Abstract
Background
Within a year of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic, vaccines inducing a robust humoral and cellular immune response were implemented worldwide. However, emergence of novel variants and waning vaccine-induced immunity led to implementation of additional vaccine boosters.
Methods
This prospective study evaluated the temporal profile of cellular and serological responses in a cohort of 639 SARS-CoV-2–vaccinated participants, of whom a large proportion experienced a SARS-CoV-2 infection. All participants were infection naïve at the time of their first vaccine dose. Proportions of SARS-CoV-2 spike–specific T cells were determined after each vaccine dose using the activation-induced marker assay, while levels of circulating SARS-CoV-2 antibodies were determined by the Meso Scale serology assay.
Results
We found a significant increase in SARS-CoV-2 spike–specific CD4+ and CD8+ T-cell responses following the third dose of a SARS-CoV-2 messenger RNA vaccine as well as enhanced CD8+ T-cell responses after the fourth dose. Furthermore, increased age was associated with a poorer response. Finally, we observed that SARS-CoV-2 infection boosts both the cellular and humoral immune response, relative to vaccine-induced immunity alone.
Conclusions
Our findings highlight the boosting effect on T-cell immunity of repeated vaccine administration. The combination of multiple vaccine doses and SARS-CoV-2 infections maintains population T-cell immunity, although with reduced levels in the elderly.
Keywords: COVID-19, vaccination, cellular response, Spike-specific T cells, longitudinal study, hybrid immunity
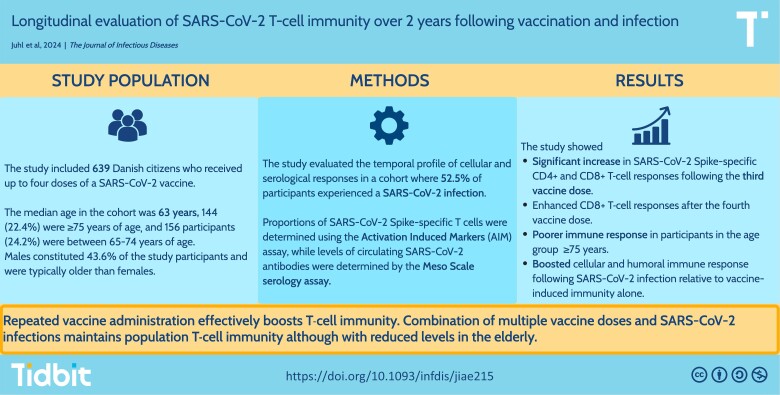
Cellular and serological responses was evaluated in a cohort of 639 SARS-CoV-2–vaccinated participants. There was a significant increase in SARS-CoV-2 spike–specific T-cell responses following each booster dose. Furthermore, SARS-CoV-2 infection boosts both the cellular and humoral immune response.
Graphical Abstract
Graphical Abstract.
This graphical abstract is also available at Tidbit: https://tidbitapp.io/tidbits/longitudinal-evaluation-of-sars-cov-2-t-cell-immunity-over-2-years-following-vaccination-and-infection?utm_campaign=tidbitlinkshare&utm_source=ITP
During the coronavirus disease 2019 (COVID-19) pandemic, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccines were rapidly developed and implemented worldwide [1–3]. The approved vaccines induce robust humoral and cellular immune responses and are therefore a highly effective means to reduce disease severity [4–6]. Neutralizing antibodies are the first line of defense against development of disease [6, 7]. However, viral antibody escape variants and the relatively fast waning of circulating antibodies have prompted enhanced focus on cellular immune memory and its role in limiting severe disease [8–11].
Variants of concern (VOCs) have evolved throughout the pandemic, and B.1.1.529 (Omicron), in particular, has resulted in high transmissibility with its multitude of mutations [12–15]. Subvariants of Omicron have continuously emerged, which led to recommendations for administering a third vaccine dose and, subsequently, updating of vaccine antigens in fourth and fifth doses [16–20]. Although mutations in the spike (S) protein of the Omicron variants have resulted in a decreased effect of neutralizing antibodies, T-cell epitopes are highly preserved from the wild-type Wuhan strain across VOCs [21–23]. T-cell immunity in vaccinated and convalescent individuals is believed to play an important role in protection against hospitalization and severe disease. However, the trajectory of cellular immunity following booster vaccinations has not been investigated to the same extent as neutralizing antibodies [9, 24]. Furthermore, the combined effect of SARS-CoV-2 infection and vaccine booster doses on T-cell immunity remains poorly characterized [25, 26].
In the current prospective study, we evaluated the temporal profile of cellular and serological responses in a study cohort of 639 participants over the course of 2 years. Induction of T-cell hybrid immunity was assessed in relation to a breakthrough infection by evaluating the impact on the circulating levels of SARS-CoV-2 S–specific T cells.
METHODS
Study Design and Data Selection
The design of the National Cohort Study of Effectiveness and Safety of SARS-CoV-2 vaccines (ENFORCE) and data selection have been previously described [4].
Study Visits and Sample Collection
The 639 participants enrolled in the study were followed for 9 study visits on days 0 (baseline), 30, 90, 190, 255, 365, 540, 570, and 730 (Supplementary Figure 1 and Supplementary Table 1). At each study visit, blood samples were collected for isolation of plasma and peripheral blood mononuclear cells (PBMCs). PBMCs were isolated from sodium citrate/Ficoll blood collection tubes from BD Vacutainer (BD CPT, catalog number: BDAM362782), as previously described [4], and stored at −150°C until analysis.
SARS-CoV-2 S–Specific T Cells
Proportions of S-specific T cells were quantified using the activation-induced marker (AIM) assay as previously described [4]. PBMCs were stimulated with PepMix SARS-CoV-2 (JPT peptides #PM-WCPV-S-2, Wuhan- Hu-1 lineage) at 2 μg/mL or negative control (dimethyl sulfoxide). The cells were stained for flow cytometry with markers enabling gating of CD4+ and CD8+ T cells and the AIMs CD69, 41BB, and OX40. Based on the flow cytometry acquisition, we performed a critical evaluation and had the following data exclusion criteria: (1) cell viability <70%, (2) total gated CD4+ or CD8+ T-cell count <10 000, and (3) percentage of CD69+CD4+ T cells >15% or percentage of CD69+CD8+ T cells >12% in negative control samples. The first 2 criteria were set to ensure adequate sample quality and a sufficient number of events in the final gate of AIM positivity. The third criterion was set to exclude samples with high background activation.
SARS-CoV-2 Antibody Profiling and Seroconversion
Serum levels of Wuhan-Hu-1 lineage SARS-CoV-2 S and nucleocapsid (N) antibodies were measured using the multiantigen serology assay (Meso Scale Diagnostics LLC, Rockville, Maryland) as previously described [6]. SARS-CoV-2 N seroconversion was defined as a level of N antibodies ≥3000 AU/mL and a fold change ≥2 from baseline.
Infection Stratification
Participants were divided into 2 groups based on whether they had been infected with SARS-CoV-2 before their 1-year study visit (day 365). This period was selected because Denmark experienced a surge in Omicron infections as society opened with continued high-capacity (polymerase chain reaction [PCR] based) infection monitoring. Infection stratification was done using SARS-CoV-2 PCR test results and N seroconversion at day 365 (Supplementary Figure 2).
Statistical Analysis
Participant demographics were tabulated showing number (%) and median (min-max). Fisher exact test was used for categorical variables, and Mann-Whitney U test for continuous variables.
Violin plots showed the kernel density estimation of the distribution of the data and were limited within the range of the observed data. Boxplots showed the quartiles of the dataset with whiskers extending to show the rest of the distribution within 1.5 × interquartile range (IQR). Outliers beyond 1.5 × IQR were not shown but still used in statistical testing. Line plots showed the median and bands the 95% confidence interval of the dataset.
Paired data were compared using paired, nonparametric Wilcoxon signed-rank test (2-tailed) with Bonferroni correction. Unpaired data were compared using unpaired, nonparametric Mann-Whitney U test (2-tailed) with Bonferroni correction.
All data analysis was done in Python version 3.9 and R version 4.2.2 with associated packages without modifications.
RESULTS
The study cohort consisted of 639 participants. All participants were SARS-CoV-2 infection naive at the time of study enrollment. All participants received 2 doses of a SARS-CoV-2 vaccine: 300 (46.9%) received 2 doses of BTN162b2 (Pfizer/BioNTech), 240 (37.6%) received 2 doses of mRNA-1273 (Moderna), and 99 (15.5%) received 1 dose of ChadOx1 (AstraZeneca) followed by 1 dose of either of the 2 mRNA vaccines (Table 1). The majority of the participants, 619 (96.9%), received a third vaccine dose, while 486 (76.1%) received a fourth vaccine dose (Supplementary Table 2). The median age in the cohort was 63 years. About a fourth, 144 (22.4%), were ≥75 years of age and 156 participants (24.2%) were between 65 and 74 years of age. Males constituted 43.6% of the study participants and were typically older than females (Table 1).
Table 1.
Participant Demographics by Age Group
| Characteristic | Age Group | P Value | |||
|---|---|---|---|---|---|
| Total (n = 639) |
<65 y (n = 341) |
65–74 y (n = 154) |
≥75 y (n = 144) |
||
| Age, y, median (min–max) | 63 (20–84) | 52 (20–64) | 69 (65–73) | 78 (74–84) | |
| Sex, No. (%) | <.0001 | ||||
| Male | 280 (44) | 114 (33) | 82 (53) | 84 (58) | |
| Female | 359 (56) | 227 (67) | 72 (47) | 60 (42) | |
| Vaccine type, No. (%) | <.0001 | ||||
| BNT162b2 | 300 (47) | 101 (30) | 73 (47) | 126 (88) | |
| mRNA-1273 | 240 (38) | 141 (41) | 81 (53) | 18 (12) | |
| ChAdOx1 + mRNA | 99 (15) | 99 (29) | 0 (0) | 0 (0) | |
| Vaccine priority group, No. (%) | <.0001 | ||||
| General population | 323 (51) | 141 (41) | 82 (53) | 100 (69) | |
| Healthcare workers | 119 (19) | 114 (33) | 5 (3) | 0 (0) | |
| Individuals at increased risk | 191 (30) | 82 (24) | 66 (43) | 43 (30) | |
| Missing | 6 (1) | 4 (1) | 1 (1) | 1 (1) | |
| CCI score, No. (%) | <.0001 | ||||
| 0 | 481 (75) | 284 (83) | 96 (62) | 101 (70) | |
| 1–2 | 126 (20) | 44 (13) | 49 (32) | 33 (23) | |
| >2 | 32 (5) | 13 (4) | 9 (6) | 10 (7) | |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: CCI, Charlson Comorbidity Index; mRNA, messenger RNA.
Vaccine-Induced Cellular Immunity Increases With Each Dose
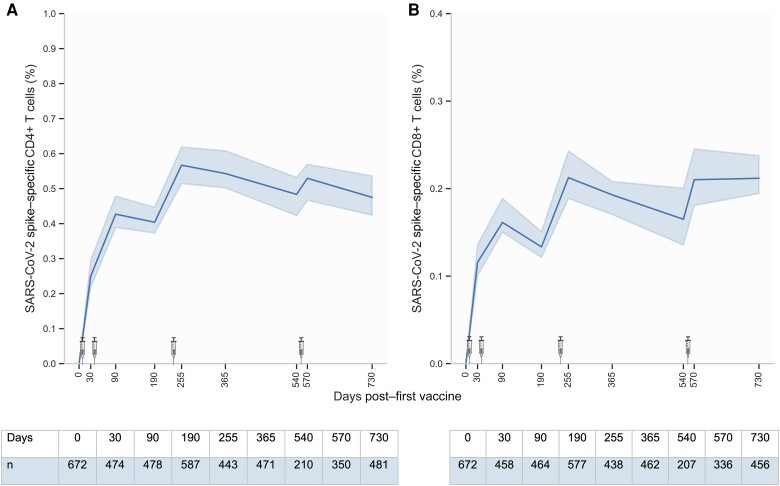
Participants were followed up to 2 years after their first vaccination (Figure 1A and 1B). As we previously reported [4], the proportion of S-specific CD4+ and CD8+ T cells increased following each dose of primary vaccination, reaching a peak response of 0.43% (IQR, 0.23%–0.71%) and 0.16% (IQR, 0.07%–0.31%), respectively. Importantly, at a median of 29 days after the third vaccine dose (day 255), the proportion of S-specific CD4+ and CD8+ T cells increased significantly to 0.57% (IQR, 0.29%–0.95%) and 0.21% (IQR, 0.10%–0.40%), respectively, which is approximately 1.3 times higher than the peak response observed after primary vaccination. Approximately 4.5 months after the third vaccine dose (day 365), the levels of S-specific CD4+ and CD8+ T cells had decreased, though not to the level observed prior to the third vaccine dose (day 190). However, T-cell responses continued to decrease between the third and fourth booster to levels observed at day 90 (after the primary vaccination). A fourth vaccine dose was administered, resulting in a slight increase in the levels of S-specific CD4+ and CD8+ T cells to 0.53% (IQR, 0.28%–0.89%) and 0.21% (IQR, 0.10%–0.43%), respectively (day 570). The level of S-specific CD8+ T cells remained stable up to the final study visit at 2 years of follow-up (day 730). Conversely, the level of S-specific CD4+ T cells decreased slightly to levels comparable to those of day 90 and day 540.
Figure 1.
Trajectory of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike (S)–specific CD4+ and CD8+ T cells from baseline to 2 years after first vaccine dose. S-specific CD4+ (A) and CD8+ (B) T cells from baseline to day 730 after first vaccine dose. Where no baseline visit was available, the percentage of S-specific T cells was set to zero. SARS-CoV-2 vaccination is shown by syringes from BioRender.com. The solid line represents the median value and bands the 95% confidence interval. The y-axis scale differs between panels.
Proportions of SARS-CoV-2 S–Specific CD4+ and CD8+ T Cells Significantly Increase Following the Third Vaccine Dose
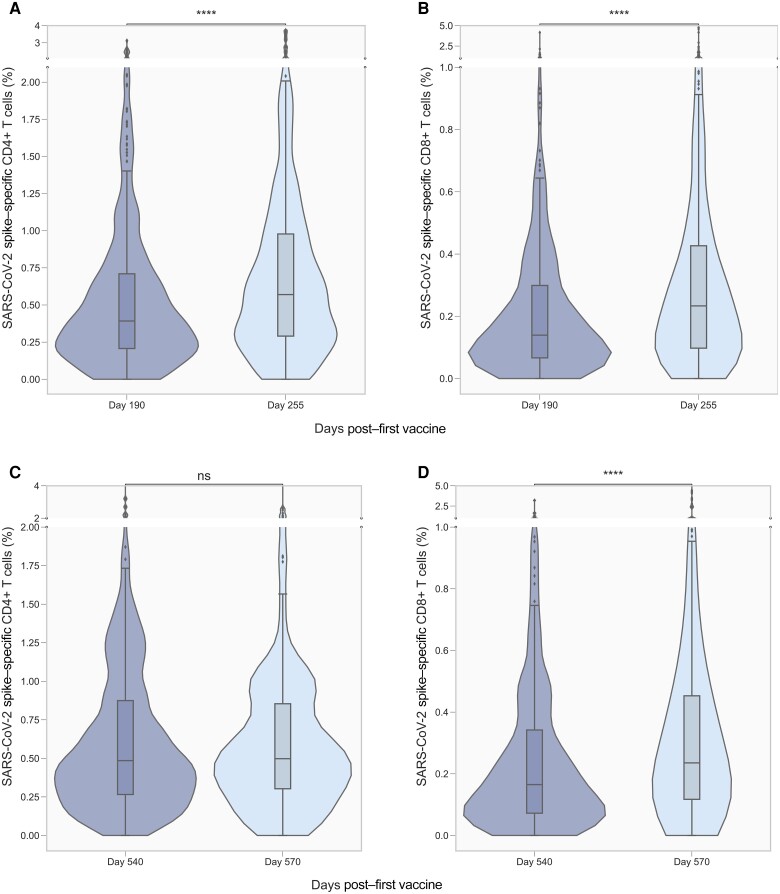
To evaluate the effect of a third and fourth vaccine dose on vaccine-induced T-cell immunity, the proportion of S-specific CD4+ and CD8+ T cells were determined before and after each vaccine dose. The proportion of S-specific CD4+ T cells increased significantly from 0.39% (IQR, 0.21%–0.71%) at day 190 to 0.57% (IQR, 029%–0.96%) after the third dose (day 255) (Figure 2A). Correspondingly, a significant increase was also observed for CD8+ T cells from 0.14% (IQR, 0.07%–0.29%) to 0.23% (IQR, 0.10%–0.43%) (Figure 2B). The proportion of S-specific CD4+ T cells did not increase after the fourth vaccine dose (Figure 2C). However, a significant increase was observed for CD8+ T cells from 0.16% (IQR, 0.07%–0.34%) to 0.24% (IQR, 0.12%–0.45%) (Figure 2D).
Figure 2.
Proportion of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike (S)–specific CD4+ and CD8+ T cells before and after third and fourth vaccine dose. S-specific CD4+ (n = 391; A) and CD8+ (n = 385; B) T cells before and after a third vaccine dose (days 190 and 255, respectively). S-specific CD4+ (n = 184; C) and CD8+ (n = 181; D) T cells before and after a fourth vaccine dose (days 540 and 570, respectively). Violin plots show the kernel density estimation of the underlying distribution. Boxplots show median, and whiskers extend to show 1.5 × interquartile range. Data are compared using the Wilcoxon signed-rank test. Not significant (ns), 5.00 × 10-2 < P; ****P ≤ 1.00 × 10-4. The y-axis scale differs between panels.
Increased Age Is Associated With Limited Induction of T-Cell Immunity Following Booster Doses
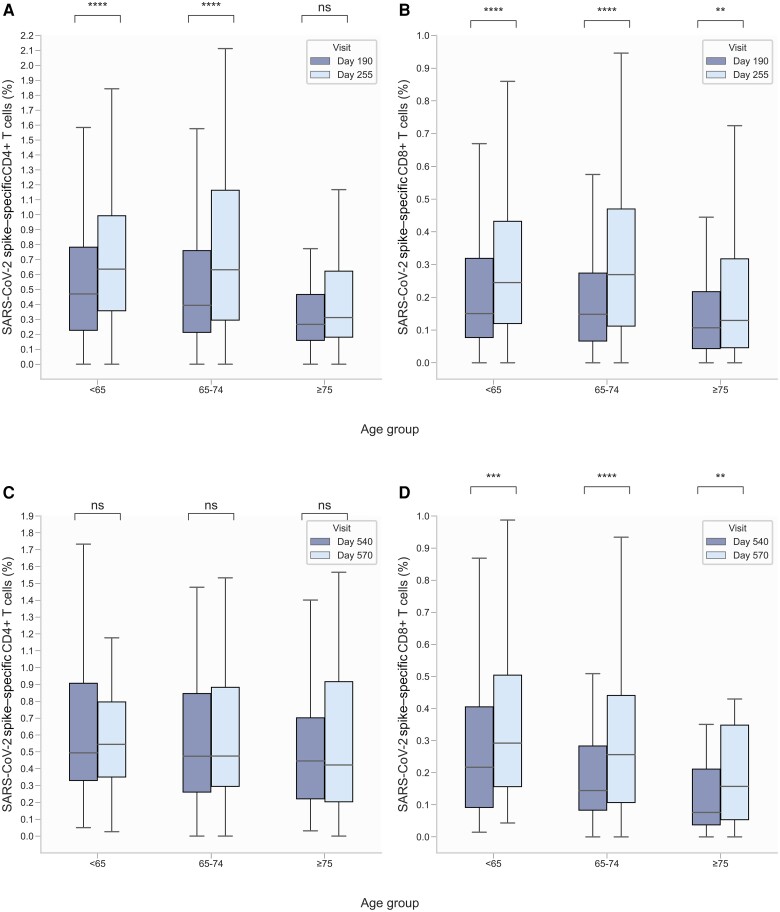
Increased age has previously been shown to negatively impact the response to primary vaccination [4, 27]. Therefore, participants were stratified by age group to evaluate the impact of age on T-cell immunity following a third and fourth vaccine dose (Figure 3).
Figure 3.
Proportion of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike (S)–specific CD4+ and CD8+ T cells before and after third and fourth vaccine dose, stratified by age group. S-specific CD4+ (age <65 y: n = 196, 65–74 y: n = 105, ≥75 y: n = 90) (A) and CD8+ (<65 y: n = 195, 65–74 y: n = 104, ≥75 y: n = 86) (B) T cells before and after a third dose (days 190 and 255, respectively). S-specific CD4+ (<65 y: n = 76, 65–74 y: n = 61, ≥75 y: n = 47) (C) and CD8+ (<65 y: n = 76, 65–74 y: n = 60, ≥75 y: n = 45) (D) T cells before and after a fourth dose (days 540 and 570, respectively). Boxplots show median, and whiskers extend to show 1.5 × interquartile range. Data are compared using the Wilcoxon signed-rank test. Not significant (ns): 5.00 × 10-2 < P; **1.00 × 10-3 < P ≤ 1.00 × 10-2; ***1.00 × 10-4 < P ≤ 1.00 × 10-3; ****P ≤ 1.00 × 10-4. The y-axis scale differs between panels.
Following a third vaccine dose, there was a significant increase in the proportion of both S-specific CD4+ and CD8+ T cells in the youngest age group (<65 years): from 0.47% (IQR, 0.23%–0.78%) to 0.64% (IQR, 0.36%–0.99%) for CD4+ T cells (n = 196) and from 0.15% (IQR, 0.08%–0.32%) to 0.24% (IQR, 0.12%–0.43%) for CD8+ T cells (n = 195). A significant increase was also evident for S-specific CD4+ and CD8+ T cells in the middle age group (65–74 years): from 0.39% (IQR, 0.21%–0.76%) to 0.63% (IQR, 0.30%–1.16%) for CD4+ T cells (n = 105) and from 0.15% (IQR, 0.07%–0.27%) to 0.27% (IQR, 0.11%–0.47%) for CD8+ T cells (n = 104). However, in the oldest age group (≥75 years), the proportion of S-specific CD4+ T cells (n = 90) did not change significantly after a third vaccine dose: from 0.27% (IQR, 0.16%–0.47%) to 0.31% (IQR, 0.18%–0.62%). Notably, there was a significant increase in the proportion of S-specific CD8+ T cells following a third vaccine dose in the oldest age group (n = 86), from 0.11% (IQR, 0.04%–0.22%) to 0.13% (IQR, 0.05%–0.32%), though less pronounced than in the 2 younger age groups.
Following a fourth vaccine dose, no significant change in the proportion of S-specific CD4+ T cells in any of the 3 age groups was observed. In contrast, the proportion of S-specific CD8+ T cells increased significantly in all 3 age groups: In the youngest age group (n = 76), from 0.22% (IQR, 0.09%–0.41%) to 0.29% (IQR, 0.16%–0.50%); in the middle age group (n = 60), from 0.14% (IQR, 0.08%–0.28%) to 0.26% (IQR, 0.11%–0.44%); and in the oldest age group (n = 45), from 0.08% (IQR, 0.04%–0.21%) to 0.16% (IQR, 0.05%–0.35%).
SARS-CoV-2 Infection Significantly Increases Vaccine-Induced Cellular and Serological Immune Response
Given the rapid spread of Omicron-fueled infections in the spring of 2022, participants were divided into 2 groups based on whether they experienced a SARS-CoV-2 infection before day 365. Of 514 participants, 270 (52.5%) participants were infected. Of the 270 infected participants, 202 (74.8%) had a positive PCR test while 68 (25.2%) had no data on positive PCR test but had seroconverted on N antibodies at their 1-year study visit (Supplementary Figure 2). The 202 positive PCR tests occurred between November 2021 and August 2022, with a median of 316 days after first vaccination.
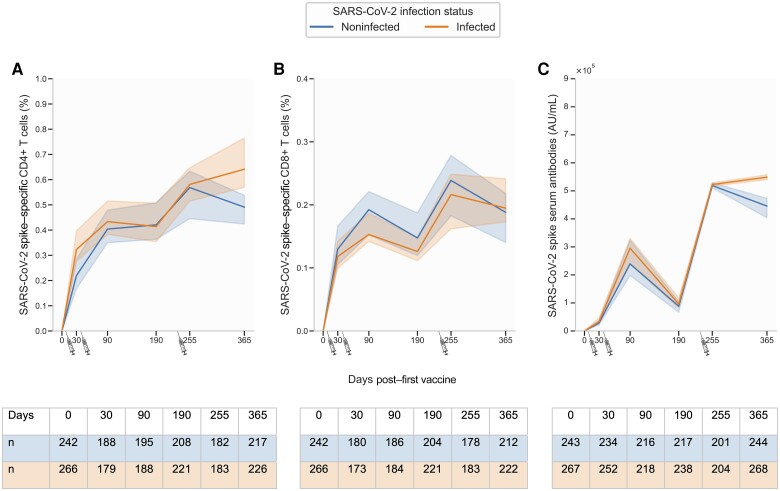
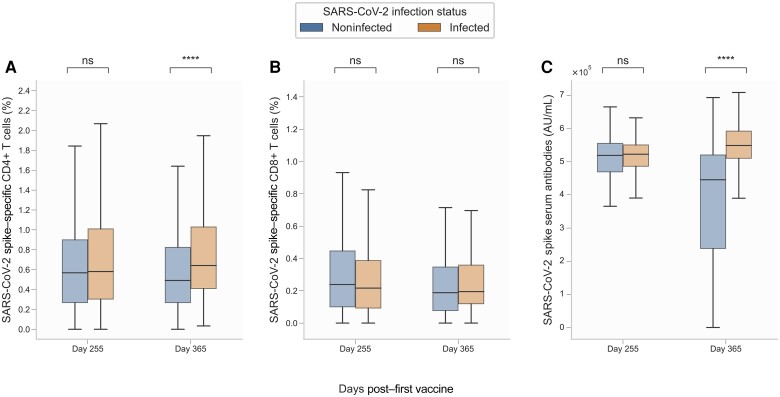
Participants in the infected group were generally younger with a median age of 60 years compared to 68 years in the noninfected group (Supplementary Table 3). No differences in the proportion of S-specific CD4+ T cells were observed between infected and noninfected participants from baseline to day 255 (Figure 4A). However, at day 365, the proportion of S-specific CD4+ T cells was significantly increased in the infected group (0.64% [IQR, 0.41%–1.03%]) compared to the noninfected group (0.49% [IQR, 0.27%–0.82%]) (Figure 5A). Interestingly, the proportion of S-specific CD8+ T cells did not differ between the 2 groups at any of the study visits, although the group not experiencing an infection had slightly higher (nonsignificant) median levels at most visits (Figures 4B and 5B). Serum levels of S antibodies followed a similar trajectory as S-specific CD4+ T cells; no difference was observed between infected and noninfected participants from baseline to day 255 (Figure 4C). However, at day 365, serum levels of S antibodies were significantly increased in the infected group (5.49 × 105 AU [IQR, 5.10–5.92 × 105]) compared to the noninfected group (4.46 × 105 [IQR, 2.38–5.20 × 105]) (Figures 4C and 5C).
Figure 4.
Trajectory of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike (S)–specific T cells and anti-S serum antibodies from baseline to day 365, stratified by infection. A, CD4+ T cells. B, CD8+ T cells. C, S serum antibodies. SARS-CoV-2 vaccination is shown by syringes. Where no baseline visit was available, the percentage of S-specific T cells was set to zero. SARS-CoV-2 vaccination is shown by syringes from BioRender.com. Data are unpaired; the solid line represents the median value and bands the 95% CI. The y-axis scale differs between panels.
Figure 5.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike (S)–specific T cells and anti-S serum antibodies at days 255 and 365, stratified by infection. A, S-specific CD4+ T cells. B, S-specific CD8+ T cells. C, Anti-S serum antibodies. Data were compared using the Mann-Whitney U test. Not significant (ns): 5.00 × 10-2 < P; ****P ≤ 1.00 × 10-4. The y-axis scale differs between panels.
Serum levels of SARS-CoV-2 N antibodies were constant from baseline to day 255 in both groups and increased significantly in the infected group compared to the noninfected group at day 365 (Supplementary Figure 3).
DISCUSSION
In this prospective study, we assessed the temporal profile of S-specific cellular and humoral immune responses in a study cohort of 639 COVID-19 vaccine recipients over the course of 2 years from first vaccine dose. The study specifically focused on the T-cell immunity trajectories following vaccine dose administration beyond the primary vaccination series. We also evaluated the impact of hybrid immunity on proportions of S-specific T cells and serum S antibody levels.
We previously reported an increased T-cell immune response following each vaccine dose of primary vaccination irrespective of vaccine type [4]. In the current study, we found that 255 days after the first vaccine dose, the proportion of S-specific T cells had decreased significantly from peak levels observed after the primary vaccination series. Importantly, following administration of a third vaccine dose, proportions of both S-specific CD4+ and CD8+ T cells increased significantly.
We analyzed the hybrid immunity induced by 3 vaccine doses and a SARS-CoV-2 infection and found a significant increase in the proportion of S-specific CD4+ T cells. Moreover, there was a significant increase in the levels of circulating antibodies in infected participants. To our surprise, this effect was not observed for S-specific CD8+ T cells, although numerous reports have identified strong and durable CD8+ T-cell responses following exposure to SARS-CoV-2 [28–30]. Our findings showed that additional exposure to SARS-CoV-2 antigen (ie, infection) further boosted humoral and CD4+ T cellular immunity to S antigen. This is in accordance with recent results by Ruhl L et al [31], who found that, compared to vaccine-only immunity, breakthrough infection enhanced humoral and cellular responses, as well as eliciting broader and more potent neutralizing antibodies. The limited boosting of CD8+ S-specific T-cell immunity following SARS-CoV-2 infection observed in our study may be explained by the fact that we determine only responses targeting the S antigen, while natural infection has been shown to include numerous non-S epitopes [28, 30, 32]. In contrast, we observed significant boosting of CD4+ T-cell responses following infection, which may be more in line with S being the dominant antibody target, where CD4+ T-cell help is required for further antibody diversification and maturation.
The significant boosting of CD4+ T-cell immunity observed in this study will be important in averting detrimental effects from future waves of SARS-CoV-2. With estimates that more than 80% of the adult population in Denmark have experienced a SARS-CoV-2 infection on top of a vaccination series, it is expected to limit the pressure on healthcare systems from SARS-CoV-2 [33].
In this study, the majority of the positive PCR tests occurred between February 2022 and April 2022 (68.8%), where B.1.1.529 (Omicron; BA.1 and BA.2) was the predominant circulating strain in Denmark [16, 34]. Multiple studies have suggested that T-cell epitope responses to Omicron are preserved [21, 35, 36]. Hence, the AIM assay used in the current study was expected to capture T-cell responses to both original Wuhan-Hu-1 as well as later sublineages, including Omicron. There is a risk of underestimating the number of infected participants, due to a lower production of N antibodies in individuals recently receiving a vaccine (within 2 months) [37]. However, because COVID-19 infection in Danish society was monitored closely by large-scale population PCR testing during the pandemic, the underestimation should be minimal.
The participants in this study represent an older segment of the general population; thus, the data are likely an underrepresentation of cellular immunity in a complete population. We observed that booster vaccine doses had limited effect on cellular immunity in the oldest age group (≥75 years), especially in the CD4+ T-cell compartment. Generally, vaccines have a decreased efficacy with increasing age, leading to a higher frequency of elderly persons displaying vaccine hyporesponsiveness [38]. The deficits in the aging immune system are due to limitations in both the innate and the adaptive immune system. While the number of T cells do not decline with increasing age, studies suggest that dendritic cells are impaired in both activating and priming T cells in the aged immune system [39, 40]. Considering the limited cellular immune response observed in the oldest age group following the third vaccine dose, it would be of great interest to investigate immune responses using a broader T-cell profile. However, the AIM assay was limited to assessing the bulk proportion of activated CD4+ and CD8+ T cells following stimulation with SARS-CoV-2 S protein, without the additional profiling of specific cytokine secretions or T-cell immune phenotypes.
In conclusion, this study reports a significant increase in T-cell immune response following consecutive booster doses of SARS-CoV-2 mRNA vaccines. However, as our study found that increased age was associated with a poorer response, continued vaccine and immune monitoring is particularly needed in older individuals. Last, we observe that SARS-CoV-2 infection boosts both the cellular (CD4+ T-cell) and humoral compartment of the immune system to levels higher than vaccination alone. Thus, the combination of multiple vaccine doses and widespread hybrid immunity results in substantial immunity in the population.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Supplementary Material
APPENDIX
ENFORCE Study Group members:
Sponsor/Data and Statistical Centre: CHIP, Rigshospitalet under the direction of Professor J. Lundgren.
Principal Investigator: Professor L. J. Østergaard, Department of Infectious Diseases, Aarhus University and Aarhus University Hospital.
Sites
Region Hovedstaden: T. Benfield (regional coordinator), L. Krohn-Dehli, D. K. Petersen, Copenhagen University Hospital–Amager and Hvidovre, Hvidovre. K. Fogh, E. H. Mikkelsen, K. Iversen (regional coordinator), Copenhagen University Hospital–Gentofte and Herlev, Herlev.
Region Midtjylland: P. Bek, V. Klastrup, F. Larsen, S. H. Rasmussen, M. H. Schleimann, S. Schieber, N. B. Stærke (regional coordinator), A. Søndergaard, B. Tarp, M. Tousgaard, Y. Yehdego, Aarhus University Hospital, Aarhus.
Region Nordjylland: J. Bodilsen, H. Nielsen (regional coordinator), K. T. Petersen, M. R. Juhl, R. K. Thisted, Aalborg University Hospital, Aalborg.
Region Sjælland: S. F. Caspersen, M. Iversen, L. S. Knudsen, J. L. Meyerhoff, L. G. Sander, L. Wiese (regional coordinator), Zealand University Hospital Roskilde, Roskilde.
Region Syddanmark: C. Abildgaard, I. K. Holden, N. E. Johansen, I. S. Johansen (regional coordinator), L. Larsen, S. O. Lindvig, L. W. Madsen, A. Øvrehus, Odense University Hospital, Odense.
Scientific Steering Committee: N. A. Kruse, H. Lomholdt, Lægemiddelstyrelsen; T. G. Krause, P. Valentiner-Branth, Statens Serum Institut; B. Søborg, Sundhedsstyrelsen; T. K. Fischer, Copenhagen University; C. Erikstrup, Aarhus University; S. R. Ostrowski, Rigshospitalet; H. Nielsen, Aalborg University Hospital; I. S. Johansen, Odense University Hospital; L. J. Østergaard (chair), M. Tolstrup, N. B. Stærke, O. S. Søgaard, Aarhus University Hospital; L. Wiese, Zealand University Hospital Roskilde; T. Benfield, Copenhagen University Hospital–Amager and Hvidovre; J. Lundgren, D. Raben, CHIP, Rigshospitalet.
Operational group: H. Nielsen, Aalborg University Hospital; I. S. Johansen, Odense University Hospital; L. J. Østergaard, M. Tolstrup, N. B. Stærke, O. S. Søgaard, Aarhus University Hospital; L. Wiese, Zealand University Hospital Roskilde; T. Benfield, Copenhagen University Hospital–Amager and Hvidovre; J. Lundgren (chair), D. Raben, CHIP, Rigshospitalet; E. Jylling, Danske Regioner; D. Hougaard, Statens Serum Institut.
Coordinating Centre: Aarhus University and Aarhus University Hospital, S. D. Andersen, K. Lykkegaard, N. B. Stærke, O. S. Søgaard, M. Tolstrup, L. J. Østergaard.
ENFORCE Laboratory: Aarhus University Hospital, S. R. Andreasen, E. Baerends, L. L. Dietz, A. K. Hvidt, A. K. Juhl, R. Olesen, M. Tolstrup.
Data and Statistical Centre: CHIP, Rigshospitalet: K. K. Andersen, W. Bannister, C. Bjernved, T. W. Elsing, F. V. Esmann, M. A. Ghafari, E. Gravholdt, S. F. Jakobsen, M. L. Jakobsen, C. M. Jensen, T. Ø. Jensen, D. Kristensen, L. R. Kumar, J. Lundgren, C. Matthews, N. Normand, C. Olsson, D. Raben, J. Reekie, A. Traytel, T. Weide.
Other contributors: A. M. Hvas, H. Støvring, Aarhus University Hospital; C. Erikstrup, University of Aarhus; T. G. Krause, Statens Serum Institut; T. K. Fischer, University of Copenhagen; S. Ostrowsky, Rigshospitalet.
Contributor Information
Anna Karina Juhl, Department of Infectious Diseases, Aarhus University Hospital; Department of Clinical Medicine, Aarhus University, Aarhus.
Lisa Loksø Dietz, Department of Infectious Diseases, Aarhus University Hospital; Department of Clinical Medicine, Aarhus University, Aarhus.
Ole Schmeltz Søgaard, Department of Infectious Diseases, Aarhus University Hospital; Department of Clinical Medicine, Aarhus University, Aarhus.
Joanne Reekie, Center of Excellence for Health, Immunity and Infections, Rigshospitalet, University of Copenhagen, Copenhagen.
Henrik Nielsen, Department of Infectious Diseases, Aalborg University Hospital; Department of Clinical Medicine, Aalborg University, Aalborg.
Isik Somuncu Johansen, Department of Infectious Diseases, Odense University Hospital; Department of Clinical Research, University of Southern Denmark, Odense.
Thomas Benfield, Department of Infectious Diseases, Copenhagen University Hospital–Amager and Hvidovre, Hvidovre; Department of Clinical Medicine, University of Copenhagen, Copenhagen.
Lothar Wiese, Department of Medicine, Zealand University Hospital, Roskilde.
Nina Breinholt Stærke, Department of Infectious Diseases, Aarhus University Hospital; Department of Clinical Medicine, Aarhus University, Aarhus.
Tomas Østergaard Jensen, Center of Excellence for Health, Immunity and Infections, Rigshospitalet, University of Copenhagen, Copenhagen.
Rikke Olesen, Department of Clinical Medicine, Aarhus University, Aarhus.
Kasper Iversen, Departments of Cardiology and Emergency Medicine, Herlev Hospital, Herlev.
Kamille Fogh, Departments of Cardiology and Emergency Medicine, Herlev Hospital, Herlev.
Jacob Bodilsen, Department of Infectious Diseases, Aalborg University Hospital; Department of Clinical Medicine, Aalborg University, Aalborg.
Lone Wulff Madsen, Department of Infectious Diseases, Odense University Hospital; Department of Regional Health Research, University of Southern Denmark, Odense.
Susan Olaf Lindvig, Department of Clinical Research, University of Southern Denmark, Odense.
Dorthe Raben, Center of Excellence for Health, Immunity and Infections, Rigshospitalet, University of Copenhagen, Copenhagen.
Sidsel Dahl Andersen, Department of Infectious Diseases, Aarhus University Hospital.
Astrid Korning Hvidt, Department of Infectious Diseases, Aarhus University Hospital.
Signe Rode Andreasen, Department of Infectious Diseases, Aarhus University Hospital.
Eva Anna Marianne Baerends, Department of Infectious Diseases, Aarhus University Hospital.
Jens Lundgren, Center of Excellence for Health, Immunity and Infections, Rigshospitalet, University of Copenhagen, Copenhagen; Department of Clinical Medicine, University of Copenhagen, Copenhagen; Department of Infectious Diseases, Copenhagen University Hospital–Rigshospitalet, Copenhagen, Denmark.
Lars Østergaard, Department of Infectious Diseases, Aarhus University Hospital; Department of Clinical Medicine, Aarhus University, Aarhus.
Martin Tolstrup, Department of Infectious Diseases, Aarhus University Hospital; Department of Clinical Medicine, Aarhus University, Aarhus.
for the ENFORCE Study Group:
J Lundgren, L J Østergaard, T Benfield, L Krohn-Dehli, D K Petersen, K Fogh, E H Mikkelsen, K Iversen, P Bek, V Klastrup, F Larsen, S H Rasmussen, M H Schleimann, S Schieber, N B Stærke, A Søndergaard, B Tarp, M Tousgaard, Y Yehdego, J Bodilsen, H Nielsen, K T Petersen, M R Juhl, R K Thisted, S F Caspersen, M Iversen, L S Knudsen, J L Meyerhoff, L G Sander, L Wiese, C Abildgaard, I K Holden, N E Johansen, I S Johansen, L Larsen, S O Lindvig, L W Madsen, A Øvrehus, N A Kruse, H Lomholdt, T G Krause, P Valentiner-Branth, B Søborg, T K Fischer, C Erikstrup, S R Ostrowski, H Nielsen, I S Johansen, L J Østergaard, M Tolstrup, N B Stærke, O S Søgaard, L Wiese, T Benfield, J Lundgren, D Raben, H Nielsen, I S Johansen, L J Østergaard, M Tolstrup, N B Stærke, O S Søgaard, L Wiese, T Benfield, J Lundgren, D Raben, E Jylling, D Hougaard, S D Andersen, K Lykkegaard, N B Stærke, O S Søgaard, M Tolstrup, L J Østergaard, S R Andreasen, E Baerends, L L Dietz, A K Hvidt, A K Juhl, R Olesen, M Tolstrup, K K Andersen, W Bannister, C Bjernved, T W Elsing, F V Esmann, M A Ghafari, E Gravholdt, S F Jakobsen, M L Jakobsen, C M Jensen, T Ø Jensen, D Kristensen, L R Kumar, J Lundgren, C Matthews, N Normand, C Olsson, D Raben, J Reekie, A Traytel, T Weide, A M Hvas, H Støvring, C Erikstrup, T G Krause, T K Fischer, and S Ostrowsky
Notes
Author contributions. O. S. S., J. R., N. B. S., D. R., J. L., L. Ø., and M. T. conceived and designed the study. J. L., L. Ø., O. S. S., D. R., N. B. S., and M. T. obtained funding. H. N., I. J., T. B., L. W., and N. B. S. provided regional oversight, recruited participants, and collected participant data. K. I., K. F., J. B., L. W. M., and S.O. L. recruited participants and provided biological material. S. D. A., A. K. H., S. R. A., E. A. M. B., R. O., L. L. D., and A. K. J. performed T-cell and antibody analysis. J. R., and T. Ø. J. provided data extract and database merging. L. L. D. and A. K. J. analyzed the data. L. L. D., A. K. J., and M. T. wrote the manuscript. All authors provided input and approved the manuscript on behalf of the ENFORCE consortium.
Financial Support . ENFORCE has received a grant from the Danish Ministry of Health (legal deeds 150 28/1 2021 and 263 3/6 2021).
References
- 1. Emborg H-D, Valentiner-Branth P, Schelde AB, et al. . Vaccine effectiveness of the BNT162b2 mRNA COVID-19 vaccine against RT-PCR confirmed SARS-CoV-2 infections, hospitalisations and mortality in prioritised risk groups. medRxiv [Preprint]. Posted online 2 June 2021. doi: 10.1101/2021.05.27.21257583 [DOI]
- 2. Pritchard E, Matthews PC, Stoesser N, et al. . Impact of vaccination on new SARS-CoV-2 infections in the United Kingdom. Nat Med 2021; 27:1370–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vasileiou E, Simpson CR, Shi T, et al. . Interim findings from first-dose mass COVID-19 vaccination roll-out and COVID-19 hospital admissions in Scotland: a national prospective cohort study. Lancet 2021; 397:1646–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dietz LL, Juhl AK, Søgaard OS, et al. . Impact of age and comorbidities on SARS-CoV-2 vaccine-induced T cell immunity. Commun Med 2023; 3:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stærke NB, Reekie J, Nielsen H, et al. . Levels of SARS-CoV-2 antibodies among fully vaccinated individuals with Delta or Omicron variant breakthrough infections. Nat Commun 2022; 13:4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Søgaard OS, Reekie J, Johansen IS, et al. . Characteristics associated with serological COVID-19 vaccine response and durability in an older population with significant comorbidity: the Danish Nationwide ENFORCE Study. Clin Microbiol Infect 2022; 28:1126–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rose R, Neumann F, Grobe O, Lorentz T, Fickenscher H, Krumbholz A. Humoral immune response after different SARS-CoV-2 vaccination regimens. BMC Med 2022; 20:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tan AT, Linster M, Tan CW, et al. . Early induction of functional SARS-CoV-2-specific T cells associates with rapid viral clearance and mild disease in COVID-19 patients. Cell Rep 2021; 34:108728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rydyznski Moderbacher C, Ramirez SI, Dan JM, et al. . Antigen-specific adaptive immunity to SARS-CoV-2 in acute COVID-19 and associations with age and disease severity. Cell 2020; 183:996–1012.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schulien I, Kemming J, Oberhardt V, et al. . Characterization of pre-existing and induced SARS-CoV-2-specific CD8(+) T cells. Nat Med 2021; 27:78–85. [DOI] [PubMed] [Google Scholar]
- 11. Sekine T, Perez-Potti A, Rivera-Ballesteros O, et al. . Robust T cell immunity in convalescent individuals with asymptomatic or mild COVID-19. Cell 2020; 183:158–68.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liu L, Iketani S, Guo Y, et al. . Striking antibody evasion manifested by the Omicron variant of SARS-CoV-2. Nature 2022; 602:676–81. [DOI] [PubMed] [Google Scholar]
- 13. DeGrace MM, Ghedin E, Frieman MB, et al. . Defining the risk of SARS-CoV-2 variants on immune protection. Nature 2022; 605:640–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hu J, Peng P, Cao X, et al. . Increased immune escape of the new SARS-CoV-2 variant of concern Omicron. Cell Mol Immunol 2022; 19:293–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Altmann DM, Boyton RJ, Beale R. Immunity to SARS-CoV-2 variants of concern. Science 2021; 371:1103–4. [DOI] [PubMed] [Google Scholar]
- 16. Hansen CH, Friis NU, Bager P, et al. . Risk of reinfection, vaccine protection, and severity of infection with the BA.5 Omicron subvariant: a nation-wide population-based study in Denmark. Lancet Infect Dis 2023; 23:167–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Barda N, Dagan N, Cohen C, et al. . Effectiveness of a third dose of the BNT162b2 mRNA COVID-19 vaccine for preventing severe outcomes in Israel: an observational study. Lancet 2021; 398:2093–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Madhi SA, Baillie V, Cutland CL, et al. . Efficacy of the ChAdOx1 nCoV-19 Covid-19 vaccine against the B.1.351 variant. N Engl J Med 2021; 384:1885–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hvidt AK, Baerends EA, Søgaard OS, et al. . Comparison of vaccine-induced antibody neutralization against SARS-CoV-2 variants of concern following primary and booster doses of COVID-19 vaccines. Front Med (Lausanne) 2022; 9:994160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Baerends EAM, Reekie J, Andreasen SR, et al. . Omicron variant-specific serological imprinting following BA.1 or BA.4/5 bivalent vaccination and previous SARS-CoV-2 infection: a cohort study. Clin Infect Dis 2023; 77:1511–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gao Y, Cai C, Grifoni A, et al. . Ancestral SARS-CoV-2-specific T cells cross-recognize the Omicron variant. Nat Med 2022; 28:472–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Choi SJ, Kim D-U, Noh JY, et al. . T cell epitopes in SARS-CoV-2 proteins are substantially conserved in the Omicron variant. Cell Mol Immunol 2022; 19:447–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Redd AD, Nardin A, Kared H, et al. . Minimal crossover between mutations associated with Omicron variant of SARS-CoV-2 and CD8+ T-cell epitopes identified in COVID-19 convalescent individuals. Mbio 2022; 13:e03617–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McMahan K, Yu J, Mercado NB, et al. . Correlates of protection against SARS-CoV-2 in rhesus macaques. Nature 2021; 590:630–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pradenas E, Marfil S, Urrea V, et al. . Impact of hybrid immunity booster vaccination and Omicron breakthrough infection on SARS-CoV-2 VOCs cross-neutralization. iScience 2023; 26:106457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Maringer Y, Nelde A, Schroeder SM, et al. . Durable spike-specific T cell responses after different COVID-19 vaccination regimens are not further enhanced by booster vaccination. Sci Immunol 2022; 7:eadd3899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wagner A, Garner-Spitzer E, Jasinska J, et al. . Age-related differences in humoral and cellular immune responses after primary immunisation: indications for stratified vaccination schedules. Sci Rep 2018; 8:9825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Minervina AA, Pogorelyy MV, Kirk AM, et al. . SARS-CoV-2 antigen exposure history shapes phenotypes and specificity of memory CD8(+) T cells. Nat Immunol 2022; 23:781–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Peng Y, Mentzer AJ, Liu G, et al. . Broad and strong memory CD4+ and CD8+ T cells induced by SARS-CoV-2 in UK convalescent individuals following COVID-19. Nat Immunol 2020; 21:1336–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hvidt AK, Guo H, Andersen R, et al. . Long-term humoral and cellular immunity after primary SARS-CoV-2 infection: a 20-month longitudinal study. BMC Immunol 2023; 24:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ruhl L, Kühne JF, Beushausen K, et al. . Third SARS-CoV-2 vaccination and breakthrough infections enhance humoral and cellular immunity against variants of concern. Front Immunol 2023; 14:1120010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lang-Meli J, Luxenburger H, Wild K, et al. . SARS-CoV-2-specific T-cell epitope repertoire in convalescent and mRNA-vaccinated individuals. Nat Microbiol 2022; 7:675–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Our World in Data Coronavirus pandemic (COVID-19) . COVID-19 data explorer vaccine doses, people vaccinated, and booster doses for Denmark. 2020. https://ourworldindata.org/coronavirus. Accessed 4 September 2023.
- 34. Baerends EAM, Hvidt AK, Reekie J, et al. . SARS-CoV-2 vaccine-induced antibodies protect against Omicron breakthrough infection. iScience 2023; 26:107621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Keeton R, Tincho MB, Ngomti A, et al. . T cell responses to SARS-CoV-2 spike cross-recognize Omicron. Nature 2022; 603:488–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Naranbhai V, Nathan A, Kaseke C, et al. . T cell reactivity to the SARS-CoV-2 Omicron variant is preserved in most but not all prior infected and vaccinated individuals. medRxiv [Preprint]. Posted online 5 January 2022. doi: 10.1101/2022.01.04.21268586 [DOI]
- 37. Mizoue T, Yamamoto S, Konishi M, et al. . Sensitivity of anti-SARS-CoV-2 nucleocapsid protein antibody for breakthrough infections during the epidemic of the Omicron variants. J Infect 2022; 85:573–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Allen JC, Toapanta FR, Chen W, Tennant SM. Understanding immunosenescence and its impact on vaccination of older adults. Vaccine 2020; 38:8264–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Panda A, Qian F, Mohanty S, et al. . Age-associated decrease in TLR function in primary human dendritic cells predicts influenza vaccine response. J Immunol 2010; 184:2518–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Grolleau-Julius A, Harning EK, Abernathy LM, Yung RL. Impaired dendritic cell function in aging leads to defective antitumor immunity. Cancer Res 2008; 68:6341–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.