Abstract
Objectives
In high-income countries hepatitis E virus (HEV) is an uncommonly diagnosed porcine-derived zoonoses. After identifying disproportionate chronic HEV infections in persons with cystic fibrosis (pwCF) postlung transplant, we sought to understand its epidemiology and potential drivers.
Design
All pwCF post-transplant attending our regional CF centre were screened for HEV. HEV prevalence was compared against non-transplanted pwCF and with all persons screened for suspected HEV infection from 2016 to 2022 in Alberta, Canada. Those with chronic HEV infection underwent genomic sequencing and phylogenetic analysis. Owing to their swine derivation, independently sourced pancreatic enzyme replacement therapy (PERT) capsules were screened for HEV.
Results
HEV seropositivity was similar between transplanted and non-transplanted pwCF (6/29 (21%) vs 16/83 (19%); p=0.89). Relative to all other Albertans investigated for HEV as a cause of hepatitis (n=115/1079, 10.7%), pwCF had a twofold higher seropositivity relative risk and this was four times higher than the Canadian average. Only three chronic HEV infection cases were identified in all of Alberta, all in CF lung transplant recipients (n=3/29, 10.3%). Phylogenetics confirmed cases were unrelated porcine-derived HEV genotype 3a. Ninety-one per cent of pwCF were taking PERT (median 8760 capsules/person/year). HEV RNA was detected by RT-qPCR in 44% (47/107) of PERT capsules, and sequences clustered with chronic HEV cases.
Conclusion
PwCF had disproportionate rates of HEV seropositivity, regardless of transplant status. Chronic HEV infection was evident only in CF transplant recipients. HEV may represent a significant risk for pwCF, particularly post-transplant. Studies to assess HEV incidence and prevalence in pwCF, and potential role of PERT are required.
Keywords: pancreatic enzymes, cystic fibrosis, hepatitis E, pancreatic disease, immunodeficiency
WHAT IS ALREADY KNOWN ON THIS TOPIC
Hepatitis E virus (HEV) is a porcine-derived zoonoses infrequently identified as a cause of hepatitis in high-income countries.
In Europe and North America, exposure has been epidemiologically linked to the consumption of pork products, although direct identification of HEV RNA in meat products marketed to humans is exceptionally uncommon.
Among heavily immunosuppressed populations, HEV can cause a chronic hepatitis (and a range of extrahepatic manifestations) that ultimately may progress to fulminant liver failure.
WHAT THIS STUDY ADDS
Chronic HEV infections are exceptionally rare, and this is the first study to report infection in persons with cystic fibrosis (pwCF) (three separate, unrelated cases in a single regional centre).
We observed rates of HEV seropositivity to be similar among pwCF, regardless of lung transplant status, and these rates were four times higher than the published Canadian national average, and even twice that of all individuals in the Province of Alberta specifically referred for HEV testing (ie, a preselected, at-risk group).
We hypothesised porcine-derived pancreatic enzyme replacement therapy (PERT), a medication taken by ~90% of individuals with CF on account of pancreatic insufficiency (median ~24 capsules/day), may represent a biologically plausible source of infection to explain HEV disproportionate occurrence in pwCF.
We found HEV RNA in 44% of PERT capsules, including all formulations from all Canadian manufacturers; moreover, PERT HEV orf1 gene sequence clustered with both CF-associated HEV infection cases and Canadian swine herds—suggesting a potential iatrogenic mechanism of infection.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
The high frequency of comorbid CF liver disease (one-third of individuals) may limit investigations into alternate liver disease aetiologies such as HEV, limiting its identification in other CF cohorts.
This study should prompt others to explore the prevalence of HEV (seropositivity and chronic infection) in other CF, and other PERT using cohorts.
Similarly, this study warrants a re-analysis of PERT safety as an agent with potential for zoonotic infection risk—especially in profoundly immunosuppressed population, such as CF lung transplant recipients.
Introduction
Hepatitis E virus (HEV) is a leading cause of viral hepatitis worldwide, particularly in low-income and middle-income countries.1 HEV is a non-enveloped, positive-sense, single-stranded RNA virus of 7.2 kb with seven genotypes; however, only genotypes 1–4 (and rarely 7) display human tropism. The WHO estimates 20 million incident infections globally, with 3.3 million symptomatic and an associated 50 000 deaths. In high-income countries, genotypes 3 and 4 are endemic in domestic swine herds and wild game, with rare sporadic zoonotic hepatitis cases reported in humans. Predominately an agent causing hepatitis, extrahepatic manifestations can occur and include neurological syndromes, renal injury and pancreatitis. Historically, HEV was considered an acute self-limited infection. However, in profoundly immunocompromised populations, chronic infections associated with genotype 3 are increasingly described.1 2 These infections are autochthonous and thought to be acquired from direct contact with or from ingestion of undercooked pork or wild game products.3 In solid organ transplant (SOT) recipients with acute HEV infection, as many as two-thirds progress to chronic hepatitis with sequalae including cirrhosis and death.1
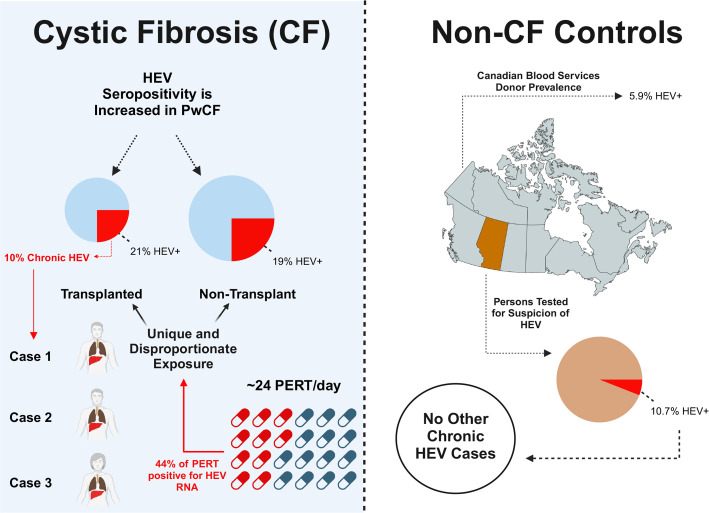
Cystic fibrosis (CF), a multisystem disorder, is the most common fatal genetic disease among Caucasians. As approximately 20% of adult persons with CF (pwCF) living in Canada are lung transplant recipients,4 understanding factors impacting this high-risk population is critical. After identifying three cases of chronic HEV infection in our CF regional centre, these being only the second, third and fourth cases of chronic HEV infection ever described in Canada, we sought to assess the prevalence and clinical outcomes across a cohort of transplanted and non-transplanted pwCF. We hypothesised that this apparent cluster of HEV chronic disease observed in pwCF was the result of disproportionate HEV exposure risk and sought to determine seroprevalence of HEV among pwCF and determine if this was increased relative to non-CF populations. As pancreatic insufficiency (PI) is among the most prominent phenotypes of CF, with afflicted individuals requiring high doses of porcine-derived pancreatic enzyme replacement therapy (PERT), we hypothesised PERT represented an HEV zoonotic exposure risk specific to pwCF and sought to determine if HEV RNA contaminated the PERT consumed by pwCF. An overview of the study rationale and results are detailed in the graphical abstract (figure 1).
Figure 1.
Graphical abstract. HEV, hepatitis E virus; PERT, pancreatic enzyme replacement therapy; pwCF, patients with cystic fibrosis.
Methods
Study design and selection of subjects
Three cohorts were included in the study (online supplemental figure 1):
gutjnl-2023-330602supp001.pdf (23.8KB, pdf)
CF-SOT recipients. After clinical cases of chronic HEV infection were identified in the Southern Alberta Adult CF Clinic, we initiated anti-HEV IgG and IgM serological, and HEV RNA screening (as positive cases can occur in the absence of seropositivity5) in all CF transplant recipients.
A prospectively enrolled CF cohort that had not previously received a SOT from the same regional CF centre.
All individuals in the Province of Alberta (total population of ~4.5 million) with clinical suspicion for HEV as a potential cause of liver disease (ie, those with elevated liver enzymes in which other causes of hepatitis were ruled out) for whom HEV testing was performed between January 2016 and August 2022 (ie, a preselected, at-risk group).
Among pwCF, demographics and treatments at time of HEV testing were recorded. PwCF with PI were identified based on clinician prescribed PERT. PERT use was classified by manufacturer, formulation and total number of capsules taken per day. For the remaining subjects, age, sex, transplant status and immunosuppressive regimen were recorded.
Clinical HEV serology and RNA testing
Serum and plasma from all transplant recipients (and stool for those under suspicion) were assessed at the National Microbiology Laboratory (Winnipeg, Manitoba, Canada) for clinical care purposes. HEV IgG and IgM were assessed using ELISA (Wantai Biopharm, Beijing, China). HEV IgG assessed for research purposes in the non-transplanted CF cohort was performed using two complementary assays (Abbexa Ca. ABX364866 and Elabscience Biotechnology Ca. E-HD-E055). Participants were considered HEV seropositive only when both IgG assays were positive. Additional details on the method for these measurements are provided in the online supplemental file.
gutjnl-2023-330602supp002.pdf (328.3KB, pdf)
Plasma samples (250 µL) received at the National Microbiology Laboratory for HEV RNA detection were extracted by silica-coated magnetic bead purification using the NUCLISENS EASYMAG instrument (Biomérieux Canada, Saint-Laurent, Quebec, Canada). In the research laboratory, nucleic acid was extracted from plasma using QIAamp MinElute Virus spin kit (Ca. 57705, CPN 1198016.6) with a modified protocol using 400 µL plasma and increasing kit reagent volumes by 2× and using carrier RNA as described by the instructions from the manufacturer.
Pancreatic enzyme detection of HEV
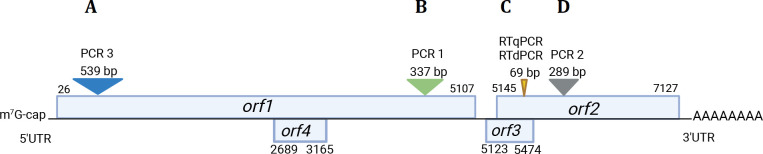
PERT was independently acquired from pwCF and pharmacies to ensure broad representation from Southern Alberta and a diverse range of formulations and lots. Eleven formulations of PERT capsules produced by four manufacturers (all manufacturers with Health Canada-approved products) were screened and lot variability determined with replicate capsules. Dissolved PERT capsules first underwent TRIzol (Thermo Fisher Scientific) extraction owing to the extreme protein-rich extract and the confounding effects of (most) PERT’s enteric coating followed by cleanup using QIAamp MinElute Virus spin columns. Assessment for molecular and genomic inhibitors in PERT extracts was assessed (see online supplemental file for details). The presence of HEV RNA was assessed using an orf3 RT-qPCR and with an orf3 RT-digital PCR (RT-dPCR) each with appropriate positive and negative controls (online supplemental table 1). Nested PCR and Sanger sequencing was performed on a subset of select HEV RNA-positive PERT samples targeting two regions of HEV orf1 (both the 5’ end and 3’ end) and orf2 (figure 2, online supplemental tables 1 and 2). HEV sequences identified from pwCF and PERT from this study are uploaded into GenBank (SUB14282691).
Figure 2.
HEV genome and the molecular and genomic targets used in this study. Of the 7.2 kb genome, four independent areas were identified including (A; PCR-3) a nested PCR yielding a 539 bp sequenced target at the 5’ end of orf1; (B; PCR-1) a nested PCR yielding a 337 bp sequenced product in orf1 at the 3’ end; (C) quantitative and digital-PCR—product of 69 bp in size from orf2/3 and (D; PCR-2) a nested PCR yielding a 289 bp sequenced product in orf2. UTR, untranslated region.
HEV RNA genotyping
Phylogenetic analysis of output and reference sequences was performed by maximum likelihood inference of a 315 bp trimmed orf1 alignment using DIVEIN web tools by the TN93+γ+I model (online supplemental material).
Statistical analyses
Descriptive statistics were completed in comparing cohorts. Differences between groups were assessed using the Wilcoxon rank-sum test or Fisher’s exact test, with p <0.05 considered statistically significant. Chi-squared test was used to compare discrete variables between groups. Relative risk (RR) for HEV was calculated between cohorts (ie, those transplanted vs non-transplanted and pwCF vs non-CF controls). Statistical analysis was performed using R, V.4.04 (R Core Team, 2021).
Results
Prevalence of HEV seropositivity and clinical disease among CF transplant recipients
After identifying the first three cases of HEV clinical disease ever reported in Alberta, Canada, all among pwCF attending the Southern Alberta Adult CF Clinic, we screened all transplanted pwCF attending the clinic for HEV seropositivity (IgG and IgM) and HEV RNA as part of an evolved clinical care pathway. Across the cohort of transplanted pwCF (n=29), all of whom had previously received life-saving lung transplants, six subjects were HEV IgG seropositive (20.7%) (table 1). Three of these individuals were also positive for HEV RNA (10.3%) and these comprised the chronic HEV infection cohort (table 2).
Table 1.
Demographics of adult CF lung transplant recipients and HEV serostatus
| HEV seropositive (n=6), N (%) | HEV seronegative (n=23), N (%) | |
| Characteristic | ||
| Female sex | 2 (33) | 13 (57) |
| Median age, years (IQR) | 40.4 (34.1–47.3) | 37.6 (29.3–49.5) |
| Rural postal code | 1 (17) | 5 (22) |
| CF-related factors | ||
| Genotype | ||
| ΔF508 homozygous | 4 (67) | 14 (61) |
| ΔF508 heterozygous | 2 (33) | 8 (35) |
| Other | 0 | 1 (4) |
| CF comorbidities | ||
| Pancreatic insufficiency | 6 (100) | 20 (87) |
| CF liver disease | 3 (50) | 7 (30) |
| GORD | 3 (50) | 16 (70) |
| Distal intestinal obstruction syndrome | 6 (100) | 21 (91) |
| CF arthropathy | 1 (17) | 2 (9) |
| Sinus disease (polyps or nasal congestion) | 6 (100) | 19 (83) |
| Pretransplant factors | ||
| Median age at time of transplant, years (IQR) | 28.6 (22.1–32.5) | 31.3 (25.6–36.1) |
| Median BMI at time of transplant (IQR, kg/m2) | 20.9 (18.7–22.1) | 19.9 (18.4–21.9) |
| Per cent predicted forced expiratory volume predicted at time of transplant, median (IQR) | 22.7 (15.1–28.3) | 23.5 (16.0–31.8) |
| CF-related diabetes (pretransplant prevalence) | 3 (50) | 11 (48) |
| Chronic Pseudomonas aeruginosa infection | 6 (100) | 21 (91) |
| Post-transplant factors | ||
| CF-related diabetes (post-transplant prevalence) | 4 (67) | 15 (66) |
| Blood product received at time of transplant | 6 (100) | 20 (87)* |
| CMV status | ||
| Donor+, recipient− | 2 (33) | 7 (30) |
| Donor+, recipient+ | 4 (67) | 15 (66) |
| Other | 0 | 1 (4) |
| Maintenance immunosuppression† | ||
| Tacrolimus | 6 (100) | 22 (96) |
| Corticosteroids | 6 (100) | 23 (100) |
| Mycophenolate | 5 (83) | 20 (87) |
| Azathioprine | 1 (17) | 3 (13) |
| Sirolimus | 0 | 1 (4) |
| Patients with ≥1 acute cellular rejection episode | 2 (33) | 9 (29) |
*Reported for those with available records at time of transplantation. Two individuals in the seronegative group did not have records available.
†Patients were often on multiple drug regimens and thus numbers greater than total sum of patients. Immunosuppressive regimen at time of HEV serostatus determination is reported.
BMI, body mass index; CF, cystic fibrosis; CMV, cytomegalovirus; HEV, hepatitis E virus.
Table 2.
Demographic features of CF transplant recipients with chronic HEV infection
| Subject 1 | Subject 2 | Subject 3 | |
| Age (years) at time of HEV detection | 40 | 41 | 36 |
| Biological sex | Male | Male | Female |
| CFTR mutation | F508del/F508del | F508del/F508del | F508del/F508del |
| Organ transplanted | Lung | Lung | Lung |
| Months since transplantation at time of HEV detection | 24 | 91 | 111 |
| Induction therapy | Rabbit antithymocyte globulins | Rabbit antithymocyte globulins | Rabbit antithymocyte globulins |
| Immunosuppressive therapy | Tacrolimus trough target 8–10 μg/mL/mycophenolate mofetil 1 g two times per day/prednisone 5 mg | Tacrolimus trough target 8–10 μg/mL/mycophenolate mofetil 1 g two times per day/prednisone 5 mg | Tacrolimus trough target 8–10 μg/mL/mycophenolate mofetil 1 g two times per day/prednisone 5 mg |
| Blood product receipt | Packed red blood cells and irradiated platelets at time of lung transplantation. None after transplant | Packed red blood cells and irradiated platelets at time of lung transplantation. None after transplant | Packed red blood cells and irradiated platelets at time of lung transplantation. None after transplant |
| CF-related comorbidities | Pancreatic insufficiency, Pseudomonas aeruginosa colonisation, DIOS, CF-related diabetes | Pancreatic insufficiency, Pseudomonas aeruginosa colonisation, DIOS | Pancreatic insufficiency, Pseudomonas aeruginosa colonisation, chronic rhinosinusitis, CF-related diabetes |
| Additional post-transplant-related comorbidities | Chronic kidney disease | Diabetes mellitus, chronic kidney disease | Chronic lung allograft dysfunction |
| Outcome | Chronic HEV encephalopathy and death | Observation due to ongoing HEV IgM+ and HEV RT-PCR+, intolerance to therapy, increasing transaminases and declining renal function | Observation due to recurrence post-treatment and reluctance to restart therapy owing to intolerance |
CF, cystic fibrosis; DIOS, distal intestinal obstruction syndrome; HEV, hepatitis E virus.
Transplanted pwCF that were determined to be HEV seropositive were younger at the time of transplant (median (IQR) 28.6 years (22.1–32.5)) as compared with HEV seronegative (31.3 years (25.6–36.1); p=0.037) (table 1). The duration of time from transplant surgery was associated with HEV seropositivity, whereas individual age was not. Those who were >3 years post-transplant had an RR of 2.53 for HEV seropositivity (1.48–4.23, p=0.023) compared with those <3 years post-transplantation. The type and amounts of transfused blood products (eg, whole blood, platelets, plasma) did not differ between groups at the time of transplantation, and no HEV seropositive individual ever received any additional blood product after the original lung transplant surgery. We determined HEV serostatus did not associate with the amount of PERT consumed among transplanted pwCF (22 PERT capsules/day (IQR 18–26) among HEV seropositive individuals vs 23 PERT capsules/day among HEV seronegative (IQR 19–26); p=0.86). Furthermore, other characteristics of transplanted pwCF, including age and sex, did not differ by HEV serostatus (table 1).
Chronic HEV infections in transplanted pwCF
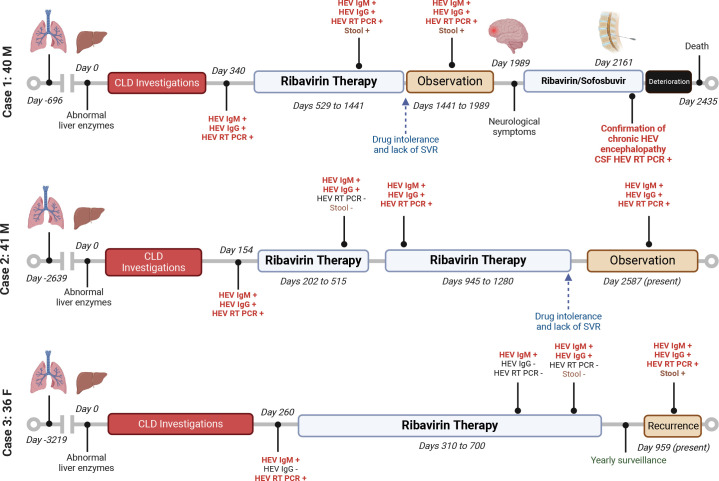
Between March 2017 and December 2022, three chronically infected HEV individuals were identified in our transplanted CF cohort (figure 3, table 2 and online supplemental figure 2).
Figure 3.
Timeline of transplanted subjects with CF with chronic HEV. Abnormal serology and viral RNA are indicated in red font. CF, cystic fibrosis; CLD, chronic liver disease; CSF, cerebrospinal fluid; SVR, sustained virological response.
gutjnl-2023-330602supp003.pdf (55.8KB, pdf)
Case 1 was a man with CF aged 40 years with no history of CF liver disease (CFLD) 2 years post-transplant when liver function abnormalities were first noted. HEV serology (IgG and IgM) and RT-qPCR were eventually found to be positive, and he was initiated on ribavirin at 600 mg (~8 mg/kg/day). He developed drug toxicity despite mitigating efforts and his viraemia was not resolved at any point through 2.5 years of therapy, leading to treatment interruption and observation. Four years following the initial HEV identification, he developed a progressive neurological syndrome including persistent nausea and weight loss and eventually gait instability and cognitive impairment. The patient was again initiated on ribavirin with subsequent addition of sofosbuvir. MRI of the brain demonstrated white matter changes and cerebrospinal fluid (CSF) analysis demonstrated a lymphocytic pleocytosis (white blood cell count 12.6×106/L, 97% lymphocytes) and HEV RNA presence was confirmed. Investigations for all other aetiologies to explain functional and cognitive decline were negative. The patient was diagnosed with HEV encephalopathy. Despite treatment, the patient’s neurological status progressively deteriorated, and he passed away 9 months later, ~6.5 years after HEV diagnosis. The family declined an autopsy.
Case 2 was a man with CF aged 41 years postlung transplantation with no history of CFLD. He developed abnormal liver enzymes 8 years after transplant and was ultimately found to be HEV IgG/IgM and RNA positive. He received ribavirin 600 mg (~8 mg/kg/day) and had documented HEV clearance (plasma and stool) after 10 months of therapy. One year later, transaminitis recurred and repeat testing demonstrated HEV RNA in plasma confirming recurrence/relapse. He was re-initiated on ribavirin but, despite mitigating efforts, was intolerant of treatment-related nausea and fatigue. This, combined with recalcitrant viral load at 9 months, led to discontinuation and observant management. Five years postdiagnosis, the patient continues to have chronic HEV infection with increasing transaminases, but unchanging liver stiffness (6.3 kPa by FibroScan, Echosens) and unwilling to consider retreatment. Because of nausea and weight loss and mood changes, neurological investigations were undertaken which did not show any MRI white matter changes and an LP did not reveal evidence of HEV RNA or lymphocytic pleocytosis.
Case 3 was a woman aged 36 years 9 years postliving-related lung transplantation. She developed abnormal liver enzymes and HEV IgM and RNA were identified. She was initiated on ribavirin therapy at 600 mg (~8 mg/kg/day) for 12 months but by 8 months was already PCR negative in plasma and stool. Repeat testing the next year demonstrated recurrence of HEV RNA, despite normal liver enzyme tests. Due to treatment-related side effects, a 2-year hiatus from repeat treatment with frequent follow-up is planned.
HEV seropositivity among non-transplanted pwCF
Given the high prevalence of HEV seropositivity in our transplanted CF cohort, we sought to explore HEV seroprevalence among a non-transplanted CF control cohort. Patients attending the same clinic were approached to participate in a prospective research protocol. Eighty-three pwCF volunteered, and 16 (19.3%) were positive for HEV IgG using two independent assays. None were positive for HEV RNA. Demographics of the control non-transplanted CF cohort are presented in table 3. No demographic or clinical factor, including patient age were found to be associated with increased risk of HEV seropositivity in this cohort. We did not observe a higher likelihood of HEV seropositivity in CF transplant recipients relative to those who had not received a transplant (RR 1.07, 95% CI 0.46 to 2.48; p=0.87).
Table 3.
Demographics of adults persons with CF without prior transplant and HEV serostatus
| HEV seropositive (n=16) N (%) |
HEV seronegative (n=67) N (%) |
|
| Characteristic | ||
| Female sex | 8 (50) | 28 (42) |
| Median age, years (IQR) | 30.9 (24.9–37.5) | 32.0 (25.0–39.3) |
| Median body mass index, median (IQR, kg/m2) | 23.2 (22.0–24.1) | 23.3 (20.9–25.1) |
| Rural postal code | 5 (31) | 22 (33) |
| Lung disease stage** | ||
| Mild | 8 (50) | 36 (54) |
| Moderate | 5 (31) | 21 (31) |
| Severe | 3 (19) | 10 (15) |
| Genotype | ||
| ΔF508 homozygous | 10 (63) | 40 (60) |
| ΔF508 heterozygous | 5 (31) | 21 (31) |
| Other | 1 (6) | 6 (9) |
| CF comorbidities | ||
| Pancreatic insufficiency | 16 (100) | 60 (90) |
| CF liver disease | 3 (19) | 16 (24) |
| GORD | 9 (56) | 41 (61) |
| Distal intestinal obstruction syndrome | 14 (88) | 42 (63) |
| CF arthropathy | 3 (19) | 9 (13) |
| Sinus disease (polyps or nasal congestion) | 15 (94) | 55 (82) |
| Respiratory infections | ||
| Pseudomonas aeruginosa | 9 (56) | 39 (58) |
| Methicillin-susceptible Staphylococcus aureus | 6 (39) | 27 (40) |
| Methicillin-resistant S. aureus | 1 (6) | 5 (8) |
| Other† | 4 (25) | 15 (22) |
*Definitions of lung stage based on forced expiratory volume in 1 s; mild ≥70%, moderate ≥40% to <70%, severe <40%.
†Other bacterial species include Escherichia coli, Enterobacter cloacae, Serratia marcescens, group C Streptococcus, Stenotrophomonas maltophilia.
CF, cystic fibrosis; HEV, hepatitis E virus.
Consistent with other CF cohorts, ~90% of individuals in each of the transplanted and non-transplanted CF cohorts were pancreatic insufficient. Furthermore, total number of PERT capsules consumed/day did not differ on the basis of transplant status (23 (IQR 19–26) vs 24 (IQR 20–27); p=0.79). Finally, PERT capsules consumed/day among non-transplanted pwCF did not differ on the basis of HEV serostatus (23 (IQR 20–27) vs 24 (IQR 22–26); p=0.63).
HEV seroprevalence in non-CF populations
Next, we sought to discern the seroprevalence of HEV in a non-CF control population using the only other dataset available—those referred for HEV testing based on clinical suspicion (ie, a preselected, at-risk population). In total, 1079 HEV serology tests were requested in all of Alberta during this 7-year period (total population of ~4.5 million residents), which was a small proportion (0.06%) of total viral hepatitides testing (hepatitis B surface antigen; total of 345 180 tests and antihepatitis C antibody; total of 1 406 224) completed by the provincial laboratory (note: much of the HBV and HCV (but not HEV) testing is also performed in regional laboratories and therefore under-reported using only the provincial health laboratory data). One hundred and fifteen Alberta residents who did not have CF (10.7%) were seropositive for HEV IgG. Thirteen (11.3%) were SOT recipients with the most prevalent being liver (n=8) followed by kidney (n=5). Despite the Southern Alberta Adult CF Clinic comprising only 0.0056% of Alberta’s population, all cases of chronic HEV hepatitis occurred in pwCF and there were no additional cases of HEV RNA positivity documented in the entirety of Alberta. Furthermore, PwCF, regardless of clinical suspicion or transplant status, had an increased HEV seropositivity risk relative to those individuals in Alberta who were specifically referred for HEV testing (22/112 (19.6%) vs 115/1079 (10.7%), RR 1.84 (95% CI 1.22 to 2.78), p=0.004).
HEV detection in pancreatic enzyme replacement therapy capsules
HEV RNA was detected by orf2 RT-qPCR in 47/107 (44%) of PERT capsules assessed based on a RT-qPCR and confirmed with RT-dPCR (figure 4, online supplemental table 3, online supplemental figure 3). Positive PERT had a median value of 50 HEV copies/capsule, IQR 23–160 copies/capsule and peak of 955 copies/capsule, by RT-qPCR. When serial samples from within the same lot were assessed by RT-qPCR, 8/16 (50%) demonstrated HEV RNA concordance among all capsules, and 50% of lots had capsules that were both positive and negative. HEV RNA was detected in PERT from all four Health Canada-approved manufacturers, although rates differed slightly (10/37 (27%), 19/33 (58%), 11/20 (55%) and 5/17 (29%), p=0.027). Three separate nested-RT-PCR assays followed by Sanger sequencing confirmed HEV RNA in qPCR-positive PERT in orf1 (including both the 5’ and tailing portion of the gene) and orf2 (figure 2). Efforts to identify HEV from PERT by whole genome sequencing, cell culture and protein assays were unsuccessful (see online supplemental file for details).
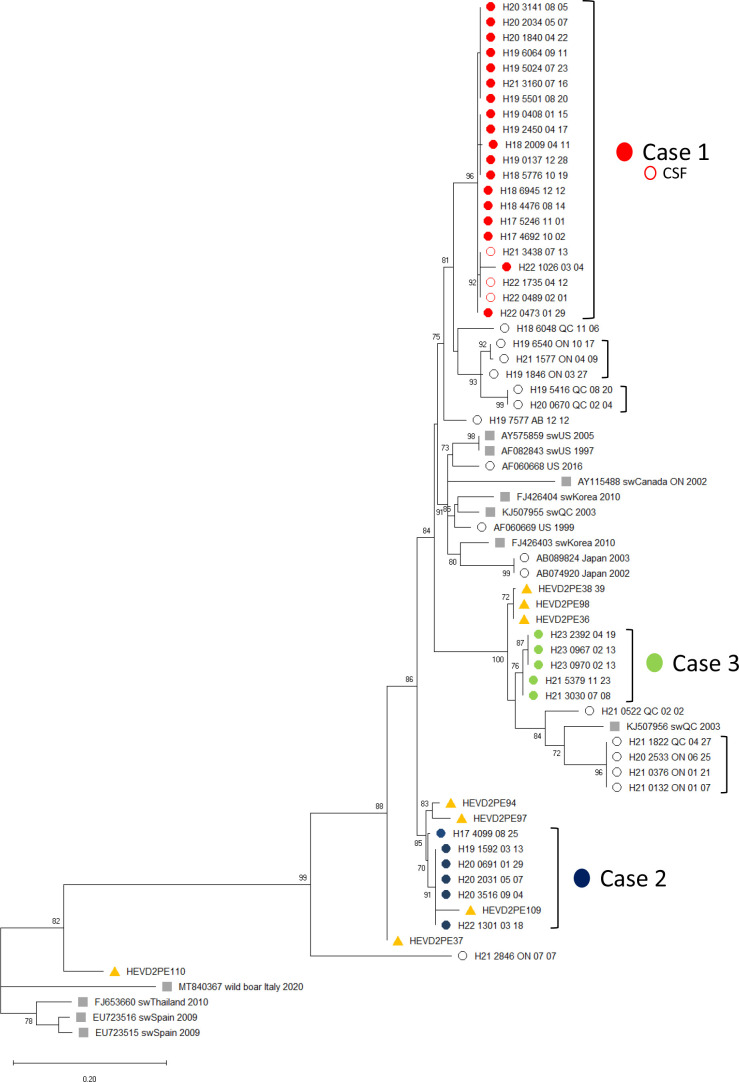
Figure 4.
Phylogenetic analysis of hepatitis E virus (HEV) sequences from lung transplant patients. HEV sequence from consecutive, longitudinal specimens from the three cystic fibrosis (CF) lung transplant recipients (filled circles derived from plasma, open circles from cerebrospinal fluid (CSF)) were aligned with reference sequences including porcine-derived HEV genotype 3 (grey squares) and non-CF human HEV genotype 3 sequences (open circle). A total of 68 orf1 sequences (8 pancreatic enzyme replacement therapy, 32 serial samples from 3 persons with CF lung transplant patients, 10 swine sequences, 1 wild boar sequence and 17 human sequences (16 acute travel-associated HEV and 1 previously not reported chronic HEV, including 13 from Canada)), were aligned, trimmed to 315 bp and analysed by maximum likelihood phylogenetic inference. Brackets denote sequences from the same individual. Those brackets without case numbers next to the cluster indicate non-CF human samples. Porcine and human HEV reference sequences show the GenBank accession number and the location and year of collection. Human sequences originating in Canada show the year (‘H’ number), followed by the patient 4-digit ID code, followed by the province and month and day of collection. The ruler shows the branch length for a pairwise distance equal to 0.2. Branch support by the approximate likelihood ratio test >70% is shown at branch nodes.
Phylogeny of HEV RNA in transplanted pwCF and PERT
The maximum likelihood phylogenetic tree of the three pwCF with chronic HEV infection and eight HEV-positive PERT was completed based on all available clinical samples in the National Microbiology Laboratory database (figure 4). The phylogenetic relationships among the different clades were confirmed by bootstrap values >70%. Based on phylogenetic analysis, the three postlung transplanted pwCF were infected with different HEV genotype 3a strains, confirming their independent acquisition. CF-associated chronic HEV cases and PERT sequences clustered with Canadian swine strains and other Canadian human HEV sequences. Notably, in cases 2 and 3 which demonstrated recurrence/relapse after apparent cure, across the 315 bp assessed there were 4 and 2 single nucleotide polymorphisms (2 and 0 differing amino acids) between the first and final isolates, respectively. As substitution rates in orf1 are expected to be <1/year, these potentially represent either ribavirin-induced mutations in occult chronically infecting populations or, less likely, potentially new independent HEV infections.
Discussion
Chronic HEV infection in SOT recipients was first reported in 2008.2 Since that time, infection in many profoundly immune suppressed cohorts have been documented.1 3 HEV is likely under-recognised as many patients are asymptomatic or have non-specific abnormal liver enzyme tests. Assessment for chronic HEV infection in immunosuppressed populations requires the simultaneous assessment of both anti-HEV antibodies (IgG and IgM) and molecular assays to detect HEV RNA (owing to the potential for false negative serology in immunosuppressed hosts).1 5 Unfortunately, chronic HEV infection can lead to rapid disease progression following acquisition.1 Whereas the majority of cases have been reported in Europe, several North American cases have more recently been described.6 7 Among chronic HEV infections in SOT, few have been reported in lung transplant recipients, including three chronic cases of HEV infection (3.2% of the total cohort reported)8 and one case of HEV-associated meningoencephalitis in antecedent idiopathic pulmonary fibrosis.9 To this point, other groups have not investigated an HEV chronic infection predilection in other cohorts such as pwCF. However, as 20% of adults with CF are lung transplant recipients, and lung transplant recipients are among the most heavily immunosuppressed SOT populations, understanding factors associated with adverse outcomes, such as HEV infection, are of the utmost importance.
HEV infection in high-income countries is predominately believed to be acquired through direct contact with pigs or the ingestion of undercooked pork, but as the vast majority of infections are asymptomatic, clinical diagnoses are rare.10 This is well illustrated by the very low frequency of clinical testing reported here among the 4.5 million individuals living in Alberta, Canada’s fourth most populous province. In contrast, a systematic review of HEV infection in the Americas observed a pooled seroprevalence ranging between 3.4% and 10.7% suggesting exposures over a lifetime do occur.11 An important study of nearly 14 000 Canadian blood donors were tested for HEV RNA.12 None of the specimens were HEV RNA positive. A subset was tested for HEV IgG and positivity rates ranged from 1.8% in Nova Scotia/New Brunswick to 6.7% in Quebec (average 5.9%). Seropositivity rates were highest in men, those working with farm animals and those born outside of Canada. In contrast, we observed uncharacteristically high rates of HEV seropositivity in pwCF, irrespective of transplant status, twice that even of those suspected of HEV (ie, those Albertan’s screened for HEV) and fourfold higher than the general Canadian population. Notably in our cohort, there was no association between rural postal code and HEV seropositivity, and all three individuals with chronic HEV infection were Canadian-born urban professionals and none reported ever eating raw or uncommon (ie, liver) pork products suggesting alternate risk factors.
Whereas blood transfusions have previously been evaluated as a risk factor for HEV transfusion-transmission in SOT recipients, we do not believe they were a contributing factor in pwCF. This is relevant as those undergoing lung transplantation almost universally receive blood products at the time of surgery.13 A recent large study in the USA and Canada of over 100 000 donations found the frequency of HEV RNA was exceedingly low in blood products, with ~1:17 000 in the USA and ~1:4600 in Canada (p=0.062).14 A risk-based decision-making process activity was undertaken by Canadian Blood Services to assess risks for HEV infection with different types of transplants. The estimated rate in heart and lung transplant recipients (lung transplant were not parsed out from heart transplants) of products in Canada (except for Quebec) was once every 711 years.15 As CF accounts for a mere 2% of SOT surgeries each year in Alberta (~20% of lung transplant surgeries are for CF, and lung transplants comprise only 11% of SOTs (n=53/495)),16 and all our observed cases of chronic HEV infection were observed in CF lung transplant recipients, this represents a significant outlier. Importantly, in the CF transplant cohort, the number of blood products received by HEV seropositive pwCF, including those with chronic HEV infection did not differ from seronegative and none received products after the transplant procedure itself. Finally, the timeline of transfusion as a risk for HEV infection is also inconsistent, as pwCF eventually diagnosed with chronic HEV infection first developed liver enzyme abnormalities many years after transplantation (the time of last transfusion) whereas median time to liver enzyme abnormality after incident infection has been estimated to be a mere 2–6 weeks in other cohorts.10
We postulated the only particularly unique feature of pwCF relative to other populations that may predispose to HEV, a zoonotic infection mostly commonly acquired from pigs, is the high frequency of PI (~90%). In fact, CF was first recognised and ultimately named on the basis of the pathological appearance of the pancreas at autopsy.17 Sixty per cent of pwCF are born with PI and up to 90% will become PI by 1 year of age.18 PI is managed through exogenous porcine-derived PERT administered in capsules where lipase, protease and amylase enzymes are packaged into granules or microspheres coated with a pH-sensitive matrix preventing enzymatic degradation in the stomach and enabling their release in the alkaline environment of the duodenum.19 Whereas PERT was initially exempt from Food and Drug Administration (FDA) approval in 1938, in 2006, new requirements for manufacturing and safety were introduced with all currently approved formulations derived from pigs.20 Adverse events attributed to PERT have rarely been described, with the exception of the potential for fibrosing colonopathy in young pwCF receiving >10 000 U lipase/kg/day.21 In fact, UK consensus guidelines for management of PI-indicated PERT is not associated with any significant complications (grade 1A, 100% agreement).22 Moreover, the FDA’s PERT guidance statement outlines ‘it is not necessary to conduct long-term safety evaluations of PERT in support of new drug applications; this is because of the long and extensive safety experience with PERT’. However, the potential for zoonotic transmission of viruses through porcine-derived PERT was always recognised.23
Potential safety mechanisms in PERT production presumed to mitigate zoonoses risk relate to certificates of animal health, acceptance criteria and viral load testing, viral inactivation studies and surveillance for animal diseases.24 However, a study of Canadian commercial swine herds (~1000 animals) demonstrated that by 6 months of age (ie, time of slaughter) 60% were HEV seropositive, with HEV RNA detected in the faeces of many.25 Furthermore, pigs infected with HEV are asymptomatic26 and can shed virus for ≥60 days.27 In porcine models of HEV infection, the pancreas, in particular the acinar cell-rich areas (from which PERT is derived), are disproportionally infected.28 HEV is highly resilient, as demonstrated by its ability to be transmitted in a range of pork products that are not properly cooked at >71°C for >20 min.29
While studies involving recipients of HEV-infected blood products suggests the lowest HEV infectious doses ranges from 7000 to 36 000 IU depending on the product,30 the required infectious dose in profoundly immunosuppressed hosts is likely to be markedly lower.
In our study of independently sourced PERT, we found 44% to be HEV RNA positive. For the average pwCF consuming 24 PERT capsules per day (median of the cohort), this would equate to 3680 HEV RNA-positive PERT capsules consumed per year. If even 1/10 000 of these RNA-positive PERT capsules had infectious HEV, it would still equate to ~0.4 exposures per person per year in our cohort. Furthermore, as multiple PERT are consumed at a time the risk posed by any individual capsule is enhanced through cumulative dosing. While our extraction protocols were designed to mitigate the extremely protein dense matrix of PERT and compensate for its enteric coating, high levels of PCR inhibition were identified suggesting that rates of PERT positivity and amount of HEV RNA identified here are likely underestimated. Indeed, we were able to sequence both HEV orf1 (multiple segments) and orf2 loci despite the relatively low levels of HEV RNA detected by RT-qPCR. We were not surprised to have been unable to confirm HEV by either whole genome sequencing, capsid protein assessment or in vitro cell culture assay given the established insensitivities of these modalities relative to their molecular counterparts and the challenging nature of the PERT matrix.31 Furthermore, in vitro testing of PERT is likely to be vastly underpowered to detect viable HEV given the fact that the assessed 107 PERT capsules are the equivalent to a mere ~4.5 days of use by the average pwCF—and we would expect the frequency of viable HEV contaminating PERT to be very rare given the few clinical cases identified. Owing to the intrinsic challenges of performing molecular and genomic testing on the protein dense and inhibitor-rich PERT capsules, we believe that the best means to definitively prove the potential of HEV acquisition from PERT would be through controlled animal challenge experiments—allowing for the longitudinal and cumulative exposure of individual subjects to the large quantities of PERT required to identify these exceedingly rare acquisition events.
Despite our hypothesis that PERT represents an HEV exposure risk disproportionate to pwCF, and our confirmation that HEV RNA does contaminate a high proportion of PERT, the risk posed by any individual PERT capsule must still be remarkably low explaining the small numbers of cases observed here. However, this does not diminish the potential scope of HEV contamination of PERT. It is estimated there are ~105 000 diagnosed individuals living with CF worldwide,32 90% of whom use PERT. Among these, 519 and 1645 individuals are transplant recipients (~97% lung) living in Canada4 and the USA,33 alone. Furthermore, there are many other conditions for which PERT are routinely prescribed (ie, postpancreatectomy and those with chronic pancreatitis). The scope of zoonotic risks from PERT may not be confined merely to HEV. Other porcine-derived human zoonoses may also be possible including established agents such as Japanese encephalitis virus, Nipah virus and swine/avian influenza strains, and others that could potentially infect humans including porcine coronaviruses, circovirus and parvovirus.34 Seroprevalence studies assessing for disproportionate exposure risk of these agents could be considered in CF and other PERT-using populations.
A potential explanation as to the absence of other reported cases of HEV infection in CF may relate to the very high frequency with which diseases of the liver exists in CF, with CFLD present in up to one-third of individuals.35 Whereas classic CFLD is attributed to ductal cholestasis, resulting in inflammation and periportal fibrosis, several other presentations are observed including hepatic steatosis and focal biliary and multilobar cirrhosis. Furthermore, abnormal liver enzymes are observed frequently through longitudinal observation, including in >90% of children with CF.36 Accordingly, clinicians may simply fail to investigate alternate causes of liver enzyme rise, and progressive liver disease, such as HEV.
The management of chronic HEV infection in SOT recipients is complex, and clinical practice guidelines have been developed to guide therapy.37 Some chronically infected individuals will clear infections spontaneously. The first step proposed by many experts is reducing immunosuppression, a strategy that has been associated with treatment success in other SOT recipients.38 Dose reduction in maintenance immunosuppression was not undertaken here owing to concerns held by lung transplant care providers about potential allograft rejection. In many cohorts, treatment with ribavirin is effective and sustained virological response rates as high as 78% have been reported within 3 months in some studies,39 despite none having achieved here. Individuals in our cohort received consistent ribavirin treatment, and dose reductions were avoided as late as possible unless haemoglobin levels fell below 90 g/L with associated prostrating fatigue. Significant nausea and vomiting were universally observed and proved challenging for the patients. While two individuals demonstrated treatment-related viral clearance (having received treatments much longer than conventional published cohorts), both relapsed/recurred or were reinfected. Moreover, the first case did not ever demonstrate any significant reduction in viral loads despite normalisation of liver function tests. Importantly, no mutations associated with ribavirin resistance, including G1634R, were identified in any patient samples.40 While it is unknown if specific HEV genotypes may show differing treatment responsiveness to ribavirin, the HEV 3a strains observed here are uncommonly identified in European populations where treatment success rates appear to be higher.41
We recognise several limitations of this work. First, as a cross-sectional, single-centre study, we were unable to determine timing of acquisition and spontaneous clearance of viraemia in those HEV with seropositivity, but negative for HEV RNA. Because of the nebulous time frame, we did not perform a formal risk exposure questionnaire across cohorts which could help identify past exposures to potential sources of HEV in future surveillance studies. While other studies have previously associated HEV seropositivity risk with ingestion of pork products (ie, bacon and cured meats),42 it is important to note that HEV RNA has not been identified in commonly consumed commercial meat products such as rib, bacon, lean ham and loin.43–46 In stark contrast, we observed HEV RNA in 44% of all PERT capsules screened (equating to 10 HEV RNA-positive capsules consumed by each pwCF daily). We acknowledge the small sample size of our CF cohort, but we sought to mitigate this by including all CF transplanted individuals and complimentary control groups including a larger non-transplanted CF population residing in the same area and >1000 non-CF controls (ie, every patient in our province, with a population of >4.5 million individuals, where HEV infection was queried over the last 7 years). Importantly, aside from our transplanted pwCF cases, no other cases of chronic HEV infection were identified in any other cohort we evaluated. As this was a single-centre study, this may not be representative of other CF and transplant centres as there may be inherent geographical differences in prevalence of HEV.11 Most importantly, while we have identified HEV RNA in 44% of all PERT capsules screened (including samples from all four Canadian manufacturers), we have not confirmed replication-competent HEV.
Conclusion
Here, we describe the first cases series of chronic HEV infection identified in Alberta (and Canada)—all in pwCF post-transplant. Only one other HEV chronic infection case has previously been published in a Canadian liver transplant recipient,47 and none other have been identified in Canada’s single National reference laboratory. Treatment proved exceedingly challenging with no case cured, one death and significant morbidity experienced by all. The identification of disproportionate HEV seropositivity among pwCF and a high prevalence of HEV RNA contaminating PERT (a commonly prescribed FDA-approved, European Medicines Agency-approved and Health Canada-approved therapeutic class) suggests a potential iatrogenic mechanism of HEV acquisition that must be further explored. Screening for HEV infections in pwCF post-transplant and establishing the seroprevalence of HEV in other cohorts of pwCF are urgently required.
gutjnl-2023-330602supp004.pdf (24KB, pdf)
Acknowledgments
The authors wish to acknowledge and are appreciative of the efforts of the patient and staff of the Southern Alberta Adult Cystic Fibrosis Clinic.
Footnotes
@cthornton32
Contributors: CST, BJW, MDP planned the study. BJW, JS carried out laboratory analysis. CST, BJW, SEC, JS, LF, RS, KF, DI, KD, SJD JB, CO and MDP gathered and analysed data. CST, BJW and MDP wrote the first draft of the manuscript. All authors contributed to revisions of the final draft. MDP is the guarantor of the work.
Funding: This work was supported in part by grants from the University of Calgary to MDP.
Competing interests: SEC: reports grants from Bristol-Myers Squibb Canada, Gilead Sciences, Genfit, Allergan, Axcella Health, and Sequana Medical and personal fees from Intercept Pharmaceuticals, Eisai, Paladin Labs, AstraZeneca and Novo Nordisk outside the submitted work.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s).
Ethics approval
This study was approved by the University of Calgary’s Conjoint Health Research Ethics Board (REB22-1216). Participants gave informed consent to participate in the study before taking part.
References
- 1. Kamar N, Dalton HR, Abravanel F, et al. Hepatitis E virus infection. Clin Microbiol Rev 2014;27:116–38. 10.1128/CMR.00057-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kamar N, Selves J, Mansuy J-M, et al. Hepatitis E virus and chronic hepatitis in organ-transplant recipients. N Engl J Med 2008;358:811–7. 10.1056/NEJMoa0706992 [DOI] [PubMed] [Google Scholar]
- 3. Wilhelm B, Waddell L, Greig J, et al. A systematic review and meta-analysis of predictors of human hepatitis E virus exposure in non-Endemic countries. Zoonoses Public Health 2020;67:391–406. 10.1111/zph.12698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cystic Fibrosis Canada . 2021 annual data report. 2023. Available: https://www.cysticfibrosis.ca/registry/2021AnnualDataReport.pdf [Accessed 23 Apr 2023].
- 5. McPherson S, Elsharkawy AM, Ankcorn M, et al. Summary of the British transplantation society UK guidelines for hepatitis E and solid organ transplantation. Transplantation 2018;102:15–20. 10.1097/TP.0000000000001908 [DOI] [PubMed] [Google Scholar]
- 6. Hansrivijit P, Trongtorsak A, Puthenpura MM, et al. Hepatitis E in solid organ transplant recipients: a systematic review and meta-analysis. World J Gastroenterol 2021;27:1240–54. 10.3748/wjg.v27.i12.1240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Samala N, Wang RY, Auh S, et al. Hepatitis E prevalence and infection in solid-organ transplant recipients in the United States. J Viral Hepat 2022;29:1134–42. 10.1111/jvh.13739 [DOI] [PubMed] [Google Scholar]
- 8. Pischke S, Greer M, Hardtke S, et al. Course and treatment of chronic hepatitis E virus infection in lung transplant recipients. Transpl Infect Dis 2014;16:333–9. 10.1111/tid.12183 [DOI] [PubMed] [Google Scholar]
- 9. Murkey JA, Chew KW, Carlson M, et al. Hepatitis E virus-associated Meningoencephalitis in a lung transplant recipient diagnosed by clinical Metagenomic sequencing. Open Forum Infect Dis 2017;4:ofx121. 10.1093/ofid/ofx121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dalton HR, Izopet J. Transmission and epidemiology of hepatitis E virus genotype 3 and 4 infections. Cold Spring Harb Perspect Med 2018;8:a032144. 10.1101/cshperspect.a032144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fernández Villalobos NV, Kessel B, Rodiah I, et al. Seroprevalence of hepatitis E virus infection in the Americas: estimates from a systematic review and meta-analysis. PLoS One 2022;17:e0269253. 10.1371/journal.pone.0269253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fearon MA, O’Brien SF, Delage G, et al. Hepatitis E in Canadian blood donors. Transfusion 2017;57:1420–5. 10.1111/trf.14089 [DOI] [PubMed] [Google Scholar]
- 13. Yoo D-W, Lee H-J, Oh S-H, et al. Transfusion requirements and blood bank support in heart and lung transplantation. Lab Med 2021;52:74–9. 10.1093/labmed/lmaa044 [DOI] [PubMed] [Google Scholar]
- 14. Delage G, Fearon M, Gregoire Y, et al. Hepatitis E virus infection in blood donors and risk to patients in the United States and Canada. Transfus Med Rev 2019;33:139–45. 10.1016/j.tmrv.2019.05.017 [DOI] [PubMed] [Google Scholar]
- 15. Ward S, Delage G, Gregoire Y, et al. Outcomes of a risk-based decision-making process for reducing the risk of hepatitis E virus transfusion transmission via the Canadian blood supply. AMMI/CACMID 2021, virtual meeting. 2021 annual conference Conférence Annuelle. Journal of the Association of Medical Microbiology and Infectious Disease Canada 2021;6:1–85. 10.3138/jammi.6.s1.abst [DOI] [Google Scholar]
- 16. Statistics Canada. Available: https://www.cihi.ca/en/summary-statistics-on-organ-transplants-wait-lists-and-donors2023 [Accessed 20 Aug 2023].
- 17. Andersen DH. Cystic fibrosis of the Pancreas and its relation to celiac disease: a clinical and pathologic study. American Journal of Diseases of Children 1938;56:344–99. 10.1001/archpedi.1938.01980140114013 [DOI] [Google Scholar]
- 18. O’Sullivan BP, Baker D, Leung KG, et al. Evolution of Pancreatic function during the first year in infants with cystic fibrosis. J Pediatr 2013;162:808–12. 10.1016/j.jpeds.2012.10.008 [DOI] [PubMed] [Google Scholar]
- 19. Freswick PN, Reid EK, Mascarenhas MR. Pancreatic enzyme replacement therapy in cystic fibrosis. Nutrients 2022;14:1341. 10.3390/nu14071341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Giuliano CA, Dehoorne-Smith ML, Kale-Pradhan PB. Pancreatic enzyme products: digesting the changes. Ann Pharmacother 2011;45:658–66. 10.1345/aph.1P770 [DOI] [PubMed] [Google Scholar]
- 21. FitzSimmons SC, Burkhart GA, Borowitz D, et al. High-dose pancreatic-enzyme supplements and fibrosing colonopathy in children with cystic fibrosis. N Engl J Med 1997;336:1283–9. 10.1056/NEJM199705013361803 [DOI] [PubMed] [Google Scholar]
- 22. Phillips ME, Hopper AD, Leeds JS, et al. Consensus for the management of pancreatic exocrine insufficiency: UK practical guidelines. BMJ Open Gastroenterol 2021;8:e000643. 10.1136/bmjgast-2021-000643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Food and Drug Administration . Center for drug evaluation and research application number: 20-725 summary review. 2009. Available: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2009/020725s000SumR.pdf [Accessed 23 Apr 2023].
- 24. Food and Drug Administration . CDER 2008 meeting documents. 2008. Available: https://web.archive.org/web/20170210022953/http://www.fda.gov/ohrms/dockets/ac//08/transcripts/2008-4402t1-Part1.pdf [Accessed 23 Apr 2023].
- 25. Yoo D, Willson P, Pei Y, et al. Prevalence of hepatitis E virus antibodies in Canadian swine herds and identification of a novel variant of swine hepatitis E virus. Clin Diagn Lab Immunol 2001;8:1213–9. 10.1128/CDLI.8.6.1213-1219.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Casas M, Pina S, de Deus N, et al. Pigs orally inoculated with swine hepatitis E virus are able to infect contact sentinels. Vet Microbiol 2009;138:78–84. 10.1016/j.vetmic.2009.03.008 [DOI] [PubMed] [Google Scholar]
- 27. Dähnert L, Eiden M, Schlosser J, et al. High sensitivity of domestic pigs to intravenous infection with HEV. BMC Vet Res 2018;14:381. 10.1186/s12917-018-1713-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jung S, Seo DJ, Yeo D, et al. Experimental infection of hepatitis E virus induces pancreatic necroptosis in miniature pigs. Sci Rep 2020;10:12022. 10.1038/s41598-020-68959-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Barnaud E, Rogée S, Garry P, et al. Thermal inactivation of infectious hepatitis E virus in experimentally contaminated food. Appl Environ Microbiol 2012;78:5153–9. 10.1128/AEM.00436-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gallian P, Lhomme S, Morel P, et al. Risk for hepatitis E virus transmission by solvent/detergent-treated plasma. Emerg Infect Dis 2020;26:2881–6. 10.3201/eid2612.191482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Papp C-P, Biedermann P, Harms D, et al. Advanced sequencing approaches detected insertions of viral and human origin in the viral genome of chronic hepatitis E virus patients. Sci Rep 2022;12:1720. 10.1038/s41598-022-05706-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Guo J, Garratt A, Hill A. Worldwide rates of diagnosis and effective treatment for cystic fibrosis. J Cyst Fibros 2022;21:456–62. 10.1016/j.jcf.2022.01.009 [DOI] [PubMed] [Google Scholar]
- 33. Cystic Fibrosis Foundation . 2021 patient registry annual data report. 2022. Available: https://www.cff.org/medical-professionals/patient-registry [Accessed 23 Apr 2023].
- 34. McLean RK, Graham SP. The pig as an amplifying host for new and emerging Zoonotic viruses. One Health 2022;14:100384. 10.1016/j.onehlt.2022.100384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kobelska-Dubiel N, Klincewicz B, Cichy W. Liver disease in cystic fibrosis. Prz Gastroenterol 2014;9:136–41. 10.5114/pg.2014.43574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Woodruff SA, Sontag MK, Accurso FJ, et al. Prevalence of elevated liver enzymes in children with cystic fibrosis diagnosed by newborn screen. J Cyst Fibros 2017;16:139–45. 10.1016/j.jcf.2016.08.002 [DOI] [PubMed] [Google Scholar]
- 37. European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu, European Association for the Study of the Liver . EASL clinical practice guidelines on hepatitis E virus infection. J Hepatol 2018;68:1256–71. 10.1016/j.jhep.2018.03.005 [DOI] [PubMed] [Google Scholar]
- 38. Kamar N, Abravanel F, Selves J, et al. Influence of immunosuppressive therapy on the natural history of genotype 3 hepatitis-E virus infection after organ transplantation. Transplantation 2010;89:353–60. 10.1097/TP.0b013e3181c4096c [DOI] [PubMed] [Google Scholar]
- 39. Kamar N, Mallet V, Izopet J. Ribavirin for chronic hepatitis E virus infection. N Engl J Med 2014;370:2447–8. 10.1056/NEJMc1405191 [DOI] [PubMed] [Google Scholar]
- 40. Todt D, Gisa A, Radonic A, et al. In vivo evidence for ribavirin-induced Mutagenesis of the hepatitis E virus genome. Gut 2016;65:1733–43. 10.1136/gutjnl-2015-311000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. von Felden J, Alric L, Pischke S, et al. The burden of hepatitis E among patients with haematological malignancies: a retrospective European cohort study. J Hepatol 2019;71:465–72. 10.1016/j.jhep.2019.04.022 [DOI] [PubMed] [Google Scholar]
- 42. Smith I, Said B, Vaughan A, et al. Case-control study of risk factors for acquired hepatitis E virus infections in blood donors, United kingdom, 2018-2019. Emerg Infect Dis 2021;27:1654–61. 10.3201/eid2706.203964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. García N, Hernández M, Gutierrez-Boada M, et al. Occurrence of hepatitis E virus in pigs and pork cuts and organs at the time of slaughter, Spain, 2017. Front Microbiol 2019;10:2990. 10.3389/fmicb.2019.02990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Leblanc D, Poitras E, Gagné M-J, et al. Hepatitis E virus load in swine organs and tissues at slaughterhouse determined by real-time RT-PCR. Int J Food Microbiol 2010;139:206–9. 10.1016/j.ijfoodmicro.2010.02.016 [DOI] [PubMed] [Google Scholar]
- 45. Feurer C, Le Roux A, Rossel R, et al. High load of hepatitis E viral RNA in pork livers but absence in pork muscle at French slaughterhouses. Int J Food Microbiol 2018;264:25–30. 10.1016/j.ijfoodmicro.2017.10.013 [DOI] [PubMed] [Google Scholar]
- 46. Krog JS, Larsen LE, Breum SØ. Tracing hepatitis E virus in pigs from birth to slaughter. Front Vet Sci 2019;6:50. 10.3389/fvets.2019.00050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Halac U, Béland K, Lapierre P, et al. Chronic hepatitis E infection in children with liver transplantation. Gut 2012;61:597–603. 10.1136/gutjnl-2011-300708 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
gutjnl-2023-330602supp001.pdf (23.8KB, pdf)
gutjnl-2023-330602supp002.pdf (328.3KB, pdf)
gutjnl-2023-330602supp003.pdf (55.8KB, pdf)
gutjnl-2023-330602supp004.pdf (24KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information.