Twenty years ago, Wald and Law1 hypothesised that, if a combination pill could be made including aspirin, folic acid, a statin, and a low-dose diuretic, beta blocker and angiotensin-converting enzyme (ACE) inhibitor (thus, allowing for the simultaneous modification of four different risk factors: low-density lipoprotein [LDL]-cholesterol, blood pressure, homocysteine, and platelet function), and administered to everyone with existing cardiovascular disease and everyone over 55 years old, there would be an 88% reduction in ischaemic heart disease events, and an 80% reduction in stroke. One third of people over the age of 55 years would benefit by gaining an average of 11 years free from a cardiac event or stroke (subsequently termed the vaccination approach). They called this pill the ‘Polypill’, and concluded that treatment would be acceptably safe and, with widespread use, would have a greater impact on the prevention of disease in the Western world than any other single intervention. They noted that, while such a preventative strategy was radical, if such a formulation existed that prevented cancer and was safe, it would be widely used. “It is time to discard the view that risk factors need to be measured and treated individually if found to be abnormal. There is much to gain and little to lose by the widespread use of these drugs.” While subsequent works have shown that folic acid is not prognostically beneficial in preventing cardiovascular disease,2 and that aspirin may not be beneficial overall in primary prevention settings,3 the concept of the combination pill was awakened in the public eye.
“The prescribed number of doses per day is inversely related to compliance.” 4
Medicines usually work when they are taken, but definitely don’t when they are not!
Adherence to medication has been widely identified as a risk factor in the development of, or the recurrence of, cardiovascular disease, good adherence being associated with positive health outcomes, and poor adherence increasing the likelihood of suffering a recurrent cardiovascular event. Naderi et al.,5 in a meta-analysis of nearly 400,000 patients receiving preventative treatment, reported that compliance with medication was about 57% after two years in secondary prevention. In developed countries, approximately 50% of patients with chronic disease do not adhere to treatment.6,7 It is reported that 45% of patients with hypertension, and 84% of those with uncontrolled hypertension, are not adherent to their antihypertensive regimen.8 Usherwood points out that non-adherence is a major reason why treatments shown to be effective in trials are often less so in clinical practice,9 and, of course, if we as physicians in our clinical practice are assuming adherence and, thus, increasing doses or number of medications for a perceived failure of control, or suboptimal response, we run the risk of dangerously escalating treatment, and it would seem important to enquire about adherence as part of any consultation, especially if there is an apparently suboptimal response to any previously chosen treatment.
Of the many influences impacting non-adherence to medications (box 1), the complexity of treatment and the daily number of prescribed pills are considered to play a significant role.10,11 Figure 1A is a typical sample prescription in a 70-year-old hypertensive man who is entirely well one year post-myocardial infarction. So what issues are likely to potentially impact his compliance. Well, assuming that he is not depressed or cognitively impaired (both negatively impact adherence to medication),12,13 there are the influences of having to take seven medications daily while feeling entirely well, and remembering to take at least one of them each evening; to order and collect medications each month at the chemist, and pay for them; to go to the doctor to get a new prescription on a regular basis, and pay for it, and he has to do this for the rest of his life. We as physicians must be engaged and convincing when we justify our prescribing; we should be mindful of cost, and should if possible be prescribing long-acting (once-a-day) medications with proven prognostic benefits. But we should also be ‘prescribing cleverly’ to reduce the complexity of the patient’s treatment, and the number of different tablets prescribed.
Box 1. Some reasons for non-adherence in patients.
|
Figure 1A. Sample prescription in post-myocardial infarction (MI) patient.
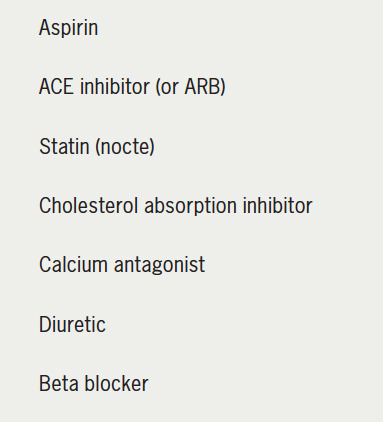
Key: ACE = angiotensin-converting enzyme; ARB = angiotensin-receptor blocker
“Patients more likely to take their medications if they normally took it in the morning than if they normally took it in the evening.” 14
Are we making an effort to prescribe cleverly?
In terms of clever prescribing, the answer appears to be NO. At the time of writing, significantly less than 15% of cardiovascular prescriptions in Ireland are combination treatments, despite having a plethora of useful agents (table 1) and despite knowing that reducing the complexity of treatment and reducing pill count increases compliance. Castellano et al. recently, in reporting the results of the SECURE trial (polypill [aspirin, ACE inhibitor and statin] versus usual care) confirmed that, in older patients following myocardial infarction, there was greater adherence to treatment in the polypill group, and a significantly lower risk of major adverse events than with usual care.15 Memon et al. reported that, in a meta-analysis of six randomised-controlled trials (13,139 patients), medication adherence was significantly higher in patients receiving a polypill (of three or four agents) compared with the control group, and the risk of cardiovascular events was significantly lower in the polypill group.16 We also know that using combination treatment at low dose (thus, reducing risk of side effects) is more effective than doubling the dose of a single agent.17 Yusuf and Pinto concluded, in their Lancet editorial last year, that the evidence for the benefits of the Polypill was substantial and should be used widely to save millions of lives each year in primary and secondary care.18
Table 1. Licenced and unlicenced drugs in Ireland.
|
Licenced |
Not licenced |
|---|---|
|
Aspirin/ACEi/statin |
Aspirin/beta blocker/ACEi/statin |
|
Statin/calcium antagonist/ACEi |
Aspirin/diuretic/ACEi/statin |
|
Calcium antagonist/ARB/diuretic |
Beta blocker/diuretic/statin ± aspirin |
|
Calcium antagonist/ACEi/diuretic |
Aspirin/ARB/beta blocker/statin |
|
Calcium antagonist/ACEi |
Calcium antagonist/ARB/diuretic/statin |
|
Calcium antagonist/ARB |
Aspirin/ACE or ARB/diuretic/statin |
|
Statin/cholesterol absorption inhibitor |
|
|
Multiple two-med combinations |
Key: ACEi = angiotensin-converting enzyme inhibitor; ARB = angiotensin-receptor blocker
Conclusion
Much evidence exists for a very significant lack of adherence to treatment, particularly in chronic disease, and especially if the patient has a complex drug regimen and high pill count. Evidence points to a significant benefit, both for secondary prevention and in high-risk primary prevention groups, from the use of combination agents. So, what are we going to do about it as prescribing physicians? If we go back to our 70-year-old man who has come to the outpatient department complaining that, while he is feeling good, he is taking far too many tablets, we should not reassure him that all is well and advise him to “keep taking the tablets”! We could attempt to simplify his treatment, for instance, by combining his aspirin, ACE inhibitor and statin as a single agent, or his calcium antagonist, ACE inhibitor (or angiotensin-receptor blocker [ARB]) and diuretic as a single agent, also combining the statin and cholesterol absorption inhibitor as a single agent (figure 1B). Medications unlicenced in the UK and Ireland include agents with four components of the following treatment options: statin, beta blocker, diuretic, ACE inhibitor or ARB and aspirin (table 1), and hopefully, in time, a steady stream of new combination therapies, if utilised, will lead to significantly improved adherence while also leading to an improved outcome for at-risk groups. But, in the meantime, there are more than enough opportunities for us to ease the (pill) burden of our patients with the agents that are already available to us. It just needs a little extra time and a relentless focus on the significant problem of non-adherence.
Figure 1B. Sample prescription in post-MI patient.
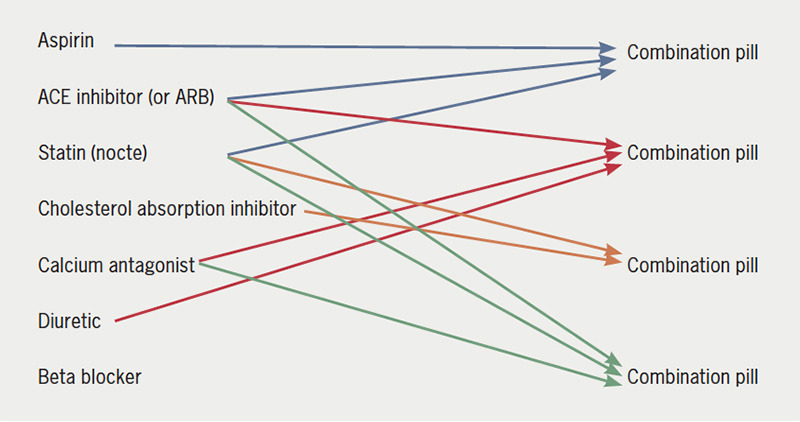
Key: ACE = angiotensin-converting enzyme; ARB = angiotensin-receptor blocker
Funding Statement
Funding None.
Footnotes
Conflicts of interest
None declared.
Contributor Information
David Mulcahy, Consultant Cardiologist Blackrock Health, Hermitage Clinic, Old Lucan Road, Dublin, D20 W722, Ireland.
Palwasha Khan, Medical Intern St Luke’s Hospital Kilkenny, Freshford Road, James Park, Kilkenny, R95 FY71, Ireland.
References
- 1.Wald NJ, Law MR. A strategy to reduce heart disease by more than 80%. BMJ. 2003;326:1419–24. doi: 10.1136/bmj.326.7404.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clarke R, Halsey J, Lewington S, et al. for the B-vitamin Treatment Trialists’ Collaboration. Effects of lowering homocysteine levels with B vitamins on cardiovascular disease, cancer, and cause-specific mortality: meta-analysis of 8 randomized trials involving 37,485 individuals. Arch Int Med. 2010;170:1622–31. doi: 10.1001/archinternmed.2010.348. [DOI] [PubMed] [Google Scholar]
- 3.Baigent C, Blackwell L, Collins R, et al. Antithrombotic Trialists’ (ATT) Collaboration. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet. 2009;373:1849–60. doi: 10.1016/S0140-6736(09)60503-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Claxton AJ, Cramer J, Pierce C. A systematic review of the associations between drug regimens and medication compliance. Clin Ther. 2001;23:1296–310. doi: 10.1016/S0149-2918(01)80109-0. [DOI] [PubMed] [Google Scholar]
- 5.Naderi SH, Bestwick JP, Wald DS. Adherence to drugs that prevent cardiovascular disease: meta-analysis on 376,162 patients. Am J Med. 2012;125:882–7. doi: 10.1016/j.amjmed.2011.12.013. [DOI] [PubMed] [Google Scholar]
- 6.Chowdhury R, Khan H, Heydon E, et al. Adherence to cardiovascular therapy: a meta-analysis of prevalence and clinical consequences. Eur Heart J. 2013;34:2940–8. doi: 10.1093/eurheartj/eht295. [DOI] [PubMed] [Google Scholar]
- 7.Vrijens B, Vincze G, Kristanto P, et al. Adherence to prescribed antihypertensive drug treatments: longitudinal study of electronically compiled dosing histories. BMJ. 2008;336:1114–17. doi: 10.1136/bmj.39553.670231.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abegaz TM, Shehab A, Gebreyohannes EA, et al. Nonadherence to antihypertensive drugs: a systematic review. Medicine (Baltimore) 2017;96:e5641. doi: 10.1097/MD.0000000000005641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Usherwood T. Encouraging adherence to long-term medication. Aust Prescr. 2017;40:147–50. doi: 10.18773/austprescr.2017.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Valmeire E, Hearnshaw H, Van Royen P, Denekens J. Patient adherence to treatment: three decades of research. A comprehensive review. J Clin Pharm Ther. 2001;26:331–42. doi: 10.1046/j.1365-2710.2001.00363.x. [DOI] [PubMed] [Google Scholar]
- 11.Caldeira D, Vaz-Carneiro A, Costa J. The impact of dosage frequency on medical adherence in chronic cardiovascular disease: systematic review and meta-analysis. Rev Port Cardiol. 2014;33:431–7. doi: 10.1016/j.repc.2014.01.013. [DOI] [PubMed] [Google Scholar]
- 12.Gehi A, Haas D, Pipkin S, Whooley MA. Depression and medication adherence in outpatients with coronary heart disease: findings from the Heart and Soul study. Arch Intern Med. 2005;165:2508–13. doi: 10.1001/archinte.165.21.2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim SJ, Kwon OD, Han EB, et al. Impact of number of medications and age on adherence to antihypertensive medications: a nationwide population-based study. Medicine (Baltimore) 2019;98:e17825. doi: 10.1097/MD.0000000000017825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Erdine S. Compliance with the treatment of hypertension: the potential of combination therapy. J Clin Hypertension. 2010;12:40–6. doi: 10.1111/j.1751-7176.2009.00200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Castellano JM, Pocock SJ, Bhatt DL, et al. Polypill strategy in secondary cardiovascular prevention. N Engl J Med. 2022;387:967–77. doi: 10.1056/NEJMoa2208275. [DOI] [PubMed] [Google Scholar]
- 16.Memon RA, Bai BR, Simran FNU, et al. Effect of the Polypill on adherence and prevention of cardiovascular diseases in patients with or at high risk of cardiovascular diseases: a meta-analysis of randomized controlled trials. Cureus. 2023;15:e34134. doi: 10.7759/cureus.34134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wald DS, Law M, Morris JK, Bestwick JP, Wald NJ. Combination therapy versus monotherapy in reducing blood pressure: meta-analysis on 11,000 participants from 42 trials. Am J Med. 2009;122:290–300. doi: 10.1016/j.amjmed.2008.09.038. [DOI] [PubMed] [Google Scholar]
- 18.Yusuf S, Pinto FJ. The polypill: from concept and evidence to implementation. Lancet. 2022;400:161–3. doi: 10.1016/S0140-6736(22)01847-5. [DOI] [PubMed] [Google Scholar]


