Abstract
Objective
Depression is a common psychiatric issue among patients with narcolepsy type 1 (NT1). Effective management requires accurate screening and prediction of depression in NT1 patients. This study aims to identify relevant factors for predicting depression in Chinese NT1 patients using machine learning (ML) approaches.
Methods
A total of 203 drug-free NT1 patients (aged 5–61), diagnosed based on the ICSD-3 criteria, were consecutively recruited from the Sleep Medicine Center at Peking University People’s Hospital between September 2019 and April 2023. Depression, daytime sleepiness, and impulsivity were assessed using the Center for Epidemiologic Studies Depression Scale for Children (CES-DC) or the Self-Rating Depression Scale (SDS), the Epworth Sleepiness Scale for adult or children and adolescents (ESS or ESS-CHAD), and the Barratt Impulse Scale (BIS-11). Demographic characteristics and objective sleep parameters were also analyzed. Three ML models-Logistic Regression (LR), Random Forest (RF), and Support Vector Machine (SVM)-were used to predict depression. Model performance was evaluated using receiver operating curve (AUC), accuracy, precision, recall, F1 score, and decision curve analysis (DCA).
Results
The LR model identified hallucinations (OR 2.21, 95% CI 1.01–4.90, p = 0.048) and motor impulsivity (OR 1.10, 95% CI 1.02–1.18, p = 0.015) as predictors of depression. Among the ML models, SVM showed the best performance with an AUC of 0.653, accuracy of 0.659, sensitivity of 0.727, and F1 score of 0.696, reflecting its effectiveness in integrating sleep-related and psychosocial factors.
Conclusion
This study highlights the potential of ML models for predicting depression in NT1 patients. The SVM model shows promise in identifying patients at high risk of depression, offering a foundation for developing a data-driven, personalized decision-making tool. Further research should validate these findings in diverse populations and include additional psychological variables to enhance model accuracy.
Keywords: narcolepsy type 1, depression, machine learning, support vector machine
Introduction
Narcolepsy type 1 (NT1) is a specific subtype of narcolepsy, characterized by excessive daytime sleepiness (EDS), cataplexy, hypnagogic/hypnopompic hallucinations (HH), sleep paralysis (SP), and disrupted nocturnal sleep (DNS).1 Importantly, neuropsychiatric manifestations are frequently observed in patients with narcolepsy.2 Despite variations in the reported prevalence of psychiatric comorbidities among patients with narcolepsy, existing empirical evidence predominantly supports a higher incidence of depression compared to other psychiatric disorders.3–6 For instance, research has shown that a higher proportion of patients with narcolepsy (32%, 95% Confidence Interval, 28–36%) experience depression or depressive symptoms.7
It has been confirmed that depression is associated with poor quality of life,8,9 low physical activity levels10 and decreased job effectiveness and daily activities11 among patients with NT1. Furthermore, comorbid depression may increase the risk of suicidality in narcolepsy patients.2 While depression is often overlooked and not adequately treated in patients with NT1. Research indicates that despite the high prevalence7 and substantial effects of depression9 in patients with narcolepsy, appropriate mental health care is not provided. European evidence-based guidelines12 recommend treating psychiatric disorders in narcolepsy using general principles, as there are limited specific studies supporting any particular treatments for depression in narcolepsy. Only a few studies have explored non-pharmacological interventions, such as cognitive behavioral therapy (CBT)13 and peer support,14 to address the psychosocial needs of these patients. For example, Ong et al13 developed a CBT for hypersomnia (CBT-H) program to address the psychosocial needs of individuals with central disorders of hypersomnolence (CDH), finding that it could reduce depressive symptoms (decrease in Patient Health Questionnaire ≥ 5) among patients with CDH. Consequently, early and accurate prediction of depression in patients with NT1 assumes paramount importance for disease management and enhancement of their overall well-being.
Several factors may contribute to the development of depression among patients with NT1, including neurobiological factors such as hypocretin deficit, cortisol levels, and corticotropin-releasing hormone (CRH) neurons;15–17 sleep-related factors like sleep quality, EDS and hallucinations;10,18 as well as psychosocial factors such as stigma or resilience.19,20 As for neurobiological factors, the deficiency in hypocretin of cerebral spinal fluid (CSF) is believed to be a potential avenue for investigating the pathological basis of depression in patients with NT1.16 Additionally, studies have demonstrated a reduction in CRH neurons in NT1,21 accompanied by hyperactivity in the remaining neurons.17 Given that paraventricular CRH neurons are crucial to the hypothalamic-pituitary-adrenal (HPA) stress axis and HPA axis overactivity is linked to depression,22 this hyperactivity may significantly contribute to mood and anxiety symptoms in NT1. Moreover, it had been reported that the disruption of white matter integrity and prevalent brain degeneration of frontal lobes were associated with depressive symptoms in narcolepsy.23 Concerning sleep-related aspects like sleep quality or EDS,10 as well as occurrences of sleep-related hallucinations,18 have been suggested to potentially correlate with depression. Compared to the former factors, there has been a limited amount of research on the psychosocial factors that impact mental illness in patients with NT1. In our prior study, we proposed a potential association between impulsivity and depressive symptoms.18 This relationship between depression and impulsivity has also been reported in other studies.24–26 Therefore, by incorporating impulsivity along with demographic and sleep-related characteristics, it may be possible to predict the presence of depression with appropriate models.
Previous studies have explored the relationship between depression risk factors among patients, which was limited to linear relationships, such as logistic models.27 However, existing clinical measures for identifying depression are time-consuming and labor-intensive, and often require specialized expertise for result evaluation.28 Besides, the overlapping symptoms of sleepiness, sleep disturbance, and fatigue,2,29 between depression and narcolepsy make it challenging to accurately detect and diagnose depression in patients with NT1 using subjective questionnaires. Polysomnography (PSG) and Multiple Sleep Latency Test (MSLT) are validated objective measures for evaluating sleep architecture, quality, and abnormal sleep patterns. Utilizing PSG and MSLT parameters may aid in differentiating depression from narcolepsy. Thus, a novel approach is needed to predict the presence of depression. In contrast to traditional statistical models and diagnostic algorithms, machine learning (ML) has the capability to analyze both linear and nonlinear relationships across high-dimensional data sets,30,31 making them well-suited for predictive modeling in clinical settings. Recent studies have leveraged ML algorithms to screen or predict diseases, such as obstructive sleep apnea,32 anxiety and depression.28,33
While depression is widely acknowledged as a common comorbidity in NT1, the specific factors that contribute to its development and the effectiveness of predictive models in this context have not been comprehensively explored. This study aims to address this gap by employing machine learning to identify novel predictors and evaluate their potential in improving the early diagnosis and management of depression in NT1 patients.
Methods
Participants
A total of 203 drug-free NT1 patients were recruited at the Sleep Medicine Center of Peking University People’s Hospital from September 2019 to April 2023. All patients were consecutively diagnosed with NT1 based on the International Classification of Sleep Disorders (ICSD-3) criteria: (1) the presence of EDS for at least 3 months; (2) the presence of clear-cut cataplexy, and a mean sleep latency ≤ 8 min with ≥ 2 sleep onset Rapid Eye Movement periods (SOREMPs) during MSLT. REM onset within 15 min of sleep onset during preceding nocturnal PSG may replace one of the SOREMPs during the MSLT. Or without cataplexy but with lower levels of hypocretin (less than 110 pg/mL or one-third of normative values) in CSF. Patients were excluded following the criteria: had a history of mental illness or were having an episode of mental illness. All participants provided written/oral informed consent (for juveniles, the permission of parents/legal guardians also were obtained). This study was approved by the ethics committees of Peking University People’s Hospital and conducted by the Declaration of Helsinki.
Input Features
We selected the following potential features based on subjective knowledge and previous studies on risk factors for comorbid depression among patients with NT1. Potential features covering five domains: (1) demographic features: age, gender, body mass index (BMI), education years; (2) clinical features: age at onset, sleep paralysis, hallucinations; (3) self-reported questionnaires: Epworth Sleepiness Scale (ESS), the total and the subscales of Barratt Impulse Scale-11 (BIS-11); (4) PSG parameters: total sleep time (TST), sleep efficiency (SE), nocturnal sleep latency (SL), wake after sleep onset (WASO), R stage (%), N1 stage (%), N2 stage (%), N3 stage (%), apnea-hypopnea index (AHI), arousal index [respiratory-related arousal index (RAI), leg movement-related arousal index (LAI), spontaneous arousal index (SAI)], periodic leg movements index (PLMI); (5) MSLT measurements: The SL in the MSLT.
Measurements
Polysomnography and Multiple Sleep Latency Test
All patients underwent one overnight polysomnography (PSG, Respironics LE-Series Physiological Monitoring System, Alice 6 LE, FL, USA) recording from 10 pm to 6 am. The more detailed information was reported in our previous research.18 Sleep stages and events were scored by experienced technicians according to the newest American Academy of Sleep Medicine (AASM) manual.34 The following PSG parameters were collected: total sleep time (TST), sleep efficiency (SE), nocturnal sleep latency (SL), wake after sleep onset (WASO), sleep stages percentages, arousals (respiratory-related arousals index, RAI; leg movement-related arousals index, LAI; spontaneous arousals index, SAI), periodic leg movements index (PLMI), and apnea-hypopnea index (AHI). The MSLT was performed at 2-hour intervals following PSG the night prior. A total of five 20-minute nap opportunities were provided. Each nap was terminated 15 minutes after sleep onset or 20 minutes after wakefulness. Sleep latency was defined as the time from the light off to the first 30-second epoch of any stage of sleep. The mean sleep latency from the 5 naps was calculated.
Demographic and Clinical Characteristics
All patients were systematically evaluated for clinical characteristics including age, gender, body mass index (BMI), age at narcolepsy onset, hypnagogic/hypnopompic hallucination (HH) and sleep paralysis (SP). HH are vivid, dreamlike experiences involving auditory, visual, or tactile sensations that occur either at sleep onset, known as hypnagogic hallucinations, or upon awakening, known as hypnopompic hallucinations.35 SP is characterized by the temporary inability to speak or move voluntary muscles, typically occurring during transitions between sleeping and waking.1
Excessive Daytime Sleepiness
Excessive daytime sleepiness was measured by the Epworth Sleepiness Scale (ESS)36 or the Epworth Sleepiness Scale for Children and Adolescents (ESS-CHAD).37 The Chinese version of ESS has been validated in Chinese adults with sleep-disordered breathing showing acceptable internal consistency (Cronbach’s α = 0.81).38 The ESS-CHAD was adapted for children and adolescents aged 7–17 years old,37 which has been used in our previous study (Cronbach’s α = 0.87).39 The ESS consists of 8 items based on retrospective reports of the likelihood of dozing off or falling asleep in a variety of different situations. Respondents were asked to rate on a 4-point scale (0–3) from would never doze to high chance of dozing. The higher the ESS score indicates the higher their daytime sleepiness.
Depression
The depressive symptoms were assessed by the Center for Epidemiologic Studies Depression Scale for Children (CES-DC)40 for adolescent NT1 patients or the Self-Rating Depression Scale (SDS)41 for adult NT1 patients. The Chinese versions of both CES-DC42 and SDS43 have been translated and validated within the Chinese population. The CES-DC is a self-administered questionnaire with 20 items. Each item is answered on a 4-point scale (0–3). Total scores range from 0 to 60, with higher scores indicating greater depressive symptomatology. Individuals with a score of 20 or more suggest depressive symptoms.44 The SDS is also a 20-item scale using a 4-point scale ranging from 1 (none, or a little of the time) to 4 (most, or all of the time). The standardized score of SDS is the raw score multiplied by 1.25, with a cut-off ≥ 53 suggesting depression symptoms.18 Depression was categorized as a dichotomous variable (depression/non-depression) according to CES-DC or SDS in this study.
Impulsivity Questionnaire
The Barratt Impulse Scale (BIS-11), revised by Patton, Stanford and Barratt, was used to assess impulsive behaviors. The Chinese version of the BIS-11 has been widely used among adolescents45,46 and adults47,48 population, showing good internal consistency (Cronbach’s α = 0.80–0.92). The BIS consists of three subscales named no-planning impulsivity, motor impulsivity and attentional impulsivity, with each subscale containing 10 items. Each item has 5-point Likert responses from 1 (never) to 5 (always). Individuals with a higher score indicate stronger impulsivity.
Sample Size
The sample size for developing the two-class prediction model was calculated using the “pmsampsize” package in R (version 4.1.1).49 The calculation was based on a target concordance index of 0.8, considering 5 predictor parameters, and assuming an outcome prevalence of 32%.7 The analysis indicated that a minimum sample size of 167, with 53 events (events per predictor parameter of at least 10.69), was required for the prediction model development.
Model Building
After partitioning the dataset into a 70:30 split, with 70% allocated for training and 30% for testing, we used 10-fold cross-validation within the training set to build and fine-tune the models. The trained models were subsequently validated on the independent test set to assess their performance. Three classification models were constructed using the Support Vector Machine (SVM), Random forest (RF), and Logistic Regression (LR) algorithms. The LR model, which is a generalized linear regression analysis model, is frequently employed in data mining and disease diagnosis. It was implemented using the “glm” function in the R programming language. The SVM is a supervised learning algorithm particularly effective for high-dimensional data classification. It was implemented using the “e1071” package in R. The optimal parameters for the SVM model are the polynomial kernel with a degree of 2, a scale of 0.01, and C of 0.25. The RF algorithm, which comprises multiple decision trees, was implemented using the R package “randomForest”. The 10-fold cross validation was carried out through the R package “caret”. Ultimately, the efficacy of the three models was evaluated in the testing sets utilizing receiver operating characteristic (ROC) curves.
Model Evaluation
Model performance metrics included the area under the receiver operating characteristic curve (AUC), accuracy, precision, recall, sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and F1 score. The DeLong test was employed to compare the differences between the ROC curves of the various models. Additionally, Decision Curve Analysis (DCA) was conducted for the three models. DCA was developed to evaluate whether the implementation of a prediction model in clinical decision-making would yield more benefits than harms. In summary, a quantitative measure known as net benefit was computed based on the true positive and false positive rates of each model, allowing for a standardized comparison of their advantages and disadvantages.50
Statistical Analysis
Descriptive statistics are presented using mean and standard deviation, frequencies, and percentages. Univariate analysis (chi-square test for categorical variables or independent samples t-test for continuous variables) was conducted to compare demographic, clinical characteristics and sleep parameters between depression and non-depression groups. Univariate analyses were conducted using IBM SPSS Statistics 26.0. In contrast, machine learning algorithms were implemented utilizing R software (http://www.R-project.org). Significant differences were found at p-value < 0.05.
Results
Demographics Characteristics of NT1 Patients
Based on the established inclusion and exclusion criteria, a total of 203 NT1 patients (131 males and 72 females), aged 5 to 61 years, were selected for inclusion in this study, comprising 106 adolescents and 97 adults. Among the NT1 patients, 47.8% (97/203) reported experiencing HH, a proportion comparable to those who reported SP (48.8%). The majority (80.8%) experienced narcolepsy onset during childhood or adolescence. The sleep latency in MLST was 1.92 ± 1.79 min. Additionally, EDS symptom was prevalent, as reflected by an ESS score of 17.04 ± 4.62, with a range of 6 to 24. The BIS score was 88.58±15.63, ranging from 41 to 138. Notably, significant differences were observed between the two groups only in the motor impulsivity subscale of the BIS and the presence of HH. Further details are presented in Table 1.
Table 1.
Univariate Analysis Between Depression and Non-Depression Groups (n=203)
| Characteristics | Total (n=203) | Non-depression (n=97) | Depression (n=106) | χ2/T value | P value |
|---|---|---|---|---|---|
| Age (at inclusion) | 21.10 ± 11.90 | 21.03 ± 12.94 | 21.16 ± 10.92 | −0.077 | 0.939 |
| Age group | 0.010 | 0.922 | |||
| Children and adolescent group | 106 (52.2%) | 51 (48.1%) | 55 (51.9%) | ||
| Adult group | 97 (47.8%) | 46 (47.4%) | 51 (52.6%) | ||
| Gender | 0.170 | 0.680 | |||
| Male | 131 (64.5%) | 64 (48.9%) | 67 (51.1%) | ||
| Female | 72 (35.4%) | 33 (45.8%) | 39 (54.2%) | ||
| Education years (years) | 9.86 ± 4.30 | 9.27 ± 4.5 | 10.41 ± 4.06 | −1.894 | 0.060 |
| Hypnagogic/hypnopompic Hallucination | 8.476 | 0.004 | |||
| Yes | 97 (47.8%) | 36 (37.1%) | 61 (62.9%) | ||
| No | 106 (52.2%) | 61 (57.5%) | 45 (42.5%) | ||
| Sleep paralysis | 3.142 | 0.076 | |||
| Yes | 99 (48.8%) | 41 (41.4%) | 58 (58.6%) | ||
| No | 104 (51.2%) | 56 (53.8%) | 48 (46.2%) | ||
| Age at onset | 13.97 ± 10.37 | 14.32 ± 11.44 | 13.64 ± 9.32 | 0.464 | 0.643 |
| Age onset group | 0.711 | 0.399 | |||
| Children and adolescent group | 164 (80.8%) | 76 (46.3%) | 88 (53.7%) | ||
| Adult group | 39 (19.2%) | 21 (53.8%) | 18 (46.2%) | ||
| BMI | 25.69 ± 5.03 | 25.26 ± 4.78 | 26.08 ± 5.24 | −1.152 | 0.251 |
| Polysomnography parameters | |||||
| Total sleep time (min) | 448.70 ± 69.74 | 458.45 ± 58.21 | 439.77 ± 78.04 | 1.919 | 0.056 |
| Wake after sleep onset (min) | 56.91 ± 47.40 | 56.9 ± 46.05 | 56.92 ± 48.82 | −0.004 | 0.997 |
| Sleep efficiency (%) | 88.24 ± 9.14 | 88.48 ± 8.67 | 88.03 ± 9.58 | 0.350 | 0.727 |
| Sleep latency (min) | 5.03 ± 8.90 | 4.69 ± 6.88 | 5.33 ± 10.43 | −0.507 | 0.613 |
| Sleep stages | |||||
| R (%) | 22.11 ± 9.55 | 21.92 ± 9.09 | 22.28 ± 9.99 | −0.262 | 0.793 |
| N1 (%) | 20.00 ± 13.30 | 20.96 ± 13.76 | 19.13 ± 12.87 | 0.979 | 0.329 |
| N2 (%) | 40.77 ± 11.40 | 39.85 ± 10.56 | 41.61 ± 12.09 | −1.100 | 0.272 |
| N3 (%) | 16.81 ± 10.08 | 17.38 ± 10.93 | 16.28 ± 9.26 | 0.777 | 0.438 |
| AHI | 6.86 ± 12.04 | 6.31 ± 9.90 | 7.37 ± 13.74 | −0.625 | 0.533 |
| Respiratory-related arousals index | 3.37 ± 7.62 | 2.82 ± 5.59 | 3.88 ± 9.10 | −0.994 | 0.322 |
| Leg movement-related arousals index | 1.37 ± 1.88 | 1.64 ± 2.22 | 1.13 ± 1.46 | 1.939 | 0.054 |
| Spontaneous arousals index | 9.99 ± 7.23 | 10.65 ± 8.23 | 9.38 ± 6.14 | 1.251 | 0.212 |
| PLMI | 7.89 ± 12.15 | 8.89 ± 14.64 | 6.98 ± 9.29 | 1.097 | 0.274 |
| Sleep latency-MSLT (min)a | 1.92 ± 1.79 | 1.81 ± 1.67 | 2.03 ± 1.90 | −0.841 | 0.401 |
| ESS | 17.28 ± 5.04 | 16.89 ± 5.40 | 17.64 ± 4.67 | −1.067 | 0.287 |
| BIS | 76.54 ± 27.03 | 75.69 ± 28.97 | 77.31 ± 25.24 | −0.426 | 0.671 |
| Attentional impulsivity score | 29.21 ± 7.34 | 28.90 ± 7.83 | 29.50 ± 6.89 | −0.584 | 0.560 |
| Motor impulsivity score | 28.10 ± 7.30 | 26.60 ± 7.03 | 29.48 ± 7.30 | −2.861 | 0.005 |
| Non-planning impulsivity score | 43.30 ± 25.92 | 43.14 ± 22.87 | 43.44 ± 28.54 | −0.082 | 0.935 |
Notes: aSleep latency refers to the mean sleep latency in the multiple sleep latency test. bP value from the Independent sample t-test (for continuous data), a Chi-square test or Fisher exact test (for categorical variables). P value in bold if < 0.05.
Abbreviations: BMI, body mass index; AHI, apnea hypopnea index; MSLT, multiple sleep latency test; BIS, Barratt Impulse Scale; PLMI, periodic leg movements index; MSLT, multiple sleep latency test; ESS, Epworth Sleepiness Scale.
Performance on Different Machine Learning Models and Logistic Regression in the Validation Dataset
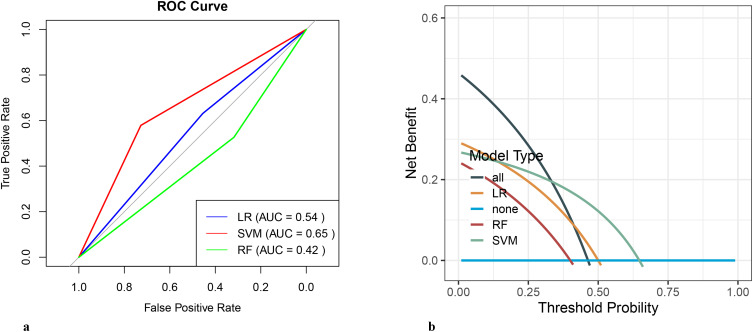
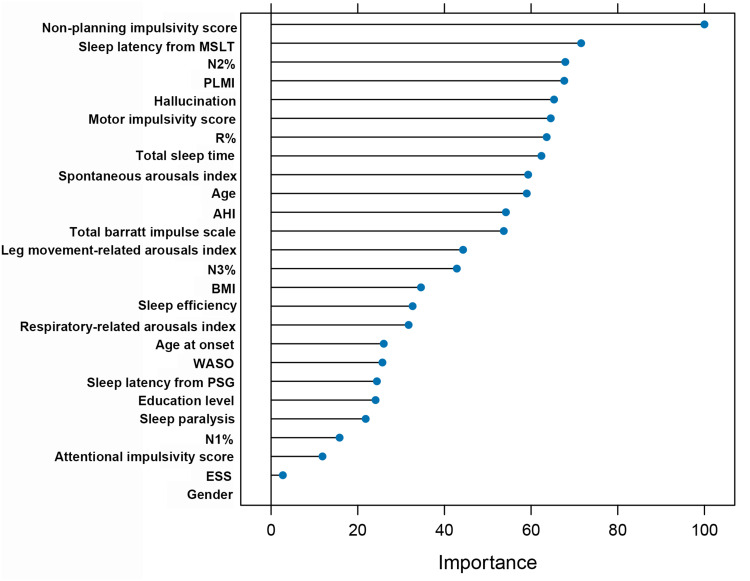
Three algorithms, namely LR, RF and SVM, were used to construct a predictive model for depression in NT1 patients. The prediction summary of the models is shown in Table 2. ROC curves and DCA of three models are shown in Figure 1. Additional information from the logistic regression model, such as odds ratio (OR) and confidence interval (CI), was shown in Supplementary Table 1. Using the clinical and sleep metrics as features of the SVM classification model achieved better performance in distinguishing depression from non-depression. Briefly, the SVM model classification of accuracy was 0.659, specificity was 0.579, sensitivity was 0.727, PPV was 0.667, and NPV was 0.647 in the test set. The DCA of the SVM model classification was also better than other models (Figure 1b). The SVM classification importance of each feature was further examined, and the results are presented in Figure 2.
Table 2.
Prediction of Different Machine Learning Models and Logistic Regression in the Validation Dataset (n = 61)
| LR | SVM | RF | |
|---|---|---|---|
| AUC (95% CI) | 0.543 (0.389–0.697) | 0.653 (0.505–0.802) | 0.422 (0.27–0.575) |
| Accuracy (95% CI) | 0.537 (0.374–0.693) | 0.659 (0.494–0.799) | 0.415 (0.263–0.579) |
| Balanced Accuracy | 0.543 | 0.653 | 0.422 |
| Sensitivity | 0.455 | 0.727 | 0.318 |
| Specificity | 0.632 | 0.579 | 0.526 |
| Positive predictive value | 0.588 | 0.667 | 0.438 |
| Negative predictive value | 0.5 | 0.647 | 0.4 |
| Prevalence | 0.537 | 0.537 | 0.537 |
| Precision | 0.588 | 0.667 | 0.438 |
| Recall | 0.455 | 0.727 | 0.318 |
| F1 Score | 0.513 | 0.696 | 0.368 |
| Kappa | 0.085 | 0.308 | −0.152 |
Abbreviations: AUC, area under receiver operating characteristic curve; CI, confidence interval; LR, logistic regression; SVM, support vector machine; RF, random forest.
Figure 1.
(a) The receiver operating characteristic curve of the models. (b) The decision curve analysis of the models.
Abbreviations: ROC, receiver operating characteristic curve; LR, logistic regression; RF, random forest; SVM, support vector machine.
Figure 2.
The importance index of all variables from the support vector machine model.
Abbreviations: BMI, body mass index; ESS, Epworth Sleepiness Scale; WASO, wake after sleep onset; PLMI, periodic leg movements index; AHI, apnea-hypopnea index.
Discussion
Depression is a prevalent comorbidity among patients with NT1. Despite many studies investigating the relationship between narcolepsy and depression, a robust predictive model for depression in NT1 patients remains elusive. This study presents evidence supporting the efficacy of ML tools in predicting depression among patients with NT1. The LR model identified hallucinations and motor impulsivity as predictors of depression. Additionally, our SVM model incorporating routinely collected clinic variables, PSG parameters, ESS scores, and BIS scores outperformed the traditional LR model in predicting depression. Consequently, using readily available data from the PSG parameters, ML algorithms offer a more efficient approach for large-scale identification of depression among patients with NT1.
In this study, 52.2% of NT1 patients (52.6% of adults and 51.9% of children and adolescents) reported experiencing depression symptoms, a rate higher than that reported in a systematic review (32%, 95% CI, 28–36%).7 This discrepancy may be due to the measurement tool we used, which could overestimate depression prevalence compared to studies employing the Diagnostic and Statistical Manual of Mental Disorders (DSM-III/IV) criteria or psychiatric interviews. The prevailing consensus in the literature is that the high prevalence of depression/depressive symptoms among narcolepsy patients is primarily attributed to hypocretin deficiency51–53 and its subsequent effects caused by narcolepsy disease, such as poor quality of life or social stigma.7,27
Motor impulsivity was also associated with depressive symptoms in children and adolescents with NT1, a relationship that we previously identified.18 This relationship may be rooted in the pathophysiology of NT1, particularly hypocretin deficiency, which is associated with emotion regulation and stress-adaptive responses.15 The lack of hypocretin may impair emotional responses, leading to increased impulsivity and a higher risk of depression. In addition, a study examining brain structure in children and adolescents has demonstrated that both depression and impulsivity are associated with cortical thickness. Similarly, our findings indicate that the depressive symptoms and impulsivity in NT1 patients are linked to morphological changes in the cerebral cortex54 and alterations in default mode network connectivity.55
The HH symptom was found to be independently associated with depressive symptoms among patients with NT1, consistent with findings from previous studies.8,27 The association between HH and depression is noteworthy and may be attributed to several overlapping physiological and psychological mechanisms. Firstly, narcolepsy is characterized by abnormal regulation of REM sleep, which is closely linked to the occurrence of hallucinations.56 The REM sleep dysregulation has also been implicated with mood disorders, particularly depression.57 Furthermore, hypocretin deficiency, the hallmark of NT1, is thought to contribute to both hallucinations and depressive symptoms.52 Finally, the distress caused by hallucinations, which can be disturbing and frightening, may exacerbate or contribute to the progression of depressive symptoms in NT1 patients.58
Our research primarily focuses on children and adolescents, who constitute 80% of patients presenting symptoms, a demographic distinct from adults in terms of depression-related factors. Additionally, our patient cohort exhibits a higher prevalence of sleep-related symptoms, such as hallucinations, compared to other studies. We have also included impulsivity as a potential non-sleep symptom factor in our analysis. Given these considerations, age-specific symptom development, the prevalence of sleep-related symptoms, and the role of impulsivity, our results may differ from those of previous research.
ML has been extensively applied in mental health for detection, diagnosis, prognosis, and treatment, particularly in depression, schizophrenia, and Alzheimer’s Disease.59 However, research on utilizing these algorithms to predict depression in patients with NT1 remains limited. Our study demonstrates that the SVM model, incorporating both clinical factors and PSG parameters, exhibited superior performance relative to other machine learning models tested (Table 2), as evidenced by its higher net benefit in decision curve analysis, in NT1 patients. Consistent with previous studies,60–62 the SVM algorithm demonstrated the optimal performance in developing ML models for depression prediction. When identifying depression in NT1 patients, it is crucial to comprehensively and precisely identify emotional difficulties. The SVM model performs better with an AUC of 0.653, showing good discriminative ability. The accuracy of 0.659 and balanced accuracy of 0.653 are strong, suggesting that the model is effective at classifying both positive and negative cases. It also exhibits high sensitivity (0.727) and recall (0.727), indicating its strength in detecting positive cases. However, the specificity (0.579) is lower compared to LR, and the F1 Score (0.696) and Kappa (0.308) suggest a generally better performance but with room for improvement. The models were trained on a subset of the available data and tested independently. However, to truly measure generalizability, it is necessary to test the models on external datasets or in real-world scenarios. Limited external validation may affect the robustness and applicability of our findings across diverse populations or settings. Although we employed 10-fold cross-validation to mitigate overfitting by validating the model on different subsets of the training data, this technique cannot eliminate the risk.
The ML model can be integrated into clinical practice to enhance early detection of depression in NT1 patients, enabling timely and personalized interventions. By serving as a decision-support tool, it can help clinicians prioritize at-risk patients for further evaluation and targeted care. Additionally, the model’s adaptability allows for continuous improvement as new biomarkers and psychological variables are incorporated, ensuring it remains a valuable resource in managing NT1 and its comorbidities.
Limitations
This study has several limitations. Firstly, this study is based on cross-sectional data, which prevents the establishment of a causal relationship between the factors investigated in our research and depression outcomes. Secondly, the sample size was relatively small; however, it should be noted that even smaller sample sizes have been used in other published studies employing SVM for clinical medicine, eg, 71 participants60 and 55 participants.61 The ML algorithm may achieve more optimal performance with larger input data;49 therefore, further validation of these models using additional data is warranted. Thirdly, the depressive symptoms were assessed by a scale, which is not the gold standard for the diagnosis of depression, although the scales have been extensively used and validated in previous studies.18,23,33 Lastly, the absence of psychological factors data among patients with NT1 poses a limitation in exploring potential causes for depression within this study. Given the multifaceted nature of depression, future research should consider integrating additional psychological variables, such as stigma, resilience, and coping strategies, into the model to improve its sensitivity and predictive accuracy. Beyond psychological factors, exploring potential biomarkers, such as inflammatory markers or neuroimaging data, and incorporating novel data sources like real-time digital health metrics or genetic information could further enhance the model’s predictive power.
Conclusion
This exploratory study demonstrates the potential of ML models in addressing clinical challenges related to depression in NT1 patients. Our findings indicate that depression in NT1 is significantly associated with sleep-related hallucinations and impulsivity. The models developed herein provide promising tools for identifying NT1 patients at elevated risk for depression, paving the way for the development of a data-driven, personalized decision-making framework. Such a tool could significantly enhance the efficiency of depression diagnoses in NT1 patients. Future research should focus on validating these findings across diverse populations and incorporating additional psychological variables to further refine and improve the model’s precision.
Acknowledgments
We would like to express our gratitude to all adult patients, juvenile patients, and their parents for their participation in this study.
Funding Statement
This study was reported by the National Natural Science Foundation of China (81700088), the National Natural Science Foundation of China (82070091), the Youth Talent Support Project from the China Association for Science and Technology, and the Leading Talents Project from Peking University School of Nursing (LJRC22YB06, LJRC22YB05).
Disclosure
The authors have no conflict of interest to declare.
References
- 1.Bassetti CLA, Adamantidis A, Burdakov D, et al. Narcolepsy - clinical spectrum, aetiopathophysiology, diagnosis and treatment. Nat Rev Neurol. 2019;15(9):519–539. doi: 10.1038/s41582-019-0226-9 [DOI] [PubMed] [Google Scholar]
- 2.BaHammam AS, Alnakshabandi K, Pandi-Perumal SR. Neuropsychiatric correlates of narcolepsy. Curr Psychiatry Rep. 2020;22(8):36. doi: 10.1007/s11920-020-01159-y [DOI] [PubMed] [Google Scholar]
- 3.Alasim H, AlQazlan S, Albanyan S, et al. Comorbid psychiatric disorders among patients with narcolepsy. Sleep Breath. 2020;24(2):629–636. doi: 10.1007/s11325-019-01890-8 [DOI] [PubMed] [Google Scholar]
- 4.Li B, Gao Z, He Y, et al. Narcolepsy and psychiatric disorders: a bidirectional Mendelian randomization study. J Psychiatr Res. 2024;169:42–48. doi: 10.1016/j.jpsychires.2023.11.034 [DOI] [PubMed] [Google Scholar]
- 5.Ohayon MM. Narcolepsy is complicated by high medical and psychiatric comorbidities: a comparison with the general population. Sleep Med. 2013;14(6):488–492. doi: 10.1016/j.sleep.2013.03.002 [DOI] [PubMed] [Google Scholar]
- 6.Ruoff CM, Reaven NL, Funk SE, et al. High rates of psychiatric comorbidity in narcolepsy: findings from the burden of narcolepsy disease (BOND) study of 9312 patients in the United States. J Clin Psychiatry. 2017;78(2):171–176. doi: 10.4088/JCP.15m10262 [DOI] [PubMed] [Google Scholar]
- 7.Li X, Sanford LD, Zong Q, et al. Prevalence of depression or depressive symptoms in patients with narcolepsy: a systematic review and meta-analysis. Neuropsychol Rev. 2021;31(1):89–102. doi: 10.1007/s11065-020-09443-7 [DOI] [PubMed] [Google Scholar]
- 8.Barateau L, Lopez R, Chenini S, et al. Depression and suicidal thoughts in untreated and treated narcolepsy. Neurology. 2020;95(20):e2755–e2768. doi: 10.1212/WNL.0000000000010737 [DOI] [PubMed] [Google Scholar]
- 9.David A, Constantino F, Santos Dos JM, Paiva T. Health-related quality of life in Portuguese patients with narcolepsy. Sleep Med. 2012;13(3):273–277. doi: 10.1016/j.sleep.2011.06.021 [DOI] [PubMed] [Google Scholar]
- 10.Parmar A, Yeh EA, Korczak DJ, et al. Depressive symptoms, sleep patterns, and physical activity in adolescents with narcolepsy. Sleep. 2019;42(8):zsz111. doi: 10.1093/sleep/zsz111 [DOI] [PubMed] [Google Scholar]
- 11.Bassi C, Biscarini F, Zenesini C, et al. Work productivity and activity impairment in patients with narcolepsy type 1. J Sleep Res. 2023;33(3):e14087. doi: 10.1111/jsr.14087 [DOI] [PubMed] [Google Scholar]
- 12.Bassetti CLA, Kallweit U, Vignatelli L, et al. European guideline and expert statements on the management of narcolepsy in adults and children. Eur J Neurol. 2021;28(9):2815–2830. doi: 10.1111/ene.14888 [DOI] [PubMed] [Google Scholar]
- 13.Ong JC, Dawson SC, Mundt JM, Moore C. Developing a cognitive behavioral therapy for hypersomnia using telehealth: a feasibility study. J Clin Sleep Med. 2020;16(12):2047–2062. doi: 10.5664/jcsm.8750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Franceschini C, Fante C, Filardi M, et al. Can a peer support the process of self-management in narcolepsy? A qualitative narrative analysis of a narcoleptic patient. Front Psychol. 2020;11:1353. doi: 10.3389/fpsyg.2020.01353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang C, Wang Q, Ji B, et al. The orexin/receptor system: molecular mechanism and therapeutic potential for neurological diseases. Front Mol Neurosci. 2018;11:220. doi: 10.3389/fnmol.2018.00220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Summers CH, Yaeger JDW, Staton CD, Arendt DH, Summers TR. Orexin/hypocretin receptor modulation of anxiolytic and antidepressive responses during social stress and decision-making: potential for therapy. Brain Res. 2020;1731:146085. doi: 10.1016/j.brainres.2018.12.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shan L, Linssen S, Harteman Z, et al. Activated Wake Systems in Narcolepsy Type 1. Ann Neurol. 2023;94(4):762–771. doi: 10.1002/ana.26736 [DOI] [PubMed] [Google Scholar]
- 18.Yang Y, Zhang J, Han F, Xiao F. Not only excessive daytime sleepiness but also depression symptoms, chronological age and onset-age were associated with impulsivity in narcolepsy type 1 patients. Nat Sci Sleep. 2022;14:1857–1866. doi: 10.2147/NSS.S377372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kapella MC, Berger BE, Vern BA, Vispute S, Prasad B, Carley DW. Health-related stigma as a determinant of functioning in young adults with narcolepsy. PLoS One. 2015;10(4):e0122478. doi: 10.1371/journal.pone.0122478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.D’Alterio A, Menchetti M, Zenesini C, et al. Resilience and its correlates in patients with narcolepsy type 1. J Clin Sleep Med. 2023;19(4):719–726. doi: 10.5664/jcsm.10418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shan L, Balesar R, Swaab DF, Lammers GJ, Fronczek R. Reduced numbers of corticotropin-releasing hormone neurons in narcolepsy type 1. Ann Neurol. 2022;91(2):282–288. doi: 10.1002/ana.26300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Menke A. The HPA axis as target for depression. Curr Neuropharmacol. 2024;22(5):904–915. doi: 10.2174/1570159X21666230811141557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park YK, Kwon OH, Joo EY, et al. White matter alterations in narcolepsy patients with cataplexy: tract-based spatial statistics. J Sleep Res. 2016;25(2):181–189. doi: 10.1111/jsr.12366 [DOI] [PubMed] [Google Scholar]
- 24.Merz EC, He X, Noble KG. Pediatric imaging, neurocognition, and genetics study. anxiety, depression, impulsivity, and brain structure in children and adolescents. NeuroImage Clin. 2018;20:243–251. doi: 10.1016/j.nicl.2018.07.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saddichha S, Schuetz C. Impulsivity in remitted depression: a meta-analytical review. Asian J Psychiatr. 2014;9:13–16. doi: 10.1016/j.ajp.2014.02.003 [DOI] [PubMed] [Google Scholar]
- 26.Guo Z, Cui Y, Qiu R, et al. The association of impulsivity with depression and anxiety symptoms: a transdiagnostic network analysis and replication. J Affect Disord. 2024;359:100–108. doi: 10.1016/j.jad.2024.05.076 [DOI] [PubMed] [Google Scholar]
- 27.Yang Y, Li C, Zhao L, Li J, Han F, Xiao F. Factors associated with depression and sub-dimension symptoms in adolescent narcolepsy. Nat Sci Sleep. 2021;13:1075–1082. doi: 10.2147/NSS.S312000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang Y, Zhu C, Feng Y, et al. Comparison of three machine learning models to predict suicidal ideation and depression among Chinese adolescents: a cross-sectional study. J Affect Disord. 2022;319:221–228. doi: 10.1016/j.jad.2022.08.123 [DOI] [PubMed] [Google Scholar]
- 29.Morse A, Sanjeev K. Narcolepsy and psychiatric disorders: comorbidities or shared pathophysiology? Med Sci. 2018;6(1):16. doi: 10.3390/medsci6010016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Y, Wang S, Hermann A, Joly R, Pathak J. Development and validation of a machine learning algorithm for predicting the risk of postpartum depression among pregnant women. J Affect Disord. 2021;279:1–8. doi: 10.1016/j.jad.2020.09.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maniaci A, Riela PM, Iannella G, et al. Machine learning identification of obstructive sleep apnea severity through the patient clinical features: a retrospective study. Life Basel Switz. 2023;13(3):702. doi: 10.3390/life13030702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marzolini S, Sarin M, Reitav J, Mendelson M, Oh P. Utility of screening for obstructive sleep apnea in cardiac rehabilitation. J Cardiopulm Rehabil Prev. 2016;36(6):413–420. doi: 10.1097/HCR.0000000000000186 [DOI] [PubMed] [Google Scholar]
- 33.Liu K, Droncheff B, Warren SL. Predictive utility of symptom measures in classifying anxiety and depression: a machine-learning approach. Psychiatry Res. 2022;312:114534. doi: 10.1016/j.psychres.2022.114534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berry RB, Brooks R, Gamaldo C, et al. AASM scoring manual updates for 2017 (Version 2.4). J Clin Sleep Med. 2017;13(5):665–666. doi: 10.5664/jcsm.6576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barateau L, Pizza F, Chenini S, Peter-Derex L, Dauvilliers Y. Narcolepsies, update in 2023. Rev Neurol. 2023;179(7):727–740. doi: 10.1016/j.neurol.2023.08.001 [DOI] [PubMed] [Google Scholar]
- 36.Johns M, Hocking B. Daytime sleepiness and sleep habits of Australian workers. Sleep. 1997;20(10):844–849. doi: 10.1093/sleep/20.10.844 [DOI] [PubMed] [Google Scholar]
- 37.Wang YG, Benmedjahed K, Lambert J, et al. Assessing narcolepsy with cataplexy in children and adolescents: development of a cataplexy diary and the ESS-Chad. Nat Sci Sleep. 2017;9:201–211. doi: 10.2147/NSS.S140143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen NH, Johns MW, Li HY, et al. Validation of a Chinese version of the Epworth sleepiness scale. Qual Life Res. 2002;11(8):817–821. doi: 10.1023/a:1020818417949 [DOI] [PubMed] [Google Scholar]
- 39.Wang M, Shen C, Liu X, et al. Executive function performance in children and adolescent patients with narcolepsy type 1. Sleep Med. 2024;119:342–351. doi: 10.1016/j.sleep.2024.05.021 [DOI] [PubMed] [Google Scholar]
- 40.Fendrich M, Weissman MM, Warner V. Screening for depressive disorder in children and adolescents: validating the center for epidemiologic studies depression scale for children. Am J Epidemiol. 1990;131(3):538–551. doi: 10.1093/oxfordjournals.aje.a115529 [DOI] [PubMed] [Google Scholar]
- 41.Zung WW. A self-rating depression scale. Arch Gen Psychiatry. 1965;12:63–70. doi: 10.1001/archpsyc.1965.01720310065008 [DOI] [PubMed] [Google Scholar]
- 42.Chen Z, Yang X, Li X. Psychometric features of CES-D in Chinese adolescents. Chin J Clin Psychol. 2009;17(4):443–448. doi: 10.16128/j.cnki.1005-3611.2009.04.027 [DOI] [Google Scholar]
- 43.Wang R, Liu L, Ge H, Han J. Study on the feasibility of the self-rating depression scale (SDS) as a diagnostic screened implement for depressive disorder in surgical inpatients. J Psychiatry. 2009;22(4):251–253. doi: 10.3969/j.issn.1009-7201.2009.04.004 [DOI] [Google Scholar]
- 44.Vilagut G, Forero CG, Barbaglia G, Alonso J. Screening for depression in the general population with the center for epidemiologic studies depression (CES-D): a systematic review with meta-analysis. PLoS One. 2016;11(5):e0155431. doi: 10.1371/journal.pone.0155431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ran H, Fang D, Donald AR, et al. Impulsivity mediates the association between parenting styles and self-harm in Chinese adolescents. BMC Public Health. 2021;21(1). doi: 10.1186/s12889-021-10386-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yao S, Yang H, Zhu X, et al. An examination of the psychometric properties of the Chinese version of the Barratt impulsiveness scale, 11th version in a sample of Chinese adolescents. Percept Mot Skills. 2007;104(3 Pt 2):1169–1182. doi: 10.2466/pms.104.4.1169-1182 [DOI] [PubMed] [Google Scholar]
- 47.Li W, Zhang W, Xiao L, Nie J. The association of internet addiction symptoms with impulsiveness, loneliness, novelty seeking and behavioral inhibition system among adults with attention-deficit/hyperactivity disorder (ADHD). Psychiatry Res. 2016;243:357–364. doi: 10.1016/j.psychres.2016.02.020 [DOI] [PubMed] [Google Scholar]
- 48.Wang Q, Wang Y, Zuo J, et al. Factors of negative affect in elderly patients with substance use disorders during COVID-19 pandemic. Front Psychiatry. 2021:12. doi: 10.3389/fpsyt.2021.697472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Riley RD, Ensor J, Snell KIE, et al. Calculating the sample size required for developing a clinical prediction model. BMJ. 2020;368:m441. doi: 10.1136/bmj.m441 [DOI] [PubMed] [Google Scholar]
- 50.Vickers AJ, Holland F. Decision curve analysis to evaluate the clinical benefit of prediction models. Spine J. 2021;21(10):1643–1648. doi: 10.1016/j.spinee.2021.02.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Abenza-Abildua MJ, Suárez-Gisbert E, Lores-Gutiérrez V, et al. Anxiety and depression in patients with narcolepsy. J Sleep Res. 2023;32(4):e13812. doi: 10.1111/jsr.13812 [DOI] [PubMed] [Google Scholar]
- 52.Dauvilliers Y, Lopez R, Ohayon M, Bayard S. Hypersomnia and depressive symptoms: methodological and clinical aspects. BMC Med. 2013;11(1):78. doi: 10.1186/1741-7015-11-78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hansen BH, Andresen HN, Gjesvik J, Thorsby PM, Naerland T, Knudsen-Heier S. Associations between psychiatric comorbid disorders and executive dysfunctions in hypocretin-1 deficient pediatric narcolepsy type 1. Sleep Med. 2023;109:149–157. doi: 10.1016/j.sleep.2023.06.021 [DOI] [PubMed] [Google Scholar]
- 54.Fulong X, Spruyt K, Xiaosong D, Zhaolong C, Jun Z, Fang H. Morphological and age-related changes in the narcolepsy Brain. Cereb Cortex N Y N 1991. 2021;31(12):5460–5469. doi: 10.1093/cercor/bhab171 [DOI] [PubMed] [Google Scholar]
- 55.Fulong X, Spruyt K, Chao L, Dianjiang Z, Jun Z, Fang H. Resting-state brain network topological properties and the correlation with neuropsychological assessment in adolescent narcolepsy. Sleep. 2020;43(8):zsaa018. doi: 10.1093/sleep/zsaa018 [DOI] [PubMed] [Google Scholar]
- 56.Scammell TE. Narcolepsy. N Engl J Med. 2015;373(27):2654–2662. doi: 10.1056/NEJMra1500587 [DOI] [PubMed] [Google Scholar]
- 57.Wang YQ, Li R, Zhang MQ, Zhang Z, Qu WM, Huang ZL. The neurobiological mechanisms and treatments of REM sleep disturbances in depression. Curr Neuropharmacol. 2015;13(4):543–553. doi: 10.2174/1570159X13666150310002540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fortuyn HAD, Lappenschaar GA, Nienhuis FJ, et al. Psychotic symptoms in narcolepsy: phenomenology and a comparison with schizophrenia. Gen Hosp Psychiatry. 2009;31(2):146–154. doi: 10.1016/j.genhosppsych.2008.12.002 [DOI] [PubMed] [Google Scholar]
- 59.Shatte ABR, Hutchinson DM, Teague SJ. Machine learning in mental health: a scoping review of methods and applications. Psychol Med. 2019;49(9):1426–1448. doi: 10.1017/S0033291719000151 [DOI] [PubMed] [Google Scholar]
- 60.Wang F, Cheung CW, Wong SSC. Use of pain-related gene features to predict depression by support vector machine model in patients with fibromyalgia. Front Genet. 2023;14:1026672. doi: 10.3389/fgene.2023.1026672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wu CT, Dillon DG, Hsu HC, Huang S, Barrick E, Liu YH. Depression detection using relative EEG power induced by emotionally positive images and a conformal kernel support vector machine. Appl Sci. 2018;8(8):1244. doi: 10.3390/app8081244 [DOI] [Google Scholar]
- 62.Yu JS, Xue AY, Redei EE, Bagheri N. A support vector machine model provides an accurate transcript-level-based diagnostic for major depressive disorder. Transl Psychiatry. 2016;6(10):e931–e931. doi: 10.1038/tp.2016.198 [DOI] [PMC free article] [PubMed] [Google Scholar]