Abstract
Background
The interleukin (IL)-1 receptor accessory protein (IL1RAP) is an essential coreceptor required for signalling through the IL-1, IL-33 and IL-36 receptors. Here, we investigate the antifibrotic potential of the combined inhibition of these cytokines by an anti-IL1RAP antibody to provide a scientific background for clinical development in systemic sclerosis (SSc).
Methods
The expression of IL1RAP-associated signalling molecules was determined by data mining of publicly available RNA sequencing (RNAseq) data as well as by imaging mass cytometry. The efficacy of therapeutic dosing of anti-IL1RAP antibodies was determined in three complementary mouse models: sclerodermatous chronic graft-versus-host disease (cGvHD), bleomycin-induced dermal fibrosis model and topoisomerase-I (topo)-induced fibrosis.
Results
SSc skin showed upregulation of IL1RAP and IL1RAP-related signalling molecules on mRNA and protein level compared with normal skin. IL-1, IL-33 and IL-36 all regulate distinct gene sets related to different pathophysiological processes in SSc. The responses of human fibroblasts and endothelial cells to IL-1, IL-33 and IL-36 were completely blocked by treatment with an anti-IL1RAP antibody in vitro. Moreover, anti-IL1RAP antibody treatment reduced dermal and pulmonary fibrosis in cGvHD-induced, bleomycin-induced and topoisomerase-induced fibrosis. Importantly, RNAseq analyses revealed effects of IL1RAP inhibition on multiple processes related to inflammation and fibrosis that are also deregulated in human SSc skin.
Conclusion
This study provides the first evidence for the therapeutic benefits of targeting IL1RAP in SSc. Our findings have high translational potential as the anti-IL1RAP antibody CAN10 has recently entered a phase one clinical trial.
Keywords: Fibroblasts; Inflammation; Scleroderma, Systemic; Pulmonary Fibrosis
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Individual members of the interleukin (IL)-1 family of cytokines have been implicated in processes relevant to the pathogenesis of systemic sclerosis (SSc) such as endothelial cell and fibroblast activation.
The IL-1 receptor accessory protein (IL1RAP) is an essential coreceptor for the IL-1 receptor, the IL-33 receptor (ST2) and the IL-36 receptor and is required for the activation of downstream cascades.
WHAT DOES THIS STUDY ADD
IL-1, IL-33 and IL-36 all activate distinct proinflammatory and profibrotic cascades relevant to the pathogenesis of SSc.
The expression of IL1RAP and of associated signalling molecules is upregulated in the skin of patients with SSc.
IL1RAP-related signalling induces fibroblast and endothelial cell activation in vitro.
Blocking IL1RAP with a monoclonal antibody ameliorates experimental dermal and pulmonary fibrosis in chronic graft-versus-host disease-induced, topoisomerase 1-induced and bleomycin-induced mouse models of fibrosis.
The inhibition of IL1RAP interferes with the activation of a transcriptional regulatory network of gene sets related to inflammation and fibrosis that are also deregulated in human SSc skin.
HOW MIGHT THIS IMPACT ON CLINICAL PRACTICE
IL1RAP offers potential as a novel antifibrotic target in SSc as it is required for IL-1, IL-33 and IL-36-dependent signalling and thus offers a potential to interfere with a network of inflammatory and fibrotic signalling pathways that are essentially required for fibrotic tissue remodelling.
A first in-human phase 1 clinical study with a humanised anti-IL1RAP antibody (CAN10, Cantargia AB) is currently ongoing.
Introduction
Fibrosis describes the accumulation of extracellular matrix in tissues and organs. Although short-term fibrogenic responses may have adaptive features, progressive fibrotic tissue remodelling with excessive parenchymal scarring and cellular dysfunction often leads to organ failure.1 2 Chronic fibrotic tissue remodelling imposes a major socioeconomic burden on modern societies as it has been estimated to account for up to 40% of the deaths in Western societies and for healthcare costs of dozens of billion Euros per year.3 Systemic sclerosis (SSc) is an autoimmune-driven systemic fibrotic disease that affects the skin, the lungs, the heart and various other internal organs.4 A major hallmark of SSc and of other fibrotic diseases is the aberrant activation of fibroblasts. Resting fibroblasts transdifferentiate into so-called myofibroblasts, which express contractile proteins such as α-smooth muscle actin (α-SMA) and release large amounts of extracellular matrix. Although the molecular mechanisms underlying the aberrant activation of fibroblasts in SSc remain incompletely understood, mediators released from activated inflammatory cells are known to play a key role in fibroblast activation in SSc, especially in earlier stages of SSc. Indeed, myofibroblasts accumulate in particular in close proximity to inflammatory infiltrates in SSc skin.5 Moreover, patients with SSc with clinical signs of inflammation (such as elevated C-reactive protein) are known to have a higher risk of disease progression.4 Several cytokines released from activated leukocytes have been shown to induce fibroblast-to-myofibroblast transition and collagen release.6
Interleukin (IL)-1, IL-33 and IL-36 are potent proinflammatory cytokines that amplify immunological responses by promoting the production and expression of adhesion molecules, cytokines, chemokines and other inflammatory mediators (reviewed in Schmitz et al; Liew, Girard and Turnquist; and Yuan et al 7–9). Interestingly, they have also been implicated in fibrotic diseases.10 11 IL-1 can induce collagen production through the activation of IL-6, transforming growth factor beta (TGFβ) and platelet-derived growth factor (PDGF). IL-1α and IL-1β have also been reported to be upregulated in lesional skin and serum from patients with SSc. IL-33 can stimulate the production of IL-13 and TGFβ1 from macrophages12 and induce α-SMA expression and collagen release in fibroblasts.13 In patients with SSc, serum levels of IL-33 are elevated and have been reported to positively correlate with the extent of skin fibrosis and the severity of pulmonary fibrosis. Blockade of the IL-36 receptor (IL36R) by IL-38 has been shown to decrease inflammation and fibrosis in models of myocardial infarction and myocarditis.14 15 Moreover, IL-36α and IL-36ɣ have recently been proposed as potential plasma biomarkers for interstitial lung disease in patients with rheumatoid arthritis.
We hypothesise that simultaneous blockade of all three cytokine pathways offers synergistic therapeutic potential in diseases with inflammation-driven fibrotic tissue remodelling such as SSc. The IL-1 receptor accessory protein (IL1RAP) offers potential as a target for simultaneous blockade of all three cytokines. IL1RAP is an essential coreceptor for the IL1R, the IL33R (ST2) and the IL36R and is required for the activation of intracellular downstream targets by these cytokines.16 CAN10 is a humanised monoclonal LALA-mutated IgG1κ antihuman IL1RAP antibody that has been designed to inhibit IL-1α/β, IL-33 and IL-36α/β/γ signalling. In this study, we used CAN10 or its surrogate antimouse IL1RAP antibody (mCAN10) to block IL-1-mediated, IL-33-mediated and IL-36-mediated signalling in three preclinical models of SSc and to explore the therapeutic potential of targeting IL1RAP in SSc and related diseases.
Material and methods
Detailed information on material and methods are provided as online supplemental information due to restrictions in word count.
ard-2023-225158supp001.pdf (17MB, pdf)
Results
Regulation of SSc-DEGs by the IL1RAP-related cytokines IL-1β, IL-33 and IL-36γ
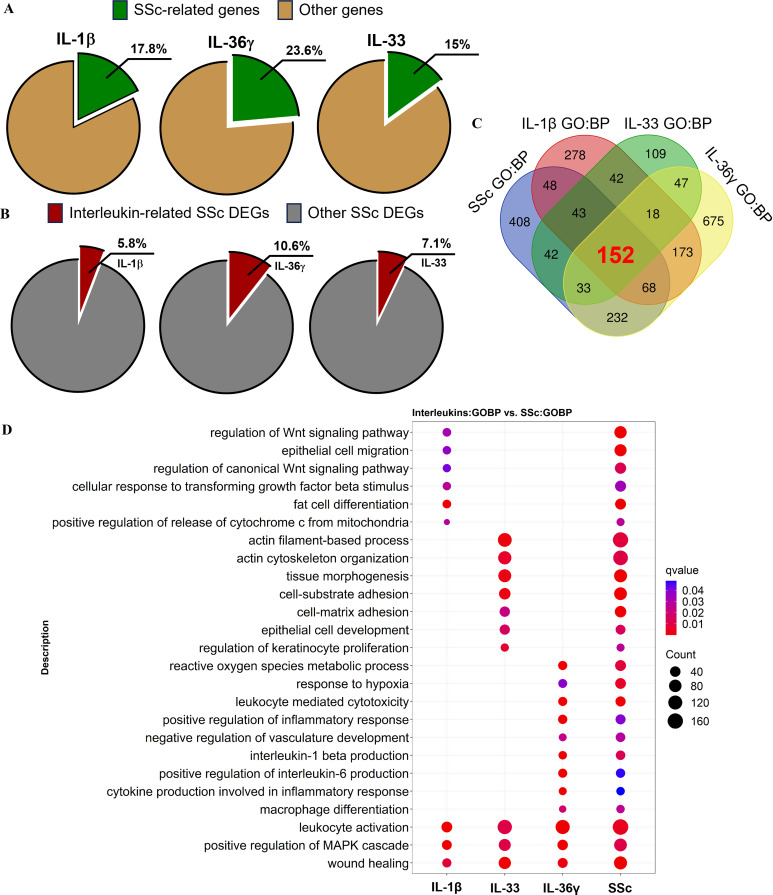
The expression patterns of IL-1β, IL-33 and IL-36γ were obtained from the publicly available RNA datasets GSE40560, GSE147235 and GSE17573217–19 and compared with the expression profile of a SSc cohort (GSE59787). Although the datasets were generated in different experimental settings, they can be used to identify core target genes of each cytokine. 18%, 15% and 24% of the signature genes within the IL-1β, IL-33 and IL-36γ datasets, respectively, exhibited overlap with 6%, 11% and 7% of the signature genes present in the SSc cohort, respectively (figure 1A,B). Although each individual cytokine showed only a modest overlap of the top genes in SSc, together the three cytokine pathways cover several of the specific aspects of the SSc pathophysiology (figure 1C,D). According to the functional analysis of the IL-1β dataset, this cytokine is mainly involved in terms related to the regulation of upstream profibrotic signalling pathways in SSc, such as the ‘regulation of Wnt signalling pathway’, ‘regulation of canonical Wnt signalling pathway’ and ‘cellular response to TGFß stimulus’. The analysis of the IL-33 dataset indicated that this cytokine is relevant for the tissue remodelling processes that characterise SSc, which involve ‘actin filament-based process’, ‘actin cytoskeleton organisation’ and ‘cell-substrate adhesion’. IL-36γ showed numerous terms related to inflammatory responses within SSc, such as ‘leucocyte-mediated cytotoxicity’, ‘macrophage differentiation’, ‘cytokine production involved in inflammatory response’ and ‘positive regulation of IL-6 production’ (figure 1C,D). Taken together, these results indicate that each cytokine has specific and relevant features of SSc pathogenesis and that targeting their common coreceptor IL1RAP may enhance the efficacy of SSc therapy by blocking multiple pathways involved in SSc.
Figure 1.
Interleukin (IL)-1β, IL-33 and IL-36ɣ signalling in systemic sclerosis (SSc). (A) Proportion of SSc-related genes among IL-1β, IL-33 and IL-36ɣ signature genes (from datasets GSE40560, GSE147235 and GSE175732, respectively), using a p-adjusted value cut-off of ≤0.1 for IL-1β and ≤0.05 for IL-33 and IL-36ɣ and a |log2 fold change| cut-off of ≥1 for all datasets. (B) Proportion of IL-1β-related, IL-33-related and IL-36ɣ-related genes among SSc signature genes in the skin (from GSE59787) using a p-adjusted value cut-off of ≤0.05 and a |log2 fold change| cut-off of ≥1. (C) Venn diagram showing the number of overlapping Gene Ontology (Biological Processes) terms among the four cohorts (GSE40560, GSE147235, GSE175732 and GSE59787), using a p-adjusted value cut-off of ≤0.05. (D) Differentially regulated biological processes among the four cohorts. DEGs, differentially expressed genes; MAPK, mitogen-activated protein kinase.
Increased mRNA levels of molecules associated with IL1RAP-related signalling in SSc
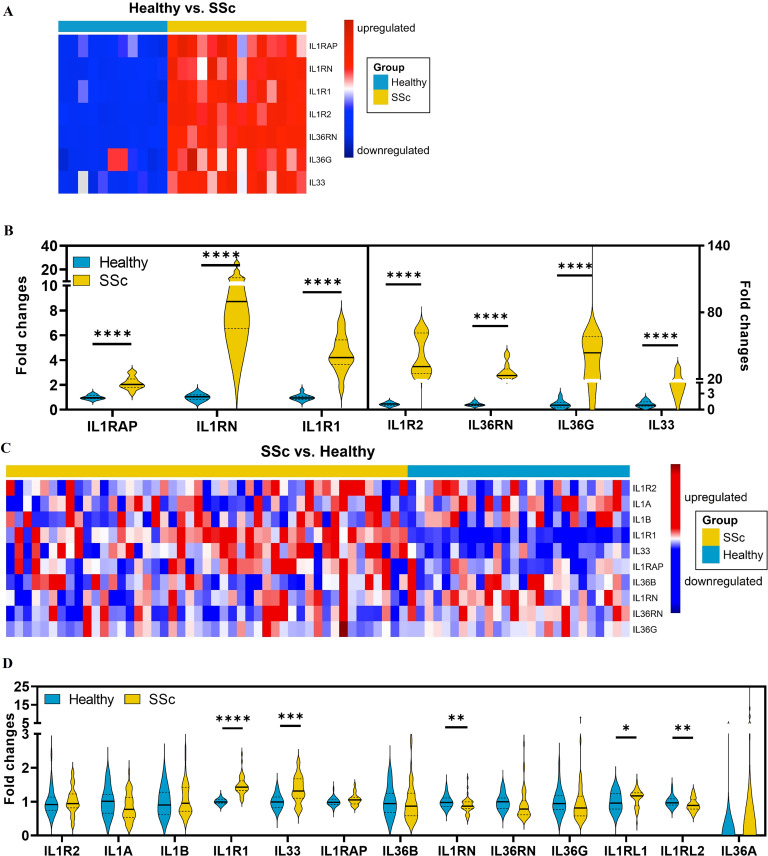
To provide further evidence for the role of the IL1RAP-related cytokines in the pathogenesis of SSc, we studied the mRNA levels of IL1RAP-related signalling molecules in SSc skin. Expression profiling of publicly available RNA datasets from skin biopsies of two North American SSc cohorts and corresponding controls demonstrated increased mRNA levels of several molecules associated with IL1RAP-related signalling. In the dataset GSE59787, which includes combined data from three monocentric SSc cohorts, the mRNA levels of IL1RAP, IL1RN, IL1R1, IL1R2, IL33, IL36RN and IL36G were significantly upregulated as compared with healthy individuals (figure 2A,B). In the multicentric PRESS cohort, composed exclusively of patients with early, diffuse-cutaneous SSc (GSE130955), the mRNA levels of IL1RL1 (IL-33R, ST2), IL1RN, IL1R1, IL33 and IL1RL2 (IL-36R) were significantly upregulated compared with healthy individuals (figure 2C,D). Together, these data demonstrate increased expression of mRNAs for different molecules associated with IL1RAP-related signalling in SSc.
Figure 2.
Increased mRNA levels of molecules associated with interleukin 1 receptor accessory protein (IL1RAP)-related signalling in systemic sclerosis (SSc). High-throughput screening for the expression of genes associated with IL1RAP-related signalling using the NCBI/GEO/GSE59787 dataset (A,B) or the NCBI/GEO/GSE130955 of SSc and healthy skin (C,D). (A,C) Representative heatmaps of mRNA expression level of detectable IL1RAP-related genes between healthy and SSc skin samples. (B,D) The comparisons of detectable IL1RAP-related genes between healthy volunteers and patients with SSc from the cohort. Only samples collected at baseline were included in the analysis. * indicates significant differences between SSc and healthy control. * p<0.05, ** p<0.01, *** p<0.001 and **** p<0.0001.
Expression profiling of IL1RAP and its associated cytokine receptors in SSc skin
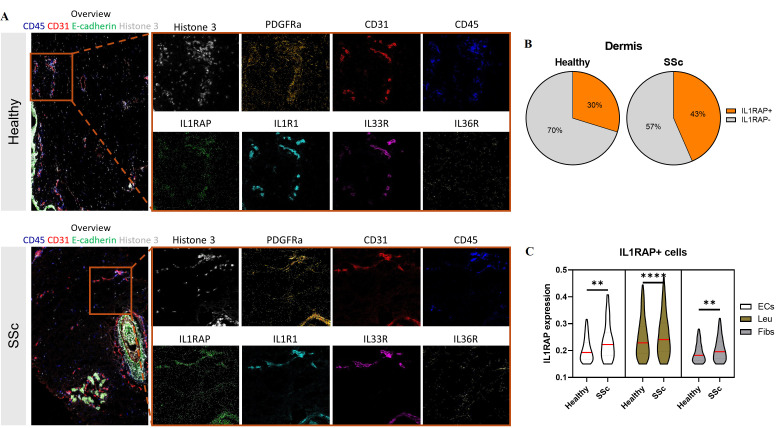
To analyse which cells express IL1RAP in SSc skin, we performed imaging mass cytometry (IMC) using a panel that included not only antibodies against IL1RAP (CAN10) and the associated receptors IL1R1, IL33R and IL36R but also antibodies against markers of different cell populations implicated in the pathogenesis of SSc (figure 3A). We observed an accumulation of IL1RAP-positive cells in the dermis of SSc skin compared with healthy skin (figure 3B). We detected three main cell types that expressed IL1RAP: fibroblasts and other mesenchymal cells (Fibs, E-cadherin-, CD31- and CD45-), leucocytes (Leu, E-cadherin-, CD31- and CD45+) and endothelial cells (ECs, E-cadherin-, CD31+ and CD45-). In SSc, all of these cell types expressed higher levels of IL1RAP than those from healthy skin (figure 3C). Further analyses showed differences in the expression of IL1RAP between different subpopulations of fibroblasts and endothelial cells. Among the different leucocyte populations, monocytes and macrophages were the dominant cellular sources of IL1RAP (online supplemental figure 1A-I). We next studied the expression of the IL1RAP-associated cytokine receptors. The percentage of cells expressing IL36R was increased in SSc skin with higher expression in fibroblasts, endothelial cells and leucocytes (online supplemental figure 2). Subsequently, we assessed the coexpression of IL1RAP with IL1R1, IL33R and IL36R and observed an elevated simultaneous expression of these receptors with IL1RAP in the dermal area of SSc skin compared with healthy skin (online supplemental figure 3).
Figure 3.
Expression patterns of interleukin 1 receptor accessory protein (IL1RAP) analysed by imaging mass cytometry (IMC) staining of systemic sclerosis (SSc) and healthy skin. (A) Representative images of IMC staining on the skin of patients with SSc and healthy donors with different cellular markers (overview, CD45, CD31, IL1RAP, IL1R1, IL33R, Il36R, platelet derived growth factor receptor alpha (PDGFRα) and histone H3). (B) Proportions of IL1RAP-positive or IL1RAP-negative cells in the dermis layer in the skin of patients SSc or healthy donors. (C) Violin plot representing the distribution of the IL1RAP intensity in IL1RAP-positive endothelial cells, leucocytes and fibroblasts or related cell populations in the dermis layer in the skin of patients with SSc or healthy donors. The red line indicates the median of data. The statistical significance was determined by two-tailed Mann-Whitney U test. * indicates significant differences between SSc and healthy control. * p<0.05, ** p<0.01, ***p<0.001 and **** p<0.0001.
We next stained for SSc and normal skin for the upstream cytokines IL-1β, IL-33 and IL-36γ using multicolour immunofluorescence. The protein levels of IL-1β, IL-33 and IL-36γ were upregulated in the skin of patients with SSc compared with healthy donors (online supplemental figure 4A–D).
IL1RAP blockade by CAN10 inhibits response to IL-1/IL-33/IL-36 signalling in vitro
In line with the expression data, a human umbilical cord endothelial cell line responds to IL-1 and IL-33 with IL-6 and IL-8 protein secretion, known markers of nuclear factor kappa B activation (online supplemental figure 5A). Similar responses to IL-1 and IL-36 were observed with human dermal fibroblasts (online supplemental figure 5B). Coincubation with CAN10, a humanised antihuman IL1RAP antibody, completely blocked the stimulatory effects of IL-1, IL-33 and IL-36 (online supplemental figure 5A,B). The CAN10 antibody also inhibited the profibrotic effects of recombinant TGFβ, a combination of IL-1β/IL-33/IL-36γ (referred to as ILs), or IL-1β/IL-33/IL-36γ plus TGFβ, on extracellular matrix accumulation and myofibroblast formation (online supplemental figure 6A,B). This shows that CAN10 effectively impairs the profibrotic and proinflammatory effects of the IL1RAP-related cytokines in vitro, in cells that are crucial to SSc pathophysiology.
Treatment of sclerodermatous cGvHD with an anti-IL1RAP antibody initiated after the onset of first clinical manifestations
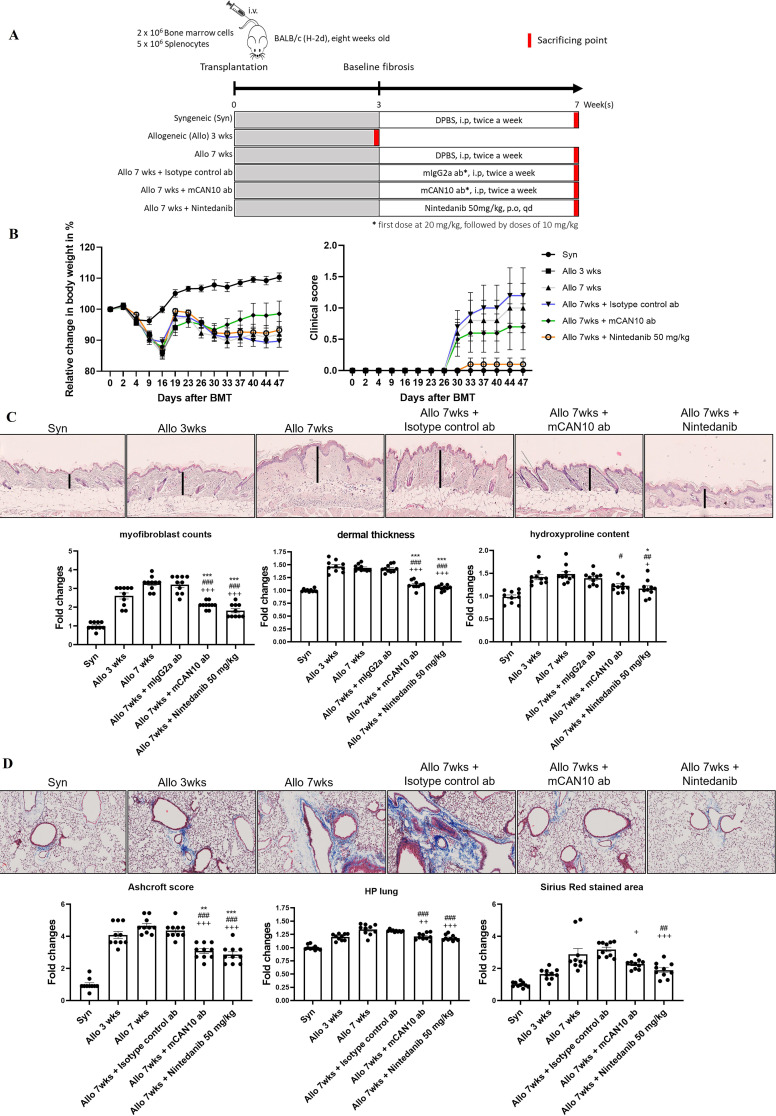
Based on the overexpression of multiple IL-1 family signalling molecules in patients with SSc, we next aimed to evaluate IL1RAP as a central signalling hub for the profibrotic effects of IL-1, IL-33 and IL-36 and the effects of therapeutic targeting by CAN10. We first employed the B10.D2 (H-2d) BALB/c (H-2d) model of sclerodermatous chronic graft-versus-host disease (cGvHD), as this model mimics patients with SSc with early, progressive, diffuse-cutaneous SSc with organ involvement, a population typically recruited for clinical trials in SSc. To demonstrate comparable pathway activation between this model and patients with SSc, we analysed the expression levels of genes within the IL-1 family signalling molecules in skin samples of mice with experimental cGvHD. We found multiple IL1RAP-dependent signalling molecules to be differentially expressed in allogeneically transplanted mice compared with syngeneically transplanted, non-fibrotic controls with upregulation of Il1a, Il36α, Il36β, Il36γ, Il1rap, Il1rl2 (IL-36R) and Il1rn but not of Il1b, Il1r2, Il1rl1 (IL-33R, ST2) and Il33 (online supplemental figure 7).
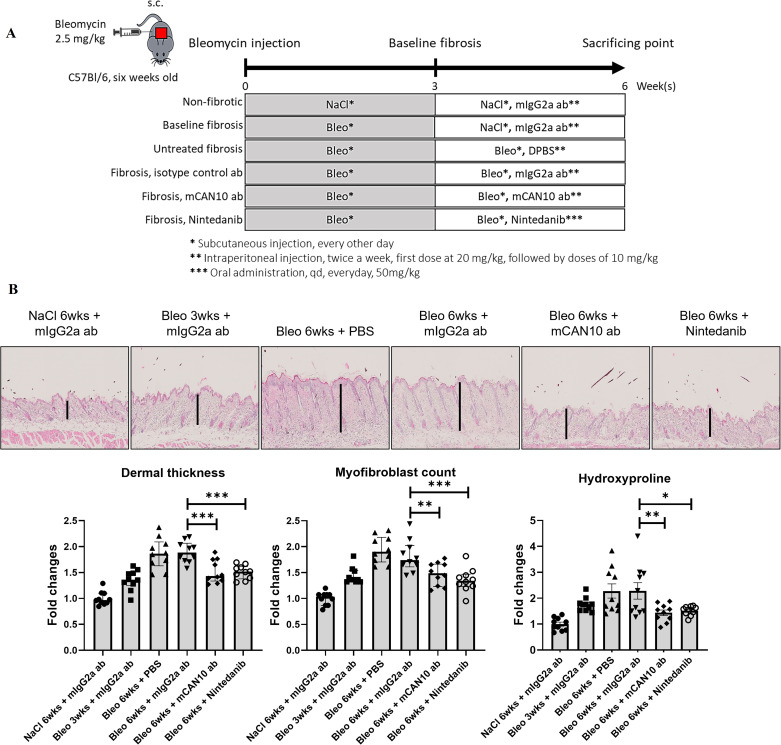
The first clinical signs of cGvHD skin disease became evident at around 30 days after allogeneic bone marrow transplantation (BMT). The composite score of cGvHD skin involvement progressively increased in vehicle-treated, allogeneically transplanted mice after BMT and in mice treated with the isotype control antibody. In contrast, syngeneically transplanted control mice did not develop any evidence of skin disease throughout the observation period.
Treatment with the mouse surrogate version of the CAN10 antibody, the antimouse IL1RAP antibody mCAN10, twice a week intraperitoneally or with daily high-dose nintedanib orally, which was used as a clinically approved control with known antifibrotic effects in cGvHD,20 ameliorated clinical signs of cutaneous cGvHD and numerically reduced the mean composite scores (figure 4A,B). The optimal dose of mCAN10 in mice was determined by pharmacokinetic studies to be 10 mg/kg (20 mg/kg first dose), and this dose was predicted to generate full receptor occupancy (online supplemental figure 8). Treatment with mCAN10 also ameliorated cGvHD-induced weight loss and did so more effectively than nintedanib (figure 4B).
Figure 4.
Treatment with the anti-IL1RAP antibody mCAN10 ameliorates clinical and histopathological signs of murine sclerodermatous cGvHD. (A) Schematic illustration of the treatment procedure of the anti-IL1RAP antibody mCAN10 on murine sclerodermatous cGvHD. (B) Effects of mCAN10 on cGvHD-induced changes in body weight and cutaneous cGvHD scores. (C) Representative images of H&E staining of skin sections at 200-fold magnification. Effects of mCAN10 on histological and biochemical fibrosis outcomes (dermal thickness, myofibroblast counts and hydroxyproline content) of dermal fibrosis in murine sclerodermatous cGvHD. Vertical black bars indicate dermal thickness. (D) Representative images of trichrome staining of lung sections at 200-fold magnification. Effects of mCAN10 on histological and biochemical fibrosis outcomes (Ashcroft scores, hydroxyproline content and fibrosis covered area) of pulmonary fibrosis in murine cGvHD. The statistical significance was determined by one-way analysis of variance with Dunnett’s multiple comparison test. P values are expressed as follows: 0.05>p > 0.01 as *; 0.01>p > 0.001 as **; p<0.001 as *** as compared with mice 3 weeks after allogeneic BMT (pretreatment). 0.05>p > 0.01 as #; 0.01>p > 0.001 as ##; p<0.001 as ### as compared with vehicle-treated mice 7 weeks after allogeneic BMT. 0.05>p > 0.01 as +; 0.01>p > 0.001 as ++; p<0.001 as +++ as compared with mice treated with control antibody 7 weeks after allogeneic BMT (pretreatment). BMT, bone marrow transplantation; cGvHD, chronic graft-versus-host disease; DPBS, Dulbecco's phosphate buffered saline.
Allogeneic BMT induced prominent skin fibrosis with increased dermal thickness, upregulated the hydroxyproline content and promoted accumulation of myofibroblasts (figure 4C). Treatment with mCAN10 started at day 21 after BMT and resulted in reduced dermal thickening, accumulation of hydroxyproline and myofibroblast differentiation as compared with vehicle-treated mice and mice treated with the isotype control antibody (figure 4C). Mice treated with mCAN10 also showed significant reductions in dermal thickening and myofibroblasts and numerical decreases in hydroxyproline content as compared with allogeneically transplanted mice sacrificed 3 weeks after transplantation, indicating that CAN10 may reverse fibrosis in this model.
Besides skin fibrosis, allogeneic BMT also induced moderate pulmonary fibrosis with increases in Ashcroft scores, in collagen-covered lung area and in hydroxyproline content (figure 4D). All of those changes progressed over time with numerically higher levels/scores at 7 weeks after BMT as compared with 3 weeks after BMT. Treatment with mCAN10 reduced the Ashcroft scores and the hydroxyproline content as compared with vehicle-treated mice followed for 7 weeks after BMT and compared with allogeneically transplanted mice treated with isotype control antibody (figure 4D). Numerical decreases were also observed for the collagen-covered lung area. Daily high-dose nintedanib, used as a positive control with previously proven efficacy in experimental cGvHD, also ameliorated fibrosis with antifibrotic effects in the range of those observed with the anti-IL1RAP antibody mCAN10.
Identification of IL1RAP-regulated target genes in experimental cGvHD
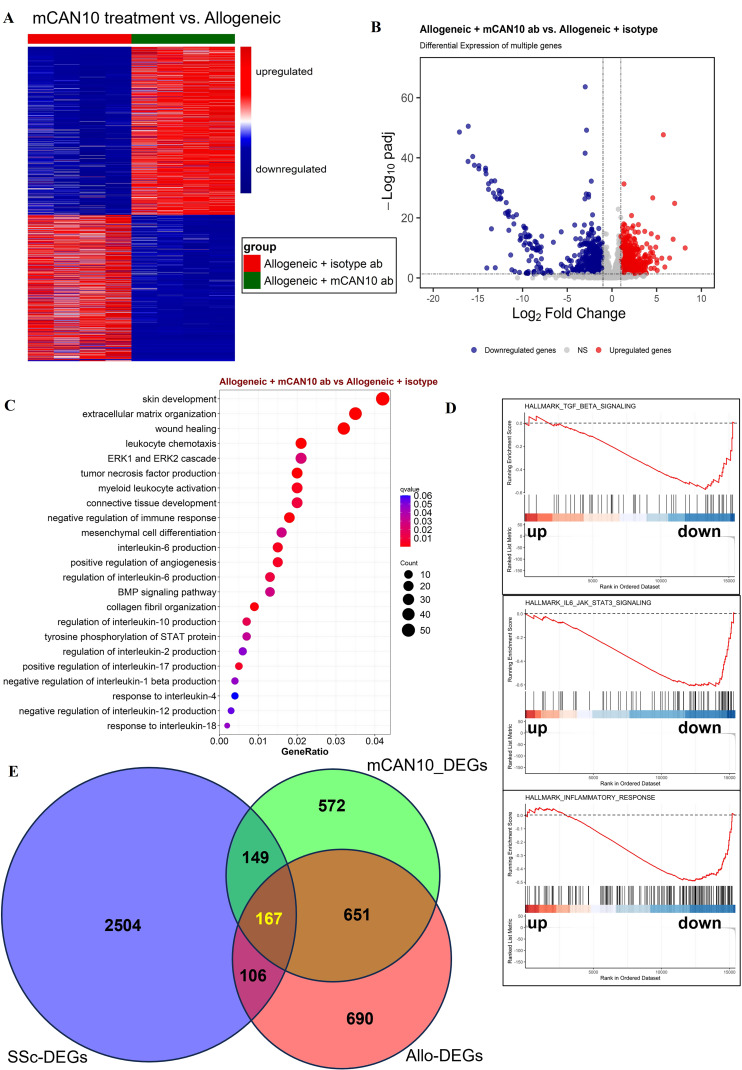
To identify downstream targets of IL1RAP, we performed RNASeq from skin samples of mice with syngeneic and allogeneic transplantation (both treated with isotype control antibody) and from allogeneically transplanted mice treated with mCAN10. A comparison of samples from allogeneically transplanted mice and mice with syngeneic transplantation using the prespecified cut-offs of false discovery rate (FDR) ≤0.05 and |log2FC|≥1.5 demonstrated 1614 differentially expressed genes (DEGs), 1093 of which were upregulated and 521 of which were downregulated (data not shown). A comparison of samples from allogeneically transplanted mice treated with mCAN10 antibody and allogeneically transplanted mice treated with isotype control antibody using the prespecified cut-offs of FDR ≤0.05 and |log2FC|≥1 demonstrated 1539 genes, 823 of which were upregulated and 716 of which were downregulated (mCAN10_DEGs) (figure 5A,B). Functional over-representation analysis from g:Profiler and Gene Set Enrichment Analysis demonstrated statistically significant modulation in particular of processes related to inflammation on IL1RAP inhibition (figure 5C,D). RNASeq data also revealed that treatment with mCAN10 modulates the expression of several profibrotic cytokines, such as Il6, Il36(a, b, g), Il1(a, b), Pdgfs, Tgfb1 and canonical Wnts (Wnt10, Wnt2, Wnt3 and Wnt3a) as well as Ifng (Ifna was not detectable, and Ifnb remained unchanged) (online supplemental figure 9).
Figure 5.
Comparison of differentially expressed genes (DEGs) in allogeneically transplanted mice treated with anti-IL1RAP or isotype control antibody. (A) Heatmap illustration showing the DEGs of allogeneic mice treated with the mCAN10 antibody (mCAN10_DEGs) compared with allogeneic mice treated with isotype control antibody (Allo_DEGs). (B) Volcano plot of mCAN10_DEGs with the expression of each gene plotted as the log2-fold change and log2-log10-FDR. (C) Differentially regulated biological processes according to over-representation analysis (ORA). (D) Selected enriched biological processes according to GSEA. (E) Venn diagrams showing the overlap between Allo-DEGs, mCAN10_DEGs and human SSc-DEGs (NCBI/GEO/GSE59787). BMP, bone morphogenetic protein; FDR, false discovery rate; ERK, extracellular signal-regulated kinase; SSc, systemic sclerosis; STAT, signal transducer and activator of transcription.
To demonstrate the relevance of these DEGs for the pathogenesis of human SSc, we assessed the overlap between Allo-DEGs (Allo vs Syn), mCAN10_DEGs (mCAN10 vs Allo) and genes differentially expressed in SSc skin (SSc-DEGs, SSc vs healthy; GSE59787). We found an overlap of 273 genes between Allo-DEGs and SSc-DEGs. Inclusion of the mCAN10_DEGs yielded 167 overlapping genes between the three groups (figure 5E), which corresponds with approximately 11% of the mCAN10_DEGs and 10% Allo-DEGs. mCAN10 treatment thus affects 61% of the human SSc-relevant genes that are also dysregulated in the cGvHD mouse model (figure 5E). In addition, mCAN10 affects another 149 genes dysregulated in patients with SSc but not recapitulated in the Allo versus Syn comparison.
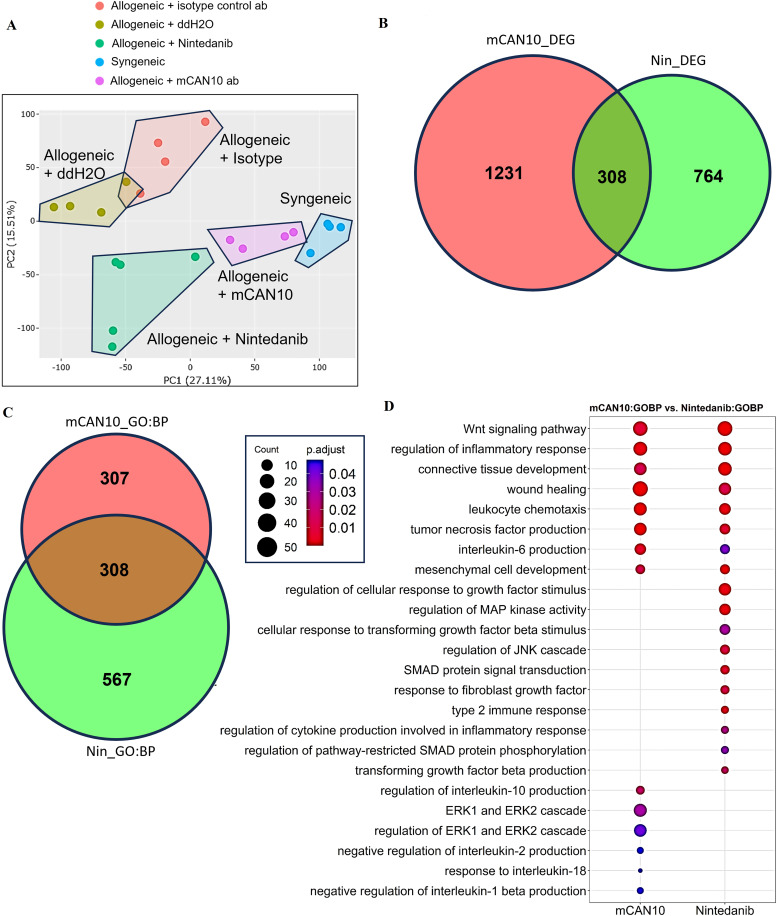
The principal component analysis demonstrated significant changes on the treatment of allogeneically transplanted mice with mCAN10 and with nintedanib compared with the respective control groups (figure 6A). Both treatments showed clearly distinct DEG profiles, suggesting different underlying mechanisms of action. mCAN10_DEGs and Nin_DEGs demonstrated an overlap of only 20% and 29%, respectively (figure 6B), demonstrating that the majority of CAN10 and nintedanib-regulated genes are distinct. Similar findings were obtained by Gene Ontology enrichment analysis. Here, the majority of function terms were specific for CAN10 and nintedanib (figure 6C). The overlapping terms commonly included downstream categories of tissue remodelling such as ‘wound healing’ or ‘mesenchymal cell development’ besides specific features of inflammation such as ‘IL-6 production’. Specific functional terms related to CAN10 treatment included several terms such as ‘regulation of IL-10 production’, ‘negative regulation of IL-2 production’, ‘response to IL-18’ and ‘negative regulation of IL-1ß production’ (figure 6D), highlighting prominent effects of CAN10 on inflammation. In contrast, nintedanib treatment affected terms related to growth factor or cytokine response, including fibroblast growth factor, TGFβ and IL-1, and their downstream signalling pathways, such as mitogen-activated protein kinase, c-Jun N-terminal kinase or SMAD. These results suggested that mCAN10 and nintedanib ameliorate fibrosis in experimental cGvHD through distinct molecular mechanisms.
Figure 6.
Comparison of differentially expressed genes (DEGs) in allogeneically transplanted mice treated with mCAN10 or nintedanib. (A) Principal component analysis depicting the distribution of various sample groups. (B) Intersection of DEGs between nintedanib-treated mice (Nin_DEGs) and mCAN10-treated mice (mCAN10_DEGs), using a p-adjusted value cut-off of ≤0.05 and a |log2 fold change| cut-off of ≥1. (C) The number of overlapping biological process-related terms between mCAN10 and nintedanib treatment. (D) Comparison plot illustrating the signature signalling pathways between nintedanib and mCAN10 treatments. ERK, extracellular signal-regulated kinase; JNK, c-Jun N-terminal kinase; MAP, mitogen-activated protein.
Treatment of pre-established bleomycin-induced and topo-induced fibrosis with an anti-IL1RAP antibody
We next aimed to confirm the antifibrotic effects of targeting IL1RAP in additional mouse models of SSc. We therefore employed the mouse model of bleomycin-induced dermal fibrosis as it is the most widely used murine model of SSc with inflammation-driven skin fibrosis. Expression profiling of the skin of mice with bleomycin-induced fibrosis (from the publicly available dataset, GSE132869) demonstrated the upregulation of multiple IL-1 family signalling molecules with an SSc-like pattern of induction within the first 2 weeks of bleomycin challenge (online supplemental figure 10A). For most of the deregulated genes, the changes persisted for 2 weeks after the last bleomycin injection, providing evidence for a prolonged deregulation on bleomycin challenge (online supplemental figure 10B).
The challenge of mice with bleomycin induced prominent local skin fibrosis with increased dermal thickness, hydroxyproline content and accumulation of myofibroblasts (figure 7A,B). Treatment with mCAN10 that started at the 4th week of bleomycin challenge resulted in reduced dermal thickening, accumulation of hydroxyproline and myofibroblast differentiation as compared with mice treated with vehicle or with control isotype antibody (figure 7B). Mice treated with mCAN10 showed comparable dermal thickness, myofibroblast counts and hydroxyproline content as mice challenged with bleomycin for only 3 weeks, suggesting that treatment with mCAN10 completely prevented the progression of bleomycin-induced skin fibrosis. The antifibrotic effects of mCAN10 were within the range of those observed with 50 mg/kg/qd nintedanib.
Figure 7.
Effects of the anti-IL1RAP antibody mCAN10 on bleomycin-induced dermal fibrosis. (A) Schematic illustration of experimental procedure. (B) Representative images of H&E staining at 200-fold magnification. Vertical black bars indicate dermal thickness. Lower panel with bar graphs for dermal thickness, myofibroblast counts and hydroxyproline content. The statistical significance was determined by one-way analysis of variance with Dunnett’s multiple comparison test. * indicates significant differences among bleomycin group treated with isotype control antibody and other groups. P-values are expressed as follows: 0.05>p > 0.01 as *; 0.01>p > 0.001 as **; p<0.001 as ***.
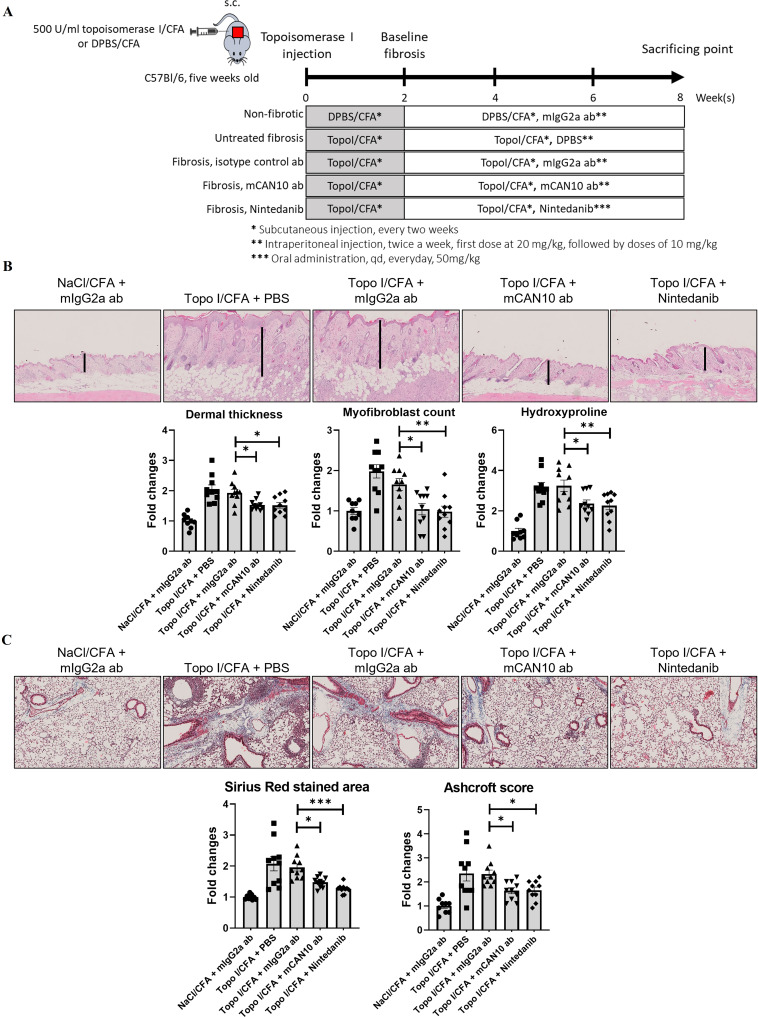
To confirm that targeted inactivation of IL1RAP is also effective in autoimmune-mediated fibrotic tissue remodelling, we next evaluated the efficacy of mCAN10 in the mouse model of topoisomerase-I-induced dermal and pulmonary fibrosis. Injection of human topo-antigen in combination with complete freund’s adjuvant (CFA) (topo mice) induced dermal and pulmonary fibrosis in mice compared with controls injected only with CFA. Topo mice demonstrated increased dermal thickness, hydroxyproline content and myofibroblasts in the skin (figure 8A,B). Treatment with mCAN10 that started on the 3rd week after the first topo-injection significantly ameliorated dermal thickening, accumulation of hydroxyproline and myofibroblast differentiation as compared with vehicle-treated mice or mice injected with the isotype control antibody (figure 8B). mCAN10 also reduced collagen deposition and histological changes as assessed by Ashcroft scoring of the lung from mice injected with topoisomerase-I (figure 8C). The antifibrotic effects of mCAN10 in the topo-model were comparable with those of daily high-dose nintedanib (figure 8B,C).
Figure 8.
Effects of the anti-IL1RAP antibody mCAN10 on histological and biochemical fibrosis outcomes of topoisomerase I (topo I)-induced dermal fibrosis. (A) Schematic illustration of the experimental procedure. (B) Representative images of H&E staining of skin sections at 200-fold magnification. Vertical black bars indicate dermal thickness. Lower panel with bar graphs for dermal thickness, myofibroblast counts and hydroxyproline content. (C) Representative images of trichrome staining of lung sections at 200-fold magnification. Lower panel with bar graphs for Sirius red stained area (collagen content) and Ashcroft score. The statistical significance was determined by one-way analysis of variance with Dunnett’s multiple comparison test. * indicates significant differences among topoisomerase I-induced fibrosis group treated with isotype control antibody and other groups. P values are expressed as follows: 0.05>p > 0.01 as *; 0.01>p > 0.001 as **; p<0.001 as ***. CFA, complete freund’s adjuvant; DPBS, Dulbecco's phosphate buffered saline; IL1RAP, interleukin 1 receptor accessory protein.
Throughout all three mouse models, treatment with mCAN10 was well tolerated without obvious signs of toxicity on clinical examination, on gross necropsy or on histology. We also did not observe an increased infection rate in our mouse experiments. Toxicity studies in preparation for clinical development did not show any treatment-related adverse events (data not shown).
Discussion
IL-1α and IL-1β are well-known key mediators of inflammation, and IL-33 and more recently also IL-36 have also been implicated in the pathogenesis of fibrotic tissue remodelling.21–25 IL1RAP is an essential coreceptor required for IL-1-dependent, IL-33-dependent and IL-36-dependent signalling, and targeting of IL1RAP thus offers potential to simultaneously inhibit several signalling pathways implicated in the pathogenesis of SSc and other fibrotic diseases. In addition to the profibrotic effects of the extracellular ligands, aberrant synthesis of intracellular precursor IL-1α in skin fibroblasts of patients with SSc has also been shown to be associated with the induction of IL-6 and procollagen.26 Mechanistically, IL-1α forms complexes with IL-1α-binding proteins that translocate to the nuclei of fibroblasts to regulate gene expression.27 Whether targeting of IL1RAP may (indirectly) interfere with this intracellular mechanism, for example, by regulations of associated signalling molecules, or whether this profibrotic mechanism may remain unaffected requires further studies.
We demonstrate in well-defined cohorts of patients with SSc that genes and functional terms regulated by IL-1, IL-33 and IL-36 are perturbed in SSc skin compared with normal skin. Of note, each of these cytokines regulates a distinct set of genes related to different pathophysiological processes in SSc, thus providing a theoretical background for synergistic effects of combined targeting of these cytokines. Moreover, mRNA of multiple central signalling components of IL1RAP-associated cytokines is differentially expressed in SSc skin. We further demonstrate by IMC that the protein levels of IL1RAP are increased in SSc. Several central cellular players in the pathogenesis of SSc such as fibroblasts, endothelial cells and different inflammatory cells express IL1RAP along with IL1R1, IL33R and IL36R and may thus be targeted by IL1RAP inhibition. Thus, IL-1, IL-33 and IL-36 signalling is activated in different pathophysiologically relevant cell types in the skin of patients with SSc across different cohorts and may be targeted by IL1RAP inhibition.
We show for the first time that combined inhibition of IL-1, IL-33 and IL-36 signalling by a neutralising anti-IL1RAP antibody exerts therapeutic effects in preclinical models of fibrosis. Therapeutic treatment with the mouse anti-IL1RAP antibody mCAN10 demonstrated potent anti-inflammatory and antifibrotic effects in the mouse models of bleomycin-induced, cGvHD-induced and topo-induced dermal and pulmonary fibrosis. These models resemble different subgroups of patients with severe, progressive SSc and different aspects of its pathogenesis. The mouse model of cGvHD-induced fibrosis emphasises the role of T cell-driven inflammation in early stages of disease development and represents patients with rapidly progressive SSc with multiorgan involvement. The model of topo-induced fibrosis recapitulates the autoimmune aspects of SSc and mimics the subgroup of patients with SSc with autoantibodies against topoisomerase I, pulmonary involvement and high risk of progression. Bleomycin-induced dermal fibrosis resembles patients with inflammation-driven, actively progressive skin fibrosis. All three models thus represent subgroups of patients having SSc with active, inflammatory SSc that are commonly recruited for clinical trials in SSc. Importantly, and similar to what was seen in human SSc skin biopsies, multiple IL1RAP-dependent signalling pathways were activated in skin biopsies from each model, strengthening the case for multiple cytokine pathway blockade in SSc by targeting IL1RAP.
RNAseq analysis of the pathways affected by the anti-IL1RAP antibody mCAN10 and subsequent comparison by transcriptional changes in the skin of patients with SSc demonstrates that the inhibition of IL1RAP regulates multiple pathways with central roles in the pathogenesis of SSc. In all three models, treatment with the anti-IL1RAP antibody was initiated on the first clinical signs of disease. We thus simulated an early onset of treatment of patients after clinical diagnosis, rather than a preventive setting, which is not realistic in most clinical settings. In both models, IL1RAP blockade demonstrated potent antifibrotic effects on dermal and pulmonary fibrosis with reduced histological and biochemical parameters of fibrosis compared with the isotype control antibody. Although these findings may benefit from confirmation in human ex vivo models, our data provide strong evidence that targeting IL1RAP may offer potential for antifibrotic therapies.
Treatment with mCAN10 was well tolerated throughout the different model systems used in our study including long-term application for up to 8 weeks without evidence of adverse effects on clinical monitoring or gross autopsy. Consistent with these findings, IL1RAP knockout mice do not show an overt phenotype.28 29 The good tolerance of IL1RAP targeting despite the inhibition of multiple IL-1 family signalling pathways may reflect the fact that most IL-1 family members are expressed at rather low levels under homeostatic conditions. Our expression profiling demonstrates that most signalling components are expressed at relatively low levels in normal healthy skin but are prominently induced in SSc skin. IL1RAP targeting may thus have only minimal effects on tissue maintenance under homeostatic conditions. A recent phase I study with a related anti-IL1RAP antibody, nadunolimab (also known as CAN04), in patients with solid tumours demonstrated a favourable safety profile,30 providing evidence that inhibition of IL1RAP might not be hindered by adverse events.
In summary, our study (1) demonstrates deregulation of IL1RAP-related signalling pathways in the skin of patients with SSc as well as regulation of specific SSc-DEGs by each of the IL1RAP-related cytokines; (2) provides evidence for profibrotic effects of the upstream cytokines IL-1, IL-33 and IL-36; and (3) demonstrates in complementary mouse models that therapeutic application of a neutralising antibody against IL1RAP ameliorates fibrosis at well-tolerated doses. These findings provide a rationale for targeted inhibition of IL1RAP in SSc and related fibrotic diseases and have high translational potential as CAN10 has recently entered a phase I clinical trial and has been granted orphan drug designation for the treatment of SSc in the USA.
ard-2023-225158supp002.xlsx (985.9KB, xlsx)
Acknowledgments
We thank Regina Kleinlein and Christoph Liebel for excellent technical assistance.
Footnotes
Handling editor: Josef S Smolen
CG, SR, JHD and TT-M contributed equally.
Contributors: CG, SR, DL, TT-M and JHWD are responsible for the overall content as the guarantors. CG, SR, DL, TT-M and JHWD designed the study. CG, SR, CT, XZ, Y-NL, ARR, A-HG, E-AM, HZ, ND, MK, PS and TT-M were involved in the acquisition and analysis of data. CG, SR, CTM, MK, PS, DL, TT-M and JHWD were involved in the interpretation of data. AK provided essential samples. All authors were involved in manuscript preparation and proofreading.
Funding: JHWD was supported by grants DI 1537/17-1, DI 1537/20-1, DI 1537/22-1, DI 1537/23-1, ZH 809/2-1 and BE 7036/2-1 of the German Research Foundation and SFB CRC1181 (project C01), SFB TR221/project number 324392634, SFB TR221/project number 324392634 (B04) and a "Großgeräteantrag" (INST 901095-1 FUGG/AOBJ:659788) of the German Research Foundation, German Federal Ministry of Education and Research (BMBF), MASCARA programme, TP 2 (01EC1903A) and a Career Support Award of Medicine of the Ernst Jung Foundation. AHG was supported by grant 21-07-23-1-Györfi of the ELAN-Foundation Erlangen. MK was supported by the German Federal Ministry of Education and Research (BMBF), CompLS programme grant 031L0262C (to MK and ND) and the German Research Foundation (SFB TR221 project number 324392634 (INF to MK and ND), RTG 2740 (project B2B)).
Competing interests: CG, SR, PS and DL are employed by and hold stocks or options in Cantargia AB. CG, SR and DL are coinventors on patents related to anti-IL1RAP monoclonal antibodies. MK is the founder and shareholder of BioInf4Life. JHWD has consultancy relationships and/or has received research funding from AbbVie, Actelion, Bristol-Myers Squibb, Celgene, Bayer Pharma, Boehringer Ingelheim, JB Therapeutics, Sanofi-Aventis, Novartis, UCB, GSK, Array Biopharma and Active Biotech in the area of potential treatments of SSc, is stock owner of 4D Science, and scientific lead and co-CEO of FibroCure. The project was supported by project-specific funding from Cantargia AB.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available in a public, open access repository. The RNASeq data have been uploaded to Gene Expression Omnibus platform with the accession number GSE255031.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was approved by an Ethics Committee(s) or Institutional Board(s) of University Hospital Duesseldorf and Erlangen. Participants gave informed consent to participate in the study before taking part.This study involves animal subjects and was approved by an Ethics Committee(s) or Institutional Board(s) of the local animal welfare committee of the Government of Mittelfranken, Unterfranken or North-Rinne-Westphalia.
References
- 1. Abraham DJ, Krieg T, Distler J, et al. Overview of pathogenesis of systemic sclerosis. Rheumatology (Oxford) 2009;48 Suppl 3:iii3–7. 10.1093/rheumatology/ken481 [DOI] [PubMed] [Google Scholar]
- 2. Distler JHW, Feghali-Bostwick C, Soare A, et al. Review: frontiers of antifibrotic therapy in systemic sclerosis. Arthritis Rheumatol 2017;69:257–67. 10.1002/art.39865 [DOI] [PubMed] [Google Scholar]
- 3. Gurtner GC, Werner S, Barrandon Y, et al. Wound repair and regeneration. Nature 2008;453:314–21. 10.1038/nature07039 [DOI] [PubMed] [Google Scholar]
- 4. Denton CP, Khanna D. Systemic sclerosis. Lancet 2017;390:1685–99. 10.1016/S0140-6736(17)30933-9 [DOI] [PubMed] [Google Scholar]
- 5. Distler JHW, Györfi A-H, Ramanujam M, et al. Shared and distinct mechanisms of fibrosis. Nat Rev Rheumatol 2019;15:705–30. 10.1038/s41584-019-0322-7 [DOI] [PubMed] [Google Scholar]
- 6. Distler JHW, Allanore Y, Avouac J, et al. EULAR scleroderma trials and research group statement and recommendations on endothelial precursor cells. Ann Rheum Dis 2009;68:163–8. 10.1136/ard.2008.091918 [DOI] [PubMed] [Google Scholar]
- 7. Schmitz J, Owyang A, Oldham E, et al. IL-33, an Interleukin-1-like cytokine that signals via the IL-1 receptor-related protein St2 and induces T helper type 2-associated Cytokines. Immunity 2005;23:479–90. 10.1016/j.immuni.2005.09.015 [DOI] [PubMed] [Google Scholar]
- 8. Liew FY, Girard JP, Turnquist HR. Interleukin-33 in health and disease. Nat Rev Immunol 2016;16:676–89. 10.1038/nri.2016.95 [DOI] [PubMed] [Google Scholar]
- 9. Yuan ZC, Xu WD, Liu XY, et al. Biology of IL-36 signaling and its role in systemic inflammatory diseases. Front Immunol 2019;10:2532. 10.3389/fimmu.2019.02532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jäger B, Seeliger B, Terwolbeck O, et al. The NLRP3-inflammasome-caspase-1 pathway is upregulated in idiopathic pulmonary fibrosis and acute exacerbations and is inducible by apoptotic A549 cells. Front Immunol 2021;12:642855. 10.3389/fimmu.2021.642855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gieling RG, Wallace K, Han YP. Interleukin-1 participates in the progression from liver injury to fibrosis. Am J Physiol Gastrointest Liver Physiol 2009;296:G1324–31. 10.1152/ajpgi.90564.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Izadi D, Layton TB, Williams L, et al. Identification of TNFR2 and IL-33 as therapeutic targets in localized fibrosis. Sci Adv 2019;5:eaay0370. 10.1126/sciadv.aay0370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. An G, Zhang X, Wang W, et al. The effects of Interleukin-33 on airways collagen deposition and matrix metalloproteinase expression in a murine Surrogate of asthma. Immunology 2018;154:637–50. 10.1111/imm.12911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. El-Awaisi J, Kavanagh DP, Rink MR, et al. Targeting IL-36 improves age-related coronary microcirculatory dysfunction and attenuates myocardial ischemia/reperfusion injury in mice. JCI Insight 2022;7:e155236. 10.1172/jci.insight.155236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mahil SK, Catapano M, Di Meglio P, et al. An analysis of IL-36 signature genes and individuals with Il1Rl2 knockout mutations Validates IL-36 as a psoriasis therapeutic target. Sci Transl Med 2017;9:411:eaan2514. 10.1126/scitranslmed.aan2514 [DOI] [PubMed] [Google Scholar]
- 16. Garlanda C, Dinarello CA, Mantovani A. The Interleukin-1 family: back to the future. Immunity 2013;39:1003–18. 10.1016/j.immuni.2013.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Boisson B, Laplantine E, Prando C, et al. Immunodeficiency, autoinflammation and amylopectinosis in humans with inherited HOIL-1 and LUBAC deficiency. Nat Immunol 2012;13:1178–86. 10.1038/ni.2457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Piyadasa H, Lloyd D, Lee AHY, et al. Characterization of immune responses and the lung transcriptome in a murine model of IL-33 challenge. Biochim Biophys Acta Mol Basis Dis 2020;1866:165950. 10.1016/j.bbadis.2020.165950 [DOI] [PubMed] [Google Scholar]
- 19. Kluwig D, Huth S, Abdallah AT, et al. Establishment of an intradermal ear injection model of IL-17A and IL-36gamma as a tool to investigate the psoriatic cytokine network. Life (Basel) 2021;11:846. 10.3390/life11080846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Huang J, Beyer C, Palumbo-Zerr K, et al. Nintedanib inhibits fibroblast activation and ameliorates fibrosis in preclinical models of systemic sclerosis. Ann Rheum Dis 2016;75:883–90. 10.1136/annrheumdis-2014-207109 [DOI] [PubMed] [Google Scholar]
- 21. Elias M, Zhao S, Le HT, et al. IL-36 in chronic inflammation and fibrosis - bridging the gap J Clin Invest 2021;131:e144336. 10.1172/JCI144336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kotsiou OS, Gourgoulianis KI, Zarogiannis SG. IL-33/St2 axis in organ fibrosis. Front Immunol 2018;9:2432. 10.3389/fimmu.2018.02432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Borthwick LA. The IL-1 cytokine family and its role in inflammation and fibrosis in the lung. Semin Immunopathol 2016;38:517–34. 10.1007/s00281-016-0559-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Artlett CM. The IL-1 family of cytokines. do they have a role in scleroderma fibrosis. Immunol Lett 2018;195:30–7. 10.1016/j.imlet.2017.11.012 [DOI] [PubMed] [Google Scholar]
- 25. O’Reilly S. Interleukin-36Alpha is elevated in diffuse systemic sclerosis and may potentiate fibrosis. Cytokine 2022;156. 10.1016/j.cyto.2022.155921 [DOI] [PubMed] [Google Scholar]
- 26. Kawaguchi Y, Nishimagi E, Tochimoto A, et al. Intracellular IL-1Alpha-binding proteins contribute to biological functions of endogenous IL-1Alpha in systemic sclerosis fibroblasts. Proc Natl Acad Sci U S A 2006;103:14501–6. 10.1073/pnas.0603545103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Werman A, Werman-Venkert R, White R, et al. The precursor form of IL-1Alpha is an Intracrine proinflammatory activator of transcription. Proc Natl Acad Sci U S A 2004;101:2434–9. 10.1073/pnas.0308705101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cullinan EB, Kwee L, Nunes P, et al. IL-1 receptor accessory protein is an essential component of the IL-1 receptor. J Immunol 1998;161:5614–20. [PubMed] [Google Scholar]
- 29. Ågerstam H, Hansen N, von Palffy S, et al. Il1Rap antibodies block IL-1-induced expansion of candidate CML stem cells and mediate cell killing in Xenograft models. Blood 2016;128:2683–93. 10.1182/blood-2015-11-679985 [DOI] [PubMed] [Google Scholar]
- 30. Robbrecht D, Jungels C, Sorensen MM, et al. First-in-human phase 1 dose-escalation study of Can04, a first-in-class Interleukin-1 receptor accessory protein (Il1Rap) antibody in patients with solid tumours. Br J Cancer 2022;126:1010–7. 10.1038/s41416-021-01657-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ard-2023-225158supp001.pdf (17MB, pdf)
ard-2023-225158supp002.xlsx (985.9KB, xlsx)
Data Availability Statement
Data are available in a public, open access repository. The RNASeq data have been uploaded to Gene Expression Omnibus platform with the accession number GSE255031.