Abstract
The varicella-zoster virus (VZV) infects >95% of the population. VZV reactivation causes herpes zoster (HZ), known as shingles, primarily affecting the elderly and individuals who are immunocompromised. However, HZ can occur in otherwise healthy individuals. We analyzed the immune signature and risk profile in patients with HZ using a genome-wide association study across different UK Biobank HZ cohorts. Additionally, we conducted one of the largest HZ human leukocyte antigen association studies to date, coupled with transcriptomic analysis of pathways underlying HZ susceptibility. Our findings highlight the significance of the major histocompatibility complex locus for HZ development, identifying 5 protective and 4 risk human leukocyte antigen alleles. This demonstrates that HZ susceptibility is largely governed by variations in the major histocompatibility complex. Furthermore, functional analyses revealed the upregulation of type I interferon and adaptive immune responses. These findings provide fresh molecular insights into the pathophysiology and activation of innate and adaptive immune responses triggered by symptomatic VZV reactivation.
Keywords: gene expression analysis, genome-wide association study, herpes zoster, HLA association, type I interferon response
Using gene expression and association studies, our research uncovered the major histocompatibility complex locus as a major risk factor for the development of herpes zoster. Additionally, a clear type I interferon and adaptive immune signature were identified in individuals with herpes zoster.
Herpes zoster (HZ; shingles) is caused by symptomatic reactivation of the varicella zoster virus (VZV) and typically presents as a painful dermatomal rash, frequently accompanied by mild symptoms such as fever, headache, and fatigue [1]. Postherpetic neuralgia (PHN; ie, long-lasting neuropathic pain) is the most common complication of HZ and significantly adds to the disease burden [2, 3]. Naturally, >95% of the population gets infected with VZV, resulting in chickenpox (varicella) [1, 4]. Upon resolution of a primary infection with VZV, the VZV particles gain access to neural ganglia where latency is established [1]. At a later age, VZV can reactivate to cause HZ [1].
The lifetime risk of developing HZ is between 25% and 30%, rising to 50% in those aged at least 80 years [2, 5]. Indeed, increasing age is a well-known risk factor for the development of HZ, thought to be a result of a decline in cellular immunity (ie, immunosenescence) [6, 7]. Yet, HZ frequently occurs in patients who are immunocompromised [8] and otherwise healthy individuals regardless of age [2, 9]. Besides waning cellular immunity due to aging, the composition of the T-cell receptor repertoire could be a determining factor for the development of HZ. The T-cell receptor repertoire reflects the major histocompatibility complex (MHC) background of an individual [10]. Thus, depending on one’s MHC constitution encoded by human leukocyte antigen (HLA) genes, each person will be able to recognize a different set of virus-derived peptides and generate a different immune response [10].
As such, a person’s HLA genetic profile could be a risk factor for the development of HZ [10]. The 9 so-called classical MHC genes are as follows: HLA-A, -B, and -C, belonging to MHC class I, and HLA-DPA1, -DPB1, -DQA1, -DQB1, -DRA, and -DRB1, belonging to MHC class II [10]. A previous pilot study including 50 Belgian individuals with a history of HZ and a control population of 25 000 Belgians obtained via the Red Cross already showed that HLA-A*11 was protective, whereas HLA-B*37 was a risk allele for the development of HZ [11]. In addition, a meta-analysis revealed that HLA-A*02 and HLA-B*40 were protective, whereas HLA-A*33 and HLA-B*44 were risk alleles for PHN in Japanese patients [12].
Besides HLA genetic variation, other genetic polymorphisms, especially related to immune system genes, can affect a person’s likeliness to develop HZ. Following VZV infection, VZV is immediately sensed by the innate immune system via pattern recognition receptors such as RNA polymerase III [13, 14]. Subsequent activation of downstream pathways leads to the production of type I interferons (IFNs) and proinflammatory cytokines that inhibit viral replication and recruit inflammatory cells to the site of infection [13, 15]. Binding of these type I IFNs to their receptor ultimately leads to the induction of IFN-stimulated genes (ISGs) with direct antiviral effector functions [16]. The importance of an adequate type I IFN response to control VZV infection is illustrated by several studies reporting that mutations in POLR3, TLR3, STAT1, STAT2, TYK2, and NEMO lead to increased susceptibility to VZV infection or even VZV viral encephalitis [17–20]. In addition to direct antiviral effector functions, type I IFNs promote T-cell expansion and activation [21]. CD4+ and CD8+ effector T cells are essential for recovery; additionally, memory T cells that develop during a primary infection are hypothesized to help prevent VZV reactivation [22]. Ultimately, to identify potential biomarkers and functional mechanisms that dictate HZ susceptibility and its associated risk profile, a dual approach of genomic studies and functional transcriptomic analyses in patients with HZ is essential.
METHODS
Participants
A total cohort of 26 patients with HZ aged 18 to 70 years (median age, 51 years; 13 men, 13 women) were prospectively recruited during an active HZ episode, as confirmed by a positive VZV polymerase chain reaction result on skin swab or saliva (n = 24) or by significantly elevated VZV IgG serum titers (>4 times; n = 2). The supplementary methods give a detailed description of the included samples.
Neutralizing Autoantibody Assay
Sera from patients with HZ and controls were coincubated in 10% plasma with IFNα2, IFNβ, or IFNω at different doses (high dose, 10 ng/mL; lower dose, 100 pg/mL). Next, a neutralization assay in a luciferase-based system was used as described by Bastard et al in Science Immunology [23]. The results were expressed as positive (1) or negative (0) for the individual cytokines, and doses and raw values were expressed as a percentage of the nonneutralizing samples of the day.
UK Biobank Data Collection
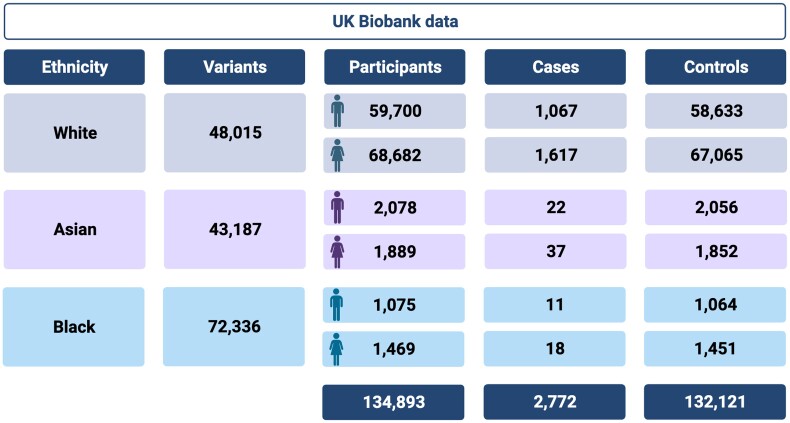
The whole exome sequencing data were obtained from the population-level exome OQFE variants (200k release) in UK Biobank [24]. These data were used to perform a genome-wide association study (GWAS; Figure 1) and HLA association study on multiple subsets of these data. Inclusion and exclusion criteria, as well as a detailed description of the statistical analysis procedure, are available in the supplementary methods.
Figure 1.
Overview of the UK Biobank data used for the genome-wide association studies. The filtered data set was divided into 3 ethnicity groups based on the metadata included in the UK Biobank. The number of variants per group are shown, as well as the gender distribution over the control participants and cases of herpes zoster. Figure made with BioRender.
Figure 1 gives an overview of the processed data used in the GWAS.
RNA Extraction, 3′ mRNA Sequencing, and NGS Data Processing
Whole blood RNA was extracted via the PaxGene blood RNA extraction kit (Qiagen) following the manufacturer's instructions. RNA samples were prepared with the QuantSeq 3' mRNA-Seq Library Prep Kit FWD for Illumina (Lexogen GmbH) for a NextSeq 500/550 sequencing run (high-output version 2.5 kit, 150 cycles, single read; Illumina). Raw data from the NextSeq were demultiplexed and processed through a 3' mRNA sequencing pipeline developed in-house, after which differential gene expression and gene ontology analyses were performed. All experimental protocols and the full NGS analysis pipeline are available in the supplementary methods.
RESULTS
Patients With HZ Show No Neutralizing Autoantibodies Against IFNα2, IFNβ, or IFNω
Since autoantibodies against cytokines have been associated with VZV central nervous system vasculopathy [25] and PHN [26] and anti-IFN antibodies have been shown to be present in a significant proportion of severe cases of COVID-19 [23, 27], we determined neutralizing autoantibodies against IFNα2, IFNβ, and IFNω in patients with HZ during the HZ episode and 1 year later and in controls. We did not detect autoantibodies against the IFNs tested here in any of the participants (Supplementary Table 3).
Genome-wide Association Analysis Shows MHC Locus Involvement in HZ Pathophysiology
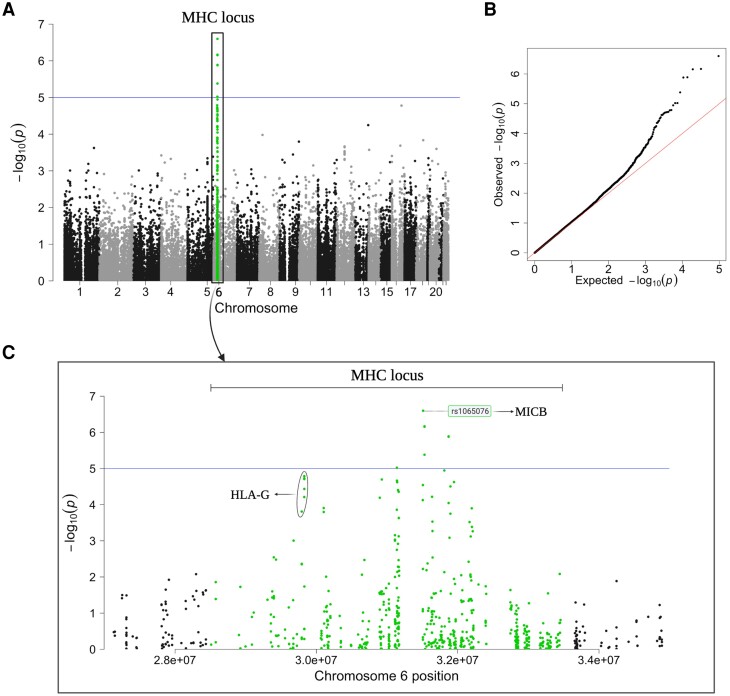
Given that the neutralizing autoantibodies did not show a host susceptibility for HZ, we conducted an extensive GWAS on the White subpopulation of the UK Biobank data. This GWAS included 2684 patients with HZ and 125 698 control participants with a total of 48 015 genetic variants that passed quality control. The results revealed a diverse variant distribution across the exome (Figure 2A). A distinct peak was observed on chromosome 6, containing a concentration of highly associated variants within the MHC locus (Figure 2C). Within this locus, 8 variants exceeded the significance threshold of 1e-05, of which 6 remained significant even after Benjamini-Hochberg multiple-testing correction (false discovery rate ≤0.05).
Figure 2.
Manhattan plot of the White subpopulation identified an association of the MHC locus (green) with HZ. A, The Manhattan plot depicts different genetic variants (n = 48 015) and their association with the development of HZ. The plot was made via the qqman package in R. B, QQ plot for the association analysis depicts the expected vs observed P values. C, Manhattan plot of the zoomed-in MHC locus on chromosome 6 identifies variants in HLA-related genes, including MICB. The most highly associated variant (P = 2.521e-07) is annotated with dbSNP. HLA, human leukocyte antigen; HZ, herpes zoster; MHC, major histocompatibility complex; MICB, MHC class I polypeptide–related sequence B.
The highest associated variant (P = 2.521e-07) is located in the MHC class I polypeptide-related sequence B (MICB) gene. Furthermore, a cluster of intron variants and synonymous mutations for the HLA-G gene was observed (Figure 2C). The QQ plot shows no population stratification (λ = 1.066; Figure 2B), which suggests a complex inheritance mechanism and provides compelling evidence for the role of HLA genes as a potential risk factor for HZ development.
Moreover, our analysis was extended to the Asian and Black subpopulations (Supplementary Figure 1). Despite revealing several significantly enriched variants, the limited number of patients with HZ in both groups (Asian, n = 59; Black, n = 29 HZ) restricts the power of these analyses. While the Asian subpopulation showed no population stratification (λ = 1.006), the lambda value of the Black subpopulation suggested multiple genetic backgrounds within the cohort (λ = 1.357). Therefore, although these findings hold promise for future research, their current reliability is limited due to the modest HZ sample sizes.
HLA Association Analysis Reveals Several Protective and Risk Alleles for HZ
The GWAS analysis revealed an enrichment of variants in the MHC locus, prompting the analysis of HLA allele frequencies between patients with HZ (n = 2826) and healthy controls (n = 135 875; Table 1). We identified 4 risk HLA alleles that were significantly enriched (P ≤ .05) in cases of HZ: HLA-A*01:01, HLA-B*07:02, HLA-C*07:02, and HLA-B*40:01. Additionally, 5 protective HLA alleles were significantly depleted (P ≤ .05) in cases of HZ: HLA-A*33:03, HLA-DRB1*11:01, HLA-A*02:01, DRB3*02:02, and HLA-DQB1*03:01.
Table 1.
All Significant HLA Alleles Related to Herpes Zoster Development in Patients vs Healthy Participants
| Adjusted P Values | ||||
|---|---|---|---|---|
| HLA Allele | Odds Ratio | 95% CI | Enrichment | Depletion |
| A*01:01 | 1.170 | 1.082–1.264 | .0130 | … |
| B*07:02 | 1.175 | 1.081–1.276 | .0130 | … |
| C*07:02 | 1.163 | 1.072–1.261 | .0153 | … |
| B*40:01 | 1.234 | 1.098–1.384 | .0195 | … |
| A*33:03a | 0.351 | .186–.602 | … | .0021 |
| DRB1*11:01a | 0.736 | .621–.867 | … | .0122 |
| A*02:01 | 0.874 | .810–.943 | … | .0264 |
| DRB3*02:02a | 0.860 | .787–.939 | … | .0264 |
| DQB1*03:01 | 0.873 | .803–.948 | … | .0381 |
All significantly enriched or depleted HLA alleles in the complete UK Biobank data set with their odds ratio and 95% CIs.
Abbreviation: HLA, human leukocyte antigen.
aSignificantly depleted in all 3 subpopulations.
The analysis was repeated for the 3 subpopulations separately to analyze the effect of population stratification on these results (Supplementary Table 4). Only 3 unique HLA alleles were consistently depleted in all 3 cohorts—HLA-A*33:03, DRB1*11:01, and DRB3*02:02—while none were consistently enriched in the 3 subpopulations. However, the Asian and Black populations consist of just a fraction of patients with HZ (1.51% and 1.14%, respectively) as compared with control participants, resulting in decreased statistical power to show significant differences.
Upregulation of ISGs Dominates the Immune Response During HZ
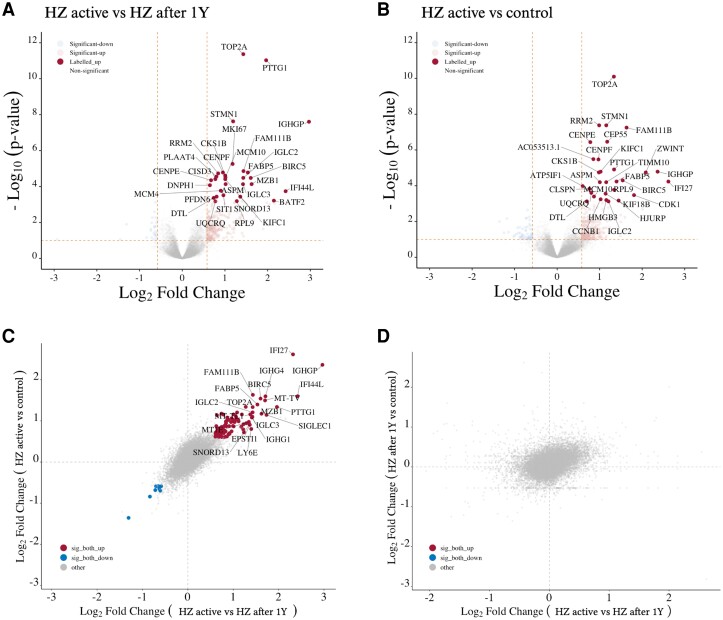
Our findings point toward a complex inheritance pattern with unknown heritability for HZ. To uncover the functional mechanisms behind this complexity, it is imperative to conduct functional genomics (ie, transcriptomics). We first compared the gene expression profile from the whole blood of patients with HZ taken during the HZ episode vs 1 year after the HZ episode and found 841 differentially expressed genes (DEGs; 596 upregulated and 245 downregulated genes; Supplementary Table 5, Figure 3A). Seven of the most upregulated genes—IFI44, IFI44L, IFI27, RSAD2, ISG15, SERPING1, and SIGLEC1—are ISGs produced by the innate immune system upon virus encounter.
Figure 3.
Differential expression analysis identifies overexpression of innate and adaptive immune transcripts in active HZ disease. Volcano plots represent top differential expression transcripts: A, HZ active vs HZ after 1 year; B, HZ active vs control. Dotted orange lines represent the cutoffs for the FDR (0.1) and logFC (±0.58). Scatter plots of logFC: C, HZ active vs HZ after 1 year and HZ active vs control; D, HZ active vs HZ after 1 year and HZ after 1 year vs control. Red, significantly upregulated in both; blue, significantly downregulated in both; orange, significantly dysregulated in both with opposite logFC; gray, nonsignificant in at least 1 analysis. Dotted gray lines represent x = 0 and y = 0 logFC. Gray dots denote nonsignificant transcripts (FDR >0.1); colored dots indicate significant dysregulation. Figures were made with ggVolcanoR. FDR, false discovery rate; HZ, herpes zoster; logFC, log2 fold change.
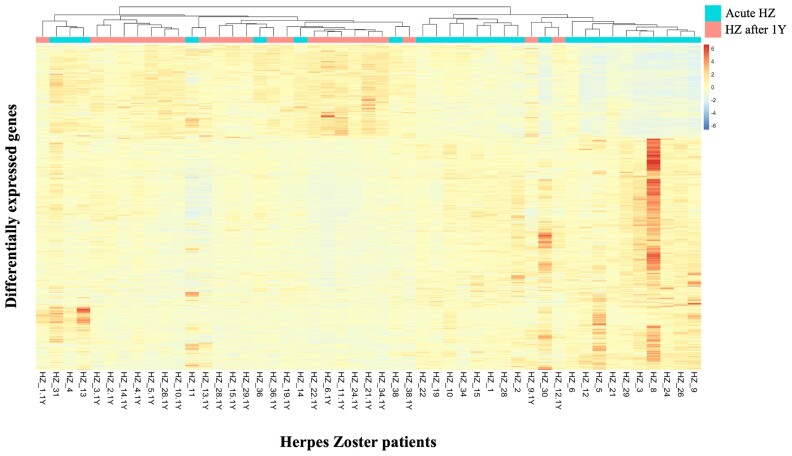
BATF2, involved in the differentiation of CD8+ thymic conventional dendritic cells following infection, was significantly upregulated during the acute HZ episode. Two genes associated with the antibody response, MZB1 and IGHG4, were significantly upregulated too, as well as RNA polymerase III (POLR3) subunit D (POLR3D) and GL (POLR3GL) and several additional ISGs: Mx1, IFIT5, OASL, IFI35, IFI27, and STAT2 (Supplementary Table 5). Finally, CXCR3, which is primarily expressed on activated T lymphocytes and NK cells, was significantly upregulated during HZ. No notable downregulated genes related to viral or immunologic functions were found. The heat map shows clustering of the gene expression profiles from blood taken during the acute HZ episode (HZ_XX, light blue) and clustering of those 1 year after HZ (HZ_XX.1Y, pink/red; Figure 4).
Figure 4.
Heat map of differentially expressed genes from blood taken during HZ vs 1 year after HZ. Heat map shows clustering of gene expression profiles from blood taken during the acute HZ episode (HZ_XX, light blue) and clustering of those 1 year after the HZ episode (HZ_XX.1Y, pink/red). HZ, herpes zoster.
Next, we compared the gene expression profiles of patients with HZ during the active HZ episode with those of control participants and found 485 DEGs (341 upregulated and 144 downregulated genes; Supplementary Table 6, Figure 3B). Ten of the most upregulated genes, including IFI27, IFI44L, and IGHG4, were also in the top upregulated genes when acute HZ was compared with 1 year after HZ. Interestingly, C4BPA, an inhibitor of the classical and lectin pathways of the complement system, was upregulated by 2 log folds. The relationship between the log2 fold change in the different conditions is shown in Figure 3C and 3D.
Finally, when the gene expression profiles of blood from patients with HZ during convalescence were compared with controls, no DEGs were found (Supplementary Table 7). In addition, for a control, we performed differential gene expression (DGE) analyses on samples from control participants taken 1 year apart from each other. Six DEGs were found, which were all related to ribosomal processes and are unlikely to reflect biologically significant differences.
Functional Enrichment Analysis Shows Activation of Host Immunity to Viral Infection
When we compared the gene expression profiles from patients during HZ with those 1 year after HZ, we found 843 DEGs. GO enrichment analysis of these DEGs revealed the involvement of 290 significant GO categories (top 200; Supplementary Table 8), including several pathways related to viral processes and host immune responses, also those specific to viral infection. Table 2 shows the first 20 significant enriched GO categories that were related to viral processes and host immune responses. These results clearly indicate that viral processes were ongoing and that host immunity to viral infection was activated. Interestingly, 6 of these categories came up in the GO enrichment analysis of the DEGs during HZ onset vs those of control participants (Table 3, Supplementary Table 9). Not surprising, viral transcription is one of the top GO terms in both analyses.
Table 2.
Top GO Categories Related to Viral Processes and Host Immune Responses of Differentially Expressed Genes During HZ vs 1 Year After HZ
| GO.ID | Term | P Value |
|---|---|---|
| GO:0019083 | Viral transcription | 1.00E-20 |
| GO:0002479 | Antigen processing and presentation of exogenous peptide antigen via MHC class I, TAP dependent | .00014 |
| GO:0050852 | T-cell receptor signaling pathway | .0002 |
| GO:0050853 | B-cell receptor signaling pathway | .00029 |
| GO:0035722 | Interleukin 12–mediated signaling pathway | .00042 |
| GO:0070498 | Interleukin 1–mediated signaling pathway | .00056 |
| GO:0060337 | Type I interferon signaling pathway | .00094 |
| GO:0051607 | Defense response to virus | .00095 |
| GO:0016032 | Viral process | .00102 |
| GO:0032020 | ISG15-protein conjugation | .00165 |
| GO:0038096 | Fc-gamma receptor signaling pathway involved in phagocytosis | .00459 |
| GO:0006955 | Immune response | .01661 |
| GO:0045059 | Positive thymic T-cell selection | .01864 |
| GO:0006910 | Phagocytosis, recognition | .02094 |
| GO:0019886 | Antigen processing and presentation of exogenous peptide antigen via MHC class II | .02287 |
| GO:0006958 | Complement activation, classical pathway | .02994 |
| GO:0002230 | Positive regulation of defense response to virus by host | .0316 |
| GO:0046596 | Regulation of viral entry into host cell | .03664 |
| GO:0019064 | Fusion of virus membrane with host plasma membrane | .03672 |
| GO:0050871 | Positive regulation of B-cell activation | .03989 |
Abbreviations: HZ, herpes zoster; MHC, major histocompatibility complex.
Table 3.
GO Categories Related to Viral Processes and Host Immune Responses During HZ vs Control
| GO.ID | Term | P Value |
|---|---|---|
| GO:0019083 | Viral transcription | 7.10E-15 |
| GO:0035722 | Interleukin 12–mediated signaling pathway | .01785 |
| GO:0016032 | Viral process | .02103 |
| GO:0002479 | Antigen processing and presentation of exogenous peptide antigen via MHC class I, TAP dependent | .02176 |
| GO:0006910 | Phagocytosis, recognition | .02291 |
| GO:0070498 | Interleukin 1–mediated signaling pathway | .03111 |
Abbreviations: HZ, herpes zoster; MHC, major histocompatibility complex.
DISCUSSION
In this project, we set out to understand why otherwise healthy individuals might develop symptomatic VZV reactivation (ie, HZ). To achieve this goal, we applied a multidisciplinary approach. Initially, no autoantibodies against IFNα2, IFNβ, and IFNω were found. Therefore, we explored germline susceptibility as a cause for HZ development and identified an enrichment of genetic variants within the MHC locus of patients with HZ. Their presence holds important value since the MHC locus harbors the HLA genes [28], which play a critical role in the adaptive immune response [29]. These results align with previous GWAS findings that also discovered genetic variation in the HLA region to be associated with HZ, including variants in the noncoding HLA complex P5 (HCP5) gene and the HLA-B gene [30, 31]. Hence, it is possible that these variants modulate immune responses, thereby significantly influencing one’s susceptibility to HZ.
The highest associated variant was located in the MICB gene, and an increase in MICB expression in herpesvirus-infected cells can lead to an enhanced T-cell response [32]. Moreover, herpesvirus proteins can intracellularly retain MICB, thereby helping the infected cells evade NK and T-cell responses [33]. These findings provide further evidence that variations in MICB expression or function could significantly affect the immune system's ability to mount an effective response against VZV reactivation [34]. Similarly, we discovered a cluster of genetic variants in the HLA-G gene, which has been associated with immune evasion of viral-infected cells through inhibition of host immune responses [35]. Interestingly, the clustering of variants within the MHC region suggests a collective contribution to the elevated risk of developing HZ through a complex inheritance pattern.
Next, we identified 4 enriched and 5 depleted HLA alleles in patients with HZ, highlighting the importance of an individual's HLA background on HZ susceptibility. Prior smaller studies have linked some of the identified alleles to PHN in cases of HZ [12, 36, 37]. Their findings show an enrichment of HLA-A*33 and HLA-B*44 in patients with PHN vs those without PHN and control groups, including a significant depletion of HLA-A*33 in PHN– HZ cases vs PHN+ HZ cases. This supports our findings showing a significant depletion of HLA-A*33 in our HZ cohort. Furthermore in these prior studies, HLA-A*02 and HLA-B*40 were significantly depleted in the PHN group while displaying enrichment in patients with HZ, similar to our findings. These studies primarily focused on alleles associated with PHN+ status, rather than examining the overall risk for HZ development; as such, it is possible that these nuanced associations indicate alleles that may confer protection against HZ while increasing the risk of developing PHN and vice versa, as reported in other studies [12, 38]. Our findings are also based on one of the largest predominantly White/Caucasian cohorts to date, which differs significantly from these prior studies, as largely conducted in Asian populations. This is supported by the fact that our analysis aligns remarkably with HLA associations identified in a European-focused study, where alleles HLA-A*02:01 and HLA-DRB1*11:01 were also depleted in HZ [39]. Our research highlights the specificity of risk and protective alleles for different genetic backgrounds [40, 41]. Additionally, it is vital to recognize that these results do not prove causality or unveil underlying biological mechanisms, proving the need for future studies to validate these findings. Studies aimed at assessing the impact of variants on HLA expression, antigen presentation machinery, and the broader immune response will shed light on the precise multifaceted molecular mechanisms underlying their association with HZ. Additionally, expanding the sample sizes and using whole genome data will increase statistical power and offer valuable insights into the functional consequences of these genetic variants.
Incorporating functional studies to help decipher the risk profile is necessary for a comprehensive understanding of the underlying biological mechanisms dictating HZ susceptibility. The pre- to peri-HZ period can span several weeks, leading to a certain degree of heterogeneity in immune response among HZ samples. Despite this variability, we are still able to identify significant differences among the gene expression profiles of the different cohorts. Notably, ISGs such as IFI44, IFI44L, IFI27, ISG15, RSAD2, SERPING1, and SIGLEC1 showed distinct upregulation during the active HZ episode vs 1 year after HZ. All of these are involved in modulation of the viral immune response through various mechanisms, including negative feedback loop [42], type I IFN–induced apoptosis [43], regulation of the complement cascade, and other pathways. Besides ISG upregulation, we found that POLR3D and POLR3GL were significantly upregulated during HZ. Mutations in POLR3A, POLR3C, POLR3E, and POLR3F have been associated with susceptibility to VZV-induced encephalitis and pneumonitis [14, 44]. However, such associations for POLR3 subtype D or GL have not been described so far. It is interesting to note that although the upregulation of POLR3D and POLR3GL might not directly indicate an increased HZ susceptibility, it does signify the importance of this pathway in controlling HZ once reactivation has been initiated.
A recent report investigated the involvement of cellular calcium disorder in the development of PHN, revealing different DEGs [45]. In our cohort, several DEGs that were related to the GO term “negative regulation of cytosolic calcium ion concentration” were upregulated during the symptomatic HZ episode: CAB39, CACNA1D, CAMK1D, and CACNB4. Conversely, only 1 gene related to calcium signaling (CIB1) was upregulated 1 year after HZ. In addition, 2 genes related to calcium signaling (S100B and CALHM6) were upregulated during acute HZ as compared with controls. Thus, our data suggest that, indeed, calcium signaling pathways are involved in HZ pathogenesis.
While we found several DEGs during HZ vs 1 year after HZ, we did not find any DEGs 1 year after HZ vs controls, indicating that the patients returned to baseline. However, some patients with HZ had a high expression of C4BPA (although not significant) as compared with controls. Indeed, a recent study showed that direct binding of C4BP to influenza A virus subtype H1N1 suppressed viral infection, whereas binding to H3N2 subtype promoted viral infection [46]. Since we see upregulation of C4BPA in patients during HZ and 1 year after the HZ episode vs the control group, further investigation is needed to determine whether C4BP may play a role, whether pro- or antiviral, in VZV infection or reactivation. Analysis of these patterns reveals a compelling similarity in expression profiles shared between HZ and other viral infections (Supplementary Table 10). Although this comparison is not exhaustive, it highlights that the HZ immune signature that we identified is present across multiple viral infections. This is not unexpected but highlights the importance of these genes and pathways in the overall antiviral immune response. Yet, to uncover the specific effects of these DEGs on the individual infections, additional targeted studies are required.
GO enrichment analysis revealed upregulation of the type I IFN signaling pathway, ISG15-protein conjugation, and complement activation during HZ. Interestingly, upregulation of the type I IFN response in patients with HZ is in line with our data that showed no neutralizing autoantibodies against IFNα2, IFNβ, or IFNω in HZ or control, implying a functional type I IFN response. Of note, in a previous study in which autoantibodies against IFNα, INFγ, GM-CSF, and IL-6 were determined in the sera of patients with HZ, neutralizing autoantibodies were identified in the HZ group with PHN but not in the HZ group without PHN [26]. GO analysis also revealed enrichment of the interleukin 1 and 12 pathway during HZ, which is involved in inflammatory cell recruitment [47] and differentiation of naive T cells as well as enhancement of NK-cell and CD8+ T-cell cytotoxicity [48, 49]. Related to the latter, the T-cell receptor signaling pathway and the MHC class I and II antigen presentation pathways were significantly enriched during HZ. Finally, B-cell receptor signaling and pathways involved in phagocytosis were significantly enriched during HZ. This accentuates the critical role of ISGs and antigen processing pathways linked to B and T cells in driving susceptibility to HZ.
One limitation of our approach would be the bulk sequencing approach, whereas a single-cell RNA sequencing approach would allow the identification of cell type–specific transcriptional changes that occur. While fully unraveling the intricate mechanisms that underlie HZ development remains beyond our current capabilities, this research has already demonstrated several crucial components tied to the genetic background and biological pathways that play an important role in the risk profile and pathophysiology of HZ development and a multitude of other viral infections in individuals who are immunocompromised or healthy.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Supplementary Material
Contributor Information
Romi Vandoren, Adrem Data Lab, Department of Computer Science, University of Antwerp; Antwerp Unit for Data Analysis and Computation in Immunology and Sequencing, University of Antwerp; Biomedical Informatics Research Network Antwerp.
Marlies Boeren, Antwerp Unit for Data Analysis and Computation in Immunology and Sequencing, University of Antwerp; Laboratory of Microbiology, Parasitology and Hygiene and Infla-Med Center of Excellence; Laboratory of Experimental Hematology, Vaccine and Infectious Disease Institute; Antwerp Center for Translational Immunology and Virology, Vaccine and Infectious Disease Institute; Centre for Health Economics Research and Modelling Infectious Diseases, Vaccine and Infectious Disease Institute.
Jolien Schippers, Antwerp Unit for Data Analysis and Computation in Immunology and Sequencing, University of Antwerp; Antwerp Center for Translational Immunology and Virology, Vaccine and Infectious Disease Institute; Centre for Health Economics Research and Modelling Infectious Diseases, Vaccine and Infectious Disease Institute.
Esther Bartholomeus, Antwerp Unit for Data Analysis and Computation in Immunology and Sequencing, University of Antwerp; Antwerp Center for Translational Immunology and Virology, Vaccine and Infectious Disease Institute; Centre for Health Economics Research and Modelling Infectious Diseases, Vaccine and Infectious Disease Institute.
Kerry Mullan, Adrem Data Lab, Department of Computer Science, University of Antwerp; Antwerp Unit for Data Analysis and Computation in Immunology and Sequencing, University of Antwerp; Biomedical Informatics Research Network Antwerp.
Nele Michels, Department of Family Medicine and Population Health, Center for General Practice/Family Medicine, University of Antwerp.
Olivier Aerts, Department of Dermatology, Antwerp University Hospital and University of Antwerp.
Julie Leysen, Department of Dermatology, Antwerp University Hospital and University of Antwerp.
An Bervoets, Department of Dermatology, Antwerp University Hospital and University of Antwerp.
Julien Lambert, Department of Dermatology, Antwerp University Hospital and University of Antwerp.
Elke Leuridan, Centre for the Evaluation of Vaccination, Vaccine and Infectious Disease Institute, University of Antwerp.
Johan Wens, Department of Family Medicine and Population Health, Center for General Practice/Family Medicine, University of Antwerp.
Karin Peeters, Antwerp Unit for Data Analysis and Computation in Immunology and Sequencing, University of Antwerp; Antwerp Center for Translational Immunology and Virology, Vaccine and Infectious Disease Institute; Centre for Health Economics Research and Modelling Infectious Diseases, Vaccine and Infectious Disease Institute.
Marie-Paule Emonds, Histocompatibility and Immunogenetic Laboratory, Rode Kruis-Vlaanderen, Mechelen.
Hilde Jansens, Department of Clinical Microbiology, Antwerp University Hospital, Belgium.
Jean-Laurent Casanova, Laboratory of Human Genetics of Infectious Diseases, Necker Branch, Institut National de la Santé et de la Recherche Médicale U1163, Necker Hospital for Sick Children, Paris; Imagine Institute, Paris Cité University, France; St Giles Laboratory of Human Genetics of Infectious Diseases, Rockefeller Branch, The Rockefeller University; Howard Hughes Medical Institute, New York, New York; Department of Pediatrics, Necker Hospital for Sick Children, Paris.
Paul Bastard, Laboratory of Human Genetics of Infectious Diseases, Necker Branch, Institut National de la Santé et de la Recherche Médicale U1163, Necker Hospital for Sick Children, Paris; Imagine Institute, Paris Cité University, France; St Giles Laboratory of Human Genetics of Infectious Diseases, Rockefeller Branch, The Rockefeller University; Pediatric Hematology-Immunology and Rheumatology Unit, Necker Hospital for Sick Children, Assistante Publique–Hôpitaux de Paris, France.
Arvid Suls, Antwerp Unit for Data Analysis and Computation in Immunology and Sequencing, University of Antwerp; Medical Genetics, University of Antwerp and Antwerp University Hospital.
Viggo Van Tendeloo, Antwerp Unit for Data Analysis and Computation in Immunology and Sequencing, University of Antwerp; Laboratory of Experimental Hematology, Vaccine and Infectious Disease Institute.
Peter Ponsaerts, Laboratory of Experimental Hematology, Vaccine and Infectious Disease Institute.
Peter Delputte, Laboratory of Microbiology, Parasitology and Hygiene and Infla-Med Center of Excellence.
Benson Ogunjimi, Antwerp Unit for Data Analysis and Computation in Immunology and Sequencing, University of Antwerp; Antwerp Center for Translational Immunology and Virology, Vaccine and Infectious Disease Institute; Centre for Health Economics Research and Modelling Infectious Diseases, Vaccine and Infectious Disease Institute; Department of Paediatrics, Antwerp University Hospital, Belgium.
Kris Laukens, Adrem Data Lab, Department of Computer Science, University of Antwerp; Antwerp Unit for Data Analysis and Computation in Immunology and Sequencing, University of Antwerp; Biomedical Informatics Research Network Antwerp.
Pieter Meysman, Adrem Data Lab, Department of Computer Science, University of Antwerp; Antwerp Unit for Data Analysis and Computation in Immunology and Sequencing, University of Antwerp; Biomedical Informatics Research Network Antwerp.
Notes
Acknowledgments. We appreciate the participation of all patients in this study. We are grateful to all unmentioned clinicians, recruiters, nurses, and laboratory colleagues. We thank Geert Mortier for his support. We extend our gratitude to the team at Columbia University Irving Medical Center for its assistance with the GWAS pipeline. This research was conducted with the UK Biobank Resource under application 70885.
Author contributions. The data used in this project were generated by M. B., J. S., E. B., N. M., O. A., J. Leysen, A. B., J. Lambert, E. L., J. W., K. P., M.-P. E., H. J., J.-L. C., P. B., and A. S. Data analysis was performed by R. V., M. B., K. M., P. B., and P. M. under the supervision of V. V. T., P. P., P. D., B. O., P. M., and K. L. The manuscript was written by R. V., M. B., E. B., K. M., B. O., P. M., and K. L. and edited by all.
Data availability. The RNA sequencing data generated and analyzed during the current study are available in the Gene Expression Omnibus repository under accession GSE242252. DGE and GO results are included in this published article and its supplementary information files. The whole exome sequencing data that support the GWAS and HLA findings of this study are available from the UK Biobank, but restrictions apply to the availability of these data, which were used under license for the current study and so are not publicly available.
Ethics approval and consent to participate. This study was approved by the ethics board of the Antwerp University Hospital and University of Antwerp (17/48/546). All methods were performed in accordance with the relevant guidelines and regulations when applicable. Written informed consent was obtained from all study participants.
Financial support. The Antwerp Unit for Data Analysis and Computation in Immunology and Sequencing consortium is supported by the Research Foundation Flanders (1861219N to B. O.; G034721N to P. P., P. D., B. O.); the Hercules Foundation; University of Antwerp Methusalem VAXINFECTIO (P. P.); Excellence Centre ASCID (P. P.); Excellence Centre Infla-Med (P. D.); Excellence Centre MST (K. L.); Industrieel Onderzoeksfonds Proof of Concept grant 46947 (P. D., P. P., B. O.); Horizon 2020 (851752-CELLULO-EPI to B. O.); Flemish Interuniversity Council iBOF MIMICRY grant (K. L.); and Chan Zuckerberg Initiative (P. M., K. L., B. O.). P. B. was supported by the Bettencourt-Schueller Foundation and the Fondation pour la Recherche Médicale (EA20170638020). The Laboratory of Human Genetics of Infectious Diseases is supported by the Howard Hughes Medical Institute; The Rockefeller University; the St Giles Foundation; the Investissement d’Avenir program, launched by the French Government and implemented by the Agence Nationale de la Recherche (ANR-10-IAHU-01); the Integrative Biology of Emerging Infectious Diseases Laboratory of Excellence (ANR-10-LABX-62-IBEID), ANR GENVIR (ANR-20-CE93-003) and ANR AI2D (ANR-22-CE15-0046) projects; the National Institutes of Health (R01AI163029); the Horizon foundation (01057100, UNDINE); the ANR-RHU COVIFERON (ANR-21-RHUS-08); the Fisher Center for Alzheimer Research Foundation; the Meyer Foundation; the JPB Foundation; the Square Foundation; Grandir–Fonds de solidarité pour l’enfance; the Fondation du Souffle; the SCOR Corporate Foundation for Science; Battersea & Bowery Advisory Group; the French Ministry of Higher Education and Research (MESRI-COVID-19); Institut National de la santé et de la reserche médicale; and the University of Paris.
References
- 1. Zerboni L, Sen N, Oliver SL, Arvin AM. Molecular mechanisms of varicella zoster virus pathogenesis. Nat Rev Microbiol 2014; 12:197–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bilcke J, Ogunjimi B, Marais C, et al. The health and economic burden of chickenpox and herpes zoster in Belgium. Epidemiol Infect 2012; 140:2096–109. [DOI] [PubMed] [Google Scholar]
- 3. Johnson RW, Alvarez-Pasquin M-J, Bijl M, et al. Herpes zoster epidemiology, management, and disease and economic burden in Europe: a multidisciplinary perspective. Ther Adv Vaccines 2015; 3:109–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brisson M, Edmunds WJ, Law B, et al. Epidemiology of varicella zoster virus infection in Canada and the United Kingdom. Epidemiol Infect 2001; 127:305–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yawn BP, Gilden D. The global epidemiology of herpes zoster. Neurology 2013; 81:928–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Levin MJ, Smith JG, Kaufhold RM, et al. Decline in varicella-zoster virus (VZV)–specific cell-mediated immunity with increasing age and boosting with a high-dose VZV vaccine. J Infect Dis 2003; 188:1336–44. [DOI] [PubMed] [Google Scholar]
- 7. Ogunjimi B, Smits E, Hens N, et al. Exploring the impact of exposure to primary varicella in children on varicella-zoster virus immunity of parents. Viral Immunol 2011; 24:151–7. [DOI] [PubMed] [Google Scholar]
- 8. Dolin R, Reichman RC, Mazur MH, Whitley RJ. NIH conference: herpes zoster-varicella infections in immunosuppressed patients. Ann Intern Med 1978; 89:375–88. [DOI] [PubMed] [Google Scholar]
- 9. Ogunjimi B, Buntinx F, Bartholomeeusen S, et al. Herpes zoster is associated with herpes simplex and other infections in under 60 year-olds. J Infect 2015; 70:171–7. [DOI] [PubMed] [Google Scholar]
- 10. Gattorno M, Martini A, et al. The immune system and the inflammatory response. In: Cassidy JT, ed. Textbook of pediatric rheumatology. 4th ed. Philadelphia: WB Saunders, 2005:19–63. [Google Scholar]
- 11. Meysman P, De Neuter N, Bartholomeus E, et al. Increased herpes zoster risk associated with poor HLA-A immediate early 62 protein (IE62) affinity. Immunogenetics 2018; 70:363–72. [DOI] [PubMed] [Google Scholar]
- 12. Meysman P, Ogunjimi B, Naulaerts S, Beutels P, Van Tendeloo V, Laukens K. Varicella-zoster virus–derived major histocompatibility complex class I–restricted peptide affinity is a determining factor in the HLA risk profile for the development of postherpetic neuralgia. J Virol 2015; 89:962–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Carter-Timofte ME, Paludan SR, Mogensen TH. RNA polymerase III as a gatekeeper to prevent severe VZV infections. Trends Mol Med 2018; 24:904–15. [DOI] [PubMed] [Google Scholar]
- 14. Ogunjimi B, Zhang S-Y, Sørensen KB, et al. Inborn errors in RNA polymerase III underlie severe varicella zoster virus infections. J Clin Invest 2017; 127:3543–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Boeren M, Van Breedam E, Buyle-Huybrecht T, et al. Activation of interferon-stimulated genes following varicella-zoster virus infection in a human iPSC-derived neuronal in vitro model depends on exogenous interferon-alpha. Viruses 2022; 14:2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Katze MG, He Y, Gale M Jr. Viruses and interferon: a fight for supremacy. Nat Rev Immunol 2002; 2:675–87. [DOI] [PubMed] [Google Scholar]
- 17. Tóth B, Méhes L, Taskó S, et al. Herpes in STAT1 gain-of-function mutation [corrected]. Lancet 2012; 379:2500. [DOI] [PubMed] [Google Scholar]
- 18. Kreins AY, Ciancanelli MJ, Okada S, et al. Human TYK2 deficiency: mycobacterial and viral infections without hyper-IgE syndrome. J Exp Med 2015; 212:1641–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hambleton S, Goodbourn S, Young DF, et al. STAT2 deficiency and susceptibility to viral illness in humans. Proc Natl Acad Sci U S A 2013; 110:3053–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sironi M, Peri AM, Cagliani R, et al. TLR3 mutations in adult patients with herpes simplex virus and varicella-zoster virus encephalitis. J Infect Dis 2017; 215:1430–4. [DOI] [PubMed] [Google Scholar]
- 21. Biron CA. Role of early cytokines, including alpha and beta interferons (IFN-alpha/beta), in innate and adaptive immune responses to viral infections. Semin Immunol 1998; 10:383–90. [DOI] [PubMed] [Google Scholar]
- 22. Weinberg A, Levin MJ. VZV T cell–mediated immunity. Curr Top Microbiol Immunol 2010; 342:341–57. [DOI] [PubMed] [Google Scholar]
- 23. Bastard P, Gervais A, Le Voyer T, et al. Autoantibodies neutralizing type I IFNs are present in ∼4% of uninfected individuals over 70 years old and account for ∼20% of COVID-19 deaths. Sci Immunol 2021; 6:eabl4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Szustakowski JD, Balasubramanian S, Kvikstad E, et al. Advancing human genetics research and drug discovery through exome sequencing of the UK Biobank. Nat Genet 2021; 53:942–8. [DOI] [PubMed] [Google Scholar]
- 25. Ansari R, Rosen LB, Lisco A, et al. Primary and acquired immunodeficiencies associated with severe varicella-zoster virus infections. Clin Infect Dis 2021; 73:e2705–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bayat A, Burbelo PD, Browne SK, et al. Anti-cytokine autoantibodies in postherpetic neuralgia. J Transl Med 2015; 13:333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bastard P, Rosen LB, Zhang Q, et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science 2020; 370:eabd4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kulski JK, Suzuki S, Shiina T. Human leukocyte antigen super-locus: nexus of genomic supergenes, SNPs, indels, transcripts, and haplotypes. Hum Genome Var 2022; 9:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mosaad YM. Clinical role of human leukocyte antigen in health and disease. Scand J Immunol 2015; 82:283–306. [DOI] [PubMed] [Google Scholar]
- 30. Crosslin DR, Carrell DS, Burt A, et al. Genetic variation in the HLA region is associated with susceptibility to herpes zoster. Genes Immun 2015; 16:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Stanaway IB, Hall TO, Rosenthal EA, et al. The eMERGE genotype set of 83,717 subjects imputed to ∼40 million variants genome wide and association with the herpes zoster medical record phenotype. Genet Epidemiol 2019; 43:63–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Groh V, Rhinehart R, Randolph-Habecker J, Topp MS, Riddell SR, Spies T. Costimulation of CD8alphabeta T cells by NKG2D via engagement by MIC induced on virus-infected cells. Nat Immunol 2001; 2:255–60. [DOI] [PubMed] [Google Scholar]
- 33. Welte SA, Sinzger C, Lutz SZ, et al. Selective intracellular retention of virally induced NKG2D ligands by the human cytomegalovirus UL16 glycoprotein. Eur J Immunol 2003; 33:194–203. [DOI] [PubMed] [Google Scholar]
- 34. Jasinski-Bergner S, Mandelboim O, Seliger B. Molecular mechanisms of human herpes viruses inferring with host immune surveillance. J Immunother Cancer 2020; 8:e000841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jasinski-Bergner S, Schmiedel D, Mandelboim O, Seliger B. Role of HLA-G in viral infections. Front Immunol 2022; 13:826074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chung HY, Song EY, Yoon JA, et al. Association of human leukocyte antigen with postherpetic neuralgia in Koreans. APMIS 2016; 124:865–71. [DOI] [PubMed] [Google Scholar]
- 37. Sumiyama D, Kikkawa EF, Kita YF, et al. HLA alleles are associated with postherpetic neuralgia but not with herpes zoster. Tokai J Exp Clin Med 2008; 33:150–3. [PubMed] [Google Scholar]
- 38. Sato M, Ohashi J, Tsuchiya N, et al. Association of HLA-A*3303-B*4403-DRB1*1302 haplotype, but not of TNFA promoter and NKp30 polymorphism, with postherpetic neuralgia (PHN) in the Japanese population. Genes Immun 2002; 3:477–81. [DOI] [PubMed] [Google Scholar]
- 39. Tian C, Hromatka BS, Kiefer AK, et al. Genome-wide association and HLA region fine-mapping studies identify susceptibility loci for multiple common infections. Nat Commun 2017; 8:599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Souquette A, Allen EK, Oshansky CM, et al. Integrated drivers of basal and acute immunity in diverse human populations. bioRxiv. Preprint posted 26 March 2023. doi: 10.1101/2023.03.25.534227 [DOI]
- 41. Gomes KF, Santos AS, Semzezem C, et al. The influence of population stratification on genetic markers associated with type 1 diabetes. Sci Rep 2017; 7:43513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. DeDiego ML, Martinez-Sobrido L, Topham DJ. Novel functions of IFI44L as a feedback regulator of host antiviral responses. J Virol 2019; 93:e01159-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gytz H, Hansen MF, Skovbjerg S, et al. Apoptotic properties of the type 1 interferon induced family of human mitochondrial membrane ISG12 proteins. Biol Cell 2017; 109:94–112. [DOI] [PubMed] [Google Scholar]
- 44. Lata E, Choquet K, Sagliocco F, Brais B, Bernard G, Teichmann M. RNA polymerase III subunit mutations in genetic diseases. Front Mol Biosci 2021; 8:696438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wu S, Yang S, Ou M, et al. Transcriptome analysis reveals the role of cellular calcium disorder in varicella zoster virus–induced post-herpetic neuralgia. Front Mol Neurosci 2021; 14:665931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Varghese PM, Murugaiah V, Beirag N, et al. C4b binding protein acts as an innate immune effector against influenza A virus. Front Immunol 2020; 11:585361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Weber A, Wasiliew P, Kracht M. Interleukin-1 (IL-1) pathway. Sci Signal 2010; 3:cm1. [DOI] [PubMed] [Google Scholar]
- 48. Zundler S, Neurath MF. Interleukin-12: functional activities and implications for disease. Cytokine Growth Factor Rev 2015; 26:559–68. [DOI] [PubMed] [Google Scholar]
- 49. Zheng H, Ban Y, Wei F, Ma X. Regulation of interleukin-12 production in antigen-presenting cells. Adv Exp Med Biol 2016; 941:117–38. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.