Abstract
Objectives
Primary mucinous ovarian carcinoma represents 3% of ovarian cancers and is typically diagnosed early, yielding favorable outcomes. This study aims to identify risk factors, focussing on the impact of age and ethnicity on survival from primary mucinous ovarian cancer.
Methods
A retrospective observational study of patients treated at Sandwell and West Birmingham Hospitals NHS Trust and University Hospital Coventry and Warwickshire. Patients included were women aged ≥16 years, with primary mucinous ovarian cancer confirmed by specialist gynecological histopathologist and tumor immunohistochemistry, including cytokeratin-7, cytokeratin-20, and CDX2. Statistical analyses were performed using R integrated development environment, with survival assessed by Cox proportional hazards models and Kaplan-Meier plots.
Results
A total of 163 patients were analyzed; median age at diagnosis was 58 years (range 16–92), 145 (89%) were International Federation of Gynecology and Obstetrics stage I and 43 (26%) patients had infiltrative invasion. Women aged ≤45 years were more likely to have infiltrative invasion (RR=1.38, 95% CI 0.78 to 2.46), with increased risk of death associated with infiltrative invasion (HR=2.29, 95% CI 1.37 to 5.83). Compared with White counterparts, South Asian women were more likely to undergo fertility-sparing surgery (RR=3.52, 95% CI 1.48 to 8.32), and have infiltrative invasion (RR=1.25, 95% CI 0.60 to 2.58). South Asian women undergoing fertility-sparing surgery had worse prognosis than those undergoing traditional staging surgery (HR=2.20, 95% CI 0.39 to 13.14). In FIGO stage I disease, 59% South Asian and 37% White women received adjuvant chemotherapy (p=0.06). South Asian women exhibited a worse overall prognosis than White women (HR=2.07, 95% CI 0.86 to 4.36), particularly pronounced in those aged ≤45 years (HR=8.75, 95% CI 1.22 to 76.38).
Conclusion
This study identified young age as a risk factor for diagnosis of infiltrative invasion. Fertility-sparing surgery in South Asian women is a risk factor for poorer prognosis. South Asian women exhibit poorer overall survival than their White counterparts.
Keywords: Surgery; Ovarian Cancer; Cystadenocarcinoma, Mucinous; Pathology
WHAT IS ALREADY KNOWN ON THIS TOPIC
Primary mucinous ovarian cancer is rare, with a generally overall favorable prognosis due to early diagnosis. In cases of advanced or recurrent disease the outcomes are very poor with poor response to adjuvant chemotherapy. Currently known risk factors for prognosis include infiltrative invasion tumors, advanced stage at diagnosis, and residual disease following surgical treatment.
WHAT THIS STUDY ADDS
This pilot study aimed to identify additional risk factors for poor prognosis to allow for better risk stratification of primary mucinous ovarian cancer. This pilot study identified young age (≤45 years) as a risk factor for infiltrative invasion (RR=1.38); South Asian ethnicity as a risk factor for poor prognosis (HR=2.07); and fertility-sparing surgery as a risk factor for poor prognosis (HR=2.74); however, these findings need confirmation in a larger study.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
The findings indicate an underlying biological mechanism may drive the potentially elevated risk of increased infiltrative invasion in young women and poor prognosis in South Asian women. These pilot results need further confirmation in a larger multicenter study. In future research, we will undertake a larger validation study and investigate the molecular landscape with genomic sequencing. Finally, the study findings underscore the need for a re-evaluation of approach and risk stratification when assessing young and, particularly South Asian, patients. Surgical approach (fertility-sparing surgery vs traditional staging surgery) should be considered carefully with thorough patient counseling, alongside liberal referral to specialist gynecological oncology services for at-risk patients while further evidence is developed in this field.
Introduction
Primary mucinous ovarian carcinoma represents 3% of all ovarian cancers. It is often diagnosed early, with at least 80% presenting in International Federation of Gynecology and Obstetrics (FIGO) stage I(), resulting in a favorable prognosis. However, several diagnostic challenges persist, specifically differentiating primary mucinous ovarian cancer as distinct from metastases to the ovary due to clinical, morphological, and immunohistochemical similarities1 2. FIGO stage I disease achieves up to 90% 5-year overall survival, whereas diseases complicated by ovarian metastases may result in a life expectancy of <6 months.3 Consequently, diagnostic accuracy is critical. This diagnostic challenge has previously contributed to over-diagnosis, impacting clinical practice and research.4 5 Datasets lacking central pathology review and rigorous immunohistochemistry may be unreliable to adequately explore novel and known prognostic factors.
For those with a ‘true’ histological diagnosis of primary mucinous ovarian cancer, adverse clinical outcomes have been found to be associated with extensive disease spread at surgery (advanced FIGO stage), tumor with infiltrative invasion pattern, and incomplete cytoreduction (residual disease)6–10 In our clinical practice, we observed that young women of South Asian ethnicity with early-stage disease appeared to have poorer survival outcomes than their White counterparts. This study aims to identify novel risk factors for poor outcome; specifically assessing the impact of age and ethnicity on primary mucinous ovarian cancer survival.
Methods
Study Design
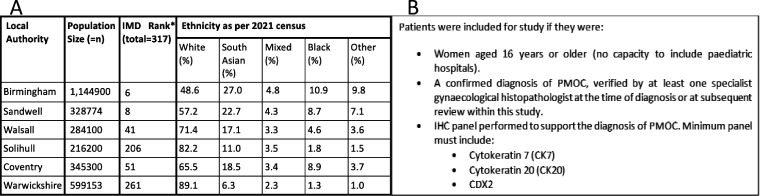
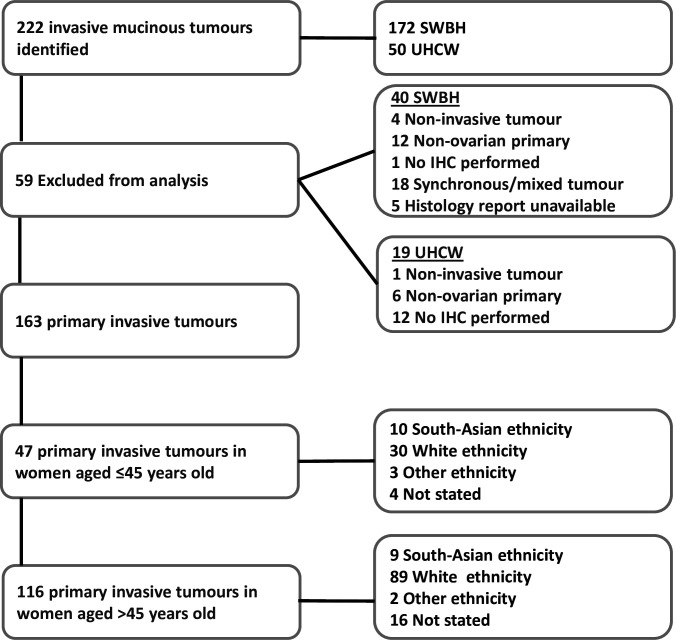
A retrospective observational study design was used, gathering consecutive data between 2005 and 2023 from the cancer registry of two neighboring gynecological cancer centers within the West Midlands, United Kingdom: Pan-Birmingham Gynecological Cancer Center and University Hospital Coventry and Warwickshire. These hospitals serve a diverse population of 2.9 million people (Figure 1A), 50% of whom reside in areas within the bottom 2.5% for deprivation.11 Study approval was obtained from the respective institutional clinical effectiveness and service improvement departments. Information on demography, histology, treatment modality, disease stage based on 1998 and 2014 FIGO classifications12 13, and clinical outcomes was abstracted from the 222 patients identified for review (Figure 1B). Ethnicity was self-reported and based on categories described by the Office of National Statistics ethnicity categories.14 15
Figure 1.
Population and patient selection methods. (A) Description of population served by cancer centers involved in this study including population size and ethnic composition.14 (B) Study inclusion criteria ensuring the selection of eligible participants. *IMD Rank, index of multiple deprivation rank of all 317 local authority councils in England: rank 1=most deprived, rank 317=least deprived.11 PMOC, primary mucinous ovarian cancer.
Initial diagnoses of primary mucinous ovarian cancer were made by gynecology specialist histopathologists. Review of histopathology reports was conducted by study authors under the supervision of expert gynecological histopathologists following specific training on the interpretation of morphological and immunohistochemical findings in primary mucinous ovarian cancer. Secondary report review by an expert histopathologist was carried out in 46/222 (21%) cases; achieving 100% interpretation concordance. Patients were included from prior to the WHO standardized reporting of primary mucinous ovarian cancer histological invasion ‘infiltrative’ or ‘expansile’16; in patients where information was insufficient to designate invasion they were recorded as ‘not stated’.
Statistical analysis
Statistical analysis used version 4.3.0 of the R integrated development environment.17 Descriptive statistics represented baseline characteristics. Fisher's exact tests were used for categorical comparisons. Relative risks comparisons based on ethnicity and age were determined using generalized linear models of the binomial family with log link. Survival analysis was conducted using Cox proportional hazards models using Firth’s correction and Kaplan-Meier plots for the respective exposure arms.
Results
Overall characteristics
A total of 222 patients were identified from the database of the two cancer centers. Overall, 59 patients did not match the histological inclusion criteria; therefore, 163 patients were included in this study (Figure 2). Within the study cohort 145 (89%) patients had FIGO stage I, 4 (2%) FIGO stage II, and 14 (9%) FIGO stage III. Invasion type was available for 144 (88%) of patients, with 43 (26%) infiltrative and 101 (62%) expansile invasion. Surgery was performed by a gynecological oncologist in 116 (71%) patients, a gynecologist in 46 (28%) of patients and not attempted in 1 (1%) patient. Surgical efforts were recorded as either traditional staging surgery, including hysterectomy and bilateral salpingo-oophorectomy omental and peritoneal biopsies (141, 86%); fertility-sparing staging surgery with retention of uterus and contralateral ovary if appropriate along side staging omental and peritoneal biopsies (19, 12%); and no surgical treatment in cases of exploratory laparotomy/laparoscopy or no surgery (5, 3%). Staging surgery excludes lymph node assessment, as this practice is not routinely performed at either of the participating sites.18 The median age at diagnosis was 58 years (range 16–92); 47 (29%) were ≤45 years old. There were 119 (73%) White, 19 (12%), South Asian, 5 (3%) other, and 20 (12%) not recorded ethnicities included. Table 1 summarizes the clinicopathological characteristics of our study population. In view of sample numbers, ethnicity comparisons were between White and South Asian patients only.
Figure 2.
Consort diagram illustrating the process of case selection for study inclusion and ethnicity and groupings within age categories. IHC, immunohistochemistry; SWBH, Sandwell and West Birmingham Hospitals; UHCW, University Hospital Coventry and Warwickshire.
Table 1.
Clinical characteristics of all patients with validated primary mucinous ovarian cancer
| Characteristics (n=163) | All ethnicities, N (%)/median (IQR) | South Asian (n=19) | White (n=119) |
| Stage at diagnosis | |||
| 1A | 76 (47%) | 5 (26%) | 58 (49%) |
| 1B | 1 (1%) | 0 (0%) | 1 (1%) |
| 1C | 68 (42%) | 13 (68%) | 45 (38%) |
| 2 | 4 (2%) | 0 (0%) | 3 (3%) |
| 3 | 14 (9%) | 1 (5%) | 12 (10%) |
| Invasion type | |||
| Infiltrative | 43 (26%) | 6 (32%) | 28 (24%) |
| Expansile | 101 (62%) | 12 (63%) | 77 (64%) |
| Not available | 19 (12%) | 1 (5%) | 14 (12%) |
| Surgeon | |||
| Gynecological oncologist | 116 (71%) | 8 (42%) | 93 (78%) |
| Gynecologist | 46 (28%) | 10 (53%) | 26 (22%) |
| No surgical attempt | 1 (1%) | 1 (5%) | 0 (0%) |
| Surgery type | |||
| Fertility-sparing surgery | 19 (12%) | 6 (32%) | 10 (8%) |
| Traditional staging surgery | 139 (85%) | 12 (63%) | 106 (89%) |
| No surgical treatment | 5 (3%) | 1 (5%) | 3 (3%) |
| Adjuvant chemotherapy | |||
| Yes | 66 (40%) | 11 (58%) | 49 (41%) |
| No | 97 (60%) | 8 (42%) | 70 (59%) |
| Survival | |||
| 5-Year overall survival | 136 (79%) | 13 (59%) | 101 (83%) |
| Median survival (IQR) | 75 (31, 132) | 46 (17, 72) | 89 (35,138) |
Impact of Recognized Risk Factors in Primary Mucinous Ovarian Cancer
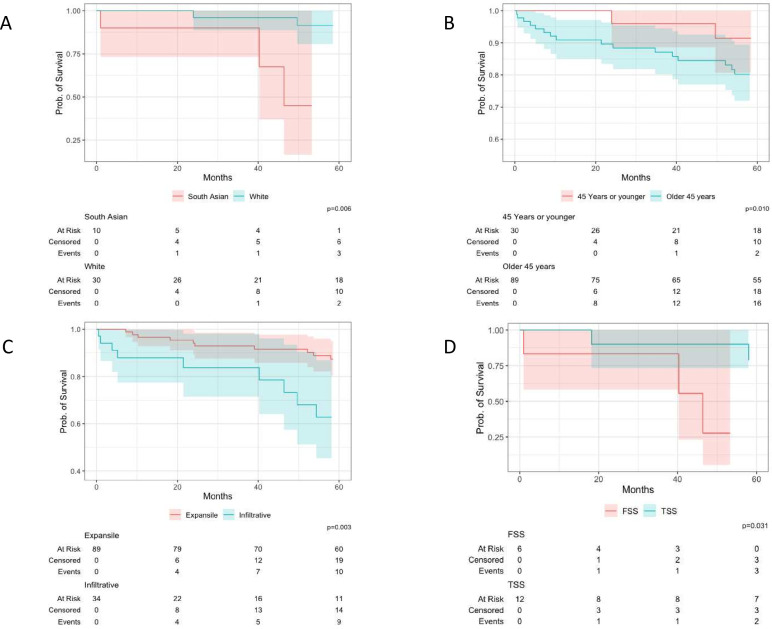
The 5-year overall survival was lower in all women diagnosed with FIGO stage IC disease than in those with FIGO stage IA, at 80% and 87%, respectively with univariate analysis showing a HR=2.29 (95% CI 1.01 to 5.55). Invasion type was not related to capsule status at diagnosis; with 76% all FIGO stage IA and 72% of FIGO stage IC being expansile invasion (p=0.74). Infiltrative invasion was associated with an increased risk of death; univariate analysis showed a HR=2.88 (95% CI 1.37 to 5.83). The 5-year overall survival of expansile invasion versus infiltrative invasion was 87% and 63%, respectively (p=0.003) as depicted in Figure 3C.
Figure 3.
Kaplan-Meier survival analysis plots of overall survival in primary mucinous ovarian cancer comparing South Asian and White ethnicity women. (A) Kaplan-Meier plot depicting 5-year overall survival in women diagnosed with primary mucinous ovarian cancer at age ≤45 years stratified by ethnicity; South Asian vs White ethnicity. (B) Kaplan-Meier plot depicting the overall survival in White ethnicity women diagnosed with primary mucinous ovarian cancer stratified by age at diagnosis: ≤45 years vs >45 years. (C) Kaplan-Meier plot depicting overall survival in women diagnosed with primary mucinous ovarian cancer stratified by invasion type: expansile vs infiltrative invasion. (D) Kaplan-Meier plot depicting 5-year overall survival in South Asian women diagnosed with primary mucinous ovarian cancer stratified by surgery type at diagnosis: fertility sparing vs traditional staging surgery (FSS vs TSS).
Impact of Age on Primary Mucinous Ovarian Cancer Outcomes
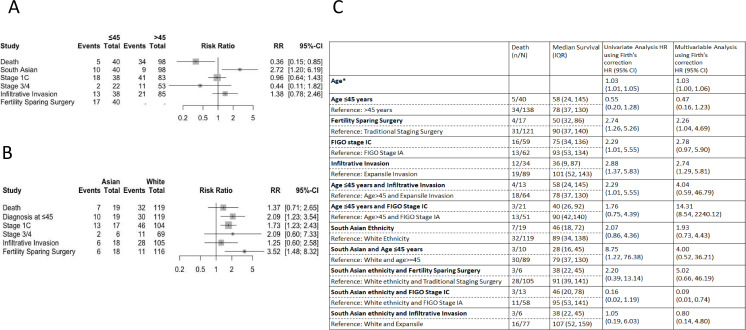
Women aged ≤45 years had surgery performed by a gynecologist more frequently (57%) than women aged >45 (17%) p<0.0001. Age at diagnosis did not affect the likelihood of tumor capsule rupture; 48% of women were diagnosed with FIGO stage IC primary mucinous ovarian cancer in both the ≤45 and >45 age groups (p=1.00, RR=0.96 (95% CI 0.64, 1.43)). In women aged≤45 the 5-year overall survival for FIGO stage IA was 100%, contrasting with 73% for FIGO stage IC (p=0.040, HR=1.76 (95% CI 0.75 to 4.39)). Women aged ≤45 years were at an increased risk of diagnosis with infiltrative invasion than women >45 years old, 35% and 28%, respectively, RR=1.38, (95% CI 0.78 to 2.46) (Figure 4A). In women aged ≤45, the 5-year overall survival of expansile invasion versus infiltrative invasion was 95% and 37% respectively p<0.002, HR=2.29 (95% CI 1.01 to 5.55). Overall, women ≤45 years had better 5-year overall survival; 83% vs 79% in the >45 years age group (p=0.049), HR=0.55 (95% CI 0.20 to 1.28). For every year older than 16 at the time of diagnosis, there was a cumulative increase in the risk of death, with a HR of 1.03 (95% CI 1.01 to 1.05).
Figure 4.
Risk analysis comparing outcomes based on age and ethnicity. (A) Relative risk (RR) forest plot comparing key prognostic risk factors in primary mucinous ovarian cancer between women aged ≤45 and women >45 years. The forest plot illustrates the relative risk in the two age groups; the risk of death, diagnosis if South Asian, diagnosis with FIGO stage 1C disease, diagnosis with FIGO stage 3/4 disease, infiltrative invasion, and fertility-sparing surgery. Each square represents the point estimate of relative risk with the horizontal line indicating the 95% CI. (B) Relative risk (RR) forest plot comparing key prognostic risk factors in primary mucinous ovarian cancer between South Asian and White ethnicity women. The forest plot illustrates the relative risk in the two ethnic groups; the risk of death, diagnosis at age ≤45 years, diagnosis with FIGO stage 1C disease, diagnosis with FIGO stage 3/4 disease, infiltrative invasion, and fertility-sparing surgery. Each square represents the point estimate of relative risk with the horizontal line indicating the 95% CI. (C) Table describing median survival, univariate and multivariable analyses of hazard ratios (HRs) with 95% confidence intervals in primary mucinous ovarian cancer. Exposures included in multivariable analyses identifiers: age, ethnicity, stage, invasion type, and surgery type. *Age assessed as a continuous variable with hazard reported for each additional year at age of diagnosis beginning at age 16 years. FIGO, International Federation of Gynecology and Obstetrics.
Impact of Fertility Sparing Surgery on Oncologic Outcomes
Nineteen women underwent fertility-sparing surgery; there was no significant difference in rates of infiltrative invasion for those undergoing fertility-sparing surgery compared with those undergoing traditional staging surgery; 8 (47%) and 5 (22%), respectively (p=0.32). In women aged ≤45 years undergoing fertility-sparing surgery, 10 (59%) were diagnosed with FIGO stage IA primary mucinous ovarian cancer compared with 10 (44%) women aged ≤45 years undergoing traditional staging surgery (p=0.78). In women aged ≤45 years there was no difference in the likelihood of receiving adjuvant chemotherapy; in those undergoing fertility-sparing surgery 6 (32%) received adjuvant chemotherapy compared with 9 (39%) of those who underwent traditional staging surgery (p=0.73). Univariate analysis indicated an increased risk of death in women undergoing fertility-sparing surgery with HR of 2.74 (95% CI 1.26 to 5.26) (see Figure 4C).
Impact of Ethnicity on Oncologic Outcomes
South Asian ethnicity women were at greater risk of being diagnosed with primary mucinous ovarian cancer aged ≤45 years old than their White counterparts, RR=2.09 (95% CI 1.23 to 3.54) (Figure 4B); with median age of 44 and 60, respectively. South Asian women exhibited no significant difference in 5-year overall survival based on age (≤45: 45%, >45: 67%; p=0.49). White women demonstrated better outcomes when diagnosed at age ≤45 years compared with those diagnosed at age >45 years, 91% vs 80% 5 year overall survival respectively (p=0.010) (Figure 3B).
South Asian women were operated on by a specialist gynecological oncologist less frequently than White women (44% vs 78%, p=0.007). A higher proportion of South Asian women were diagnosed at FIGO stage IC in comparison with White ethnicity women: 68% (n=13) vs 38% (n=45) (p=0.039, RR=1.73 (95% CI 1.23 to 2.43)). In FIGO stage I disease, 10 (59%) South Asian women and 38 (37%) White women received adjuvant chemotherapy (p=0.06). South Asian ethnicity women opted for fertility-sparing surgery in greater proportions than White ethnicity women 6 (32%) and 11 (9%), respectively (p=0.012). Women of South Asian ethnicity undergoing fertility-sparing surgery had worse 5-year overall survival rates of 28% compared with 79% in traditional staging surgery (p=0.031; Figure 3D). In White ethnicity women undergoing fertility-sparing surgery the 5-year overall survival was 88% vs 85% in traditional staging surgery (p=0.38). Univariate analysis of fertility-sparing surgery in South Asian women demonstrated an increased risk of death HR of 2.20 (95% CI 0.39 to 13.14) (Figure 4C).
An increased risk of infiltrative invasion was identified in South Asian women, RR=1.25 (95% CI 0.60 to 2.58). Infiltrative invasion was identified in 6 (32%) South Asian women versus 22 (18%) in White women. This observed trend persisted in women aged ≤45 years; infiltrative disease was diagnosed in 5 (50%) South Asian women versus 6 (20%) White women.
The 5-year overall survival for the entire study population was 83%, with a notable difference between South Asian and White women. The 5-year overall survival was 59% in South Asian women and 83%, in white women (HR=2.07 95% CI 0.86 to 4.36). This disparity was greater in women aged ≤45 years, with 5-year overall survival in South Asian women being 45% vs 91% in White ethnicity women (p=0.006) (Figure 3A). Young South Asian women were shown to be at particular risk, with univariate analysis of the interaction being HR=of 8.75 (95% CI 1.22 to 76.38) (Figure 4C).
Discussion
Summary of the Main results
Our study has revealed differences in survival outcomes in women of South Asian and White ethnicity. In each postulated risk exposure, young age, infiltrative invasion, capsule rupture, and fertility-sparing surgery, South Asian women consistently fared worse than their White counterparts.
Results in the Context of Published Literature
This study identified an increased risk of infiltrative invasion in all women aged ≤45 years. Infiltrative invasion is associated with a significant increased risk of death in this age group, with no differential impact based on ethnicity. Overall, age ≤45 years exhibited a protective effect in terms of mortality; however, South Asian women aged ≤45 years did not share this protective advantage. It is noteworthy that South Asian women were more likely to receive a diagnosis of primary mucinous ovarian cancer at age ≤45 years.
The overall risk of extensive disease spread (FIGO stage III/IV) at diagnosis remained low, with progression of disease stage correlating with a decline in overall survival consistently across all ethnicity groups.19 FIGO stage IC was identified as a predictor of lower 5-year overall survival compared with FIGO stage IA. South Asian women were more like to be diagnosed with FIGO stage IC than their White counterparts; importantly surgery type/age at diagnosis is not related to this increased risk. Fertility-sparing surgery was associated with an increased risk of death within this cohort. The finding of increased risk associated with fertility-sparing surgery had a differential impact within the cohort. South Asian ethnicity women experienced worse outcomes following fertility-sparing surgery compared with traditional staging surgery; this was not the case in White women.
This study corroborated findings that infiltrative invasion was associated with poorer prognosis than expansile invasion.20 Not only were young women more likely to be diagnosed with infiltrative invasion, but the impact of infiltrative invasion also appeared to be more detrimental in this subgroup. South Asian women demonstrated a trend of increased risk of infiltrative invasion, and this subgroup exhibited the poorest 5-year overall survival.
The rarity of primary mucinous ovarian cancer and the challenge in distinguishing it from metastases to the ovary pose a significant challenge in acquiring sufficient large-volume data regarding its outcomes. Following central pathology review, up to 50–70% of cases are reclassified as metastasis to the ovary.4 5 Up to 38% of metastatic ovarian tumors will precede the detection of the true primary tumor,21therefore, creating a dataset of this size of validated cases contributes significantly to the body of literature regarding primary mucinous ovarian cancer and its outcomes.
We have demonstrated for the first time that South Asian ethnicity, and young age are prognostic factors in primary mucinous ovarian cancer. Our study’s findings have corresponded with previously published data reporting high risk of recurrence and death associated with infiltrative invasion, with apparent FIGO stage I infiltrative invasion carrying a substantial risk of recurrence (40–52%) and death (33%).21 In apparent FIGO stage I tumors with infiltrative invasion, 17–30% exhibit lymph node metastasis7 22 23 suggesting that occult metastasis may contribute to the adverse outcomes associated with this disease. Our study has also corroborated more advanced stage at diagnosis as a poor prognostic indicator.5 6 24–26
Variations in incidence of ovarian tumors based on ethnic origin has been broadly reported within the literature.27–29 Biological mechanisms linked to ethnic variations in cancer incidence are already understood—for instance, clear cell ovarian carcinoma is associated with endometriosis, both clear cell ovarian carcinoma and endometriosis are more frequently diagnosed in East Asia.27 28 Variations in outcome based on ethnicity have previously been reported30 31 32 ; Qi et al demonstrated that Black ethnicity women have a relative risk of death in primary mucinous ovarian cancer of 2.22 compared with White women.33
Overall, young age (≤45 years) exhibited a protective effect for mortality, consistent with findings in the literature33 34; however, the differential outcome noted in the South Asian ethnicity subgroup is a novel finding. It has been widely regarded that fertility-sparing surgery is safe in early-stage primary mucinous ovarian cancer,35 5 36 a notion we sought to validate in this study. Contrary to expectations, our findings do not corroborate this assumption. Notably, while we did not observe a specific impact on 5-year overall survival in White women, a prognostic impact was identified in South Asian women. This difference is notable and underscores the emergence of a clinically significant risk factor in this population.
Strengths and Weaknesses
This is a consecutive dataset from two neighboring cancer centers which serve a population of 2.9 million people and provide tertiary referral care for eight district general hospital gynecology units. All diagnoses of primary mucinous ovarian cancer are made within regional multidisciplinary team meetings, with extensive expert histopathology input. Long-term robust clinical follow-up data are available and allow insight into survival. Analyzing this large database has allowed for novel factors to be investigated—if validated in other studies, this will have significant clinical implications.
Despite this being a large dataset for this disease, the numbers are small as primary mucinous ovarian cancer is rare. Given the very small numbers (three Black, two other ethnicity) we were unable to investigate other ethnicities. In this cohort we were only able to identify the ethnicity in 88% of patients based on hospital recorded demographic data. It is important to note this is in keeping with national ethnicity reporting in healthcare; within NHS digital Hospital Episode Statistics (HES) 82% of patients have ethnicity recorded.37
The evaluation of invasion type was achieved in only 88% of cases owing to insufficient information in certain cases, rendering it impossible to complete the assessment based on the original histological report. The primary objective of this research was to investigate whether age and/or ethnicity could serve as prognostic indicators in patients with primary mucinous ovarian cancer. Initially, univariate analysis was conducted to address this specific question. However, given the available data and the potential for a broader evaluation, we also sought to assess the additional prognostic value of ethnicity and/or age while adjusting for established prognostic risk factors; consequently, a multivariable analysis was performed. It is important to note that the relatively small number of cases in this series resulted in wide confidence intervals, highlighting the inherent limitations of this type of assessment within this dataset.
Implications for Practice and Future Research
South Asian women appear to have poorer survival outcomes than their White ethnicity counterparts. A distinct underlying biological mechanism may drive the suggested elevated risk; this requires further investigation. We plan to perform genomic analyses of primary mucinous ovarian cancer tumors using next-generation sequencing to gain further understanding of the molecular landscape of the disease. Clarifying variations in biology related to variations in outcome will give greater opportunity to identify significant drivers of poor outcomes and potentially identify points of access for targeted treatment.
Both young women and South Asian women are more likely to have surgery performed by a non-specialist gynecological oncologist and are at increased risk of infiltrative invasion, which has implications for survival. The study findings underscore the need for a re-evaluation of approach and risk stratification when assessing young, and particularly, South Asian patients. Currently, it is difficult to establish the relative impacts of disease biology and surgical treatment provider in determining the poor overall survival experienced by South Asian women. The presence of two known risk factors for poor outcome, infiltrative invasion and surgery by non-specialist gynecologists38, renders this assessment challenging. Surgical approach (fertility-sparing surgery vs traditional staging surgery) should be considered very carefully with thorough patient counseling, alongside liberal referral to specialist gynecological oncology services for at-risk patients while further evidence is developed in this field.
Conclusion
In conclusion, this study identified South Asian ethnicity and fertility-sparing surgery in specific subgroups as novel risk factors. South Asian women, particularly those aged ≤45 years, exhibit an overall poorer prognosis than their White counterparts. Intriguingly, young age is associated with the higher-risk infiltrative phenotype.
Despite the challenges posed by the rarity of primary mucinous ovarian cancer, the rigorous validation of cases provides robust insights. Going forward, a larger multicenter study will investigate these findings, allowing for deeper exploration of variations in outcomes. Genomic analyses aim to identify distinct biological mechanisms, offering opportunities for targeted treatments. This study marks a crucial step toward understanding the complexities of primary mucinous cancer and improving tailored interventions.
Acknowledgments
This research was supported in part by the International Centre for Theoretical Sciences (ICTS) for participating in the program - Machine Learning for Health and Disease (code: ICTS/mlhd2023/7) and the Birmingham Women's Health Global Research Programme. We extend our gratitude to Professor Richard Riley for his invaluable assistance with the statistical analyses, particularly in prognostic evaluations in rare cancers.
Footnotes
@TejumolaOlaoye1, @holyhauri, @elaineleung, @sundar_sudha
Contributors: Guarantor of integrity of the entire study: SSS. Study concepts and design: TO, SSS. Literature research: TO. Clinical studies: TO, SSS, KSu, KSi, SK, AG, EL, JY, JB, RG, AW, WB, RC. Data analysis: TO. Statistical analysis: TO, A. Manuscript preparation: TO, A. SSS. Manuscript editing: TO, SSS, KS, AW, JB, JY, EL. Manuscript review: TO, A, WB, AW, RG, KSu, AG, EL, RC, JP, SW, JY, JB, SK, KSi, and SSS.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data are available upon reasonable request. To minimize the unnecessary dissemination of patient clinical information, data will be provided in an anonymized form only for specific requests.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was approved by Sandwell and West Birmingham NHS Trust Clinical Effectiveness Department, University Hospitals Coventry and Warwickshire Clinical Audit and Effectiveness Department. Aggregated anonymized clinical data held within departmental cancer registries were used as the basis of the study.
References
- 1. Dundr P, Singh N, Nožičková B, et al. Primary mucinous ovarian tumors vs. ovarian metastases from gastrointestinal tract, pancreas and biliary tree: a review of current problematics. Diagn Pathol 2021;16:20. 10.1186/s13000-021-01079-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lerwill MF, Young RH. Mucinous tumours of the ovary. Diagnostic Histopathology 2008;14:366–87. 10.1016/j.mpdhp.2008.06.010 [DOI] [Google Scholar]
- 3. Perren TJ. Mucinous epithelial ovarian carcinoma. Ann Oncol 2016;27 Suppl 1:i53–7. 10.1093/annonc/mdw087 [DOI] [PubMed] [Google Scholar]
- 4. Gore M, Hackshaw A, Brady WE, et al. An International, phase III randomized trial in patients with Mucinous epithelial ovarian cancer (mEOC/GOG 0241) with long-term follow-up: and experience of conducting a clinical trial in a rare gynecological tumor. Gynecol Oncol 2019;153:541–8. 10.1016/j.ygyno.2019.03.256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gouy S, Arfi A, Maulard A, et al. Results from a monocentric long-term analysis of 23 patients with ovarian Sertoli-Leydig cell tumors. Oncologist 2019;24:702–9. 10.1634/theoncologist.2017-0632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hada T, Miyamoto M, Ishibashi H, et al. Survival and biomarker analysis for ovarian mucinous carcinoma according to invasive patterns: retrospective analysis and review literature. J Ovarian Res 2021;14:33. 10.1186/s13048-021-00783-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Muyldermans K, Moerman P, Amant F, et al. Primary invasive Mucinous ovarian carcinoma of the intestinal type: importance of the Expansile versus Infiltrative type in predicting recurrence and lymph node metastases. Eur J Cancer 2013;49:1600–8. 10.1016/j.ejca.2012.12.004 [DOI] [PubMed] [Google Scholar]
- 8. Pisano C, Greggi S, Tambaro R, et al. Activity of chemotherapy in mucinous epithelial ovarian cancer: a retrospective study. Anticancer Res 2005;25:3501–5. [PubMed] [Google Scholar]
- 9. Wu SG, Li FY, Lei J, et al. Histological tumor type is associated with one-year cause-specific survival in women with stage III-IV epithelial ovarian cancer: A surveillance, epidemiology, and end results (SEER) database population study, 2004-2014. Med Sci Monit 2020;26:e920531. 10.12659/MSM.920531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lee KR, Scully RE. Mucinous tumors of the ovary: a clinicopathologic study of 196 borderline tumors (of intestinal type) and carcinomas, including an evaluation of 11 cases with 'Pseudomyxoma peritonei'. Am J Surg Pathol 2000;24:1447–64. 10.1097/00000478-200011000-00001 [DOI] [PubMed] [Google Scholar]
- 11. Chen VW, Ruiz B, Killeen JL, et al. Pathology and classification of ovarian tumors. Cancer 2003;97:2631–42. 10.1002/cncr.11345 [DOI] [PubMed] [Google Scholar]
- 12. Pecorelli S, Benedet JL, Creasman WT, et al. 1994-1997 FIGO staging of gynecologic cancer FIGO Committee on gynecologic oncology International Federation of Gynecology and obstetrics. Int J Gynaecol Obstet 1999;65. [DOI] [PubMed] [Google Scholar]
- 13. Prat J, FIGO Committee on Gynecologic Oncology . Staging classification for cancer of the ovary, Fallopian tube, and Peritoneum. International Journal of Gynaecology and Obstetrics 2014;124:1–5:S0020-7292(13)00520-1. 10.1016/j.ijgo.2013.10.001 [DOI] [PubMed] [Google Scholar]
- 14. Bristow RE, Powell MA, Al-Hammadi N, et al. Disparities in ovarian cancer care quality and survival according to race and socioeconomic status. J Natl Cancer Inst 2013;105:823–32. 10.1093/jnci/djt065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Office for National Statistics . Ethnic group, England and Wales [https://www.ons.gov.uk/peoplepopulationandcommunity/culturalidentity/ethnicity/bulletins/ethnicgroupenglandandwales/census2021#ethnic-groups-in-england-and-wales]. 2021.
- 16. WHO Classification of Tumours Editorial Board . Tumours of the ovary. In: WHO Classification of Tumours, 5th Edition, Volume 4: Female Genital Tumours. Lyon: International Agency for Research on Cancer, 2020: 48–53. [Google Scholar]
- 17. R Core team . Vienna, Austria: R Foundation for Statistical Computing; R: A Language and Environment for Statistical Computing, 2023. Available: https://www.R-project.org/ [Google Scholar]
- 18. Hoogendam JP, Vlek CA, Witteveen PO, et al. Surgical lymph node assessment in mucinous ovarian carcinoma staging: a systematic review and meta-analysis. BJOG 2017;124:370–8. 10.1111/1471-0528.14226 [DOI] [PubMed] [Google Scholar]
- 19. Mackay HJ, Brady MF, Oza AM, et al. Gynecologic Cancer Intergroup. Prognostic relevance of uncommon ovarian histology in women with stage III/IV epithelial ovarian cancer. Int J Gynecol Cancer 2010;20:945–52. 10.1111/IGC.0b013e3181dd0110 [DOI] [PubMed] [Google Scholar]
- 20. Gouy S, Saidani M, Maulard A, et al. Staging surgery in early-stage ovarian mucinous tumors according to expansile and Infiltrative types. Gynecol Oncol Rep 2017;22:21–5. 10.1016/j.gore.2017.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ackroyd SA, Goetsch L, Brown J, et al. Pancreaticobiliary metastasis presenting as primary mucinous ovarian neoplasm: a systematic literature review. Gynecol Oncol Rep 2019;28:109–15. 10.1016/j.gore.2019.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schmeler KM, Tao X, Frumovitz M, et al. Prevalence of lymph node metastasis in primary mucinous carcinoma of the ovary. Obstet Gynecol 2010;116:269–73. 10.1097/AOG.0b013e3181e7961d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gouy S, Saidani M, Maulard A, et al. Characteristics and prognosis of stage I ovarian mucinous tumors according to expansile or Infiltrative type. Int J Gynecol Cancer 2018;28:493–9. 10.1097/IGC.0000000000001202 [DOI] [PubMed] [Google Scholar]
- 24. Babaier A, Ghatage P. Mucinous cancer of the ovary: overview and current status. Diagnostics (Basel) 2020;10:52. 10.3390/diagnostics10010052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hess V, A’Hern R, Nasiri N, et al. Mucinous epithelial ovarian cancer: a separate entity requiring specific treatment. J Clin Oncol 2004;22:1040–4. 10.1200/JCO.2004.08.078 [DOI] [PubMed] [Google Scholar]
- 26. Rodríguez IM, Prat J. Mucinous tumors of the ovary: a clinicopathologic analysis of 75 borderline tumors (of intestinal type) and carcinomas. Am J Surg Pathol 2002;26:139–52. 10.1097/00000478-200202000-00001 [DOI] [PubMed] [Google Scholar]
- 27. Matz M, Coleman MP, Sant M, et al. The histology of ovarian cancer: worldwide distribution and implications for International survival comparisons (CONCORD-2). Gynecol Oncol 2017;144:405–13. 10.1016/j.ygyno.2016.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lee AW, Navajas EE, Liu L. Clear differences in ovarian cancer incidence and trends by Ethnicity among Asian Americans. Cancer Epidemiol 2019;61:142–9. 10.1016/j.canep.2019.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Matz M, Coleman MP, Carreira H, et al. Worldwide comparison of ovarian cancer survival: histological group and stage at diagnosis (CONCORD-2). Gynecol Oncol 2017;144:396–404. 10.1016/j.ygyno.2016.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ross J, Braswell KV, Madeira da Silva L, et al. Unraveling the etiology of ovarian cancer racial disparity in the deep South: is it nature or nurture. Gynecol Oncol 2017;145:329–33. 10.1016/j.ygyno.2017.02.025 [DOI] [PubMed] [Google Scholar]
- 31. Han L, Husaiyin S, Liu J, et al. Period analysis of Intraracial differences in incidence and survival rates in epithelial ovarian cancer. Comput Math Methods Med 2021;2021:8032209. 10.1155/2021/8032209 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 32. Wu J, Sun H, Yang L, et al. Improved survival in ovarian cancer, with widening survival gaps of races and socioeconomic status: a period analysis, 1983-2012. J Cancer 2018;9:3548–56. 10.7150/jca.26300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Qi X, Xu L, Wang J, et al. Nomograms for primary Mucinous ovarian cancer: a SEER population-based study. J Gynecol Obstet Hum Reprod 2022;51:102424. 10.1016/j.jogoh.2022.102424 [DOI] [PubMed] [Google Scholar]
- 34. Yang SP, Su HL, Chen XB, et al. Long-term survival among histological subtypes in advanced epithelial ovarian cancer: population-based study using the surveillance, epidemiology, and end results database. JMIR Public Health Surveill 2021;7:e25976. 10.2196/25976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Crafton SM, Cohn DE, Llamocca EN, et al. Fertility-sparing surgery and survival among reproductive-age women with epithelial ovarian cancer in 2 cancer registries. Cancer 2020;126:1217–24. 10.1002/cncr.32620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gouy S, Saidani M, Maulard A, et al. Results of fertility-sparing surgery for expansile and infiltrative mucinous ovarian cancers. Oncologist 2018;23:324–7. 10.1634/theoncologist.2017-0310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shiekh SI, Harley M, Ghosh RE, et al. Completeness, agreement, and Representativeness of Ethnicity recording in the United Kingdom's clinical practice research Datalink (CPRD) and linked hospital episode Statistics (HES). Population Health Metrics 2023;21::3. 10.1186/s12963-023-00302-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Junor EJ, Hole DJ, McNulty L, et al. Specialist Gynaecologists and survival outcome in ovarian cancer: a Scottish national study of 1866 patients. British Journal of Obstetrics and Gynaecology 1999;106:1130–6. 10.1111/j.1471-0528.1999.tb08137.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon reasonable request. To minimize the unnecessary dissemination of patient clinical information, data will be provided in an anonymized form only for specific requests.