):647–651. doi: 10.1016/0092-8674(80)90428-6. [DOI] [PubMed] [Google Scholar]
- Lo Y. M., Mehal W. Z., Fleming K. A. False-positive results and the polymerase chain reaction. Lancet. 1988 Sep 17;2(8612):679–679. doi: 10.1016/s0140-6736(88)90487-4. [DOI] [PubMed] [Google Scholar]
- Lo Y. M., Mehal W. Z., Fleming K. A. Rapid production of vector-free biotinylated probes using the polymerase chain reaction. Nucleic Acids Res. 1988 Sep 12;16(17):8719–8719. doi: 10.1093/nar/16.17.8719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono Y., Onda H., Sasada R., Igarashi K., Sugino Y., Nishioka K. The complete nucleotide sequences of the cloned hepatitis B virus DNA; subtype adr and adw. Nucleic Acids Res. 1983 Mar 25;11(6):1747–1757. doi: 10.1093/nar/11.6.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prevention of primary liver cancer. Report on a meeting of a W.H.O. Scientific Group. Lancet. 1983 Feb 26;1(8322):463–465. [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Shafritz D. A., Kew M. C. Identification of integrated hepatitis B virus DNA sequences in human hepatocellular carcinomas. Hepatology. 1981 Jan-Feb;1(1):1–8. doi: 10.1002/hep.1840010102. [DOI] [PubMed] [Google Scholar]
- Shibata D. K., Arnheim N., Martin W. J. Detection of human papilloma virus in paraffin-embedded tissue using the polymerase chain reaction. J Exp Med. 1988 Jan 1;167(1):225–230. doi: 10.1084/jem.167.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata D., Fu Y. S., Gupta J. W., Shah K. V., Arnheim N., Martin W. J. Detection of human papillomavirus in normal and dysplastic tissue by the polymerase chain reaction. Lab Invest. 1988 Oct;59(4):555–559. [PubMed] [Google Scholar]
- Slusarczyk J., Hess G., Hansson B. G., Meyer Zum Büschenfelde K. H. Lack of hepatitis B virus DNA sequences in sera from patients with acute and chronic liver diseases diagnosed as non-A, non-B-hepatitis. Liver. 1986 Dec;6(6):337–340. doi: 10.1111/j.1600-0676.1986.tb00301.x. [DOI] [PubMed] [Google Scholar]
- Thiers V., Nakajima E., Kremsdorf D., Mack D., Schellekens H., Driss F., Goudeau A., Wands J., Sninsky J., Tiollais P. Transmission of hepatitis B from hepatitis-B-seronegative subjects. Lancet. 1988 Dec 3;2(8623):1273–1276. doi: 10.1016/s0140-6736(88)92891-7. [DOI] [PubMed] [Google Scholar]
- Tiollais P., Pourcel C., Dejean A. The hepatitis B virus. Nature. 1985 Oct 10;317(6037):489–495. doi: 10.1038/317489a0. [DOI] [PubMed] [Google Scholar]
- Yokosuka O., Omata M., Imazeki F., Ito Y., Okuda K. Hepatitis B virus RNA transcripts and DNA in chronic liver disease. N Engl J Med. 1986 Nov 6;315(19):1187–1192. doi: 10.1056/NEJM198611063151903. [DOI] [PubMed] [Google Scholar]
- Ziemer M., Garcia P., Shaul Y., Rutter W. J. Sequence of hepatitis B virus DNA incorporated into the genome of a human hepatoma cell line. J Virol. 1985 Mar;53(3):885–892. doi: 10.1128/jvi.53.3.885-892.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Franchis R., Cross N. C., Foulkes N. S., Cox T. M. A potent inhibitor of Taq polymerase copurifies with human genomic DNA. Nucleic Acids Res. 1988 Nov 11;16(21):10355–10355. doi: 10.1093/nar/16.21.10355. [DOI] [PMC free article] [PubMed] [Google Scholar]