Abstract
Background
Cerebrospinal fluid (CSF)-venous fistulas (CVFs) are increasingly identified as a cause of spontaneous intracranial hypotension (SIH). Lateral decubitus digital subtraction myelography (LD-DSM) and CT myelography (LD-CTM) are mainly used for detection, but the most sensitive method is yet unknown.
Objective
To compare LD-DSM with LD-CTM for diagnostic yield of CVFs.
Methods
Patients with SIH diagnosed with a CVF between January 2021 and December 2022 in which the area of CVF(s) was covered by both diagnostic modalities were included. LD-CTM immediately followed LD-DSM without repositioning the spinal needle, and the second half of the contrast agent was injected at the CT scanner. Patients were awake or mildly sedated. Retrospectively, two neuroradiologists evaluated data independently and blinded for the presence of CVF.
Results
Twenty patients underwent a total of 27 combined LD-DSM/LD-CTM examinations (4/20 with follow-up and 3/20 with bilateral examinations). Both raters identified significantly more CVFs with LD-CTM than with LD-DSM (rater 1: 39 vs 9, P<0.001; rater 2: 42 vs 12, P<0.001). Inter-rater agreement was substantial for LD-DSM (κ=0.732) and LD-CTM (κ=0.655). The results remained significant after considering the senior rating for cases of disagreement (39 vs 10; P<0.001), and no CVF detected on LD-DSM was missed on LD-CTM.
Conclusion
In this study, LD-CTM has a higher diagnostic yield for the detection of CVFs than LD-DSM and should supplement LD-DSM, but further studies are needed. LD-CTM can be easily acquired in awake or mildly sedated patients with the second half of contrast injected just before CT scanning, or it may be considered as a stand-alone investigation.
Keywords: CT, Intervention, Spine
WHAT IS ALREADY KNOWN ON THIS TOPIC
Imaging of cerebrospinal fluid (CSF)-venous fistulas is challenging—one reason why this entity was not even known as a cause of spontaneous intracranial hypotension before its initial description in 2014. As the most sensitive diagnostic modality for their detection is not yet known, a direct comparison of the two main diagnostic methods is warranted.
WHAT THIS STUDY ADDS
This study is the first to compare digital subtraction myelography and CT myelography in the lateral decubitus position in awake patients and demonstrates the superiority of CT myelography in detecting CSF-venous fistulas.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
As CT is also widely available and relatively easy to use, the detection rate of CSF-venous fistulas may further increase.
Introduction
A cerebrospinal fluid (CSF)-venous fistula (CVF) is an abnormal connection between the CSF and a paraspinal vein (internal or external vertebral venous plexus), usually at the level of a nerve root sleeve. Next to ventral and lateral dural tears, it is increasingly identified as a cause of spontaneous intracranial hypotension (SIH) in patients typically presenting with orthostatic headaches but also with a variety of other symptoms, including coma, bibrachial amyotrophy, or frontotemporal brain sagging syndrome, respectively.1 Technical improvements, such as lateral decubitus myelography, allowed for increased detection of CVFs, and only a few years after its initial description by Schievink et al 2 in 2014, CVF is considered to be the cause of SIH in every fourth patient.3
Lateral decubitus digital subtraction myelography (LD-DSM) and lateral decubitus CT myelography (LD-CTM) are the methods most commonly used in the search for CVF. Sensitivity for CVF detection has been little studied for DSM and CTM,4 5 but comparative studies of both methods are lacking. Other techniques such as fluoroscopic dynamic myelography6 7 and MR myelography8 with the application of intrathecal gadolinium have been reported, but with low significance for diagnostic workup.
DSM for CVF detection was initially performed under general anesthesia to avoid motion artifacts.2 Several modifications in awake or sedated patients have been added.9 10 Since it is not yet clear which modality is the most sensitive, we routinely perform LD-DSM immediately followed by LD-CTM in awake patients. In this procedure, the spinal needle is kept in place during transport in the lateral decubitus position, and the second half of the contrast agent can be applied at the CT scanner. This new approach has been shown to be safe and allows for a proper comparison of two fully fledged modalities in the search for CVF, which, to the best of our knowledge, has not been carried out before. Identifying the most sensitive modality could further increase the detection rate of CVFs.
This retrospective study is the first to evaluate the diagnostic yield of CVF in awake patients with SIH, directly comparing LD-DSM and LD-CTM. We discuss advantages and disadvantages of both techniques in terms of routine daily use and feasibility.
Methods and materials
Data selection
The local ethics committee approved the trial. Written informed consent was obtained from each patient.
We retrospectively identified a consecutive cohort of patients with SIH according to the International Classification of Headache Disorders, third edition,11 between January 2021 and December 2022. Patients diagnosed with a CVF by LD-DSM and/or LD-CTM were included if the region of interest (one or more CVFs) was covered by both examinations (LD-CTM and LD-CTM).
Imaging
We routinely administered 1 mg lorazepam orally for mild sedation and to protect against seizures. A board-certified neuroradiologist with 9 years of experience either performed the examinations or supervised two neuroradiology fellows with 3 and 5 years of experience, each of whom was familiar with myelography in patients with SIH.
Technique/procedure of LD-DSM
All DSM were done in an angiography unit (Philips Allura Clarity, Philips Medical Systems, Nederland B.V.; Siemens Artis Icono biplane, Siemens Erlangen, Germany). Lumbar puncture was performed under fluoroscopic guidance in the lateral decubitus position with a 20 gauge spinal needle. The table was tilted head down by 6–8° to obtain a homogeneous layer of contrast along the thecal sack. The flat detector was positioned anterior-posteriorly to cover, if possible, an area from L1/2 to the upper thoracic spine (usually Th 2/3), where most fistulas are known to occur.9 Patients were instructed to continue shallow breathing and not to move during the procedure. After confirmation of intrathecal needle position, 7–8 mL of an iodine-containing contrast agent (300 mg iodine/ml; iomeprol 300 M, Bracco, Germany) was injected, and digital subtraction images were obtained. Angiography suite settings were: 1 frame per second for monoplanar acquisition (average run time of DSM was 49 s; range 25 to 60 s; median 60 s), detector coverage: 49 cm. Additional dynamic fluoroscopy and single radiographs were usually added. Postprocessing was mainly performed to reduce artifacts due to respiratory motion, using manual or automatic pixel shift.
Patient transport to the CT scanner
The lumbar needle was kept in place after DSM, while the syringe with the infusion extension line was wrapped into a sterile surgical drape that was attached to the patient’s back. From the lateral decubitus position in which the patient was examined, he was transferred to the bed with the assistance of 2–3 people using a rollboard (Transaroll Silverboard-Standard, Transatlantic, Neu-Anspach, Germany) and then transferred to the nearby CT scanner. The patient then returned to the CT table in the same manner. Special care was taken to ensure that the patient consequently remained in a lateral decubitus position and did not return to the supine position.
This procedure has been shown to be safe (applied to >100 examinations at our institution without complications).
Technique/procedure of LD-CTM
For the LD-CTM, we used a custom-made wooden table with a gradual tilt function. The table was tilted 7° head down with the patient still in the lateral decubitus position and the arms elevated above the head. The investigator then instructed the patient to stop moving and inhale slowly through a straw (3 mm diameter) to ensure continuous inspiration during LD-CTM, which has previously been reported to promote CVF outflow.12–14 Immediately after injection of the second half of the contrast agent of 7–8 mL (the maximum dose of 15 mL per examination was not exceeded), a single CT scan was performed in the caudocranial direction covering an area from the spinal needle to the upper cervical spine.
LD-CTM was performed using a Somatom Definition AS 64 scanner (Siemens, Erlangen, Germany) in a single energy mode applying the following settings: helical scan mode, rotation time 1.0 s, pitch 0.8, tube voltage 80–140 kV depending on the patient’s constitution, tube current 320 mA, automated care control. Axial reconstructions were made at 0.75 mm, bone kernel, and 3 mm sagittal, bone kernel.
This procedure was usually repeated the next day on the other side if no CVF was found or if there were suspicious findings on the other side.
Imaging analysis
Data were extracted from a local picture archiving and communication system.
Inclusion criteria were: (a) patients with SIH previously diagnosed with at least one CVF by LD-DSM and/or LD-CTM, and (b) CVF location being covered by both modalities, LD-DSM and LD-CTM.
Demographic (sex and age) and radiological data (initial ‘Bern SIH score’ and presence of ‘spinal longitudinal extradural CSF collection’) were collected and analyzed.
Both datasets, for LD-DSM (including fluoroscopy and radiographs, if available) and LD-CTM, were pseudonymized. Two board-certified neuroradiologists with 5 and 8 years of experience in neuroradiology (both familiar with the imaging features of SIH and CVF but not involved in the diagnostic work-up), blinded to patient data and clinical history, independently reviewed the examinations and assessed the following criteria: evidence, number, and exact spinal level(s) of CVF(s) according to LD-DSM and/or LD-CTM. As the DSM covers a shorter area of the spinal axis (usually 49 cm), evaluation of the CTM was limited to the range of the DSM in each case. In addition to the DSM dataset, the raters were allowed to use additional material: unsubtracted myelographic series (same data as DSM but without subtraction) and dynamic fluoroscopic images and single radiographs, if available. For the LD-CTM dataset after transvenous embolization (n=3), an additional unenhanced CT scan was submitted to ensure that hyperdensities of onyx glue and contrast agent could be distinguished.
In cases of disagreement between raters 1 and 2, the senior rater (5 years of experience in neuroradiology and specialized in diagnostics and treatment of SIH) adjudicated. The senior rater was blinded to the patients' data and the raters' results, but allowed to use all information from a combined examination in one patient (LD-DSM and LD-CTM), as was the case in our clinical practice.
Statistical analysis
All statistical analyses were performed using SPSS 29 (IBM, Armonk, New York, USA). Descriptive statistics included calculation of the mean, SD, and range for normally distributed data. Inter-rater agreement between rater 1 and rater 2 was assessed by calculating Cohen’s κ. Differences between CVFs found in DSM and CTM were compared using McNemar’s test for dependent dichotomous variables. An α level of 0.05 was considered statistically significant.
Results
Patient population
Of 154 patients with SIH identified between January 2021 and December 2022, 26 patients were diagnosed with one or multiple CVFs. Six patients were excluded owing to the lack of combined LD-DSM/LD-CTM examinations. Of the 20 patients, four had combined follow-up after therapy (three had transvenous onyx embolization, one had surgery), and bilateral combined examinations (one or more CSF fistulas on both sides) from three patients were included in the analysis. Thus, a total of 27 combined datasets were analyzed in the study (online supplemental figure S1).
jnis-2023-020789supp001.pdf (141.2KB, pdf)
Twelve of the 20 patients included in this study were women. The mean age was 53±15.4 years (range 28–88 years). The mean Bern SIH score on initial MRI of the head was 6.4±1.9 (range 1–8), based on a 9-point Likert Scale indicating the probability of SIH: ≥5 points=high probability, 3–4 points=intermediate probability, and 0–2 points=low probability.15 All patients were negative for spinal longitudinal extradural CSF collection on spinal MRI, with one exception (concurrent presentation of a lateral dural tear and CVF).
Diagnostic yield for CSF-venous fistulas (LD-DSM vs LD-CTM)
Both raters identified significantly more CVFs on LD-CTM than with LD-DSM: Rater 1 found 9 CVFs on LD-DSM and 39 CVFs on LD-CTM (P<0.001). Rater 2 identified 12 CVFs on LD-DSM and 42 CVFs on LD-CTM (P<0.001). Inter-rater agreement regarding the exact spinal level of each detected CVF was substantial for LD-DSM (κ=0.732) and LD-CTM (κ=0.655). After accounting for senior rating for cases of disagreement between rater 1 and rater 2, the results remained highly significant (10 vs 39 ; P<0.001).
Imaging examples of the raters' decisions for both modalities are shown in figure 1.
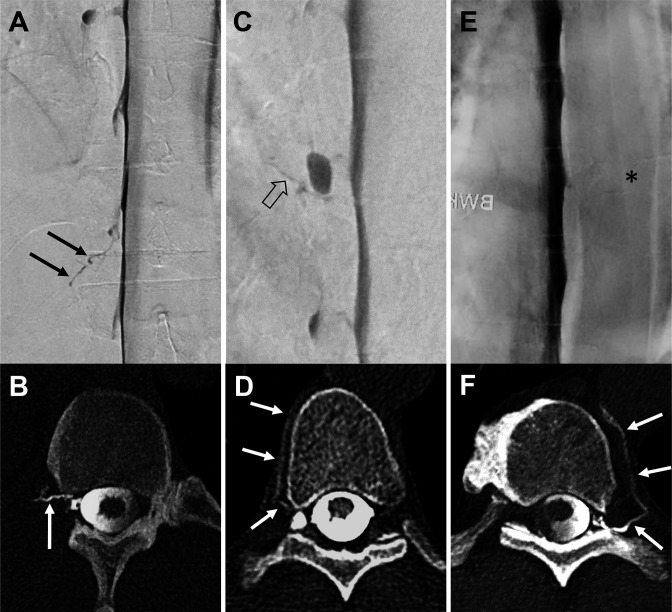
Figure 1.
Three exemplary patients with a combined examination (A+B, C+D, and E+F): lateral decubitus digital subtraction myelography (LD-DSM; A, C, E) in anterior-posterior view followed in each case by lateral decubitus CT myelography (LD-CTM; B, D, F) with a second half of contrast application in axial view. In the first example, a contrasted paraspinal vein at the Th 12/L1 level on the right side is visible in both examinations, LD-DSM (black arrows in A) and LD-CTM (white arrow in B): rated as positive for a CSF-venous fistula (CVF) by raters 1 and 2 in each modality. The second example shows a tiny hyperdense line on LD-DSM (open black arrow in C) adjacent to a nerve root diverticulum at the level of Th 8/9 on the right side (rater 1: CVF-negative, rater 2: CVF-positive, senior rater adjudicated: CVF-positive). On LD-CTM of the same patient, a contrasted paraspinal vein (white arrows in D) is visible at the same level (raters 1 and 2: CVF-positive). The third example does not show a contrasted vein on LD-DSM (asterisk in E) at the level Th 9/10 left (raters 1 and 2: CVF-negative). The LD-CTM of the same patient clearly demonstrates a contrasted paravertebral vein (white arrows in F) at the same level (raters 1 and 2: CVF-positive).
Rater 1 identified a CVF in two patients each on LD-DSM at a spinal level that he had not described on LD-CTM. Rater 2 did not find any CVF on LD-DSM that he had not detected on the corresponding LD-CTM. According to the senior rater, none of the CVFs detected on the LD-DSM were missed on the LD-CTM. The first rater identified 32, the second rater 30, and the senior rater 28 CVFs only on LD-CTM that were missed on LD-DSM (examples shown in figure 2).
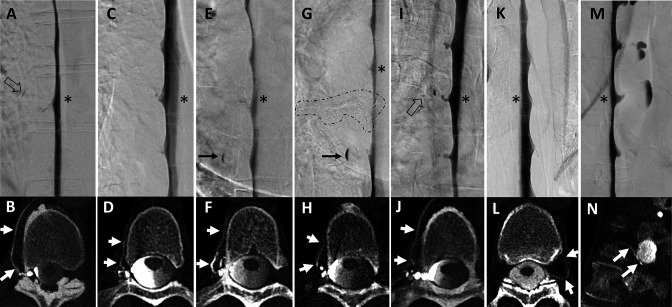
Figure 2.
Seven examples of combined lateral decubitus digital subtraction myelography (LD-DSM) and lateral decubitus CT myelography (LD-CTM) studies (A+B, C+D, E+F, G+H, I+J, K+L, M+N), in which DSM was rated negative but CTM was rated positive for CSF-venous fistulas (CVFs) after senior rating in cases of disagreement between raters 1 and 2. The asterisk in each LD-DSM (A, C, E, G, I, K, M) indicates the spinal level at which a CVF was found at LD-CTM (white arrows in B, D, F, H, J, L, N). Retrospectively, the DSM shows a small lesion as hint of a potential CVF in two examples: a tiny tubular and a dot-like structure in (A) and (I; open black arrows). In example E+F, a patient detected with a CVF only at CTM (F) was treated by transvenous embolization (onyx cast circled by dashed line in G) and was evaluated at follow-up with a de novo CVF at the spinal level above (asterisk in G), also seen only at CTM (white arrows in H). The black arrow in (E) and (G) shows a spinal diverticulum filled with contrast agent only in the lower aspect.
For the 20 patients, raters found (after senior rating) at least one CVF in 7/20 patients with DSM and in 19/20 patients with CTM (online supplemental table S1). One patient was rated as CVF negative by raters 1 and 2.
The distribution of CVF after senior rating along the spine was as follows: On LD-DSM, six CVFs occurred on the left side between spinal levels Th 5/6 and Th 12/L1, and four CVFs occurred on the right side between levels Th 5/6 and Th 12/L1. On LD-CTM, 20 CVFs were identified on the left side between the Th 4/5 and Th 12/L1 levels and 18 CVFs were identified on the right side between the Th 2/3 and L1/2 levels.
On the LD-DSM, there was no clustering of CVF at specific spinal levels with the exception of Th 12/L1 on the left side (n=2). The most frequent location of CVF on the LD-CTM (n≥3) was as follows: Th 8/9, Th 10/11, Th 11/12, and Th 12/L1 on the left side and Th 10/11 on the right side.
Multiple synchronous CVFs occurred in six patients: four patients had two CVFs (at adjacent spinal levels), one patient had three CVFs (at adjacent spinal levels; see figure 3), and one patient had six CVFs (four at remote and two at adjacent spinal levels).
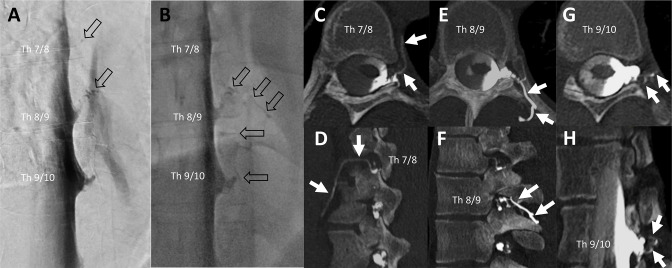
Figure 3.
Example of a patient evaluated as having multiple synchronous CSF-venous fistulas (CVFs; n=3) at the initial examination on the left side (Th 7/8, 8/9, and 9/10). Lateral decubitus digital subtraction myelography (LD-DSM) (A) and lateral decubitus fluoroscopy (B) indicated a CVF at level Th 8/9 by rater 1 and at Th 7/8, 8/9, and 9/10 by rater 2 and the senior rater (open black arrows in A and B). At the level Th 7/8 was a dot-like structure only visible at DSM (open black arrow in A at level Th 7/8). Raters 1 and 2, both identified three CVFs at lateral decubitus CT myelography (LD-CTM) at adjacent spinal levels, shown on axial (C, E, G) and sagittal (D, F) and oblique sagittal (H) CTM by white arrows. As the contrasted veins shown here each have contact with the nerve root sleeve, it is more likely that three CVFs are present than that they originate from one point of fistula only.
The results are presented in table 1 and in online supplemental table S1).
Table 1.
Results of CSF-venous fistula (CVF) detection after pseudonymization of 27 combined examinations and independent reviewing by raters 1 and 2. A senior rater adjudicated in cases of disagreement between raters 1 and 2.
| Number of detected CVFs on | Rater 1 | Rater 2 | Senior rater* |
| LD-DSM | 9 | 12 | 10 |
| LD-CTM | 39 | 42 | 39 |
| LD-DSM only (missed on LD-CTM) | 2 | 0 | 0 |
| LD-CTM only (missed on LD-DSM) | 32 | 30 | 28 |
* Results after adjudication of senior rater in cases of disagreement between raters 1 and 2.
CVF, CSF-venous fistula; LD-CTM, lateral decubitus CT-myelography; LD-DSM, lateral decubitus digital subtraction myelography.
Discussion
This study reveals the superiority of CT myelography (CTM) over digital subtraction myelography (DSM) for the detection of CVFs in lateral decubitus position in awake or mildly sedated patients. We report on a further refinement of the myelographic technique with a second half of contrast agent administration at LD-CTM (referred to as ‘dual contrast CT myelography’), directly following LD-DSM (given the first half of contrast). This procedure allows a direct comparison of both modalities in the detection of CVFs.
As CVFs have poor response to untargeted epidural blood patch,3 16 it is critical to visualize the exact location of the CVF to offer appropriate treatment, such as transvenous-catheter guided embolization, ligation by open surgery, or targeted CT-guided fibrin patch. According to recent literature, the detection rate of CVFs has dramatically increased in recent years due to advancements in imaging techniques—in particular, by examining patients in the lateral decubitus position: Schievink et al 4 reported a fivefold higher diagnostic rate in lateral decubitus compared with prone position for DSM, whereas Kranz et al 17 demonstrated better visibility for subtle CVFs in lateral versus prone position for CTM. The next step now seems to clarify which of the two methods is most sensitive for detecting CVFs in the lateral decubitus position.
Currently, the sensitivity for detecting CVF in patients with SIH negative for a spinal longitudinal extradural CSF collection is reported to be 74% (using general anesthesia and biplane acquisition)4 and 50% with LD-CTM in a procedural approach (performed in awake patients),5 but comparative studies are lacking. In our study, the detection rate with LD-CTM is approximately threefold higher than with LD-DSM, in awake patients and with monoplanar acquisition. A recent study showed that cross-sectional imaging (using cone-beam CT) may better detect CVF than DSM in lateral decubitus position in selected cases18: in 15 patients with indeterminate findings at LD-DSM, a CVF was detected in seven patients on immediately following with cone-beam CT.
DSM is a relatively new technique, which may accurately display a CVF under optimal conditions with high spatial and very high temporal resolution. However, there are some drawbacks to be mentioned: DSM requires an angiography or myelography suite and handling may be complex. The success of this examination substantially depends on the experience of the operator, usually reflected by a steep learning curve. Several factors may influence the image quality positively or negatively.
Potential reasons for missing a CVF on LD-DSM include the following: (a) patient movement and breathing artifacts may be a problem in awake patients. Some institutions use general anesthesia, others apply only mild to moderate sedation. In our experience, motion artifacts can be minimized when the patient is placed in a stable and relaxed position and continues to breath shallowly and steadily; however, motion artifacts cannot be completely avoided. (b) Superimpositions, such as of the contrast column, may obscure a CVF on DSM (especially for CVFs draining into the internal epidural venous plexus). (c) A CVF with a contrast drain in alignment to the anterior-posterior projection of the flat panel detector (along the paravertebral vein, for example) can be easily overlooked since it sometimes appears only as a flickering contrast spot (figure 2I).18 In case of (b) and (c), a biplane acquisition on DSM may be beneficial. (d) Insufficient duration of the DSM application, as recently reported by Mark et al.19 (e) Small and therefore low-contrast CVFs may have better visibility on cross-sectional imaging. In summary, LD-DSM is a challenging technique. However, the detection rate for CVF with DSM may be more sensitive under general anesthesia than in awake patients due to artifact reductions.
LD-CTM has been reported with a 50% diagnostic yield for CVF,5 but this has rarely been investigated so far. There are some approaches reporting on a consecutive use of LD-CTM following LD-DSM, demonstrating either an equal20 or incremental diagnostic yield at CTM.17 21 However, injection of a second dose of contrast at LD-CTM has not been described so far but it is likely to be beneficial because the time that elapses before the CT scan can dilute or drain the contrast agent. Our results show a significantly higher detection rate of CVF with LD-CTM and none of the CVF detected with LD-DSM were missed with LD-CTM (table 1).
CT scanners are widely used, thus, handling is well known and less complex. A simple wedge-shaped foam (20 cm high) is sufficient to lift the hips and make the contrast run upside down towards the head.5 22 In our experience, a 30 s injection of contrast agent (7–8 mL) immediately followed by the CT scan in a caudocranial direction is a good guide to ensure that the contrast layer is distributed evenly throughout all spinal segments. Unlike LD-DSM, the field of view is not restricted at LD-CTM and the entire spine can be covered. In addition, consecutive contralateral CTM scans can safely be performed in a single diagnostic session,22 facilitating diagnostic workup. Based on our experience, motion artifacts during LD-CTM are not an issue, even if CT is performed under continuous inspiration (using a straw at our institution), a positive effect previously described to promote fistula outflow12 and recently reinforced under the term ‘resisted inspiration’.13 14 Compared with DSM, CTM has a lower but still high spatial resolution of 0.4–0.6 mm but offers cross-sectional imaging that even may show CVFs that are small or drain into the internal epidural venous plexus.23 On the other hand, temporal resolution in dynamic CTM is low compared with DSM (and not present at our LD-CTM, since we only use a monophasic CT scan). Consequently, the exit point of a CVF could remain undetected or, on the other hand, a single CVF might be mistaken for multiple CVFs if the veins are contrasted across multiple spinal levels. Schievink et al reported on two cases where the hyperdense paraspinal vein at CTM did not correspond to the site of the leak.24
The most important downside of CTM is radiation exposure which, although few data are available so far, has been reported as being up to threefold higher than with DSM in a diagnostic workup for patients with CSF leaks.25 Radiation exposure can be reduced with monophasic CTM, as being performed at our institution. However, further reduction of radiation exposure during CTM remains an important task in the future. In this regard, photon counting CT seems promising: in addition to potentially reduced radiation exposure, it also offers advantages in detecting CVF providing higher spatial resolution and spectral analysis.26
This study has some limitations: (a) Data were retrospectively assessed in a monocentric setting. (b) This study is based on radiological assessment only, and to date no gold standard for CVF detection exists. Since further confirmation of CVFs, such as follow-up examinations and clinical outcome after treatment, is lacking in this study, CVFs could be considered as potential CVFs. (c) The rating includes a bias since at least one underlying CVF had to be expected per case and the total number of rated CVFs could thus be overstated. (d) In some of the cases rated as multiple CVFs at adjacent spinal levels, the CVF may arise only from a single fistula point. (e) The number of patients and CVF is relatively low in this study and further investigations should follow. (f) The consecutive DSM/CTM examinations partially differed in the way in which they were conducted: Patients continued to breathe during DSM while CTM was performed under continuous inspiration, and the latter may be beneficial for CVF detection. Intrathecal pressure was probably higher at CTM (after the second dose of contrast) than with DSM in this study; however, the value and benefit of pressurization of the thecal sac for CVF outflow has not been studied so far. Finally, it should be noted that the contrast agent had a larger volume at LD-CTM, on the one hand, and had more time to fill even large spinal diverticula between LD-DSM and LD-CTM, on the other hand, which might have affected fistula outflow and visibility.
Conclusion
In this study, LD-CTM is superior to LD-DSM regarding the CVF detection rate in awake or mildly sedated patients with SIH, and no CVF detected on LD-DSM was missed on LD-CTM, but further confirmation is needed.
If equipment for DSM is available, LD-CTM should supplement LD-DSM by injection of a second half of contrast just before the CT scan. Otherwise, LD-CTM may be considered as a stand-alone modality. The use of LD-CTM could further increase the detection rate of CVF.
Footnotes
@Niklas_Luetzen, @NFBelachew, @enriqueduardoMD, @KathaDCwolf, @JrgenBeck
Contributors: NL: conception and design of the study, analysis of data and manuscript writing, guarantor for the overall content of the study. TD, UW, EBA: data evaluation NFB: performed statistical analysis. All co-authors contributed to acquisition of data and critical reviewed the manuscript. HU: study conception and critical review of the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information. ‘Not applicable’.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was approved by the ethics committee of University Hospital Freiburg (vote number: 22-1249-S1-retro). Participants gave informed consent to participate in the study before taking part.
References
- 1. Urbach H. Intracranial hypotension: clinical presentation, imaging findings, and imaging-guided therapy. Curr Opin Neurol 2014;27:414–24. 10.1097/WCO.0000000000000105 [DOI] [PubMed] [Google Scholar]
- 2. Schievink WI, Moser FG, Maya MM. CSF-venous fistula in spontaneous intracranial hypotension. Neurology 2014;83:472–3. 10.1212/WNL.0000000000000639 [DOI] [PubMed] [Google Scholar]
- 3. Roytman M, Salama G, Robbins MS, et al. CSF-venous fistula. Curr Pain Headache Rep 2021;25:5. 10.1007/s11916-020-00921-4 [DOI] [PubMed] [Google Scholar]
- 4. Schievink WI, Maya MM, Moser FG, et al. Lateral decubitus digital subtraction myelography to identify spinal CSF-venous fistulas in spontaneous intracranial hypotension. J Neurosurg Spine 2019:1–4. 10.3171/2019.6.SPINE19487 [DOI] [PubMed] [Google Scholar]
- 5. Mamlouk MD, Ochi RP, Jun P, et al. Decubitus CT myelography for CSF-venous fistulas: a procedural approach. AJNR Am J Neuroradiol 2021;42:32–6. 10.3174/ajnr.A6844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kranz PG, Amrhein TJ, Schievink WI, et al. The ‘hyperdense paraspinal vein’ sign: a marker of CSF-venous fistula. AJNR Am J Neuroradiol 2016;37:1379–81. 10.3174/ajnr.A4682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kranz PG, Amrhein TJ, Gray L. CSF venous fistulas in spontaneous intracranial hypotension: imaging characteristics on dynamic and CT myelography. AJR Am J Roentgenol 2017;209:1360–6. 10.2214/AJR.17.18351 [DOI] [PubMed] [Google Scholar]
- 8. Madhavan AA, Carr CM, Benson JC, et al. Diagnostic yield of intrathecal gadolinium MR myelography for CSF leak localization. Clin Neuroradiol 2022;32:537–45. 10.1007/s00062-021-01060-y [DOI] [PubMed] [Google Scholar]
- 9. Kranz PG, Gray L, Malinzak MD, et al. CSF-venous fistulas: anatomy and diagnostic imaging. AJR Am J Roentgenol 2021;217:1418–29. 10.2214/AJR.21.26182 [DOI] [PubMed] [Google Scholar]
- 10. Kim DK, Brinjikji W, Morris PP, et al. Lateral decubitus digital subtraction myelography: tips, tricks, and pitfalls. AJNR Am J Neuroradiol 2020;41:21–8. 10.3174/ajnr.A6368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Headache classification committee of the International headache society (IHS) the International classification of headache disorders. Cephalalgia 2018;38:1–211. 10.1177/0333102417738202 [DOI] [PubMed] [Google Scholar]
- 12. Amrhein TJ, Gray L, Malinzak MD, et al. Respiratory phase affects the conspicuity of CSF-venous fistulas in spontaneous intracranial hypotension. AJNR Am J Neuroradiol 2020;41:1754–6. 10.3174/ajnr.A6663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mark IT, Amans MR, Shah VN, et al. Resisted inspiration: a new technique to aid in the detection of CSF-venous fistulas. AJNR Am J Neuroradiol 2022;43:1544–7. 10.3174/ajnr.A7636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kranz PG, Malinzak MD, Gray L, et al. Resisted inspiration improves visualization of CSF-venous fistulas in spontaneous intracranial hypotension. AJNR Am J Neuroradiol 2023;44:994–8. 10.3174/ajnr.A7927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dobrocky T, Grunder L, Breiding PS, et al. Assessing spinal cerebrospinal fluid leaks in spontaneous intracranial hypotension with a scoring system based on brain magnetic resonance imaging findings. JAMA Neurol 2019;76:580–7. 10.1001/jamaneurol.2018.4921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kumar N, Diehn FE, Carr CM, et al. Spinal CSF venous fistula: a treatable etiology for CSF leaks in craniospinal hypovolemia. Neurology 2016;86:2310–2.:2310. 10.1212/WNL.0000000000002776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kranz PG, Gray L, Amrhein TJ. Decubitus CT myelography for detecting subtle CSF leaks in spontaneous intracranial hypotension. AJNR Am J Neuroradiol 2019;40:754–6. 10.3174/ajnr.A5995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Madhavan AA, Cutsforth-Gregory JK, Benson JC, et al. Conebeam CT as an adjunct to digital subtraction myelography for detection of CSF-venous fistulas. AJNR Am J Neuroradiol 2023;44:347–50. 10.3174/ajnr.A7794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mark I, Madhavan A, Oien M, et al. Temporal characteristics of CSF-venous fistulas on digital subtraction myelography. AJNR Am J Neuroradiol 2023;44:492–5. 10.3174/ajnr.A7809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Madhavan AA, Kim DK, Brinjikji W, et al. Diagnosis of a cerebrospinal fluid-venous fistula associated with a venous malformation using digital subtraction and computed tomography myelography. World Neurosurg 2020;135:262–6. 10.1016/j.wneu.2019.12.075 [DOI] [PubMed] [Google Scholar]
- 21. Shlapak DP, Mark IT, Kim DK, et al. Incremental diagnostic yield and clinical outcomes of lateral decubitus CT myelogram immediately following negative lateral decubitus digital subtraction myelogram. Neuroradiol J 2023:19714009231173110. 10.1177/19714009231173110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Carlton Jones L, Goadsby PJ. Same-day bilateral decubitus CT Myelography for detecting CSF-venous fistulas in spontaneous intracranial hypotension. AJNR Am J Neuroradiol 2022;43:645–8. 10.3174/ajnr.A7476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lützen N, Kremers N, Fung C, et al. The ‘hyperdense basivertebral vein’ sign: another marker of a CSF-venous fistula. Neuroradiology 2022;64:627–30. 10.1007/s00234-022-02908-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schievink WI, Maya MM, Moser FG. False Localizing signs of spinal CSF-venous fistulas in spontaneous intracranial hypotension: report of 2 cases. J Neurosurg Spine 2019:1–4. 10.3171/2019.4.SPINE19283 [DOI] [PubMed] [Google Scholar]
- 25. Nicholson PJ, Guest WC, van Prooijen M, et al. Digital subtraction myelography is associated with less radiation dose than CT-based techniques. Clin Neuroradiol 2021;31:627–31. 10.1007/s00062-020-00942-x [DOI] [PubMed] [Google Scholar]
- 26. Madhavan AA, Yu L, Brinjikji W, et al. Utility of photon-counting detector CT myelography for the detection of CSF-venous fistulas. AJNR Am J Neuroradiol 2023;44:740–4. 10.3174/ajnr.A7887 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jnis-2023-020789supp001.pdf (141.2KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information. ‘Not applicable’.