Abstract

Restricted fetal growth (RFG) is a leading contributor to perinatal mortality and has been associated with gestational exposure to air pollution, such as fine particulate matter (PM2.5), nitrogen dioxide (NO2), and polycyclic aromatic hydrocarbons (PAHs). This study examines the association between trimester-specific and weekly means of air pollution throughout gestation and placental inflammatory markers at delivery. In a prospective cohort study of 263 pregnant women in Rochester, NY, we measured interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α) in placental tissue and estimated gestational exposure to PM2.5 and NO2 using a high-resolution spatial-temporal model. Exposure to PAHs was estimated using urinary 1-hydroxypyrene (1-OHP) concentrations collected once per trimester. Using distributed lag models with a penalized spline function, each interquartile range (2.6 μg/m3) increase in PM2.5 concentration during gestational weeks 6–11 was associated with decreased placental IL-6 levels (−22.2%, 95% CI: −39.0%, −0.64%). Using multiple linear regression models, each interquartile range increase of 1-OHP was associated with an increase in TNF-α in the first trimester (58.5%, 95% CI: 20.7%, 74.2%), third trimester (22.9%, 95% CI: 0.04%, 49.5%), and entire pregnancy (29.6%, 95%CI: 3.9%,60.6%). Our results suggest gestational exposure to air pollution may alter the inflammatory environment of the placenta at delivery.
Keywords: pregnancy, air pollution, PAHs, placenta, tumor necrosis factor, interleukin
Introduction
Restricted Fetal Growth (RFG) has been associated with later-in-life health effects, including diabetes mellitus, coronary heart disease, and neurodevelopment delay.1 Recent epidemiolocal studies, including ours, have associated reduced fetal growth (measured via ultrasound) or reduced size at birth (e.g., birth weight, length, or head circumference) with increased exposure to combustion-originated air pollutants such as fine particles (PM2.5), nitrogen dioxide (NO2), and polycyclic aromatic hydrocarbons (PAHs), during pregnancy.2−4 Our previous findings highlighted that decreased gestational exposure to PM2.5 due to clean air actions during the 2008 Beijing Olympics Games was associated with increased birth weight.3 However, the molecular mechanisms by which air pollution contributes to RFG are not fully understood.
Placental insufficiency is a key contributor to RFG.5 Alterations in the inflammatory status of the placenta contribute to placental insufficiency, where the vasculature and nutrient delivery are impaired, leading to fetal deprivation.6,7 For example, El-Shazly et al. found placentas collected from preterm birth had lower Th2-derived cytokines such as Interleukin-6 (IL-6) than those from term deliveries.8 In another study, an increase in TNF- α was seen in the placentas of those with preterm birth.9 However, placental inflammation as an outcome is less examined in studies of air pollution exposure during pregnancy but may contribute to reduced fetal growth and preterm birth induced by air pollution.
In our previous study, we found that exposure to air pollution sources in a pregnancy cohort in Rochester, New York, U.S.A., was correlated with a urinary marker of PAHs,10 which has been associated with systemic inflammatory responses in previous studies.11 In addition, combustion-originated pollutants and cytokine inflammatory mediators can translocate the lung and enter systemic circulation,12 which may further cross the placental barrier.13 While air pollution exposure during pregnancy has been linked to inflammatory alterations in maternal and cord blood,14 epidemiology studies have also found an association between air pollution and placental inflammatory complications such as preeclampsia.15,16 Herein we aim to examined to what extent air pollution exposure during pregnancy negatively affects placental function, specifically placental inflammation at delivery.
In a pregnancy cohort study in Rochester, New York, we examined whether gestational week PM2.5 and NO2 concentrations and trimester-specific PAHs exposure (i.e., urinary 1-hydroxypyrene) were associated with changes in interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α) measured in postpartum placental tissues in term deliveries. We hypothesized that increases in each pollutant would be associated with increased IL-6 and TNF-α and explored when, during pregnancy, any such associations were observed.
Methods
Study participants and sample collection. We used data from a prospective longitudinal pregnancy cohort at the University of Rochester, the UPSIDE (Understanding Pregnancy Signals and Infant Development) cohort, part of the NIH Environmental Influences on Child Health Outcomes (ECHO) program. Further details can be found in a previous publication.17 Briefly, recruitment occurred during the first trimester of pregnancy from University of Rochester Medical Center-associated clinics between December 2015 and April 2019. To be eligible for the study, participants had to be at least 18 years old, pregnant with only one fetus, without any substance abuse or psychotic illness history, proficient in English, with no significant endocrine disorders, such as polycystic ovary syndrome or obstetric issues that threatened the pregnancy upon enrollment. Of the 326 participants enrolled, 263 participants had a singleton pregnancy, a first-trimester study visit, a placenta sample collected at delivery, and at least one urine sample and did not have a medical screen failure, miscarriage, preterm birth (<37 gestational weeks), or polycystic kidney disease. Preterm births were excluded to avoid confounding factors unrelated to air pollution and to ensure consistent exposure measurements across the study group (0–37 weeks of gestation). Participants attended in-person study visits once per trimester, where they completed questionnaires and provided biospecimen, including spot urine samples. Participants received compensation for each study visit and were offered transportation to study visits when necessary. The University of Rochester Research Subjects Review Board and Duke University Institutional Review Board approved the study. A flowchart of participants included in this study can be found in the Supporting Information (Figure S1). A comparison of the demographic characteristics between participants who were included versus excluded from the analysis is also available in Table S1.
Placental Inflammation Measurement
A small section of the flash-frozen placenta was obtained immediately upon delivery from the villus region of the placenta, excluding maternal decidua and chorionic plate. Placental tissue samples were stored at −80 °C until laboratory analysis. A small section of a placenta was cut, weighed, and placed in Lysing Matrix A tubes (MP Biomedicals 6910–500). The tissue was then homogenized with the Fast Prep 24 Homogenizer (MP Biomedical, 6VFV9) using Tissue Extraction Reagent 1 (Thermo Fisher, FNN007) combined with Pierce Protease Inhibitor Mini Tablets (Thermo Fisher, A32955). Homogenized samples were centrifuged, and the protein was extracted and stored at −80 °C. Total protein concentrations were determined using the Pierce Detergent Compatible Bradford Assay Kit (Thermo Fisher, 23246). Following the manufacturer’s instructions, IL-6 and TNF-α measurements were conducted using the Human IL-6 ELISA Kit (Thermo Fisher, KHC0061) and TNF-α Human ELISA Kit, High Sensitivity (Thermo Fisher, BMS223HS). IL-6 and TNF-α concentrations were normalized by total protein concentrations prior to analysis.
Exposure Estimates
Exposure to PM2.5 and NO2 was estimated using random forest models as described previously.18 Briefly, the model was generated using monitoring station data, satellite data, meteorological data, and land use variables as predictors. Furthermore, a network of low-cost sensors was also used to generate the model. The external validation (R2) for PM2.5 was 0.65, and the root mean squared error of the model was 2.96 μg/m3. The external validation (R2) for NO2 was 0.43, and the root mean squared error of the model was 4.20 parts per billion (ppb). The model generated daily residential PM2.5 and NO2 concentrations across the study area with a 1 km × 1 km spatial resolution. Daily concentrations from the 1 km2 grid containing each participant’s residence were assigned to that participant/pregnancy. Gestational week concentrations were then calculated from the daily concentrations, starting with gestational week 0 (calculated using either the date of the last menstrual period or crown-rump length, depending on the participant) to gestational week 37 to capture the gestational week concentrations from conception through 37 weeks (full-term). Exposures to PAHs were estimated based on urinary 1-hydroxypyrene (1-OHP) measurements, as described previously.10 We used a high-performance liquid chromatograph with a fluorometric detector to quantify 1-OHP levels in a total of 640 urine samples collected over each trimester from the 263 participants. Specific gravity was measured to normalize 1-OHP concentrations.
Statistical Analysis
We examined the distribution of each variable and conducted a natural log transformation for any variables that were right-skewed. N = 263 participants were included in the distributed lag nonlinear model (DLNM) analysis to explore the effects of gestational week PM2.5 and NO2 concentrations on inflammatory markers (TNF-α and IL-6) measured in placental tissue at delivery. The lag model assumed a linear association between PM2.5 or NO2 and each cytokine in a given gestational week (0 to 37) and used cubic regression penalized splines with penalties on the second derivatives to allow this relationship to vary smoothly across weeks.19 Week 37 was chosen as the cutoff for exposure because it represents a full-term pregnancy. Individuals who gave birth > 37 were included in the study. However, their exposures were only estimated up to 37 weeks. Effect estimates were scaled to the interquartile range (IQR) of PM2.5 and NO2 throughout pregnancy, 2.6 μg/m3 and 4.5 μg/m3, respectively. We also used linear regression models to examine the trimester-specific and average associations between 1-OHP and placenta inflammation at delivery. N = 224 participants were included in the first trimester, 206 in the second, and 210 in the third. Average 1-OHP was calculated by averaging at least two measurements across each trimester N = 257. From which we calculated the estimated change in each outcome associated with each interquartile range increase in the biomarker. A number of potential confounders, including maternal education (less than high school or high school, some college or bachelor’s degree, postgraduate degree), Medicaid use (yes, no), maternal employment (yes, no), infant sex (male, female), maternal age (years), smoking (yes, no), and prepregnancy body mass index (BMI, numeric) were assessed for their effects on placental inflammation using univariate analysis. Covariate data was collected from the questionnaire given at each trimester. Each variable was included in the final model if it resulted in a > 8.0% change in TNF-α associated with each interquartile range increase in 1-OHP concentration in a stepwise model-building procedure. The final model includes prepregnancy BMI, age, and smoking status.
We conducted several sensitivity analyses to assess if our main findings were robust to several assumptions, such as labor and delivery complications, outliers, and individuals who moved throughout pregnancy. The first set of sensitivity analyses (A-I) removed participants with labor and delivery complications, including preeclampsia (A), prolonged labor: >20 h for first-time mothers and >14 h for mothers who have had children previously (B), postpartum hemorrhage (C), chorioamnionitis (D), preterm rupture of the membrane (ROM): any ROM <37 weeks that did not result in preterm birth (E), premature rupture of the membrane: ROM before the onset of labor, (F), prolonged rupture of the membrane: ROM for > 18 h before delivery (G), precipitous delivery (H), and all labor and delivery complications (I). The following set of sensitivity analyses investigated the role of outliers among the 1-OHP measurements and cytokine measurements separately as well as compete cases (J–L). Outliers were identified using the confidence interval method; briefly, cut-offs were generated ± 1.5(IQR).20 Following outlier identification, 1-OHP outliers per trimester were removed (J), and TNF-α outliers were removed (K). Complete case analysis excluded participants missing three trimesters of 1-OHP data (L). Delivery mode was also addressed (V). Three sensitivity analyses were completed to validate the findings of PM2.5 on IL-6 concentrations by (1) excluding participants with preeclampsia, (2) excluding participants with potential exposure misclassification defined as moved during the study period were removed, and (3) removing participant with IL-6 outliers (cutoff generated by ± 1.5(IQR). Table S2 includes definitions of the labor and delivery complications. We then compared findings from these sensitivity analyses to those from the main analyses described above. Linear regression models were fitted using the lm function, and distributed lag models were fit using the dlnm package within R (www.r-project.org).
Results
Table 1 summarizes the maternal characteristics. Briefly, 263 participants provided data for this analysis; over 50% of our population self-reported as non-Hispanic White, while 54.8% reported Medicare use and had a smoking prevalence of 7.0%.
Table 1. Demographic Characteristics of Study Participants (N = 263).
| demographic characteristics | mean ± standard deviation (or N) |
|---|---|
| race ethnicity | |
| non-hispanic white | 156 (59.3%) |
| hispanic | 27 (10.3%) |
| non-hispanic black | 59 (22.4%) |
| othera | 21 (8.0%) |
| highest education | |
| less than high school or high school | 90 (34.2%) |
| some college or bachelors degree | 107 (40.7%) |
| post graduate degree | 65 (24.7%) |
| age mean ± SD (years) | 29 ± 4.6 |
| medicare use | |
| yes | 144 (54.8%) |
| no | 119 (45.2%) |
| smoking during pregnancy | |
| yes | 18 (6.8%) |
| no | 237 (90.1%) |
| BMI mean ± SD (kg/m2) | 27.9 ± 7 |
| infant sex | |
| male (0) | 128 (48.7%) |
| female (1) | 135 (51.3%) |
| placental weight mean ± SD (g) | 413.0 ± 87.4 |
| birth weight mean ± SD (g) | 3400.2 ± 512.3 |
| gestational age at delivery mean ± SD (weeks) | 39.7 ± 1.2 |
| exposure misclassificationb | |
| yes | 30 (11.4%) |
| no | 233 (88.6%) |
“Others” includes American Indian, Alaska Native, and participants self-reporting as “other”
Exposure misclassification refers to participants who moved during the study.
Levels of Air Pollution Exposure
The mean ± standard deviation concentrations across all trimesters were 6.74 ± 1.82 μg/m3 for PM2.5 and 10.6 ± 5.0 ppb for NO2. These exposure levels were below the current nationally established EPA limits for PM2.5 (9 μg/m3) and NO2 (53 ppb). The urinary 1-OHP geometric mean (IQR) was 63.7 (65.6) pg/mL. Using a previously established method to estimate the creatinine-adjusted 1-OHP levels based on specific gravity-adjusted 1-OHP levels,21 the geometric mean 1-OHP levels in our study (41.2 ng/g creatinine) was lower than that of nationally representative women in National Health and Nutrition Examination Survey (NHANES) in 2015–2016 (150 ng/g creatinine).22 Trimester-specific descriptive statistics for 1-OHP, PM2.5, and NO2 are summarized in Table 2. Trimesters were defined as 0–12, 13–26, and 27–37 gestational weeks.
Table 2. Summary Statistics of Exposure Estimates and Placental Cytokine Concentrations.
| variable, unit | time period | median | SD | IQR |
|---|---|---|---|---|
| 1-OHP, pg/mL | trimester 1 | 52.2 | 57. 5 | 53.7 |
| trimester 2 | 54.1 | 84.0 | 66.5 | |
| trimester 3 | 65.7 | 80.0 | 65.0 | |
| average | 63.7 | 62.7 | 65.3 | |
| PM2.5, μg/m3 | trimester 1 | 6.72 | 0.9 | 1.3 |
| trimester 2 | 6.67 | 1.0 | 1.5 | |
| trimester 3 | 6.86 | 1.0 | 1.4 | |
| average | 6.74 | 1.8 | 2.6 | |
| NO2, ppb | trimester 1 | 11.2 | 4.8 | 7.8 |
| trimester 2 | 10.4 | 4.8 | 8.0 | |
| trimester 3 | 10.2 | 5.0 | 7.9 | |
| average | 10.6 | 5.1 | 4.5 | |
| IL-6, ng/μg protein | at delivery | 5.3 | 7.6 | 4.6 |
| TNF-α, ng/μg protein | at delivery | 0.4 | 1.5 | 0.6 |
Air Pollution and Placental Inflammatory Markers
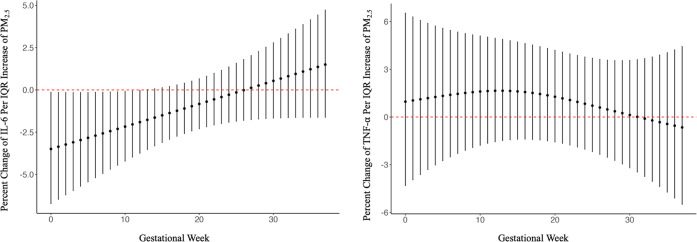
Summary statistics of the placental inflammatory markers can be seen in Table 2. Figure 1 shows the gestational week associations between PM2.5 and placental concentrations of IL-6 (A) and TNF-α (B) at birth from the distributed lag nonlinear models. A negative association was observed between each IQR increase in PM2.5 in the gestational weeks 6–11 in IL-6 (−22.2%, 95% CI: −39.0%, −0.64%). The estimated cumulative effect of exposure to an IQR increase in PM2.5 each week during the pregnancy was associated with a decrease in IL-6 −49.4% (95% CI: −61.7%, 19.1%). Furthermore, the estimated cumulative effect of exposure to an IQR increase in PM2.5 each week during pregnancy was associated with a 40.3% (95% CI: −42.3% to 241%) increase in TNF-α concentrations (Table S3).
Figure 1.
Estimated distributed lag effect of PM2.5 throughout pregnancy and placental inflammatory markers IL-6 (left panel) and TNF-α (right panel). Starting at gestational week 0, defined as the date of the last menstrual period or crown-rump length, depending on the participant, to gestational week 37. Adjusted for prepregnancy BMI, smoking during pregnancy, and maternal age. Lag effects are presented as the percent change in placental inflammatory marker per IQR increase of PM2.5 (2.6 μg/m3) (N = 263).
Figure 2 shows the time-dependent associations of NO2 with placental concentrations of IL-6 (A) and TNF-α (B). Within the DLNM analyses, we did not observe any associations between increased NO2 concentrations during individual gestational weeks and placental cytokine levels. Exposure to IQR increases in NO2 concentration during the entire pregnancy was estimated to be associated with a −0.13% (95% CI: −13.8%, 15.8%, and 4.45% (95% CI: −16.2%, 30.2%) differences in IL-6 and TNF-α concentrations, respectively, suggesting null effects of NO2 on placental inflammation (Table S3).
Figure 2.
Estimated distributed lag effect of NO2 and placental inflammatory markers IL6 (left panel) and TNF-α (right panel). Starting at gestational week 0 defined as the date of the last menstrual period or crown-rump length depending on participant to gestational week 37. Adjusted for prepregnancy, BMI, smoking during pregnancy, and maternal age. Lag effects are presented as the percent change in placental inflammatory marker and 1 IQR increase of NO2 (4.5 ppb). N = 263.
PAHs Exposure and Placental Inflammatory Markers
We examined the separate associations between 1-OHP in each trimester and placental levels of IL-6 and TNF-α (Figure 3 and Table S4). Each IQR increase in 1-OHP levels during the first and third trimester was associated with an increase in TNF-α 58.5% (95% CI: 20.7%, 74.2%) and 22.9% (95% CI: 0.04%, 49.5%) respectively. Each IQR increase in the average 1-OHP over the three trimesters was also associated with an increase in TNF-α levels 29.6% (95%CI: 3.9%, 60.6%). In contrast, no associations were observed between IL-6 and 1-OHP in any trimester. Each IQR increase in the average 1-OHP over the three trimesters was associated with an increase in IL-6 3.91% (95% CI: −10.0%, 20.0%).
Figure 3.
Associations of urinary 1-OHP concentrations with placental inflammatory marker IL-6 (left panel), and TNF-α (right panel). Associations were tested using linear models. All models were adjusted for smoking status, maternal age, and prepregnancy BMI. The association is presented as percent changes of 1-OHP concentrations per one interquartile increase in 1-OHP concentration, averaged over three trimesters: first, second, and third trimester. Significant was determined as p < 0.05.
Sensitivity Analysis
We conducted rigorous sensitivity analyses to assess the robustness of our primary findings. Specifically, we examined the impact of labor and delivery complications (A-I), removal of 1-OHP outliers on our results (J), removal of cytokine outliers (K), and complete cases (L) (Figures S2–S4 and Tables S5–S7). These sensitivity analyses showed minimal alterations in the main effects of 1-OHP and TNF-α. Excluding all participants with labor and delivery complications resulted in a decrease in the size of the effect estimates between average 1-OHP and first trimester 1-OHP 12.2% increase of TNF-α (95% CI: −11.7%, 45.4), (34.3% increase in TNF-α (95% CI: 7.9%–68.6% respectively. These changes may be attributable to the smaller sample size in the subset analysis (N = 257 and N = 199 for average 1-OHP and N = 224 and N = 165 for first-trimester 1-OHP). Furthermore, removing TNF-α outliers reduced the overall effect size of the trimester TNF-α and 1-OHPs relationship; however, the results remained robust at 34.3% (95% CI: 14.2–57.8). Additional sensitivity analyses were completed to test the robustness of the lagged effect of interquartile range increases of PM2.5 on IL-6 concentrations (−22.2%, 95% CI: −39.0%, −0.64%). However, the initial sensitive time windows disappeared in each sensitivity analysis. However, the overall direction stayed the same (Table S8).
Discussion
This study aimed to fill the knowledge gap on whether low-level air pollution exposures during pregnancy were associated with increased placental inflammation at birth. Increased PM2.5 concentrations below current NAAQS standards were associated with lower concentrations of placental inflammation biomarkers, specifically IL-6. Furthermore, PAH concentrations in the first trimester and averaged across the entire pregnancy were associated with higher TNF-α concentrations in term placentas. These results suggest that exposure to combustion-originated air pollutants such as PAHs may alter the inflammatory status of the placenta within our study population. Furthermore, our results suggest that exposures during early pregnancy may be more important in contributing to placental inflammation at delivery than during later pregnancy time points.
PM2.5 is a complex mixture containing PAHs.23 1-Hydroxypyrene is a urinary marker for pyrene exposure and is associated with short-term exposure to PM2.5 among the study population.18 Once inhaled, PAHs can translocate into the bloodstream, and numerous studies have shown that PAHs can induce systemic inflammation.24−26 Herein, we extended the pro-inflammatory effects of PAHs to the placenta by showing an association between placental TNF-α concentrations and 1-OHP in maternal urine. Increased inflammation in maternal blood due to PAH exposure may reach the syncytiotrophoblast where TNF-α mRNA is expressed, which could alter TNF-α expression and lead to an excess of TNF-α in the placental tissue.27
Placental development is a complex and intricate mechanism involving a highly conserved immunological environment. Implantation and placentation during the first trimester include an evolutionarily conserved upregulation of (IL-6).28 Additionally, TNF-α is imperative for normal placental and fetal development but, in excess, can lead to preterm birth, preeclampsia, and late pregnancy loss.27 In our study, we observed higher placental TNF-α associated with PAH exposure and lower IL-6 associated with increased PM2.5 concentrations during pregnancy. These results suggest that exposure to air pollution may perturb the immunological hemostasis in the placenta, which may adversely affect placental development. The underlying biological mechanisms are yet to be investigated. It is well established, however, that the proinflammatory response in the respiratory tract and the circulatory system to air pollution exposure is acute and can fluctuate with daily variations in air pollution levels.29 In this study, we observed that air pollution in early pregnancy was associated with the two proinflammatory cytokines in the placenta at delivery. One of our hypothesized mechanisms is that early pregnancy exposure may alter epigenetic regulations that shift the immune hemostasis toward a heightened Th-1 proinflammatory state of the placenta throughout the pregnancy.
Numerous studies have supported the hypothesis that exposure to air pollution contributes to low birth weight and fetal growth restriction.2−4 However, results are mixed regarding the critical time window since the evidence is available supporting stronger associations during preconception windows30,31 as well as the first-,32 second-,33 and third-trimesters.34 Remarkably, a recent muti-center randomized trial (i.e., HAPIN), the use of a clean cook stove, in comparison with the use of more polluting biomass stove, from gestational week 18 until the birth, resulted in a small (5.3 g) increase in birth weight,3536 while a subgroup of participants who started to use the clean stove during early pregnancy (before gestational week 18) had a substantially larger (33.8 g) increase in birth weight. This is consistent with our findings suggesting that the first trimester may be a more susceptible time window of exposure.
A limited number of prior studies have examined the effects of air pollution on placental inflammation. Studies in vitro using human first-trimester trophoblast cells found that exposure to wood smoke and a metabolite of benzo(a)pyrene (benzo(a)pyrene-7,8-dihydrodiol-9,10-epoxide) increased IL-637,38 and TNF-α secretion.38 A cohort that compared clinically recognized early pregnancy loss (CREPL) to elective termination in the first trimester found that higher ambient PM2.5 concentrations eight days before the end of pregnancy were associated with increased villus IL-6 in the CREPL group.39 Furthermore, higher concentrations of IL-6 and TNF-α were associated with each IQR increase in PM2.5 three weeks after ovulation in both groups compared to the weeks prior and just after ovulation.39 In the Boston Birth Cohort, Nachman et al. found the odds ratio of intrauterine inflammation (assessed via placental pathology, was highest with PM2.5 exposure in the first trimester) (OR = 1.93; 95% CI: 1.55, 2.40) compared to second (OR = 1.67; 95% CI: 1.35, 2.08), and third trimester (OR = 1.53; 95% CI: 1.24, 1.90).40 Our study contributes to this growing body of evidence and shows that exposure to PAHs, even at low levels, may contribute to higher placental TNF-α levels. Our findings on IL-6, while inconsistent with previous air pollution studies in the placenta, studies looking at maternal serum and cord blood found a decrease in IL-6 associated with 10 μg/m3 PM2.5 in the first trimester and reduced IL-6 levels among women who smoked during pregnancy.41,42 It was hypothesized that the reduced IL-6 levels were partly due to the PAH-induced reduction of T-cells, which would reduce the amount of IL-6 produced.42 Furthermore, El-Shazly et al. found a decrease in placental IL-6 in placentas from preterm births compared to placentas from term births.8 Additional studies, however, are warranted to confirm our findings on IL-6.
Several things need to be considered when interpreting the effect size of our study. There are dietary and other environmental sources of pyrene, such as barbequed or grilled food and environmental tobacco smoke. Moreover, these sources of pyrene may be correlated to predictors of socioeconomic disparity.32 Therefore, these unmeasured sources may result in an overestimate of the effect due to air pollution. Second, since PM2.5 and NO2 concentrations were estimated for the 1-km2 grid containing a subject’s residence and not the residence itself, there may be some exposure measurement error and reduced the statistical power. It is also important to note while our study did not find statistically significant associations with NO2, our study had a relatively small sample size, and therefore, future studies with a larger sample size should be conducted to confirm our null results for NO2 and placental inflammatory markers at delivery. Further, the inference was made based on the magnitude, direction, and pattern of associations observed across the lag times for each pollutant examined and not solely on the statistical significance of a hypothesis test p-value.
This study’s strengths include the use of a well-established internal biomarker of PAH exposure (urinary 1-hydroxypyrene) to provide insight into participants’ PAH exposure, the application of the DLNM to estimate the effect of air pollution over pregnancy, and the use of a high-resolution spatiotemporal model to generate daily PM2.5 and NO2 concentrations within 1-km2 grids across the study area. This approach minimizes exposure misclassification and reduces bias toward the null in our effect estimates compared to studies relying solely on central-site monitoring data. Additionally, our study found associations with low levels of air pollution, which may apply to many other parts of the United States.
There are limitations of our study. First, the internal biomarker of exposure to pyrene has a short half-life (∼12–24 h), which means that a single spot measurement per trimester may not accurately reflect the average exposure for the entire trimester. Second, other factors (e.g., maternal acetaminophen) may influence placental TNF-α and IL-6 in addition to air pollution. While we did our best to address the potential influence of those factors, some factors (e.g., diet) were not measured in this study. Third, the placenta samples were collected at delivery, providing a snapshot of inflammation at the end of pregnancy only, without examining earlier markers of inflammation. Last, while our cohort is clinically representative of the centers recruited from, it is not regionally representative of Rochester, New York, or nationally representative.
Conclusions
This study highlights the importance of understanding the effect of air pollution exposure during pregnancy on placental inflammation in the placenta at birth, a factor pivotal to fetal growth and development. Findings underscore that the first trimester may be a period of particular susceptibility to the effects of air pollution exposure, with 1-OHP levels showing exposure positively associated with TNF-α concentrations in the placenta. We also found an increase in the third trimester, as well as an average of 1-OHP levels across all trimesters associated with TNF-α concentrations in the placenta, suggesting a potential influence during the last trimester of pregnancy of PAH exposure on placental inflammation as well. Furthermore, this study showed lower IL-6 concentrations in term placenta associated with higher first-trimester PM2.5. These findings suggest that air pollution exposure during pregnancy may perturb the immunological hemostasis of the placenta.
Acknowledgments
The authors thank Grace Zhang for her contribution to the measurement of placental total protein. This study is funded by the National Institutes of Health (R01ES027495, UG3 OD023349, HD083369), The Wynne Center for Family Research. The National Center for Advancing Translational Sciences of the National Institutes of Health (UL1 TR002001) and the University of Rochester CTSA. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Glossary
Abbreviations Used
- IL-6
Interleukin 6
- TNF-α
Tumor Necrosis Factor Alpha
- 1-OHP
1-hydroxypyrene
- PM2.5
Particulate Matter less than 2.5 μm in aerodynamic diameter
- NO2
Nitrogen Dioxide
- RFG
Restricted Fetal Growth
- PAH
Polyaromatic Hydrocarbons
- OR
Odds Ratio
- CREPL
cohort that compared clinically recognized early pregnancy loss
- NAAQS
National Ambient Air Quality Standards
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/envhealth.4c00077.
Forest plots, tables of the sensitivity analysis conducted, results from regression models used to determine associations of 1-OHP and inflammatory cytokines, PM2.5 and NO2, definitions of the labor and delivery complications, and a diagram of the number of participants included in each analysis (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Kamphof H. D.; Posthuma S.; Gordijn S. J.; Ganzevoort W. Fetal Growth Restriction: Mechanisms, Epidemiology, and Management. Matern.-Fetal Med. 2022, 4 (3), 186. 10.1097/FM9.0000000000000161. [DOI] [Google Scholar]
- Malmqvist E.; Liew Z.; Källén K.; Rignell-Hydbom A.; Rittner R.; Rylander L.; Ritz B. Fetal Growth and Air Pollution - A Study on Ultrasound and Birth Measures. Environ. Res. 2017, 152, 73–80. 10.1016/j.envres.2016.09.017. [DOI] [PubMed] [Google Scholar]
- Rich D. Q.; Liu K.; Zhang J.; Thurston S. W.; Stevens T. P.; Pan Y.; Kane C.; Weinberger B.; Ohman-Strickland P.; Woodruff T. J.; Duan X.; Assibey-Mensah V.; Zhang J. Differences in Birth Weight Associated with the 2008 Beijing Olympics Air Pollution Reduction: Results from a Natural Experiment. Environ. Health Perspect. 2015, 123 (9), 880–887. 10.1289/ehp.1408795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao X.; Cheng H.; Zhou J.; Zhang J.; Zhu Y.; Yang C.; Di Narzo A.; Yu J.; Shen Y.; Li Y.; Xu S.; Zhang Z.; Chen J.; Cheng J.; Hao K. Prenatal Exposure to Ambient Air Multi-Pollutants Significantly Impairs Intrauterine Fetal Development Trajectory. Ecotoxicol. Environ. Saf. 2020, 201, 110726. 10.1016/j.ecoenv.2020.110726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardozza L. M. M.; Caetano A. C. R.; Zamarian A. C. P.; Mazzola J. B.; Silva C. P.; Marçal V. M. G.; Lobo T. F.; Peixoto A. B.; Araujo Júnior E. Fetal Growth Restriction: Current Knowledge. Arch. Gynecol. Obstet. 2017, 295 (5), 1061–1077. 10.1007/s00404-017-4341-9. [DOI] [PubMed] [Google Scholar]
- Baker B. C.; Heazell A. E. P.; Sibley C.; Wright R.; Bischof H.; Beards F.; Guevara T.; Girard S.; Jones R. L. Hypoxia and Oxidative Stress Induce Sterile Placental Inflammation in Vitro. Sci. Rep. 2021, 11 (1), 7281. 10.1038/s41598-021-86268-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C. J.; Romero R.; Chaemsaithong P.; Kim J.-S. Chronic Inflammation of the Placenta: Definition, Classification, Pathogenesis, and Clinical Significance. Am. J. Obstet. Gynecol. 2015, 213 (4), S53–S69. 10.1016/j.ajog.2015.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Shazly S.; Makhseed M.; Azizieh F.; Raghupathy R. Increased Expression of Pro-Inflammatory Cytokines in Placentas of Women Undergoing Spontaneous Preterm Delivery or Premature Rupture of Membranes. Am. J. Reprod. Immunol. N. Y. N 2004, 52 (1), 45–52. 10.1111/j.1600-0897.2004.00181.x. [DOI] [PubMed] [Google Scholar]
- Holcberg G.; Huleihel M.; Sapir O.; Katz M.; Tsadkin M.; Furman B.; Mazor M.; Myatt L. Increased Production of Tumor Necrosis Factor-Alpha TNF-Alpha by IUGR Human Placentae. Eur. J. Obstet. Gynecol. Reprod. Biol. 2001, 94 (1), 69–72. 10.1016/S0301-2115(00)00321-3. [DOI] [PubMed] [Google Scholar]
- Lin Y.; Craig E.; Liu X.; Ge Y.; Brunner J.; Wang X.; Yang Z.; Hopke P. K.; Miller R. K.; Barrett E. S.; Thurston S. W.; Murphy S. K.; O’Connor T. G.; Rich D. Q.; Zhang J. J. Urinary 1-Hydroxypyrene in Pregnant Women in a Northeastern U.S. City: Socioeconomic Disparity and Contributions from Air Pollution Sources. J. Expo. Sci. Environ. Epidemiol. 2024, 34, 407. 10.1038/s41370-023-00555-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y.; Ramanathan G.; Zhu Y.; Yin F.; Rea N. D.; Lu X.; Tseng C.-H.; Faull K. F.; Yoon A. J.; Jerrett M.; Zhu T.; Qiu X.; Araujo J. A. Pro-Oxidative and Proinflammatory Effects After Traveling From Los Angeles to Beijing: A Biomarker-Based Natural Experiment. Circulation 2019, 140 (24), 1995–2004. 10.1161/CIRCULATIONAHA.119.042054. [DOI] [PubMed] [Google Scholar]
- Wang W.; Lin Y.; Yang H.; Ling W.; Liu L.; Zhang W.; Lu D.; Liu Q.; Jiang G. Internal Exposure and Distribution of Airborne Fine Particles in the Human Body: Methodology, Current Understandings, and Research Needs. Environ. Sci. Technol. 2022, 56 (11), 6857–6869. 10.1021/acs.est.1c07051. [DOI] [PubMed] [Google Scholar]
- Bové H.; Bongaerts E.; Slenders E.; Bijnens E. M.; Saenen N. D.; Gyselaers W.; Van Eyken P.; Plusquin M.; Roeffaers M. B. J.; Ameloot M.; Nawrot T. S. Ambient Black Carbon Particles Reach the Fetal Side of Human Placenta. Nat. Commun. 2019, 10 (1), 3866. 10.1038/s41467-019-11654-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman C.; Dabelea D.; Thomas D. S. K.; Peel J. L.; Adgate J. L.; Magzamen S.; Martenies S. E.; Allshouse W. B.; Starling A. P. Exposure to Ambient Air Pollution during Pregnancy and Inflammatory Biomarkers in Maternal and Umbilical Cord Blood: The Healthy Start Study. Environ. Res. 2021, 197, 111165. 10.1016/j.envres.2021.111165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira G.; Haggar F.; Shand A. W.; Bower C.; Cook A.; Nassar N. Association between Pre-Eclampsia and Locally Derived Traffic-Related Air Pollution: A Retrospective Cohort Study. J. Epidemiol. Community Health 2013, 67 (2), 147–152. 10.1136/jech-2011-200805. [DOI] [PubMed] [Google Scholar]
- Jia L.; Liu Q.; Hou H.; Guo G.; Zhang T.; Fan S.; Wang L. Association of Ambient Air Pollution with Risk of Preeclampsia during Pregnancy: A Retrospective Cohort Study. BMC Public Health 2020, 20 (1), 1663. 10.1186/s12889-020-09719-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor T.; Best M.; Brunner J.; Ciesla A. A.; Cunning A.; Kapula N.; Kautz A.; Khoury L.; Macomber A.; Meng Y.; Miller R. K; Murphy H.; Salafia C. M; Vallejo Sefair A.; Serrano J.; Barrett E. Cohort Profile: Understanding Pregnancy Signals and Infant Development (UPSIDE): A Pregnancy Cohort Study on Prenatal Exposure Mechanisms for Child Health. BMJ Open 2021, 11 (4), e044798 10.1136/bmjopen-2020-044798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge Y.; Yang Z.; Lin Y.; Hopke P. K.; Presto A. A.; Wang M.; Rich D. Q.; Zhang J. Generating High Spatial Resolution Exposure Estimates from Sparse Regulatory Monitoring Data. Atmos. Environ. 2023, 313, 120076. 10.1016/j.atmosenv.2023.120076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparrini A.; Scheipl F.; Armstrong B.; Kenward M. G. A Penalized Framework for Distributed Lag Non-Linear Models. Biometrics 2017, 73 (3), 938–948. 10.1111/biom.12645. [DOI] [PubMed] [Google Scholar]
- Hansen J.; Ahern S.; Earnest A. Evaluations of Statistical Methods for Outlier Detection When Benchmarking in Clinical Registries: A Systematic Review. BMJ Open 2023, 13 (7), e069130 10.1136/bmjopen-2022-069130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrieri M.; Trevisan A.; Bartolucci G. B. Adjustment to Concentration-Dilution of Spot Urine Samples: Correlation between Specific Gravity and Creatinine. Int. Arch. Occup. Environ. Health 2000, 74 (1), 63–67. 10.1007/s004200000190. [DOI] [PubMed] [Google Scholar]
- Biomonitoring Data Tables for Environmental Chemicals; CDC. https://www.cdc.gov/exposurereport/data_tables.html (accessed 2023. –10–06). [Google Scholar]
- Jahedi F.; Dehdari Rad H.; Goudarzi G.; Tahmasebi Birgani Y.; Babaei A. A.; Ahmadi Angali K. Polycyclic Aromatic Hydrocarbons in PM1, PM2.5 and PM10 Atmospheric Particles: Identification, Sources, Temporal and Spatial Variations. J. Environ. Health Sci. Eng. 2021, 19 (1), 851–866. 10.1007/s40201-021-00652-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H.; Liu R.; Yang L.; Cheng H.; Wang S.; Zhang B.; Shao J.; Ma S.; Norbäck D.; Zhang X.; An T. Exposure to Polycyclic Aromatic Hydrocarbons (PAHs) in Outdoor Air and Respiratory Health, Inflammation and Oxidative Stress Biomarkers: A Panel Study in Healthy Young Adults. Sci. Total Environ. 2023, 899, 165582. 10.1016/j.scitotenv.2023.165582. [DOI] [PubMed] [Google Scholar]
- Mallah M. A.; Changxing L.; Mallah M. A.; Noreen S.; Liu Y.; Saeed M.; Xi H.; Ahmed B.; Feng F.; Mirjat A. A.; Wang W.; Jabar A.; Naveed M.; Li J.-H.; Zhang Q. Polycyclic Aromatic Hydrocarbon and Its Effects on Human Health: An Overeview. Chemosphere 2022, 296, 133948. 10.1016/j.chemosphere.2022.133948. [DOI] [PubMed] [Google Scholar]
- Altemose B.; Robson M. G.; Kipen H. M.; Ohman Strickland P.; Meng Q.; Gong J.; Huang W.; Wang G.; Rich D. Q.; Zhu T.; Zhang J. Association of Air Pollution Sources and Aldehydes with Biomarkers of Blood Coagulation, Pulmonary Inflammation, and Systemic Oxidative Stress. J. Expo. Sci. Environ. Epidemiol. 2017, 27 (3), 244–250. 10.1038/jes.2016.38. [DOI] [PubMed] [Google Scholar]
- Haider S.; Knöfler M. Human Tumour Necrosis Factor: Physiological and Pathological Roles in Placenta and Endometrium. Placenta 2009, 30 (2), 111–123. 10.1016/j.placenta.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yockey L. J.; Iwasaki A. Role of Interferons and Cytokines in Pregnancy and Fetal Development. Immunity 2018, 49 (3), 397–412. 10.1016/j.immuni.2018.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camarinho R.; Garcia P. V.; Choi H.; Rodrigues A. S. Overproduction of TNF-α and Lung Structural Remodelling Due to Chronic Exposure to Volcanogenic Air Pollution. Chemosphere 2019, 222, 227–234. 10.1016/j.chemosphere.2019.01.138. [DOI] [PubMed] [Google Scholar]
- Ghozikali M. G.; Ansarin K.; Naddafi K.; Nabizadeh R.; Yaghmaeian K.; Jaafari J.; Dehghanzadeh R.; Atafar Z.; Faraji M.; Mohammadi A.; Goudarzi G.; Yunesian M. Status of TNF-α and IL-6 as pro-Inflammatory Cytokines in Exhaled Breath Condensate of Late Adolescents with Asthma and Healthy in the Dust Storm and Non-Dust Storm Conditions. Sci. Total Environ. 2022, 838, 155536. 10.1016/j.scitotenv.2022.155536. [DOI] [PubMed] [Google Scholar]
- Ha S.; Zhu Y.; Liu D.; Sherman S.; Mendola P. Ambient Temperature and Air Quality in Relation to Small for Gestational Age and Term Low Birthweight. Environ. Res. 2017, 155, 394–400. 10.1016/j.envres.2017.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu Z.; Xie C.; Wen X.; Tian F.; Yuan S.; Jia D.; Chen W.-Q. Potential Pathways by Which Maternal Second-Hand Smoke Exposure during Pregnancy Causes Full-Term Low Birth Weight. Sci. Rep. 2016, 6, 24987. 10.1038/srep24987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.; Xu J.; Chen D.; Sun P.; Ma X. The Association between Air Pollution and Preterm Birth and Low Birth Weight in Guangdong, China. BMC Public Health 2019, 19 (1), 3. 10.1186/s12889-018-6307-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji N.; Johnson M.; Eckel S. P.; Gauderman W. J.; Chavez T. A.; Berhane K.; Faham D.; Lurmann F.; Pavlovic N. R.; Grubbs B. H.; Lerner D.; Habre R.; Farzan S. F.; Bastain T. M.; Breton C. V. Prenatal Ambient Air Pollution Exposure and Child Weight Trajectories from the 3rd Trimester of Pregnancy to 2 Years of Age: A Cohort Study. BMC Med 2023, 21 (1), 341. 10.1186/s12916-023-03050-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta A.; Alexander D.; Karrison T.; Morhasson-Bello O.; Wilson N.; Atalabi O. M.; Adu D.; Ibigbami T.; Adekunle S.; Adepoju D.; Olamijulo J.; Akinwunmi O.; Afolabi O. S.; Deji-Abiodun O.; Adedokun B.; Aschebrook-Kilfoy B.; Ojengbede O.; Olopade C. O. Household Air Pollution, Ultrasound Measurement, Fetal Biometric Parameters and Intrauterine Growth Restriction. Environ. Health 2021, 20 (1), 74. 10.1186/s12940-021-00756-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clasen T. F.; Chang H. H.; Thompson L. M.; Kirby M. A.; Balakrishnan K.; Díaz-Artiga A.; McCracken J. P.; Rosa G.; Steenland K.; Younger A.; Aravindalochanan V.; Barr D. B.; Castañaza A.; Chen Y.; Chiang M.; Clark M. L.; Garg S.; Hartinger S.; Jabbarzadeh S.; Johnson M. A.; Kim D.-Y.; Lovvorn A. E.; McCollum E. D.; Monroy L.; Moulton L. H.; Mukeshimana A.; Mukhopadhyay K.; Naeher L. P.; Ndagijimana F.; Papageorghiou A.; Piedrahita R.; Pillarisetti A.; Puttaswamy N.; Quinn A.; Ramakrishnan U.; Sambandam S.; Sinharoy S. S.; Thangavel G.; Underhill L. J.; Waller L. A.; Wang J.; Williams K. N.; Rosenthal J. P.; Checkley W.; Peel J. L. Liquefied Petroleum Gas or Biomass for Cooking and Effects on Birth Weight. N. Engl. J. Med. 2022, 387 (19), 1735–1746. 10.1056/NEJMoa2206734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlandsson L.; Lindgren R.; Nääv Å.; Krais A. M.; Strandberg B.; Lundh T.; Boman C.; Isaxon C.; Hansson S. R.; Malmqvist E. Exposure to Wood Smoke Particles Leads to Inflammation, Disrupted Proliferation and Damage to Cellular Structures in a Human First Trimester Trophoblast Cell Line. Environ. Pollut. 2020, 264, 114790. 10.1016/j.envpol.2020.114790. [DOI] [PubMed] [Google Scholar]
- Wang W.; Wang R.; Zhang Q.; Mor G.; Zhang H. Benzo(a)Pyren-7,8-Dihydrodiol-9,10-Epoxide Induces Human Trophoblast Swan 71 Cell Dysfunctions Due to Cell Apoptosis through Disorder of Mitochondrial Fission/Fusion. Environ. Pollut. Barking Essex 2018, 233, 820–832. 10.1016/j.envpol.2017.11.022. [DOI] [PubMed] [Google Scholar]
- Gong C.; Chu M.; Yang J.; Gong X.; Han B.; Chen L.; Bai Z.; Wang J.; Zhang Y. Ambient Fine Particulate Matter Exposures and Human Early Placental Inflammation. Environ. Pollut. 2022, 315, 120446. 10.1016/j.envpol.2022.120446. [DOI] [PubMed] [Google Scholar]
- Nachman R. M.; Mao G.; Zhang X.; Hong X.; Chen Z.; Soria C. S.; He H.; Wang G.; Caruso D.; Pearson C.; Biswal S.; Zuckerman B.; Wills-Karp M.; Wang X. Intrauterine Inflammation and Maternal Exposure to Ambient PM2.5 during Preconception and Specific Periods of Pregnancy: The Boston Birth Cohort. Environ. Health Perspect. 2016, 124 (10), 1608–1615. 10.1289/EHP243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozzoni P.; Iodice S.; Persico N.; Ferrari L.; Pinelli S.; Corradi M.; Rossi S.; Miragoli M.; Bergamaschi E.; Bollati V.; Alinovi R.; Biggeri A.; Borghi F.; Cantone L.; Catelan D.; Cattaneo A.; Cavallo D.; Dioni L.; Dolo V.; Giusti I.; Grisotto L.; Hoxha M.; Ischia B.; Mariani J.; Monticelli D.; Rota F.; Rota I.; Rovelli S.; Spinazzè A.; Stoppa G.; Vicenzi M. Maternal Air Pollution Exposure during the First Trimester of Pregnancy and Markers of Inflammation and Endothelial Dysfunction. Environ. Res. 2022, 212, 113216. 10.1016/j.envres.2022.113216. [DOI] [PubMed] [Google Scholar]
- Latzin P.; Frey U.; Armann J.; Kieninger E.; Fuchs O.; Röösli M.; Schaub B. Exposure to Moderate Air Pollution during Late Pregnancy and Cord Blood Cytokine Secretion in Healthy Neonates. PloS One 2011, 6 (8), e23130 10.1371/journal.pone.0023130. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.