Abstract
Delirium in critical ill patients is a complex and common neurological syndrome in the intensive care unit (ICU) that is caused by a range of structural or functional abnormalities. ICU Delirium is associated with reduced compliance, prolonged hospital stays, greater use or delayed withdrawal of sedatives, higher rates and durations of mechanical ventilation, and higher rates of mortality. The aetiology and pathogenesis of ICU delirium are unclear, and the lack of better prediction, prevention, and treatment measures leads to a non-standardized control of delirium. By searching the relevant literature, we aim in this narrative review to describe progress in the pathogenesis, predictive biomarkers, diagnosis, and treatment of ICU delirium.
Keywords: Delirium, intensive care unit, biomarkers, diagnosis, treatment
1. Foreword
Delirium is a common acute disturbance of mental status, with an incidence rate of 20–50% in the intensive care unit (ICU) [1] and up to 80% in patients on mechanical ventilation [2]. Once delirium occurs, patient compliance is severely compromised. Severity of illness, prolonged sedation and mechanical ventilation are independent risk factors associated with in-hospital mortality in patients with delirium [2,3]. Although local recommendations and international guidelines have been published [4,5], they are often not followed [6,7]. A worldwide online survey disclosed that although respondents acknowledged the need for delirium monitoring, more than 58% of them did not use specific tools to monitor delirium [7]. For a long time, insufficient attention has been given to the normative diagnosis and management of delirium in the ICU, in both adult and paediatric patients [6–8]. Second, the tools used for diagnosis and treatment evaluation differ between countries, regions, hospitals, and even departments [9–11]. In recent years, with the continued development of diagnostic criteria for delirium and the gradual exploration of treatment interventions, clinicians have gradually paid more attention to delirium in the ICU [5,12]. This narrative review summarizes the pathogenesis, predictive biomarkers, diagnostic criteria, prevention and treatment measures, and potential therapeutic targets of ICU delirium by systematically reviewing recent works to provide a theoretical basis for further research and clinical practice.
2. Pathogenesis and biomarkers
The pathophysiology of delirium is complex, involving multiple interactions between aetiologies and precipitating factors that are still poorly explored. Understanding the mechanisms will help to predict the risk of delirium as early as possible and will encourage clinicians to implement interventions to reduce the incidence of delirium.
2.1. Neurological disease and imaging biomarkers
Aging and neurological diseases are important susceptibility factors for delirium. Patients with delirium observed more brain atrophy, white matter lesions, and ischemic and hypoxic vascular lesions, as indicated by different MRI sequences [13–15]. About 54.9% of patients with delirium have brain lesions, and 16.1% have brain atrophy on MRI; these abnormal MRI findings are associated with reduced C5a and IC3b levels and increased tau levels [15]. Decreased arousal network activity and an imbalance in cortico-subcortical hemispheric connectivity have also been associated with the onset of delirium in patients with MRI abnormalities [16]. Compared with other biomarkers, preexisting brain atrophy and brain lesions before delirium onset may help to rapidly identify individuals at high risk of delirium. However, the relationship between these imaging changes and the development of delirium remains unclear. The value of imaging findings in predicting ICU delirium needs further study.
2.2. Metabolic disorders and humoral biomarkers
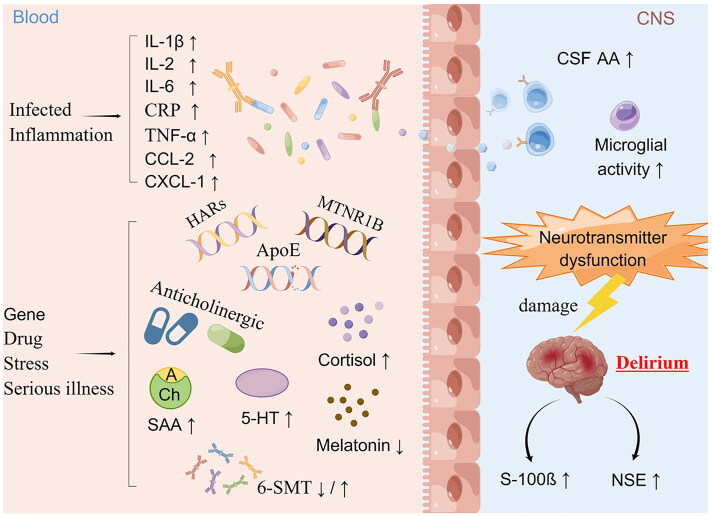
Metabolic disorders contribute to delirium onset or act as mediators between drugs [17,18], systemic inflammation [19,20], sleep deprivation [21], physical restraints [21,22], and other changes in the surrounding environment and acute abnormalities of the nervous system (Figure 1).
Figure 1.
Potential pathophysiology of delirium. IL-1β, interleukin 1β; IL-2, interleukin 2; IL-6, interleukin 6; CRP, C-reactive protein; TNF-α, tumour necrosis factor α; CCL-2, C-C chemokine ligand 2; CXCL-1, chemokine C-X-C motif ligand 1; HARs, human accelerated regions; MTNR1B, melatonin receptor 1B gene; Apo-E, apolipoprotein E; SAA, serum anticholinergic activity; CSF AA, cerebrospinal fluid anticholinergic activity; 5-HT, serotonin; 6-SMT, 6-hydroxymelatonin sulfate; S-100β, S100 calbindin B; NSE, neuron-specific enolase. Drawn with Figdraw.
Neurotransmitter imbalances, especially of acetylcholine, are among the most reported risk biomarkers for delirium [23]. In patients with respiratory failure or shock, a higher daily plasma acetylcholinesterase was associated with an increased risk of delirium compared with a normal mental status on the same day [24]. In patients with sepsis, approximately 90% exhibit statistically significant decreases in acetylcholinesterase activity over a period of at least 5 consecutive days from baseline [25], while longitudinal changes were observed only in patients with suspected septic-associated encephalopathy and could be used to diagnose septic-associated encephalopathy in patients with delirious symptoms [25]. In addition, postoperative plasma gamma-aminobutyric acid was independently associated with delirium in critical illness patients [26]. Although neurotransmitter testing has not been included in the scope of laboratory testing, making bedside testing difficult to achieve at present. However, it has been gradually incorporated into disease screening in hospitals, and may become a bedside monitoring biomarker in the future.
Systemic inflammation is another common risk factor for delirium in critical illness patients. Inflammatory mediators may cross the blood–brain barrier, leading to functional or structural impairment in the central nervous system [27–29]. Significantly higher serum neutrophil–lymphocyte ratios were found in elderly patients with critical illness with delirium than in those with a normal mental status [30]. A prospective study enrolled 78 patients admitted over 24 h in the ICU and collected blood samples within 12 h of enrolment. The results showed that soluble tumour necrosis factor (TNF) receptor-1 and -2, adiponectin, and interleukin (IL)-1β levels were higher in patients with delirium occurrence during the first 72 h of ICU admission [31]. IL-6, IL-8, IL-10, IL-18, TNF-α, and chemokines (CCL2, CCL3, CXCL1, and CXCL10) have also been reported to be elevated in ICU delirium patients and are associated with delirium severity [19,31,32]. Therefore, inflammatory markers and cytokines are potential biomarkers for the prediction of ICU delirium.
Other metabolic biomarkers have also been related to ICU delirium. One of the most important stress hormones is cortisol. Under stress conditions, increased adrenal axis reactivity, excessive secretion of cortisol, and the use of glucocorticoids can cause cognitive and mental disorders such as mood and memory disturbances [17,33]. Patients with septic delirium had significantly higher plasma cortisol levels than patients without delirium [34].
No delirium-specific serum markers have been identified. Analyses of relevant humoral metabolic biomarkers after ICU admission and before the onset of delirium are useful for identifying the prevalence of delirium, so these may become therapeutic targets for reducing the risk of delirium, especially the levels of neurotransmitters and inflammatory mediators.
3. Tools for screening, diagnosis, and therapeutic effect assessment
Delirium can be divided into hyperactive, hypoactive, and mixed delirium according to the characteristics of the symptoms [35]. In the clinic, hyperactive delirium and mixed delirium are more easily identified, while hypoactive delirium is often overlooked due to the lower state of consciousness. Many methods for screening and diagnosing delirium are often used in the clinic and in research [9,11,36–44] (Table 1). The Statistical Diagnostic Manual of Mental Disorders (DSM) published by the American Psychiatric Association and the International Classification of Diseases are the gold standard for diagnosing mental disorders worldwide [45] and are the strictest criteria based on symptoms and aetiology; this standard mostly needs to be applied by neurologists and psychiatrists. The DRS-R-98 assessment method is more rigorous and detailed, and can be used to distinguish between hyperactive and hypoactive delirium, with a sensitivity of 92% and specificity of 95% [41], though it still has shortcomings and suffers from too little evidence in ICU patients [46]. As delirium assessment in the ICU is carried out mostly by bedside nurses, a simple and fast score based on symptoms is more applicable for screening. Compared with CAM [36,47], the Confusion Assessment Method for the Diagnosis of Delirium in the ICU (CAM-ICU) and Intensive Care Delirium Screening Checklist (ICDSC) are more often used for the diagnosis of critical illness and are recommended by ICU guidelines [5,8]. CAM-ICU is superior in ruling out patients without ICU delirium and in detecting delirium in patients with ventilation and has higher summary specificity than ICDSC [48,49]. In randomized controlled trials, CAM-ICU is the most used tool to evaluate the effects of pharmacological and nonpharmacological therapies on the primary outcome of delirium incidence in randomized controlled trials (RCTs) [50–52].
Table 1.
Common diagnostic methods for ICU delirium in the clinic and related studies.
| Type of tools | Methods | Abbreviations |
|---|---|---|
| Diagnosis | Diagnostic and statistical manual of mental disorders-5 | DSM-5 |
| Screening | Confusion assessment method for the intensive care unit | CAM-ICU |
| Intensive care delirium screening checklist | ICDSC | |
| The confusion assessment method | CAM | |
| Delirium severity | Confusion assessment method-severity | CAM-S |
| Confusion assessment method for the intensive care unit-7 | CAM-ICU-7 | |
| Delirium rating scale-revised-98 | DRS-R-98 | |
| Memorial delirium assessment score | MDAS | |
| Sedative level | Richmond agitation and sedation scale | RASS |
| Sedation-agitation scale | SAS | |
| Ramsay sedation scale | Ramsay | |
| Observer’s assessment of alertness/sedation scale (OAA/S) | (OAA/S) |
ICU, intensive care unit.
For adult ICU patients with hyperactive delirium, sedatives such as continuous IV infusions of dexmedetomidine rather than benzodiazepine infusions are preferred by clinicians according to guideline recommendation [53]. These patients may need to be evaluated for both sedation level and delirium control. Two tools are most often used to assess the effect of sedation: the Richmond Agitation and Sedation Scale (RASS) [54] and the Sedation-Agitation Scale (SAS), which are also recommended by ICU guidelines [4,5]. RASS is supported by the most evidence from RCTs [55–57]. CAM-ICU combines the degree of sedation and can be assessed at the same time as RASS [10]. Sedatives can be titrated to maintain either light or deep sedation. Multiple studies support lighter sedation levels in adult ICU patients to improve outcomes, including a shortened duration of mechanical ventilation, a shorter hospital stay, and less long-term cognitive dysfunction [53], and it was beneficial for delirium screening and monitoring.
Based on the above evidence, CAM-ICU is the most appropriate tool for screening and monitoring ICU delirium, especially for patients under sedation.
4. Prevention and treatment
There is a comprehensive management system of nonpharmacological and pharmacological interventions for the prevention and treatment of delirium in the ICU. Intervention of primary disease and the reduction of medical triggers, such as inflammatory responses [27–29], abnormal energy metabolism caused by hypoxia and ion disturbance [58,59], an uncomfortable environment [21], and drugs, such as the intraoperative application of dopamine and analgesic ketamine [60], sedative midazolam [61], and benzodiazepines [62,63] (Table 2), are important measures for preventing delirium.
Table 2.
Drugs often used in the ICU that increase the risk of delirium.
| Type of drugs | Measures to avoid delirium onset or recurrent if must be used | Typical drugs |
|---|---|---|
| Corticosteroids | Low-dose | Glucocorticoids |
| Benzodiazepine, benzodiazepine receptor antagonist | Low-dose, avoid sudden withdrawal, and weaned over several days | Alprazolam, Lorazepam, Midazolam, flumazenil |
| Opioids, opioid antagonist, naloxone, or mixed agonist/antagonists | Low-dose, avoid sudden withdrawal, and weaned over several days | Sufentanil, Nalbuphine |
| Anaesthetic | Low-dose, avoid sudden withdrawal, and weaned over several days | Propofol, Ketamine |
| Anticholinergic drugs | Low-dose | Atropine |
Nonpharmacological interventions, which mainly include control of the environment (avoiding noise, confusing stimuli, continuous light stimulation, sleep deprivation, maintaining circadian rhythm, etc.), cognitive functional rehabilitation training, family nursing knowledge training, and music training, are the cornerstone of delirium prevention/management and are recommended first line by all guidelines published to date, both adult and paediatric [4,5,64–70]. If the effectiveness of nonpharmacological interventions I limited, pharmacologic treatment with antipsychotics (or other agents) should be limited to those with severe symptoms and/or those with nonpharmacologic interventions that have failed. The treatment of elderly critically ill patients is often complicated by the presence of multiple diseases, an attention should be given to controlling the types or standard use of drugs (Table 2) in high-risk patients. Reducing or avoiding the use of analgesic, delirium-active sedatives such as benzodiazepines, psychotropic and hormonal drugs during procedures and after ICU admission can reduce the onset or recurrence of delirium [18,71,72], which need a cooperated attention of anaesthesiologists, surgeons, and practicing intensivists, as an exposure to these drugs such as benzodiazepine has been reported to be correlated with an increased risk of delirium [73,74]. If drug application cannot be avoided, low-dose, serological drug concentration testing or the avoidance of sudden withdrawal may be important preventive measures [18].
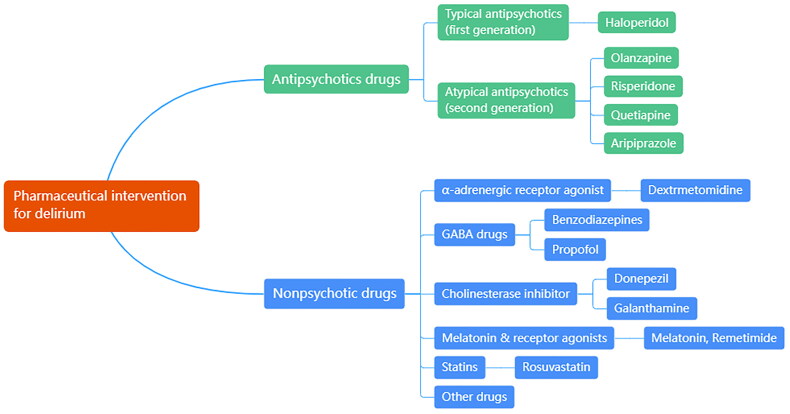
Drugs used for delirium prevention and treatment are mainly divided into antipsychotic drugs and nonpsychotic drugs (Figure 2). Antipsychotics have been reported to improve clinical symptoms, shorten duration, and reduce the severity of delirium [75]. However, antipsychotics such as haloperidol and ziprasidone have not been shown to reduce the rate of delirium occurrence [72,76,77] or shorten the duration of ICU delirium, and their high incidence of adverse reactions has also been criticized, as confirmed in recent years, by numerous large-scale placebo-controlled RCTs and meta-analyses [78–81]. For nonpsychotic drugs, the use dexmedetomidine was related with reduced risk of ICU delirium [72,73,82], duration of mechanical ventilation and ICU length of stay comparing to other sedatives, due to its low risk of respiratory depression [73]. Among atypical antipsychotic drugs, ICU patients with delirium who received quetiapine had a reduced duration of delirium [83]. A recent meta-analysis showed that olanzapine did not have a clear beneficial effect compared with other delirium drugs [84]. At present, few scholars have studied the safety of delirium drugs in nonmechanically ventilated ICU populations [3]. This field requires further exploration.
Figure 2.
Classification of pharmaceutical interventions for delirium.
Several studies have developed and tested other delirium drugs with potential therapeutic targets. The application of exogenous melatonin and melatonin receptor agonists such as ramelteon was associated with improved sleep, reduced the incidence of delirium, and shortened period of ventilators [85–87]. The inflammatory response is another important target of current research, and critically ill patients often have complications such as organ infection or systemic inflammatory responses. Patients with delirium have been reported to benefit from statins, probably through their anti-inflammatory effects [88–90]. Further high-quality studies focused on delirium prevention and treatment are still needed.
5. Summary and outlook
Delirium is a state of abnormal brain function due mostly to pathological or functional changes in the brain parenchyma. Different patients may face different contributing factors, such as sleep deprivation and abnormal sleep rhythm caused by continuous ECG monitoring; light stimulation and noise; the application of hormones, anaesthetics and cholinergic drugs; ischemia; hypoxia; and cerebrovascular disease. Early assessment of risk factors in high-risk patients to predict the occurrence of delirium and avoid the contributions of known factors is an ideal management strategy for such patients. There is currently no good method for predicting and preventing delirium. Whether delirium can be predicted and how to develop a good prediction and prevention method will continue to challenge intensivists and neurologists. Further research into delirium treatment drugs and neurocritical care practice strategies is warranted to determine both their efficacy and safety.
Acknowledgments
None declared.
Funding Statement
This work was supported by the Dalian Medical Key Specialty ‘Climbing the Peak Plan’ [Grant No. 2022ZZ267] and the Dalian High-Level Talents Innovation Support Plan [Grant Nos. 2021RQ029 and 2023RY019].
Authors contributions
S.B.L.: conceptualization, software, formal analysis, investigation, writing-original draft. H.Y.W.: software, formal analysis, investigation. M.L.D.: formal analysis, investigation. R.L.Y.: visualization, supervision. C.H.J.: visualization, supervision. J.J.L. and H.Z.: writing-reviewing and editing, project administration, funding acquisition.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
Data availability is not applicable to this article, as no new data were created or analyzed in this study.
References
- 1.Salluh JI, Soares M, Teles JM, et al. Delirium epidemiology in critical care (DECCA): an international study. Crit Care. 2010;14(6):R210. doi: 10.1186/cc9333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mesa P, Previgliano IJ, Altez S, et al. Delirium in a Latin American intensive care unit. A prospective cohort study of mechanically ventilated patients. Rev Bras Ter Intensiva. 2017;29:337–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu S, Zhao R, Yang R, et al. Are dexmedetomidine and olanzapine suitable to control delirium in critically ill elderly patients? A retrospective cohort study. Biomed Pharmacother. 2021;139:111617. doi: 10.1016/j.biopha.2021.111617. [DOI] [PubMed] [Google Scholar]
- 4.Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50(12):e344–e418. doi: 10.1161/STR.0000000000000211. [DOI] [PubMed] [Google Scholar]
- 5.Devlin JW, Skrobik Y, Gélinas C, et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. 2018;46(9):e825–e873. doi: 10.1097/CCM.0000000000003299. [DOI] [PubMed] [Google Scholar]
- 6.Genoni F, Guerrini M, Sannino P, et al. Italian pediatric intensive care units need to improve the assessment of delirium, like many other Countries. Minerva Anestesiol. 2023;89(11):1060–1061. doi: 10.23736/S0375-9393.23.17452-9. [DOI] [PubMed] [Google Scholar]
- 7.Morandi A, Piva S, Ely EW, et al. Worldwide survey of the "assessing pain, both spontaneous awakening and breathing trials, choice of drugs, delirium monitoring/management, early exercise/mobility, and family empowerment" (ABCDEF) bundle. Crit Care Med. 2017;45(11):e1111–e1122. doi: 10.1097/CCM.0000000000002640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galazzi A, Giusti GD, Pagnucci N, et al. Assessment of delirium in adult patients in Intensive Care Unit: Italian critical care nurses best practices. Intensive Crit Care Nurs. 2021;66:103072. doi: 10.1016/j.iccn.2021.103072. [DOI] [PubMed] [Google Scholar]
- 9.Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU). JAMA. 2001;286(21):2703–2710. doi: 10.1001/jama.286.21.2703. [DOI] [PubMed] [Google Scholar]
- 10.Gusmao-Flores D, Salluh JI, Chalhub R, et al. The confusion assessment method for the intensive care unit (CAM-ICU) and intensive care delirium screening checklist (ICDSC) for the diagnosis of delirium: a systematic review and meta-analysis of clinical studies. Crit Care. 2012;16(4):R115. doi: 10.1186/cc11407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bergeron N, Dubois MJ, Dumont M, et al. Intensive care delirium screening checklist: evaluation of a new screening tool. Intensive Care Med. 2001;27(5):859–864. doi: 10.1007/s001340100909. [DOI] [PubMed] [Google Scholar]
- 12.Smith HAB, Besunder JB, Betters KA, et al. 2022 society of critical care medicine clinical practice guidelines on prevention and management of pain, agitation, neuromuscular blockade, and delirium in critically ill pediatric patients with consideration of the ICU environment and early mobility. Pediatr Crit Care Med. 2022;23(2):e74–e110. doi: 10.1097/PCC.0000000000002873. [DOI] [PubMed] [Google Scholar]
- 13.Song R, Song G, Xie P, et al. [Diffusion tensor imaging and resting-state functional magnetic resonance imaging in patients with delirium in intensive care unit]. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2020;32(1):88–93. doi: 10.3760/cma.j.cn121430-20190905-00016. [DOI] [PubMed] [Google Scholar]
- 14.Haggstrom L, Welschinger R, Caplan GA.. Functional neuroimaging offers insights into delirium pathophysiology: a systematic review. Australas J Ageing. 2017;36(3):186–192. doi: 10.1111/ajag.12417. [DOI] [PubMed] [Google Scholar]
- 15.Orhun G, Esen F, Özcan PE, et al. neuroimaging findings in sepsis-induced brain dysfunction: association with clinical and laboratory findings. Neurocrit Care. 2019;30(1):106–117. doi: 10.1007/s12028-018-0581-1. [DOI] [PubMed] [Google Scholar]
- 16.Boukrina O, Kowalczyk M, Koush Y, et al. Brain network dysfunction in poststroke delirium and spatial neglect: an fMRI study. Stroke. 2022;53(3):930–938. doi: 10.1161/STROKEAHA.121.035733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schreiber MP, Colantuoni E, Bienvenu OJ, et al. Corticosteroids and transition to delirium in patients with acute lung injury. Crit Care Med. 2014;42(6):1480–1486. doi: 10.1097/CCM.0000000000000247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pun BT, Badenes R, Heras La Calle G, et al. Prevalence and risk factors for delirium in critically ill patients with COVID-19 (COVID-D): a multicentre cohort study. Lancet Respir Med. 2021;9(3):239–250. doi: 10.1016/S2213-2600(20)30552-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chan CK, Song Y, Greene R, et al. Meta-analysis of ICU delirium biomarkers and their alignment with the NIA-AA research framework. Am J Crit Care. 2021;30(4):312–319. doi: 10.4037/ajcc2021771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lei W, Ren Z, Su J, et al. Immunological risk factors for sepsis-associated delirium and mortality in ICU patients. Front Immunol. 2022;13:940779. doi: 10.3389/fimmu.2022.940779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang J, Ji Y, Wang N, et al. Risk factors for the incidence of delirium in cerebrovascular patients in a neurosurgery intensive care unit: a prospective study. J Clin Nurs. 2018;27(1–2):407–415. doi: 10.1111/jocn.13943. [DOI] [PubMed] [Google Scholar]
- 22.Li X, Zhang L, Gong F, et al. Incidence and risk factors for delirium in older patients following intensive care unit admission: a prospective observational study. J Nurs Res. 2020;28(4):e101. doi: 10.1097/jnr.0000000000000384. [DOI] [PubMed] [Google Scholar]
- 23.Hshieh TT, Fong TG, Marcantonio ER, et al. Cholinergic deficiency hypothesis in delirium: a synthesis of current evidence. J Gerontol A Biol Sci Med Sci. 2008;63(7):764–772. doi: 10.1093/gerona/63.7.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hughes CG, Boncyk CS, Fedeles B, et al. Association between cholinesterase activity and critical illness brain dysfunction. Crit Care. 2022;26(1):377. doi: 10.1186/s13054-022-04260-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zujalovic B, Mayer B, Hafner S, et al. AChE-activity in critically ill patients with suspected septic encephalopathy: a prospective, single-centre study. BMC Anesthesiol. 2020;20(1):287. doi: 10.1186/s12871-020-01204-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoshitaka S, Egi M, Kanazawa T, et al. The association of plasma gamma-aminobutyric acid concentration with postoperative delirium in critically ill patients. Crit Care Resusc. 2014;16(4):269–273. doi: 10.1016/S1441-2772(23)01618-6. [DOI] [PubMed] [Google Scholar]
- 27.Cortese GP, Burger C.. Neuroinflammatory challenges compromise neuronal function in the aging brain: postoperative cognitive delirium and Alzheimer’s disease. Behav Brain Res. 2017;322(Pt B):269–279. doi: 10.1016/j.bbr.2016.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neerland BE, Hall RJ, Seljeflot I, et al. Associations between delirium and preoperative cerebrospinal fluid C-reactive protein, interleukin-6, and interleukin-6 receptor in individuals with acute hip fracture. J Am Geriatr Soc. 2016;64(7):1456–1463. doi: 10.1111/jgs.14238. [DOI] [PubMed] [Google Scholar]
- 29.Kealy J, Murray C, Griffin EW, et al. Acute inflammation alters brain energy metabolism in mice and humans: role in suppressed spontaneous activity, impaired cognition, and delirium. J Neurosci. 2020;40(29):5681–5696. doi: 10.1523/JNEUROSCI.2876-19.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Egberts A, Mattace-Raso FU.. Increased neutrophil-lymphocyte ratio in delirium: a pilot study. Clin Interv Aging. 2017;12:1115–1121. doi: 10.2147/CIA.S137182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khan BA, Perkins AJ, Prasad NK, et al. Biomarkers of delirium duration and delirium severity in the ICU. Crit Care Med. 2020;48(3):353–361. doi: 10.1097/CCM.0000000000004139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith RJ, Lachner C, Singh VP, et al. Cytokine profiles in intensive care unit delirium. Acute Crit Care. 2022;37(3):415–428. doi: 10.4266/acc.2021.01508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harris MA, Cox SR, Brett CE, et al. Cognitive ability across the life course and cortisol levels in older age. Neurobiol Aging. 2017;59:64–71. doi: 10.1016/j.neurobiolaging.2017.07.012. [DOI] [PubMed] [Google Scholar]
- 34.Nguyen DN, Huyghens L, Zhang H, et al. Cortisol is an associated-risk factor of brain dysfunction in patients with severe sepsis and septic shock. Biomed Res Int. 2014;2014:712742–712747. doi: 10.1155/2014/712742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krewulak KD, Stelfox HT, Leigh JP, et al. Incidence and prevalence of delirium subtypes in an adult ICU: a systematic review and meta-analysis. Crit Care Med. 2018;46(12):2029–2035. doi: 10.1097/CCM.0000000000003402. [DOI] [PubMed] [Google Scholar]
- 36.Inouye SK, van Dyck CH, Alessi CA, et al. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113(12):941–948. doi: 10.7326/0003-4819-113-12-941. [DOI] [PubMed] [Google Scholar]
- 37.Arbanas G. Diagnostic and statistical manual of mental disorders (DSM-5). Codas. 2015;25:591–644. [DOI] [PubMed] [Google Scholar]
- 38.World Health Organization. The ICD-10 classification of mental and behavioural disorders: clinical descriptions and diagnostic guidelines[J]. World Health Organization, 1992, 362. [Google Scholar]
- 39.Breitbart W, Rosenfeld B, Roth A, et al. The memorial delirium assessment scale. J Pain Symptom Manage. 1997;13(3):128–137. doi: 10.1016/s0885-3924(96)00316-8. [DOI] [PubMed] [Google Scholar]
- 40.Psychiatric CSo . CCMD-3 classification and diagnostic criteria of mental disorders in China. Shandong Science and Technology Press. 2001. [Google Scholar]
- 41.Trzepacz PT, Mittal D, Torres R, et al. Validation of the delirium rating scale-revised-98: comparison with the delirium rating scale and the cognitive test for delirium. J Neuropsychiatry Clin Neurosci. 2001;13(2):229–242. doi: 10.1176/jnp.13.2.229. [DOI] [PubMed] [Google Scholar]
- 42.Inouye SK, Kosar CM, Tommet D, et al. The CAM-S: development and validation of a new scoring system for delirium severity in 2 cohorts. Ann Intern Med. 2014;160(8):526–533. doi: 10.7326/M13-1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hart RP, Levenson JL, Sessler CN, et al. Validation of a cognitive test for delirium in medical ICU patients. Psychosomatics. 1996;37(6):533–546. doi: 10.1016/S0033-3182(96)71517-7. [DOI] [PubMed] [Google Scholar]
- 44.MacLullich AM, Shenkin SD.. The 4 ‘A’s test for detecting delirium in acute medical patients: a diagnostic accuracy study. Health Technol Assess. 2019;23:1–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sachdev PS, Blacker D, Blazer DG, et al. Classifying neurocognitive disorders: the DSM-5 approach. Nat Rev Neurol. 2014;10(11):634–642. doi: 10.1038/nrneurol.2014.181. [DOI] [PubMed] [Google Scholar]
- 46.Gross AL, Tommet D, D’Aquila M, et al. Harmonization of delirium severity instruments: a comparison of the DRS-R-98, MDAS, and CAM-S using item response theory. BMC Med Res Methodol. 2018;18(1):92. doi: 10.1186/s12874-018-0552-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.De J, Wand AP.. Delirium screening: a systematic review of delirium screening tools in hospitalized patients. Gerontologist. 2015;55(6):1079–1099. doi: 10.1093/geront/gnv100. [DOI] [PubMed] [Google Scholar]
- 48.Chen TJ, Chung YW, Chang HR, et al. Diagnostic accuracy of the CAM-ICU and ICDSC in detecting intensive care unit delirium: a bivariate meta-analysis. Int J Nurs Stud. 2021;113:103782. doi: 10.1016/j.ijnurstu.2020.103782. [DOI] [PubMed] [Google Scholar]
- 49.Ewers R, Bloomer MJ, Hutchinson A.. An exploration of the reliability and usability of two delirium screening tools in an Australian intensive care unit: a pilot study. Intensive Crit Care Nurs. 2021;62:102919. doi: 10.1016/j.iccn.2020.102919. [DOI] [PubMed] [Google Scholar]
- 50.Rosa RG, Falavigna M, da Silva DB, et al. Effect of flexible family visitation on delirium among patients in the intensive care unit: the ICU visits randomized clinical trial. JAMA. 2019;322(3):216–228. doi: 10.1001/jama.2019.8766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wibrow B, Martinez FE, Myers E, et al. Prophylactic melatonin for delirium in intensive care (Pro-MEDIC): a randomized controlled trial. Intensive Care Med. 2022;48(4):414–425. doi: 10.1007/s00134-022-06638-9. [DOI] [PubMed] [Google Scholar]
- 52.Khan BA, Perkins AJ, Campbell NL, et al. Pharmacological management of delirium in the intensive care unit: a randomized pragmatic clinical trial. J Am Geriatr Soc. 2019;67(5):1057–1065. doi: 10.1111/jgs.15781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Barr J, Fraser GL, Puntillo K, et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med. 2013;41(1):263–306. doi: 10.1097/CCM.0b013e3182783b72. [DOI] [PubMed] [Google Scholar]
- 54.Ely EW, Truman B, Shintani A, et al. Monitoring sedation status over time in ICU patients: reliability and validity of the Richmond Agitation-Sedation Scale (RASS). JAMA. 2003;289(22):2983–2991. doi: 10.1001/jama.289.22.2983. [DOI] [PubMed] [Google Scholar]
- 55.Hughes CG, Mailloux PT, Devlin JW, et al. Dexmedetomidine or propofol for sedation in mechanically ventilated adults with sepsis. N Engl J Med. 2021;384(15):1424–1436. doi: 10.1056/NEJMoa2024922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dallı ÖE, Yıldırım Y, Aykar F, et al. The effect of music on delirium, pain, sedation and anxiety in patients receiving mechanical ventilation in the intensive care unit. Intensive Crit Care Nurs. 2023;75:103348. doi: 10.1016/j.iccn.2022.103348. [DOI] [PubMed] [Google Scholar]
- 57.Hui D, De La Rosa A, Wilson A, et al. Neuroleptic strategies for terminal agitation in patients with cancer and delirium at an acute palliative care unit: a single-centre, double-blind, parallel-group, randomised trial. Lancet Oncol. 2020;21(7):989–998. doi: 10.1016/S1470-2045(20)30307-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Han Y, Zhang W, Liu J, et al. Metabolomic and lipidomic profiling of preoperative CSF in elderly hip fracture patients with postoperative delirium. Front Aging Neurosci. 2020;12:570210. doi: 10.3389/fnagi.2020.570210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tripp BA, Dillon ST, Yuan M, et al. Targeted metabolomics analysis of postoperative delirium. Sci Rep. 2021;11(1):1521. doi: 10.1038/s41598-020-80412-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Erstad BL, Patanwala AE.. Ketamine for analgosedation in critically ill patients. J Crit Care. 2016;35:145–149. doi: 10.1016/j.jcrc.2016.05.016. [DOI] [PubMed] [Google Scholar]
- 61.Gile J, Scott B, Eckle T.. The period 2 enhancer nobiletin as novel therapy in murine models of circadian disruption resembling delirium. Crit Care Med. 2018;46(6):e600–e608. doi: 10.1097/CCM.0000000000003077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Han JH, Chen A, Vasilevskis EE, et al. Supratherapeutic psychotropic drug levels in the emergency department and their association with delirium duration: a preliminary study. J Am Geriatr Soc. 2019;67(11):2387–2392. doi: 10.1111/jgs.16156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lu Y, Chen L, Ye J, et al. Surgery/Anesthesia disturbs mitochondrial fission/fusion dynamics in the brain of aged mice with postoperative delirium. Aging (Albany NY). 2020;12(1):844–865. doi: 10.18632/aging.102659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Enomoto R, Lee-Hiraiwa E.. Elimination of the causes of poor sleep underlying delirium is a basic strategy to prevent delirium. Curr Mol Pharmacol. 2021;14(2):132–137. doi: 10.2174/1874467213666200424150709. [DOI] [PubMed] [Google Scholar]
- 65.Baron R, Binder A, Biniek R, et al. Evidence and consensus based guideline for the management of delirium, analgesia, and sedation in intensive care medicine. Revision 2015 (DAS-Guideline 2015) - short version. Ger Med Sci. 2015;13:doc19. doi: 10.3205/000223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vincent JL, Shehabi Y, Walsh TS, et al. Comfort and patient-centred care without excessive sedation: the eCASH concept. Intensive Care Med. 2016;42(6):962–971. doi: 10.1007/s00134-016-4297-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Godfrey M, Green J, Smith J, et al. Process of implementing and delivering the Prevention of Delirium system of care: a mixed method preliminary study. BMC Geriatr. 2019;20(1):1. doi: 10.1186/s12877-019-1374-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kyeong S, Choi SH, Eun Shin J, et al. Functional connectivity of the circadian clock and neural substrates of sleep-wake disturbance in delirium. Psychiatry Res Neuroimaging. 2017;264:10–12. doi: 10.1016/j.pscychresns.2017.03.017. [DOI] [PubMed] [Google Scholar]
- 69.Wang S, Hammes J, Khan S, et al. Improving recovery and outcomes every day after the ICU (IMPROVE): study protocol for a randomized controlled trial. Trials. 2018;19(1):196. doi: 10.1186/s13063-018-2569-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Khan SH, Xu C, Purpura R, et al. Decreasing delirium through music: a randomized pilot trial. Am J Crit Care. 2020;29(2):e31–e38. doi: 10.4037/ajcc2020175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hayhurst CJ, Farrin E, Hughes CG.. The effect of ketamine on delirium and opioid-induced hyperalgesia in the Intensive Care Unit. Anaesth Crit Care Pain Med. 2018;37(6):525–527. doi: 10.1016/j.accpm.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 72.Burry LD, Cheng W, Williamson DR, et al. Pharmacological and non-pharmacological interventions to prevent delirium in critically ill patients: a systematic review and network meta-analysis. Intensive Care Med. 2021;47(9):943–960. doi: 10.1007/s00134-021-06490-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lewis K, Alshamsi F, Carayannopoulos KL, et al. Dexmedetomidine vs other sedatives in critically ill mechanically ventilated adults: a systematic review and meta-analysis of randomized trials. Intensive Care Med. 2022;48(7):811–840. doi: 10.1007/s00134-022-06712-2. [DOI] [PubMed] [Google Scholar]
- 74.Mody K, Kaur S, Mauer EA, et al. Benzodiazepines and development of delirium in critically ill children: estimating the causal effect. Crit Care Med. 2018;46(9):1486–1491. doi: 10.1097/CCM.0000000000003194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Burry L, Mehta S, Perreault MM, et al. Antipsychotics for treatment of delirium in hospitalised non-ICU patients. Cochrane Database Syst Rev. 2018;6(6):Cd005594. doi: 10.1002/14651858.CD005594.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Neufeld KJ, Yue J, Robinson TN, et al. Antipsychotic medication for prevention and treatment of delirium in hospitalized adults: a systematic review and meta-analysis. J Am Geriatr Soc. 2016;64(4):705–714. doi: 10.1111/jgs.14076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Marra A, Vargas M, Buonanno P, et al. Haloperidol for preventing delirium in ICU patients: a systematic review and meta-analysis. Eur Rev Med Pharmacol Sci. 2021;25(3):1582–1591. doi: 10.26355/eurrev_202102_24868. [DOI] [PubMed] [Google Scholar]
- 78.Andersen-Ranberg NC, Poulsen LM, Perner A, AID-ICU Trial Group, et al. Haloperidol for the Treatment of Delirium in ICU Patients. N Engl J Med. 2022;387(26):2425–2435. doi: 10.1056/NEJMoa2211868. [DOI] [PubMed] [Google Scholar]
- 79.Zayed Y, Barbarawi M, Kheiri B, et al. Haloperidol for the management of delirium in adult intensive care unit patients: A systematic review and meta-analysis of randomized controlled trials. J Crit Care. 2019;50:280–286. doi: 10.1016/j.jcrc.2019.01.009. [DOI] [PubMed] [Google Scholar]
- 80.van den Boogaard M, Slooter AJC, Brüggemann RJM, et al. Effect of haloperidol on survival among critically ill adults with a high risk of delirium: the REDUCE randomized clinical trial. JAMA. 2018;319(7):680–690. doi: 10.1001/jama.2018.0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Girard TD, Exline MC, Carson SS, et al. Haloperidol and ziprasidone for treatment of delirium in critical illness. N Engl J Med. 2018;379(26):2506–2516. doi: 10.1056/NEJMoa1808217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Herling SF, Greve IE, Vasilevskis EE, et al. Interventions for preventing intensive care unit delirium in adults. Cochrane Database Syst Rev. 2018;11(11):Cd009783. doi: 10.1002/14651858.CD009783.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Devlin JW, Roberts RJ, Fong JJ, et al. Efficacy and safety of quetiapine in critically ill patients with delirium: a prospective, multicenter, randomized, double-blind, placebo-controlled pilot study. Crit Care Med. 2010;38(2):419–427. doi: 10.1097/CCM.0b013e3181b9e302. [DOI] [PubMed] [Google Scholar]
- 84.Liu SB, Liu S, Gao K, et al. Olanzapine for the treatment of ICU delirium: a systematic review and meta-analysis. Ther Adv Psychopharmacol. 2023;13:20451253231152113. doi: 10.1177/20451253231152113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Campbell AM, Axon DR, Martin JR, et al. Melatonin for the prevention of postoperative delirium in older adults: a systematic review and meta-analysis. BMC Geriatr. 2019;19(1):272. doi: 10.1186/s12877-019-1297-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang Q, Gao F, Zhang S, et al. Prophylactic use of exogenous melatonin and melatonin receptor agonists to improve sleep and delirium in the intensive care units: a systematic review and meta-analysis of randomized controlled trials. Sleep Breath. 2019;23(4):1059–1070. doi: 10.1007/s11325-019-01831-5. [DOI] [PubMed] [Google Scholar]
- 87.Jaiswal SJ, McCarthy TJ, Wineinger NE, et al. Melatonin and sleep in preventing hospitalized delirium: a randomized clinical trial. Am J Med. 2018;131(9):1110–1117.e1114. doi: 10.1016/j.amjmed.2018.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Page VJ, Casarin A, Ely EW, et al. Evaluation of early administration of simvastatin in the prevention and treatment of delirium in critically ill patients undergoing mechanical ventilation (MoDUS): a randomised, double-blind, placebo-controlled trial. Lancet Respir Med. 2017;5(9):727–737. doi: 10.1016/S2213-2600(17)30234-5. [DOI] [PubMed] [Google Scholar]
- 89.Agus A, Phair G, Page VJ, et al. Simvastatin for the prevention and treatment of delirium in critically ill, mechanically ventilated patients (MoDUS): a cost-effectiveness analysis. Lancet Respir Med. 2018;6(3):e9–e10. doi: 10.1016/S2213-2600(18)30070-5. [DOI] [PubMed] [Google Scholar]
- 90.Needham DM, Colantuoni E, Dinglas VD, et al. Rosuvastatin versus placebo for delirium in intensive care and subsequent cognitive impairment in patients with sepsis-associated acute respiratory distress syndrome: an ancillary study to a randomised controlled trial. Lancet Respir Med. 2016;4(3):203–212. doi: 10.1016/S2213-2600(16)00005-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data availability is not applicable to this article, as no new data were created or analyzed in this study.