Abstract
In radiotherapy for pediatric abdominal tumors, determining the effect of concurrent chemotherapy on polyglycolic acid (PGA) spacers is crucial; yet this effect has not been validated. Therefore, we aimed to evaluate the impact of cyclophosphamide (CPA) chemotherapy on the PGA spacer using a rat model. Twenty-four rats were implanted with the spacer, and morphological changes in the spacer were assessed on CT for both the CPA-dosed group (40 mg/kg) and the control group. The size and volume of the spacer were quantified using CT, while the degree of adhesion and microscopic examination of the tissue were determined using pathology specimens. Morphologically, the size of the spacer decreased over time in both the CPA-dosed and control groups, with no significant differences observed between groups. No significant differences in adhesion were observed between the two groups. Macrophages were observed around the PGA fibers, suggesting their involvement in the degradation of the PGA spacer. These results suggest that CPA does not cause significant clinically problematic degradation or adverse tissue reactions to the PGA spacer. This study reinforced the benefits of PGA spacers; however, future research focusing on in vivo longitudinal monitoring of individual rats, as well as on humans, is required.
Keywords: spacer, polyglycolic acid, PGA, cyclophosphamide, chemotherapy
INTRODUCTION
In radiation therapy, increasing the radiation dose is desirable for enhancing local treatment efficacy. However, when treating cancers near tissues at risk, the dose is often limited to protect surrounding healthy tissues. Even modalities like particle therapy cannot entirely eliminate adverse events. Developments in radiation therapy include techniques for artificially creating a ‘space’ between tumors and healthy organs [1–4]. Hydrogel spacers, known for their biocompatibility and ability to create space temporarily, are extensively utilized to distance the rectum from the radiation field, particularly in prostate cancer treatment. However, the injection area is limited [5, 6]. GORE-TEX® spacers (W. L. Gore & Associates, Inc., Newark, DE, USA) have been used to protect radiosensitive organs, such as the small and large intestines, when in close proximity to malignant tumors [7–9]. While these methods are effective in certain contexts, they often present limitations in terms of biodegradability, long-term safety and patient comfort. Polyglycolic acid (PGA) spacers have been developed and clinically adopted to overcome these limitations. In animal studies, PGA spacers have demonstrated biological safety, shielding efficacy and durability [10–14]. They represent a major advance in terms of reducing the long-term complications associated with conventional spacers and providing a biodegradable, patient-friendly alternative.
Chemotherapy prior to or in combination with radiation therapy is commonly used to treat pediatric malignancies, particularly Ewing sarcoma and rhabdomyosarcoma. There is concern that radiotherapy or particle therapy may cause significant disruption in later life, including growth retardation and infertility, if organs such as the spine, gastrointestinal tract and reproductive organs, are in close proximity to the tumor [14, 15]. Previous studies on PGA spacers have been validated primarily in specimens without chemotherapy. Although our institution has some experience with spacer implantation in combination with chemotherapy, it remains unknown how the PGA spacer is adversely affected when combined with chemotherapy in children (Fig. 1). Therefore, understanding how chemotherapy affects PGA spacers was the main focus of this study.
Fig. 1.
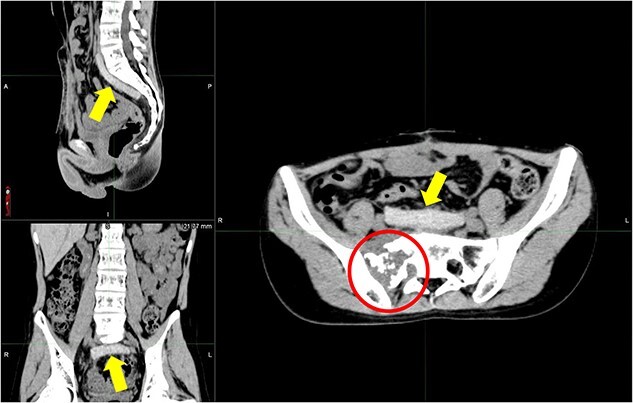
Clinical application of PGA spacers. CT image of a 14-year-old boy with a primary Ewing-like tumor in the sacrum, a case of recurrence (circle) after previous proton therapy in the primary sacrum. A PGA spacer (arrow) was implanted to reduce the dose irradiated to the gastrointestinal tract as much as possible during re-proton therapy with irinotecan and CPA chemotherapy combination. PGA, polyglycolic acid; CT, computed tomography; CPA, cyclophosphamide.
PGA spacers are rarely removed, and abdominal computed tomography (CT) is rarely performed to avoid radiation exposure. This leaves little opportunity for clinical evaluation of implanted PGA spacers and necessitates animal studies. In the present study, rats were selected for two reasons: they possess sufficient space in the abdominal cavity to implant the PGA spacer and for ease of experimentation.
Cyclophosphamide (CPA) is an alkylating agent that inhibits the synthesis of nucleic acids in cells and is used in many cancer types, including Ewing’s sarcoma, rhabdomyosarcoma, multiple myeloma, malignant lymphoma and leukemia. It can be taken orally. CPA was chosen for chemotherapy because of its ease of administration in experimental animals and its common clinical use in pediatric malignancies. The main side effects are myelosuppression and hemorrhagic cystitis, with the former being used to determine the optimal dosage in this study [16, 17]. Therefore, this study aimed to investigate the effects of CPA on the PGA spacer in a rat model.
MATERIALS AND METHODS
Rats
This experiment was conducted after deliberation and approval by the Nagoya City University Animal Experiment Committee (Approval No. 20–039) and was conducted in compliance with the Code of Ethics. The experimental design necessitated considerations for the ease of blood collection, drug administration and the capacity of the abdominal cavity to accommodate the spacer. We utilized 24 male rats (Sprague–Dawley; closed colony, aged nine weeks). Three rats only with CPA and three controls were prepared in addition to the 24 rats with implanted spacers to determine the effect of CPA administration on the rats. A week-long acclimatization period to the laboratory environment was conducted prior to the study to minimize stress-related variables. The rats were housed under controlled 12-hour light/dark cycle conditions, with unrestricted access to food and water. Continuous monitoring of their health status and behavior was performed to ensure their welfare and to identify any signs of distress or illness. To maintain research standards as well as ethical considerations, humane euthanasia was performed on six rats every four weeks using carbon dioxide asphyxiation. This method was chosen to mitigate suffering and was performed within each housing cage to reduce stress among the cohort.
PGA spacer
The PGA spacer (NESKEEP®, Alfresa Pharma Corporation, Osaka, Japan) was designed to create a gap between normal organs and tumors, thereby facilitating increased dosage delivery to the cancer during radiotherapy and particle therapy. It is a 5-mm-thick non-woven fabric featuring numerous cavities. To remove internal air, the PGA spacer was previously injected with saline solution into its interior with a syringe. Subsequently, it was cut into 3-cm squares using a scalpel or surgical scissors. The prepared PGA spacer was meticulously wrapped on all sides with Seplafilm® (Kaken Pharmaceutical Co., Ltd., Japan) [18], a bioresorbable membrane comprising sodium hyaluronate and carboxymethyl cellulose, to prevent adhesion between the spacer and surrounding organs. This procedure was performed on all spacers.
The volume of the PGA spacer per body weight used in the rats was 7.5–9.0 cm3/kg, which is comparable to implanting a 500–600 cm3 spacer in a 70 kg adult and is considered to be clinically relevant volume.
Cyclophosphamide administration
In this experiment, 24 rats were randomly and equally divided into two groups: a CPA-dosed group and a control group. CPA was administered orally to the CPA-dosed group using a catheter. This method was chosen for its dosage accuracy and minimal stress imposition on the animals. Doses (40 mg/kg) were administered once every 4 weeks for 16 weeks. This dose was chosen to ensure the safety of the rats and to prevent acute side effects. This CPA dose is the same dose given to pediatric patients with malignant tumors [19]. Furthermore, this dose was also validated in this experiment.
Blood sample
Blood samples were drawn from the tail artery of sedated rats. They were collected before each dose and at 0, 3-, 7-, 14-, 21- and 28-days post-dose in each cycle. The primary aim of these blood analyses was to monitor signs of pancytopenia, focusing on white blood cell counts (neutrophils), hemoglobin levels and platelet counts.
Surgery
The PGA spacer is typically handled in a sterile environment, such as an operating room; however, in this study, the constraints of the animal experimental setting necessitated the creation of a clean area, and the operation was performed within a quasi-sterile environment. For sedation, we administered inhalation anesthesia using isoflurane, which is known for its efficacy and safety in small rodents [20, 21]. A 6–7 cm incision was made in the midline of the rat’s abdomen, and the PGA spacer was placed between the intestinal tract and the abdominal wall, with several sutures (blue nylon) securing it to the abdominal wall.
CT
CT was conducted using the Optima CT 580 (GE Healthcare, Japan). Imaging parameters were standardized with an X-ray tube voltage of 120 kV, gantry rotation time of 0.5 seconds, field of view of 10 × 10 cm, slice thickness of 0.625 mm, pixel size of 0.02 cm, and a matrix size of 512 × 512 pixels. These settings optimized image quality and consistency for precise assessments of the PGA spacers. Owing to our facility’s limitations, continuous weekly CT and temporal monitoring of the PGA spacers in living rats were not feasible. To ensure hygienic conditions during the CT procedure, euthanized rats were meticulously prepared to prevent contamination of the CT scanner and the environment. CT was performed within 2 hours post-euthanasia to assess in vivo changes effectively. The rats underwent CT at 4, 8, 12 and 16 weeks to evaluate the dimensional changes of the PGA spacer over time.
Autopsy
All rats were dissected within 3 hours after CT. The abdominal cavity was opened via an incision, exposing the PGA spacer for initial observation. Observations were focused on assessing the extent of adhesions on the spacer’s external surface; thereafter, its internal structure was examined through pathological analysis. A modified version of the adhesion scoring system, typically employed during surgical evaluations (Table 1), was utilized to assess the extent of dissection and adhesion of the extracted spacer [22]. The evaluation was performed by two people: the person who performed the dissection and an assistant dissector. In addition, photographs and other digital data were taken and acquired from two directions (back and front) to allow for reconfirmation and review of the evaluation. PGA spacers that were too small for accurate recognition and evaluation through CT or visual inspection were assigned a negative (−) rating. For spacers rated negatively, the presence of the blue nylon suture, used to secure the spacer to the peritoneum, served as an important marker for locating the reduced PGA spacer.
Table 1.
Adhesion score system for PGA spacer
| Score | Description of score: separability, adhesion surface area (%) |
|---|---|
| 0 | No adhesion |
| 1 | Partially filmy adhesion: easy to separate with gentle traction, 1%–25% |
| 2 | Partially thick adhesion: separated with moderate traction, 26%–50% |
| 3 | Dense adhesion: not separate, 51%–75% |
| 4 | Very dense adhesion: not separate, 76%–100% |
| ― | Obvious reduction, fibrous adhesions |
Pathology
Pathology specimens were fixed in formalin with the PGA spacer and adherent surrounding tissues (intestinal tract, peritoneum, liver, etc.), and hematoxylin–eosin staining was used to evaluate cell and tissue responses to the spacer. These specimens were prepared by the Pathology Department staff as well-preserved tissue samples to reproduce in vivo conditions accurately. Pathology experts performed histological evaluations and shared information on all pathology specimens. Particular attention was paid to identifying macrophages, inflammatory responses and histological changes.
Image-based measurement
The assessment focused on the spacer’s length, width, thickness and volume. Following imaging, the CT data were imported into RayStation® (Raysearch Laboratories, Sweden), a radiation therapy planning system, to confirm the spacer’s position and interaction with adjacent tissues, perform contouring, and measure the spacer’s size and volume. To ensure uniformity, one of the study members, a radiation oncologist, performed all spacer contouring and size measurements.
Statistical analysis
Blood cell counts before and after CPA administration for each rat, as well as spacer size every 4 weeks were recorded and analyzed separately for the CPA-dosed and control groups. To eliminate individual differences, at least three sets of data at each time point were used. Given the data followed a non-parametric distribution, the Kruskal–Wallis test was chosen to compare variables between the defined groups. Statistical significance was set at P < 0.05. Data were analyzed using SPSS version 28 (IMB corp., Armonk, NY).
RESULTS
Assessment of cyclophosphamide dosage
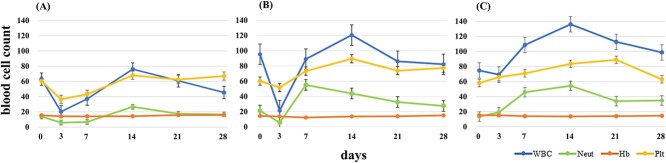
Neutrophil counts in the CPA-dosed group were lower than those in the control group on day three after dosing. The decrease in neutrophils from day 0 to day 3 with CPA was 19% and 53%, respectively (Fig. 2A and B), while without CPA, the increase was 45% (Fig. 2C) (P = 0.01).
Fig. 2.
Results of blood sampling in rats and controls following CPA administration. (A) CPA-dosed group without spacer surgery; (B) CPA-dosed group with PGA spacer surgery; (C) control group with PGA spacer surgery. Blood tests were performed immediately before and on day 3 and weeks 1, 2, 3 and 4 after the experiment began. The measurements include white blood cells (blue; ×100/mL), neutrophils (green; ×100/mL), hemoglobin (orange; g/dL), and platelets (yellow; ×103/mL). The CPA-dosed group shows a decrease in neutrophils on the third day compared with the control group. CPA, cyclophosphamide; PGA, polyglycolic acid.
Comparative analysis of PGA spacer reduction on CT
Figure 3A illustrates the representative CT images of the control group. After 4 weeks of PGA spacer implantation, its morphology remained unchanged and appeared as a highly absorbent object on CT. After 8 weeks, it became thicker and slightly rounded, with reduced absorbency; by 12 weeks, it had become spherical with visible shrinkage; and by 16 weeks, the shrinkage was so pronounced that the spacer was no longer recognizable and had nearly vanished.
Fig. 3.
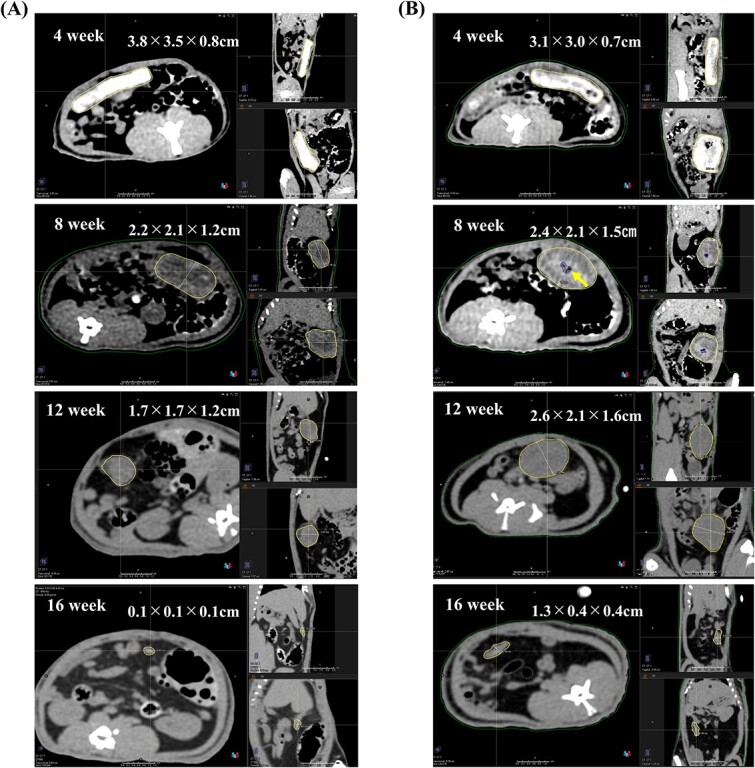
Comparison of the two groups by CT. (A) CT of the control group, taken at four-week intervals, showed the PGA spacer within the rat’s abdominal cavity. Over time, the spacer decreases in size, with noticeable morphological changes between weeks 4 and 8, including an increase in thickness and a more spherical shape. (B) CT of the CPA-dosed group, taken at four-week intervals, demonstrates a similar pattern of size reduction in the spacer. Notably, a 0.05-cm3 pocket of air (arrow) is observed within the spacer at week 8. CT, computed tomography; PGA, polyglycolic acid; CPA, cyclophosphamide.
Figure 3B displays the CT images of the CPA-dosed group. The PGA spacer’s shrinkage pattern was similar to that observed in the control group, with air pockets visible within the spacer at week 8.
Figure 4 presents a graph depicting the size changes of the PGA spacer in both groups. The difference in size parameters between the two groups appeared to be more pronounced from 12 to 16 weeks; however, there was no statistically significant difference in spacer thickness between the two groups (P = 0.12).
Fig. 4.
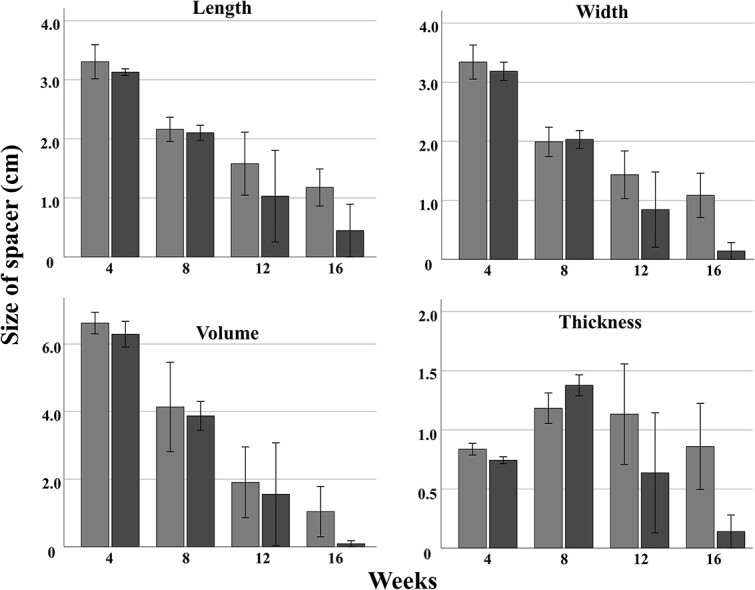
Size change of PGA spacer in CPA-dosed and control groups. Gray: Control group with PGA spacer surgery; black: CPA-dosed group with PGA spacer surgery. Data are presented as mean measurements from three rats per group, showing no significant difference in size reduction between the groups (P > 0.12). PGA, polyglycolic acid; CPA, cyclophosphamide.
Evaluation of adhesions
Small bands of remnants were observed on some of the spacers examined between 12 and 16 weeks, showing a decrease. In these instances, adhesions received a negative (−) rating. A trend toward fewer adhesion scores was observed in the CPA-dosed group relative to the control group (Fig. 5), although this difference was not statistically significant (P = 0.21).
Fig. 5.
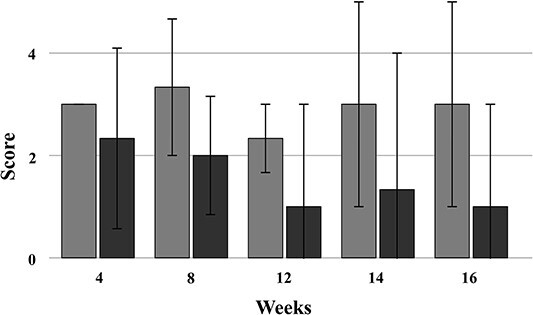
Results of PGA spacer adhesion evaluation in CPA-dosed and control groups. Gray: Control group with PGA spacer surgery; black: CPA-dosed group with PGA spacer surgery. The figure shows the average adhesion score for spacers from both groups, obtained at four-week intervals, based on assessments from three rats in each group. PGA, polyglycolic acid; CPA, cyclophosphamide.
Pathological analysis of microscopic changes in PGA spacers
The pathological examination elucidated the microscopic alterations occurring within the PGA spacer at different time points. Histological analyses revealed that macrophages enveloped the PGA fibers within the spacer (Fig. 6). Notably, marked fibrotic changes were observed on the spacer’s surface, alongside adhesions to adjacent organs, including the peritoneum, the liver and muscle tissues. While inflammatory cells, such as neutrophils, were identified, there was no evidence of acute or chronic inflammation.
Fig. 6.
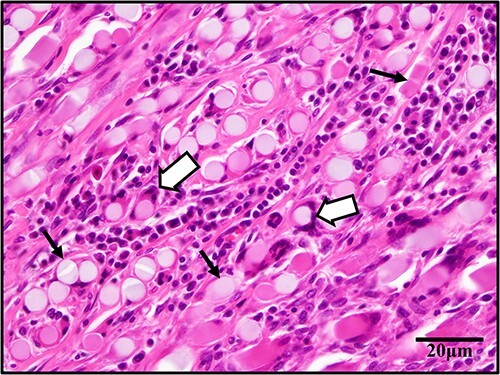
Tissue specimen (hematoxylin–eosin stain) around the PGA spacer. The image displays PGA fibers (black arrows) contained within a circular or rectangular structure with a relatively homogeneous interior. These are surrounded by macrophages and multinucleated cells (white arrows) at 400× magnification. PGA, polyglycolic acid.
DISCUSSION
The PGA spacer is intended for implantation in the human body; therefore, ecological compatibility, actual contraction processes and behavior should be evaluated during chemotherapy. Although, in some cases, PGA spacers have been used clinically in combination with chemotherapy, existing studies have shown that in vivo evaluation using chemotherapy has not been adequately performed [10, 11, 14]. In the present study, using a rat model, we compared the CPA-dosed group with a control group and analyzed morphological changes in the PGA spacer over a 16-week period using direct observations of abdominal CT and extracted spacers. This provided a unique perspective for evaluating changes in the PGA spacer in the combined chemotherapy setting.
Although CPA was selected for chemotherapy because of its versatility in the treatment of pediatric tumors, the optimal CPA dose for rats has not been determined in existing experiments. Considering myelosuppression was observed on day three of CPA administration to the rats in the current study at a dose of 40 mg/kg based on pediatric malignancy dosing guidelines [19], it was determined that an appropriate pharmacological effect could be achieved in rats.
Similar changes in spacer dimensions, such as length, width, thickness and volume, were observed with and without CPA administration, suggesting that CPA does not influence PGA spacer shrinkage. Given the long duration of radiation and particle therapy in pediatric malignancies, ensuring that spacers do not degrade prematurely is critical. A characteristic change was observed both in the CPA-dosed group and the control group; specifically, the thickness of the PGA spacer transiently increased during degradation in the rat’s body. This change may be due to the smaller size of the implanted spacer compared to that used in humans. The PGA spacer used in the current experiment was the same as that used in clinical practice, suggesting there may be periods of temporary increases or decreases in spacer thickness in spacers implanted in humans.
Contrary to our initial hypothesis that CPA administration may increase susceptibility to infection and adhesion formation, we observed a trend toward fewer adhesions in the CPA-dosed group compared to the control group. Although this difference was not statistically significant, it is possible that macrophages and other factors found in the surrounding area may be involved.
The results of the experiments suggest that hydrolysis plays a notable role in the degradation of the PGA spacer. However, the presence of macrophages around the PGA fiber also indicates that macrophages may contribute to the degradation process of the spacer, possibly as part of the body’s response to the PGA fiber [23]. Based on pathological images, the absence of inflammatory reactions indicated no incompatibility reactions to the surroundings, including adherent organs, further emphasizing the biocompatibility of PGA. Additionally, macrophages are assumed to be involved in the regulation of fibrosis [24]. This supports the safety profile of PGA spacers, although further detailed pathological studies and validation are necessary to consolidate these findings.
There were several limitations and constraints to the present study, the most significant being facility limitations, such as the inability to perform CT on live rats. Given it is desirable to evaluate the same spacer using CT over time to eliminate individual differences, consideration should be given to coordinating other facilities for additional experiments. Another limitation is that chemotherapy was limited to CPA. Other anticancer drugs may affect the PGA spacer through different mechanisms. The current results may not apply to other chemotherapy regimens, and thus, further extensive evaluation is needed. Moreover, additional in vivo longitudinal studies in rats are needed to address the above limitations and confirm the clinical efficacy and safety of the PGA spacer. In addition, an ongoing Phase I study (UMIN 000039288) is expected to provide further insight and potentially expand the use of PGA spacers in cancer treatment protocols.
This study provides crucial insight into the impact of CPA chemotherapy on the morphological and histological characteristics of PGA spacers in a pediatric abdominal tumor model. Our results show that CPA administration does not significantly influence the degradation process of the PGA spacer nor cause adverse tissue reactions. These outcomes highlight the efficacy of the PGA spacer and confirm its compatibility with CPA chemotherapy and radiation therapy. Future research should expand on this investigation by further comparing outcomes between CPA-dosed and non-dosed groups in rats.
PRESENTATION AT A CONFERENCE
Presentation at the 35th annual meeting of the Japanese Society for Radiation Oncology, Hiroshima, 21–24 October 2022.
ACKNOWLEDGEMENTS
We sincerely thank the Alfresa Pharma Corporation for providing the PGA spacers essential for our research. Their generous contributions were instrumental in achieving the outcomes of this study. We would like to thank Editage (www.editage.jp) for English language editing.
Contributor Information
Yusuke Tsuzuki, Department of Radiation Oncology, Nagoya Proton Therapy Center, Nagoya City University West Medical Center, 1-1-1 Hirate-cho, Kita-ku, Nagoya 462-8508, Japan; Department of Radiology, Nagoya City University Graduate School of Medical Sciences, 1 Kawasumi, Mizuho-cho, Mizuho-ku, Nagoya 467-8601, Japan.
Michi Kamei, Department of Pediatrics and Neonatology, Nagoya City University Graduate School of Medical Sciences, 1 Kawasumi, Mizuho-cho, Mizuho-ku, Nagoya 467-8601, Japan.
Hiromitsu Iwata, Department of Radiation Oncology, Nagoya Proton Therapy Center, Nagoya City University West Medical Center, 1-1-1 Hirate-cho, Kita-ku, Nagoya 462-8508, Japan.
Risa Takeda, Department of Pediatrics and Neonatology, Nagoya City University Graduate School of Medical Sciences, 1 Kawasumi, Mizuho-cho, Mizuho-ku, Nagoya 467-8601, Japan.
Hiroaki Kimura, Department of Orthopedic Surgery, Nagoya City University Graduate School of Medical Sciences, 1 Kawasumi, Mizuho-cho, Mizuho-ku, Nagoya 467-8601, Japan.
Hisaki Aiba, Department of Orthopedic Surgery, Nagoya City University Graduate School of Medical Sciences, 1 Kawasumi, Mizuho-cho, Mizuho-ku, Nagoya 467-8601, Japan.
Takayuki Murase, Department of Pathology and Molecular Diagnostics, Nagoya City University Graduate School of Medical Sciences, 1 Kawasumi, Mizuho-cho, Mizuho-ku, Nagoya 467-8601, Japan.
Takahiro Tsuchiya, Central Department of Radiology, Nagoya City University Hospital, 1 Kawasumi, Mizuho-cho, Mizuho-ku, Nagoya 467-8602, Japan.
Ryohei Sasaki, Division of Radiation Oncology, Kobe University Graduate School of Medicine, 7-5-2 Kusunoki-cho, Chuo-ku, Kobe 650-0017, Japan.
Akio Hiwatashi, Department of Radiology, Nagoya City University Graduate School of Medical Sciences, 1 Kawasumi, Mizuho-cho, Mizuho-ku, Nagoya 467-8601, Japan.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
FUNDING
This work was supported by the Japan Society for the Promotion of Science, KAKENHI [grant numbers 21 K07682 and 21 K07758].
REFERENCES
- 1. Matsumoto Y, Fukumitsu N, Ishikawa H et al. A critical review of radiation therapy: from particle beam therapy (proton, carbon, and bnct) to beyond. J Pers Med 2021;11:825. 10.3390/jpm11080825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Baskar R, Lee KA, Yeo R, Yeoh KW. Cancer and radiation therapy: current advances and future directions. Int J Med Sci 2012;9:193–9. 10.7150/ijms.3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dörr W, Herrmann T, Baumann M. Application of organ tolerance dose-constraints in clinical studies in radiation oncology. Strahlenther Onkol 2014;190:621–7. 10.1007/s00066-014-0613-5. [DOI] [PubMed] [Google Scholar]
- 4. Noël G, Antoni D. Organs at risk radiation dose constraints. Cancer Radiother 2022;26:59–75. 10.1016/j.canrad.2021.11.001. [DOI] [PubMed] [Google Scholar]
- 5. Harvey M, Ong WL, Chao M et al. Comprehensive review of the use of hydrogel spacers prior to radiation therapy for prostate cancer. BJU Int 2023;131:280–7. 10.1111/bju.15821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sakuramachi M, Murakami N, Nagao A et al. Hydrogel spacer injection to the meso-sigmoid to protect the sigmoid colon in cervical cancer brachytherapy: a technical report. J Contemp Brachytherapy 2023;15:465–9. 10.5114/jcb.2023.134174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fukumoto T, Komatsu S, Hori Y et al. Particle beam radiotherapy with a surgical spacer placement for advanced abdominal leiomyosarcoma results in a significant clinical benefit. J Surg Oncol 2010;101:97–9. 10.1002/jso.21417. [DOI] [PubMed] [Google Scholar]
- 8. Ogino T, Sekimoto M, Nishimura J et al. Intraluminal migration of a spacer with small bowel obstruction: a case report of rare complication. World J Surg Onc 2012;10:30. 10.1186/1477-7819-10-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Komatsu S, Terashima K, Matsuo Y et al. Validation of combination treatment with surgical spacer placement and subsequent particle radiotherapy for unresectable hepatocellular carcinoma. JSurgOncol 2019;120:214–22. 10.1002/jso.25495. [DOI] [PubMed] [Google Scholar]
- 10. Sasaki R, Demizu Y, Yamashita T et al. First-in-human phase 1 study of a nonwoven fabric bioabsorbable spacer for particle therapy: space-making particle therapy (SMPT). Adv Radiat Oncol 2019;4:729–37. 10.1016/j.adro.2019.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Akasaka H, Sasaki R, Miyawaki D et al. Preclinical evaluation of bioabsorbable polyglycolic acid spacer for particle therapy. Int J Radiat Oncol Biol Phys 2014;90:1177–85. 10.1016/j.ijrobp.2014.07.048. [DOI] [PubMed] [Google Scholar]
- 12. Shiba S, Okamoto M, Sakai M, Ohno T. Visualizing bioabsorbable spacer effectiveness by confirming the distal-tail of carbon-ion beams: first-in-human report. Tomography 2022;8:2339–46. 10.3390/tomography8050195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Serizawa I, Kusano Y, Kano K et al. Three cases of retroperitoneal sarcoma in which bioabsorbable spacers (bioabsorbable polyglycolic acid spacers) were inserted prior to carbon ion radiotherapy. J Radiat Res 2022;63:296–302. 10.1093/jrr/rrac002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kimura M, Asai K, Iwata H et al. Impact on dose distribution and volume changes of a bioabsorbable polyglycolic acid spacer during chemo-proton therapy for a pediatric Ewing sarcoma. J Radiat Res 2020;61:952–8. 10.1093/jrr/rraa087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cotterill SJ, Ahrens S, Paulussen M et al. Prognostic factors in Ewing’s tumor of bone: analysis of 975 patients from the European intergroup cooperative Ewing’s sarcoma study group. J Clin Oncol 2000;18:3108–14. 10.1200/JCO.2000.18.17.3108. [DOI] [PubMed] [Google Scholar]
- 16. Dalia OS, Abo El-Nasr NME, Fayez AM et al. Uro-protective role of chrysin against cyclophosphamide-induced hemorrhagic cystitis in rats involving the turning-off NF-κB/P38-MAPK, NO/PARP-1, and STAT-3 signaling cascades. Chem Biol Interact 2023;382:110585. 10.1016/j.cbi.2023.110585. [DOI] [PubMed] [Google Scholar]
- 17. Cash T, Jonus HC, Tsvetkova M et al. A phase 1 study of simvastatin in combination with topotecan and cyclophosphamide in pediatric patients with relapsed and/or refractory solid and CNS tumors. Pediatr Blood Cancer 2023;70:1–10. 10.1002/pbc.30405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Okubo S, Shindoh J, Kobayashi Y, Hashimoto M. Safety of bioabsorbable membrane (Seprafilim®) in hepatectomy in the era of aggressive liver surgery. HPB 2021;23:528–32. 10.1016/j.hpb.2020.08.008. [DOI] [PubMed] [Google Scholar]
- 19. McCowage GB, Mrongovius R, Alvaro F et al. Treatment of children with poor risk solid tumors by further escalation of the VETOPEC regimen including very high-dose cyclophosphamide and peripheral stem cell support: an Australian and New Zealand Children's Hematology and oncology group study. Pediatr Blood Cancer 2011;57:958–64. 10.1002/pbc.23042. [DOI] [PubMed] [Google Scholar]
- 20. Diven K. Inhalation anesthetics in rodents. Lab Animal 2003;32:44–7. 10.1038/laban0303-44. [DOI] [PubMed] [Google Scholar]
- 21. Ahmadi-Noorbakhsh S, Farajli Abbasi M, Ghasemi M et al. Anesthesia and analgesia for common research models of adult mice. Lab Animal Research 2022;38:40. 10.1186/s42826-022-00150-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kang H, Chung YS, Kim SW et al. Effect of temperature-sensitive poloxamer solution/gel material on pericardial adhesion prevention: supine rabbit model study mimicking cardiac surgery. PLoS One 2015;10:e0143359. 10.1371/journal.pone.0143359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhang J, Xie B, Xi Z et al. A comparable study of polyglycolic acid’s degradation on macrophages’ activation. Mater Sci 2020;109:110574. 10.1016/j.msec.2019.110574. [DOI] [PubMed] [Google Scholar]
- 24. Wynn TA, Barron L. Macrophages: master regulators of inflammation and fibrosis. Semin Liver Dis 2010;30:245–57. 10.1055/s-0030-1255354. [DOI] [PMC free article] [PubMed] [Google Scholar]