Abstract
Administration of IL-2 to HIV-infected patients leads to expansion of a unique subset of CD4+CD45RO–CD25+ cells. In this study, the origin, clonality, and function of these cells were investigated. Analysis of TCR excision circles revealed that the CD4+CD45RO–CD25+ cells were the product of peripheral expansion but remained polyclonal as determined by TCR repertoire analysis. Phenotypically, these cells were distinct from naturally occurring Tregs; they exhibited intermediate features, between those of memory and naive cells, and had lower susceptibility to apoptosis than CD45RO–CD25– or memory T cells. Studies of intracellular cytokine production and proliferation revealed that cytokine-expanded naive CD25+ cells had low IL-2 production and required costimulation for proliferation. Despite elevated expression of forkhead transcription factor P3 (foxP3), they exerted only weak suppression compared with CD45RO+CD25+high cells (Tregs). In summary, in vivo IL-2 administration to HIV-infected patients leads to peripheral expansion of a population of long-lived CD4+CD45RO–CD25+ cells that express high levels of foxP3 but exert weak suppressive function. These CD4+CD25+ cytokine-expanded naive cells, distinct from antigen-triggered cells and Tregs, play a role in the maintenance of a state of low turnover and sustained expansion of the CD4+ T cell pool.
Introduction
Randomized controlled clinical trials have shown that intermittent administration of IL-2 in combination with antiretroviral therapy to HIV-infected patients leads to significant and sustained expansion of CD4+ T cells (1–3) of naive and central memory phenotype (4). We have previously shown that the preferential increase of naive CD4+ cells by IL-2 is predominantly the result of the expansion of a unique subset of naive CD4+ T cells expressing the α chain of the trimeric IL-2 receptor (CD25) (5). Evaluation of basal T cell proliferation in patients treated with IL-2 has shown significant decreases in cell proliferation as measured by the expression of the nuclear antigen Ki67 or BrdU incorporation that strongly correlated with the increases in the CD4+CD25+ T cell counts (4), suggesting that these cells have a fundamental role in the observed CD4+ T cell expansion.
The expansion of a CD4+ T cell subset with naive phenotype in HIV-infected patients treated intermittently with IL-2 could represent increased thymic production or peripheral expansion of preexisting naive cells or even reversion of expanded memory T cells to a naive phenotype. Polyclonal expansion of naive cells would certainly be a desirable clinical effect of any immunotherapy targeting immunodeficient hosts such as HIV-infected individuals. The induction of CD4+ T cells expressing CD25+ cells with in vivo IL-2 immunotherapy also raises the question of a possible relationship of these cells to naturally occurring Tregs, particularly since IL-2 is considered essential for the maintenance, expansion, and functional capacity of these cells (6, 7). Tregs have been described in animal models (8) and in humans (9–11) as anergic CD4+ T cells expressing high levels of CD25 and exerting strong suppression after TCR stimulation. Tregs seem to represent a population with a fundamental role in preservation of normal T cell homeostasis as has been demonstrated in CD25 knockout animals (12). Although natural Tregs originate from the thymus, cells with similar characteristics can also be generated in the periphery under appropriate conditions (13). Forkhead transcription factor P3 (foxP3) has been described as the critical gene for Tregs’ development and function and is the most widely accepted signature of these cells even in the absence of CD25 expression (14, 15). The role of Tregs in HIV immunopathogenesis is currently unclear, and it would be hard to speculate as to whether their expansion (or functional restoration) would be an important therapeutic goal or an undesirable effect of an immune-based therapy in HIV infection. Given the well-established role of increased T cell turnover in HIV immunopathogenesis (16, 17), it has been suggested that dysfunction of Tregs in HIV-infected patients may contribute to the dysregulation of immune activation (18). On the other hand, it is possible that Tregs could hamper protective anti-HIV–specific immune responses (19, 20).
In the current study, the origin, phenotype, clonality, and function of this subset of CD4+CD45RO–CD25+ cells were investigated by comparing these cells with conventional naive cells (CD4+CD45RO–CD25–) and with memory cells. Detailed analysis of their phenotypic characteristics, functional properties, and gene expression profile showed that these cells were also distinct from Tregs. This is, to our knowledge, the first demonstration of in vivo emergence, by IL-2 immunotherapy in humans, of a cytokine-expanded naive (CEN) CD4 population expressing high levels of foxP3. The emergence of this cell population could potentially be playing an important role in T cell homeostasis.
Results
IL-2 induces polyclonal peripheral expansion of CD4+CD45RO–CD25+ cells.
We have previously shown that the CD4+ T cell expansions induced by IL-2 are preferential for cells expressing CD25 and include CD45RO– cells as well as cells with intermediate CD45RO expression (5) (Figure 1A). In the CD45RO+ subset, cells with intermediate CD25 expression also increase while the CD25+high subset remains stable.
Figure 1.
Expansion of naive CD4+CD25+ cells after IL-2 administration. (A) Representative example of phenotypic changes occurring after IL-2 administration. Dot plots of CD45RO and CD25 expression on CD4+ T cells of an HIV patient at baseline and after 3 cycles of IL-2. CD25-expressing CD45RO– and CD45RO+ cells increased after IL-2 without significant change in the CD4+CD45RO+CD25+high subset. (B) An example of the bead separation of studied CD45RO– subsets is shown. Naive cells were defined as CD45RO– and CD27+.
The division history of the CD4+CD45RO–CD25+ cells (Figure 1B) was studied by measuring their T cell receptor excision circle (TREC) content. The TREC content of the CD45RO–CD25+ cells was significantly lower than that of the CD45RO–CD25– cells (6,600 versus 70,000 signal joint TREC (sjTREC) copies/106 cells; P < 0.01, n = 14) but consistently higher than that of the memory (CD45RO+) cells (6,600 versus 3,400; P < 0.01, n = 7) (Figure 2A), strongly suggesting that these cells were not new thymic emigrants. Within the CD45RO+ subset, there were no significant differences between the TREC content of CD25– and that of CD25+high subsets (data not shown). Despite their significant differences in replicative history, the 2 CD45RO– subsets (CD25+ and CD25–) contained similar levels of proviral DNA (11.5 and 11 copies/106 cells respectively), which were lower than the levels detected in the memory subset (185 copies/106 cells, n = 11; Figure 2B). CD45RO+CD25– and CD45RO+CD25+high cells had similar proviral DNA levels (data not shown). Taken together, the TREC and proviral DNA data suggest that the CD45RO–CD25+ cells were predominantly derived from peripheral expansion of the naive pool rather than reversion of memory cells. Further supporting this hypothesis were data generated by studying the TCR repertoire of the above separated subsets by analysis of the CDR3 region length polymorphisms. Polyclonality was observed in the TCR repertoire of the CD45RO–CD25+ fraction that mimicked the patterns of the CD45RO–CD25– cells and did not reproduce the skewed patterns that can characteristically be seen in subfamilies of memory cells (Figure 2C).
Figure 2.
CD4+CD45RO–CD25+ cells in IL-2–treated patients do not represent primary thymic emigrants but polyclonal CEN cells. (A) CD4+CD45RO–CD25+ cells had a TREC content significantly lower than naive CD4+CD45RO–CD25– cells but higher than the memory cell subset. (B) Proviral DNA levels were similar in CD4+CD45RO– subsets regardless of CD25 expression and consistently lower than in the memory cells (median values from 11 independent donors). (C) The TCR repertoire patterns of CD4+CD45RO–CD25+ cells were similar to those of CD4+CD45RO–CD25– cells. Representative patterns from 3 different patients are shown.
In order to evaluate whether IL-2 can lead to polyclonal expansion of cells that are not expressing CD25, we also studied in vitro the effects of IL-2 stimulation on CD25-depleted cells from IL-2–treated patients. In these experiments, IL-2 induced CD25 expression (median CD25 expression 9.6% after 5 days on CD4 cells versus 1.2% in unstimulated cultures from 4 independent donors) and proliferation of the CD25-depleted CD4+ cells (CFSE-dim CD4 cells; median 5.8% versus 0.9% in unstimulated cultures from 4 independent donors) (see Supplemental Figure 1A; supplemental material available online with this article; doi:10.1172/JCI24307DS1). The degree of proliferation in response to IL-2 was similar in the presence or absence of added CFSE-unstained CD25+ cells (data not shown). In addition, the degree of proliferation of CD25– cells in response to IL-2, as measured by intracellular Ki67, was similar to that of the CD25+ fraction (median increases above control 14.3% and 14.6% respectively, data not shown).
CEN CD25+ cells are phenotypically distinct from CD45RO–CD25– and CD45RO+ cells and show low rates of spontaneous and fas-mediated apoptosis.
Having characterized the cells expanded in vivo by IL-2 as predominantly the product of peripheral expansion of preexisting naive cells with a unique phenotype, we elected to refer to these cells as cytokine-expanded naive (CEN) cells.
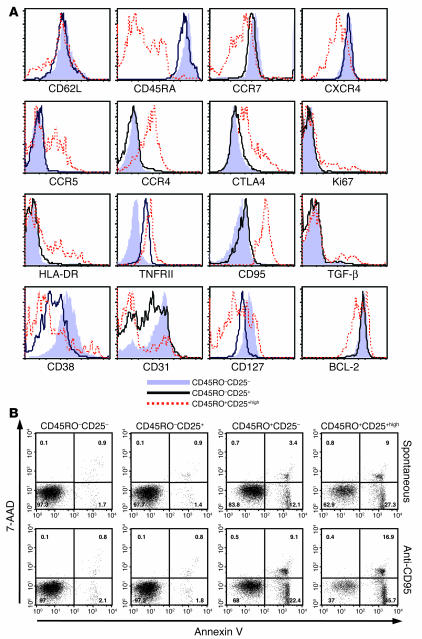
The phenotype of these CEN cells was further studied with a variety of markers that relate to the activation and differentiation state of T lymphocytes and are used to characterize cells as naive cells, memory cells, and/or Tregs. No differences were noted compared with CD4+CD45RO–CD25– cells in the expression of CD69, CD28, CD45RA (data not shown), CD27 (Figure 1A), human leukocyte antigens (HLA-DR), CCR7, or CD62L (Figure 3A). Consistent with a naive phenotype, CEN cells were CD27+, CCR7+, CD62L+, CD45RA+, CXCR4+, CCR5– with very low Ki67 expression (Figures 1B and 3A). Interestingly, the median fluorescent intensity (MFI) was lower for CD38, CD127, CD31, and CD132 and was higher for CD122 in the CEN cell subset (Figure 3A and data not shown) compared with that for naive CD25– cells. In particular, a strikingly increased expression of TNF receptor II (TNFRII) and a higher MFI for CD95 was noted in CEN cells compared with the naive CD25– cells. The expression of CD31, considered a marker for primary thymic emigrants (21), was lower in the CD25+ fraction than in the CD25– fraction of CD45RO– cells, supporting the TREC data that the CEN cells had undergone additional post-thymic proliferation. In most patients, a slightly higher MFI for CD45RO and intracellular cytotoxic T lymphocyte–associated protein 4 (CTLA-4) was observed, and in a few patients, a slightly lower level of B cell CLL/lymphoma 2 (BCL-2) was also noted (Figures 1 and 3A). The CEN cell fraction was also distinct from the memory subset with clearly lower expression of CD45RO (Figure 1), CCR5, surface TGF-β, and CCR4 and higher expression of CD62L, CXCR4, CD38, CCR7, and CD31 (Figure 3A and Supplemental Figure 1A). CD45RO+CD25+high cells had phenotypic features consistent with Tregs, including high expression of HLA-DR, CD62L, CD95, intracellular CTLA-4, CCR5, and CCR4 (Figure 3A).
Figure 3.
CEN cells in IL-2–treated patients have distinct phenotypic features compared with CD45RO–CD25– and memory cells and are less susceptible to apoptosis. (A) Representative overlay histograms from 1 patient (of 10 with similar findings) are shown. CD4+CD45RO–CD25– T cells are represented by solid light blue profiles, CD4+CD45RO–CD25+ T cells by black lines, and CD4+CD45RO+CD25+high cells by dashed red lines. (B) Annexin V and 7-AAD staining of separated CD4+CD45RO–CD25+ and CD25– and CD4+CD45RO+CD25– and CD25+high subsets from 1 representative patient (of 7). Both spontaneous and fas-mediated apoptosis were lower in CEN cells than in naive CD25– or memory CD4 subsets.
Because of the high expression of CD95 and TNFRII and the lower expression of CD127 and CD132, the apoptosis of the different subsets was studied. Both spontaneous and fas-induced apoptosis were lower in the CEN cells than in either the memory (CD25– and CD25+high) or naive CD25– cells (Figure 3B). This is in clear contrast to the increased susceptibility to apoptosis of naturally occurring Tregs (Figures 3B and Supplemental Figure 2B) that has previously been reported (10).
A lower proportion of CEN cells produce IL-2 compared with naive CD25– cells.
After in vitro stimulation for 4 hours with PMA and ionomycin, CEN cells produced IL-2 at significantly lower levels than did the naive CD4+CD25– cells (1.9% versus 17.7%, P < 0.001; Figure 4A). The lower proportion of IL-2 production was not associated with lower activation, since CD69 upregulation was noted in both subsets upon stimulation (Figure 4B). Consistent with CD25– and CEN cells’ naive phenotype (CD45RO–CD45RA+CD62L+), production of IFN-γ or IL-4 was found in less than 0.5–1% of either CEN or naive CD25– cell subsets. A very low proportion of CD45RO+CD25+high cells produced IL-2 compared with CD45RO+CD25– cells (0.8% versus 37.3%, P = 0.03; Figure 4C). The proportion of CD25+high cells that produced IL-2 was 34-fold lower (range: 7- to 56-fold) than the proportion of CD25– cells in the memory subset that did so. In the naive subset, the proportion of CD25+ cells that produced IL-2 was 9-fold lower (range: 3- to 35-fold) than the proportion of CD25– cells that did so (median values from 6 independent experiments using different donors). An example indicating the proportion of Treg-enriched (CD4+CD25+high) cells producing IL-2 from a healthy volunteer under similar conditions is shown in Supplemental Figure 2C.
Figure 4.
A lower proportion of CEN cells than of naive CD4+CD25– cells produce IL-2. (A) IL-2 production was studied with intracellular cytokine staining after stimulation of separated subsets with PMA and ionomycin for 4 hours (n = 11, P = 0.001). (B) Separated CD45RO– subsets showed similar degrees of activation (as measured by CD69 expression) despite significant differences in IL-2 production. (C) CD45RO+CD25+high cells did not produce IL-2 after similar stimulation (n = 6).
Following a 96-hour stimulation in vitro with anti-CD3/anti-CD28 beads, IL-2 production significantly increased, and cells positive for IFN-γ and IL-4 emerged from both CD45RO–CD25+ and CD25– subsets (data not shown).
CEN cells respond poorly to anti-CD3 in the absence of costimulation and weakly suppress anti-CD3–induced proliferation of naive CD25– cells.
In lymphocyte proliferation assays, separated CEN cells were found to be hyporesponsive when stimulated by anti-CD3 alone in the absence of costimulation (26,089 in naive CD25– versus 2,454 net cpm in CEN cells, P = 0.02, median values from 9 independent donors; Figure 5A). Addition of costimulation with anti-CD28 led to similar degrees of proliferation in both subsets (median 133,102 versus 118,892, P = 0.2). Response to phytohemagglutinin (PHA) was also higher in the naive CD25– pool compared with that in CEN CD25+ cells (86,581 versus 38,059, P = 0.02; Figure 5A). In experiments designed to address the presence of suppressive function, with naive CD25– cells used as responder cells and the addition of CEN cells at different ratios, a weak to moderate degree of suppression (25–50%) was detected (Figure 5B). This is considerably lower than the degree of suppression seen with Tregs from healthy volunteers (as shown in Supplemental Figure 2, D and E).
Figure 5.
CEN cells require costimulation for proliferation and can weakly suppress CD4+CD45RO–CD25– cells. (A) Separated CEN (black bars) or naive CD25– cells (white bars) from IL-2–treated patients were cultured in the presence of media, anti-CD3, or PHA for 96 hours. Media (background) proliferation was subtracted. Median values from 9 independent experiments using different donors are shown. (B) Separated CD4+CD25– cells were cultured at fixed numbers either alone (white bars) or in the presence of CD4+CD25+ cells at ratios of 1:2 (black bars) or 1:1 (gray bars). Median values from 7 independent experiments using different donors are shown.
CEN cells express high levels of foxP3 that are similar to those observed in cells of the same phenotype in healthy volunteers and lower than those observed in CD45RO+CD25+high cells.
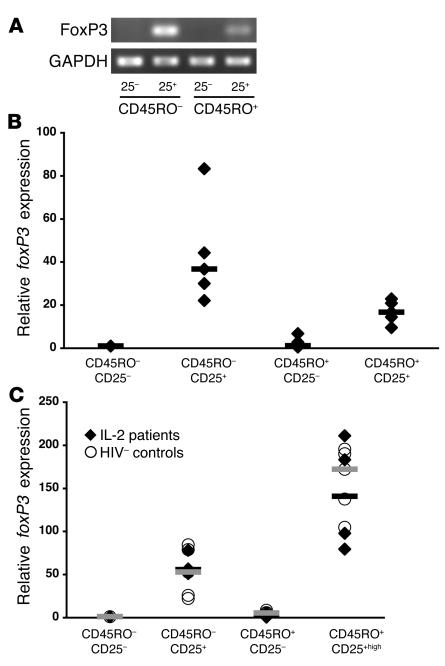
Because of the similarities of CEN cells to Tregs, analysis of foxP3 gene expression was performed in CD4 subsets further separated by CD45RO and CD25 expression. foxP3 expression was higher in CEN cells than in the CD45RO+CD25+ pooled subset (including cells with intermediate as well as high CD25 expression) in 5 patients treated with IL-2 as shown by RT-PCR (Figure 6A) and real-time PCR (Figure 6B).
Figure 6.
CEN cells express high levels of foxP3. (A) Levels of foxP3 mRNA by RT-PCR in CD4+ T cells, from a representative IL-2–treated patient, separated by CD45RO and CD25 into 4 subsets (CD45RO–CD25– and CD25+, CD45RO+CD25– and CD25+ [low and high]). (B) Real-time PCR was used to study the relative expression of foxP3 in CD45RO–CD25+ versus CD45RO+CD25+ cells. For 5 independent donors (IL-2–treated patients; diamonds), foxP3 expression was higher in the CD45RO–CD25+ subset than in the CD45RO+CD25+ subset (including cells with both low and high CD25 expression). Median values are shown as black bars. (C) foxP3 expression in CD4+CD45RO–CD25+ cells and CD4+CD45RO+CD25+high cells from IL-2–treated patients (diamonds, median values as black bars) is similar to that observed in identical CD4 subsets from healthy volunteers (circles, median values as gray bars).
Since Treg activity is enriched in CD45RO+CD25+high cells, additional patients and healthy volunteers were studied by looking specifically at this subset. Similar expression of foxP3 was seen in the IL-2–treated patients and healthy volunteers in all separated subsets, with the highest levels of expression found in the CD45RO+CD25+high cells in both groups (Figure 6C).
CEN cells exhibit only weak suppression compared with CD45RO+CD25+high cells.
Because of the high levels of foxP3 expression in the CEN subset, a direct comparison of the proliferative and suppressive capacity of CEN versus CD45RO+CD25+high cells from the same patients was performed. In lymphocyte proliferation assays, CD45RO+CD25+high cells were anergic while CEN cells were hyporesponsive with significant proliferation after costimulation (Figure 7A, median values from 4 experiments). In CFSE assays, CD4+CD45RO–CD25– cells were stained with CFSE and used as responder cells with CEN or CD45RO+CD25+high cells added at different ratios. In this system, the CD45RO+CD25+high cells, consistent with their higher foxP3 expression, exerted much more potent suppression than CEN cells, as shown in Figure 7. In a series of 8 experiments using different donors, CD45RO+CD25+high cells were able to suppress the anti-CD3 proliferative response of the CD45RO–CD25– by 65% (range: 44–93%) at 1:1 ratios and by 40% at 1:2 ratios (range: 0–62%). In contrast, CEN cells exerted 25% (range: 3–57%) suppression at 1:1 ratios that was not observed consistently at 1:2 ratios. CEN cells did not exert any suppression when CD45RO+CD25– cells were used as responder cells (data not shown).
Figure 7.
CEN cells from IL-2–treated patients exert weak suppression compared with that of Tregs. (A) Proliferative responses of separated subsets to anti-CD3 as measured by tritiated thymidine incorporation. Median values from 4 independent experiments using different donors are shown. (B) CD4+CD45RO–CD25– cells were stained with CFSE and stimulated for 96 hours with anti-CD3 (1 μg/ml) with or without the addition of either CD45RO–CD25+ or CD45RO+CD25+high subsets from the same patient at different ratios. Gating was done on CD4+CFSE+ cells. One of 8 experiments from independent donors is shown.
Discussion
In this study, the in vivo administration of intermittent 5-day cycles of IL-2 to patients infected with HIV was found to lead to the induction of a long-lived population of CEN CD4+CD25+ T cells expressing high levels of foxP3. These CEN cells were hyporesponsive or anergic in the absence of costimulation but were clearly distinct from the naturally occurring Tregs with respect to phenotype and suppressive potential. Their weak suppression of polyclonal naive T cell activation suggests that although these cells are not classic Tregs, they may play a role in the regulation of naive T cell activation and proliferation.
Previous in vitro studies have clearly shown that human naive cells stimulated by specific cytokines (IL-2 or IL-7 in the presence of proinflammatory cytokines) can proliferate in the absence of antigen and preserve a naive phenotype, but can express some markers of memory cells such as CD25 (22, 23). A subset of CEN cells expressing CD25 has been previously described in low numbers in healthy volunteers (5), and a report on patients with rheumatoid arthritis suggested that this phenotype could exist in higher numbers under conditions of intense inflammation (24).
The phenotypic analysis of the CEN cells studied here demonstrated some features that were intermediate between memory and naive cells (CD38, CD95, TNFRII, CD31, and intracellular CTLA-4) while most other features were consistent with the conventional markers of naive T cells (CD45RO– with high expression of CD62L, CD45RA, CD27, and CCR7). These features may help differentiate intense cytokine-driven homeostatic proliferation of naive cells with maintenance of a naive phenotype from antigen-mediated expansion and conversion to memory cells. In irradiated mice, naive cells can masquerade as memory cells after intense homeostatic proliferation in lymphopenic hosts (25, 26). Kimmig et al. (21) recently described the presence of peripherally expanded human naive CD4 cells that downregulated CD31 secondary to MHC triggering from self antigens. Thus, it seems possible that CD25+ and CD31– naive CD4+ T cells in healthy adults represent 2 distinct pathways of homeostatic proliferation as suggested by Geginat et al. (23): (a) cytokine-mediated and (b) stimulated by MHC triggering from self-antigens. The lack of complete overlap between the naive CD25+ and CD31– cells supports this hypothesis and suggests that these 2 subsets of naive cells could potentially be representative of 2 distinct pathways of peripheral expansion. Preliminary results in HIV-seronegative volunteers are also supportive of this notion (our unpublished observations).
The peripheral origin of the majority of CEN CD4+CD25+ cells seems evident, but it is difficult to exclude entirely the possibility that some memory cells proliferate during IL-2 cycles and revert to a more naive phenotype after the cytokine stimulus is withdrawn. The evidence against a significant degree of reversion is the observation that the TREC content in the CEN cells was higher and the proviral DNA content was lower compared with that of the memory cells in all tested patients, although the lower proviral DNA content could be explained by preferential death of proliferating infected cells. In addition, TCR repertoire analysis did not show similarities between CEN CD25+ and memory cells. Finally, IL-2 was the only cytokine that these cells were able to produce, albeit at low levels. The fact that CD45RO can be upregulated by IL-2 alone (27) seems adequate to explain the presence of the intermediate CD45RO+ subset.
Despite some similarities to the described Tregs, the CEN CD4+CD25+ T cells showed clear differences, as summarized in Table 1. It is unclear at this point whether foxP3 upregulation is a unique feature of IL-2–expanded naive cells or a common characteristic of cytokine-expanded T cells that retain their naive phenotype. The generation of foxP3-expressing T cells with regulatory function and memory phenotypes has been described as occurring in vitro when naive CD25– cells were activated by anti-CD3 in the presence of TGF-β (28). Although it is known that in vivo IL-2 administration leads to the induction of proinflammatory cytokines (29), increases in TGF-β have not been detected in the same setting (our unpublished observations). It seems feasible that the significant division history of the CEN cells, as shown by their diluted TREC content, could explain their distinct functional characteristics. Some studies have demonstrated that, in animal lymphopenic hosts, naive cells after homeostatic proliferation functioned comparably to memory cells (26, 30), but others showed long-lasting anergy of naive cells after homeostatic proliferation (31). On the other hand, human naive T cells after in vitro cytokine-mediated expansion can acquire a split naive-memory phenotype as well as properties of memory cells with respect to cytokine production (32).
Table 1.
Comparison of CEN cells to Treg cells
The potential clinical significance of the expansion of these CEN cells and their in vivo functionality and fate remain unclear. Current ongoing phase III studies will be the best means of definitively answering this question (33). Our findings suggest that these cells are phenotypically and functionally closer to conventional naive cells than memory cells or Tregs since they can be activated to memory by TCR stimulation together with costimulation but have a higher threshold for TCR activation and can weakly suppress polyclonal stimulation of naive CD25– cells. It is also plausible that the CEN cells may have a role in decreasing the persistent activation and pronounced activation-induced cell death leading to the depletion of the naive T cell pool of HIV-infected patients (34). Recently, Godfrey et al. described a phenotypically similar cell subset (CD45RO–CD25+high) in cord blood with clear Treg activity after stimulation, suggesting that recent thymic emigrants with this phenotype have strong suppressive activity (35). Although our data show that the CEN cells are clearly distinct from Tregs, it is possible that a small fraction of these cells may be maturing into Tregs after stimulation and conversion to a memory phenotype. It is also unclear what effects IL-2 therapy may have on survival or expansion of preexisting naturally occurring Tregs, given the fundamental role of this cytokine in development and maintenance of this T cell subset in animal models (36, 37). Our data suggest that the overall pool of CD45RO+CD25+ cells after IL-2 contains only a small fraction of cells (CD45RO+CD25+high) with foxP3 levels and function consistent with those of Tregs. Longitudinal studies will be needed to address potential functional changes that are induced in this subset by IL-2.
In summary, in this study, we have analyzed the origin, clonality, and function of a novel subset of CEN CD4+CD25+ T cells in HIV-1–infected patients treated with intermittent IL-2. The data suggest that these cells represent a long-lived polyclonal population of peripherally expanded preexisting naive cells that are phenotypically and functionally distinct from naturally occurring Tregs. High levels of foxP3 expression and weak suppression of polyclonal stimulation of naive cells suggest a potential role of these cells in naive T cell homeostasis. Finally, these findings reveal a potential pathway for inducing in vivo foxP3 expression in T cell subsets.
Methods
Study participants.
Patients received IL-2 (Proleukin; Chiron) and underwent lymphapheresis or large-volume blood draw (150 ml) in 2001–2004 under protocols approved by the Institutional Review Board at the National Institute of Allergy and Infectious Diseases (NIAID). Studied patients (n = 40) were participants of either the long-term follow-up IL-2 cohort at NIAID (38) or of the ongoing randomized controlled phase III study known as ESPRIT (Evaluation of Subcutaneous Proleukin in a Randomized International Trial; ref. 33). Participants received IL-2 cycles at 3–7.5 million units s.c. twice a day for 5 days, initially every 2 months and subsequently at intervals determined by their CD4+ T cell count. Viral burden was tested by Ultra-Sensitive Branched DNA version 3 (Bayer Health Care — Diagnostics Division; sensitivity < 50 copies/ml). All patients received antiretroviral therapy. The characteristics of study participants are shown in Table 2.
Table 2.
Characteristics of study participantsA
Immunophenotyping.
The immunofluorescence staining of human cells by lysed whole blood method (BD Biosciences — Immunocytometry Systems) was followed as previously described (29). The monoclonal antibodies used were: CD4 peridinin-chlorophyll-protein complex (PerCP); FITC or allophycocyanin (APC) (clone SK3); CD8 FITC or PerCP (clone SK1); CD3 FITC, PerCP, or APC (clone SK7); CD45RO APC or PE (clone UCHL-1); CD62L PE (clone SK11); CD45RA FITC or PE (clone L48); CD27 FITC or PE (clone L128); CD28 (CD28.2); CD38 (HB-7); HLA-DR (L243); CD95 PE (clone DX2); CD69 PE (clone L78); CCR5 (2D7); CXCR4 (12G5); TNFRII (911B3H10); CTLA-4 (BN13); CD25 PE or FITC or APC (clone 2A3); CD122 PE (clone Tu-27); CD132 (clone AG184); and IgG1 FITC, PE, or APC (clone X40) (all from BD Biosciences — Pharmingen and BD Biosciences — Immunocytometry Systems). CD127 (clone R34.34) was obtained from Beckman Coulter Inc. and CXCR3 (49801), CXCR5 (clone 51505), and biotinylated chicken anti-TGFβ 1 from R&D Systems. The samples were analyzed on a 4-color multiparameter flow cytometer (FACSCalibur; BD Biosciences — Immunocytometry Systems). Approximately 1–1.5 × 105 total events and a minimum of 5,000 events in the CD4+ gate were collected per sample.
Intracellular staining for the nuclear antigen Ki67 was performed as previously described (39) with Ki67 PE antibody (clone B56; BD Biosciences — Pharmingen). Intracellular cytokine staining of freshly isolated separated subsets after 4 hours of PMA/ionomycin stimulation was performed as described by Prussin et al. (40). Staining of live cells with annexin V and 7-aminoactinomycin D (7-AAD) was performed as previously described (29) in cells that were incubated at 37°C overnight in complete media with or without anti-CD95 (BD Biosciences — Pharmingen) at 1 μg/ml. FlowJo software was used for all flow cytometric data analysis (version 6; Tree Star Inc.).
Separation of naive CD4+CD25+ and naive CD4+CD25– cells by magnetic beads.
Fresh PBMCs were obtained from lymphapheresis and isolated by Ficoll-Hypaque lymphocyte separation. Selection of CD4+ cells was performed by multisort CD4+ separation or CD4+ T cell isolation procedure (Miltenyi Biotec), according to the manufacturer’s recommended protocols. The resulting population was on average 95% CD4+ positive and was further depleted of CD45RO+ cells with the use of CD45RO beads. The CD4+CD45RO– fraction was further separated into CD25+ and CD25– fractions by CD25 microbeads (Miltenyi Biotec). The purity of the separated CD4+CD25– and CD4+CD25+ subsets was on average 90% (range: 80–95%) (Figure 1). A modified version of the protocol for positive selection was used to separate CD4+CD45RO+CD25+high cells that have been described as highly enriched in Tregs (41); CD4+CD45RO+ cells were first incubated with 5 μl of CD25 beads/107 cells and underwent 3 consecutive simple positive selections (autoMACS; Miltenyi Biotec) to obtain the CD25+high population. The initial negative fraction was reincubated with CD25 beads at 20 μl/107 cells and underwent “sensitive depletion” (an autoMAC option) to obtain the CD25-negative subset (example from a healthy volunteer is shown in Supplemental Figure 1A). In a few experiments, cell subsets were alternatively obtained by cell sorting (FACSAria; BD Biosciences — Pharmingen).
TREC analysis.
TREC in purified cell subsets was quantitated by real-time PCR by the cell-lysis method as described previously (42). The consistency of the DNA content of the cell lysate was checked by real-time PCR using a ribosomal protein gene and the TaqMan Gene Expression Assay kit from Applied Biosystems.
HIV-1 proviral DNA measurement.
HIV-1 proviral DNA was quantitated by PCR using primers from the gag region. 8E5 cells containing one copy of the proviral DNA were used to obtain the standard graph as previously described (43).
TCR-repertoire analysis.
Separated CD4+ subsets (3.5–6.0 × 106) from 9 patients were stored in RNAlater (Ambion) at –70°C until use. Cells were washed using cold PBS, and total cellular RNA was isolated from the cells using the RNeasy Isolation Kit (QIAGEN). Total cellular RNA (5 μg) served as a template for cDNA synthesis using the SuperScript First Strand Synthesis system for RT-PCR (Invitrogen Corp.) with oligonucleotide deoxythymidine (dT). The synthesized cDNA (5 μg) was used to amplify the TCR Vβ genes by PCR. A total of 24 aliquots of the synthesized cDNA were amplified for 40 cycles with 1 of 24 TCR Vβ-specific primers and unlabelled Cβ primer (44). Aliquots of the 24 PCR products (2 μl) were then labeled by 7 cycles of PCR amplification with 6-carboxyfluorescein–conjugated (6-FAM–conjugated) Cβ primer. The labeled products (2.5 μl) were then resolved by electrophoresis on 6% polyacrylamide gels with a size standard, GeneScan 500 TAMRA (Applied Biosystems). Data were analyzed with the Applied Biosystems PRISM 377 automated sequencing system using GeneScan Analysis software (version 3.1.2) (Applied Biosystems).
CFSE-mixing experiments.
CD4+CD25+ and CD4+CD25– cells were separated from PBMCs of 4 independent donors and stained with CFSE as previously described (45). The CFSE-stained subset (CD25– or CD25+) was cultured with CFSE-unstained CD4-depleted PBMCs in the presence or absence of CFSE-unstained opposite CD4 subset (CD25+ or CD25– respectively). In vitro culture with complete medium with or without IL-2 100 IU/ml (aldesleukin; kindly provided by Chiron) was performed for 5–7 days. The initial phenotype of CD4+ T cells was traced according to CFSE-staining. At day 5–7, proliferation of the CFSE-stained fraction was evaluated by CFSE dilution. CD25 and intracellular Ki67 expression were evaluated in both CD4 subsets after gating on CFSE-stained CD4 T cells, which were compared to unstained CD4 T cells.
In the mixing experiments studying suppression, CFSE-stained CD4+CD45RO–CD25– cells were cocultured at different ratios with CFSE-unstained CD4+CD45RO–CD25+ or CD4+CD45RO+CD25+high cells.
Real-time quantitative PCR and RT-PCR for foxP3 detection.
Total RNA was extracted by an RNeasy Mini Kit (QIAGEN) and treated with DNase 1 to eliminate possible genomic DNA contamination. cDNA was prepared with random hexamers using Superscript ΙΙ reverse transcriptase (Invitrogen Corp.). Messenger RNA levels were quantified by real-time PCR using the ABI 7000 Sequence Detection System (Applied Biosystems). FoxP3, IL-2 receptor α (IL-2–RA), glucocorticoid-induced TNF receptor (GITR), CTLA-4, and IL-10 quantitative analyses were performed using TaqMan gene expression assays (Applied Biosystems). Samples were run in triplicate, and their expression levels were determined by comparing experimental levels to standard curves generated using serial dilutions of the same positive sample. 18S rRNA was used for normalization. Data were expressed as fold-change values using CD4+CD45RO–CD25– as a reference sample.
RT-PCR was performed in a 50-μl reaction containing 1XPCR Gold Buffer, 0.2 mM dNTPs, 1.5 mM MgCl2, 20 pmol of primers, and 1.25 U of AmpliTaq Gold (Applied Biosystems). cDNA from each population was subjected to nonsaturating PCR (32 cycles) using the following primer pairs: foxP3, 5′-GAAACAGCACATTCCCAGAGTTC-3′ and 5′-ATGGCCCAGCGGATGAG-3′; and GAPDH, 5′-CCACATCGCTCAGACACCAT-3′ and 5′-GGCAACAATATCCACTTTACCAGAGT-3′ (46).
Statistical methods.
Medians and distributions of the data for the different cell subsets (CD4+CD45RO–CD25+, CD4+CD45RO–CD25– and CD4+CD45RO+ cells) were compared by the Wilcoxon 2-sample method.
Supplementary Material
Acknowledgments
The authors would like to thank all the participating patients and the staff of the National Institute of Allergy and Infectious Diseases/Critical Care Medicine Department for their enthusiastic support, Anthony S. Fauci for his ongoing encouragement, and Zvi Bentwich for his insightful comments and suggestions. This project has been funded in part with federal funds under National Cancer Institute contract NO1-CO-12400. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organization imply endorsement by the US government.
Footnotes
Nonstandard abbreviations used: 7-AAD, 7-aminoactinomycin D; APC, allophycocyanin; CEN, cytokine-expanded naive; CTLA-4, cytotoxic T lymphocyte–associated protein 4; foxP3, forkhead transcription factor P3; HLA-DR, human leukocyte antigen; MFI, median fluorescent intensity; PerCP, peridinin-chlorophyll-protein complex; PHA, phytohemagglutinin; sjTREC, signal joint TREC; TNFRII, TNF receptor II; TREC, T cell receptor excision circle.
Conflict of interest: The US Government has been granted a use patent for intermittent IL-2 therapy (“Immunologic enhancement with intermittent interleukin-2 therapy,” US Patent 6,548,055, issued April 15, 2003) wherein H. Clifford Lane and Joseph A. Kovacs are named as inventors. All other authors have declared that no conflict of interest exists.
References
- 1.Kovacs JA, et al. Controlled trial of interleukin-2 infusions in patients infected with the human immunodeficiency virus. N. Engl. J. Med. 1996;335:1350–1356. doi: 10.1056/NEJM199610313351803. [DOI] [PubMed] [Google Scholar]
- 2.Davey RT, Jr, et al. A randomized trial of high- versus low-dose subcutaneous interleukin-2 outpatient therapy for early human immunodeficiency virus type 1 infection. J. Infect. Dis. 1999;179:849–858. doi: 10.1086/314678. [DOI] [PubMed] [Google Scholar]
- 3.Davey RT, Jr, et al. Immunologic and virologic effects of subcutaneous interleukin 2 in combination with antiretroviral therapy: a randomized controlled trial. JAMA. 2000;284:183–189. doi: 10.1001/jama.284.2.183. [DOI] [PubMed] [Google Scholar]
- 4.Sereti I, et al. IL-2–induced CD4+ T-cell expansion in HIV-infected patients is associated with long-term decreases in T-cell proliferation. Blood. 2004;104:775–780. doi: 10.1182/blood-2003-12-4355. [DOI] [PubMed] [Google Scholar]
- 5.Sereti I, et al. Long-term effects of intermittent interleukin 2 therapy in patients with HIV infection: characterization of a novel subset of CD4(+)/CD25(+) T cells. Blood. 2002;100:2159–2167. [PubMed] [Google Scholar]
- 6.Thornton AM, Piccirillo CA, Shevach EM. Activation requirements for the induction of CD4+CD25+ T cell suppressor function. Eur. J. Immunol. 2004;34:366–376. doi: 10.1002/eji.200324455. [DOI] [PubMed] [Google Scholar]
- 7.Malek TR. The main function of IL-2 is to promote the development of T regulatory cells. J. Leukoc. Biol. 2003;74:961–965. doi: 10.1189/jlb.0603272. [DOI] [PubMed] [Google Scholar]
- 8.Shevach EM. Certified professionals: CD4(+)CD25(+) suppressor T cells. J. Exp. Med. 2001;193:F41–F46. doi: 10.1084/jem.193.11.f41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jonuleit H, et al. Identification and functional characterization of human CD4(+)CD25(+) T cells with regulatory properties isolated from peripheral blood. J. Exp. Med. 2001;193:1285–1294. doi: 10.1084/jem.193.11.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taams LS, et al. Human anergic/suppressive CD4(+)CD25(+) T cells: a highly differentiated and apoptosis-prone population. Eur. J. Immunol. 2001;31:1122–1131. doi: 10.1002/1521-4141(200104)31:4<1122::aid-immu1122>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 11.Stephens LA, Mottet C, Mason D, Powrie F. Human CD4(+)CD25(+) thymocytes and peripheral T cells have immune suppressive activity in vitro. Eur. J. Immunol. 2001;31:1247–1254. doi: 10.1002/1521-4141(200104)31:4<1247::aid-immu1247>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 12.Almeida AR, Legrand N, Papiernik M, Freitas AA. Homeostasis of peripheral CD4+ T cells: IL-2R alpha and IL-2 shape a population of regulatory cells that controls CD4+ T cell numbers. J. Immunol. 2002;169:4850–4860. doi: 10.4049/jimmunol.169.9.4850. [DOI] [PubMed] [Google Scholar]
- 13.Akbar AN, Taams LS, Salmon M, Vukmanovic-Stejic M. The peripheral generation of CD4+ CD25+ regulatory T cells. Immunology. 2003;109:319–325. doi: 10.1046/j.1365-2567.2003.01678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 15.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- 16.Mohri H, et al. Increased turnover of T lymphocytes in HIV-1 infection and its reduction by antiretroviral therapy. J. Exp. Med. 2001;194:1277–1287. doi: 10.1084/jem.194.9.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kovacs JA, et al. Identification of dynamically distinct subpopulations of T lymphocytes that are differentially affected by HIV. J. Exp. Med. 2001;194:1731–1741. doi: 10.1084/jem.194.12.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oswald-Richter K, et al. HIV infection of naturally occurring and genetically reprogrammed human regulatory T-cells. PLoS Biol. 2004;2:E198. doi:10.1371/journal.pbio.0020198. doi: 10.1371/journal.pbio.0020198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kinter AL, et al. CD25(+)CD4(+) regulatory T cells from the peripheral blood of asymptomatic HIV-infected individuals regulate CD4(+) and CD8(+) HIV-specific T cell immune responses in vitro and are associated with favorable clinical markers of disease status. J. Exp. Med. 2004;200:331–343. doi: 10.1084/jem.20032069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weiss L, et al. Human immunodeficiency virus-driven expansion of CD4+CD25+ regulatory T cells which suppress HIV-specific CD4 T-cell responses in HIV-infected patients. Blood. 2004;104:3249–3256. doi: 10.1182/blood-2004-01-0365. [DOI] [PubMed] [Google Scholar]
- 21.Kimmig S, et al. Two subsets of naive T helper cells with distinct T cell receptor excision circle content in human adult peripheral blood. J. Exp. Med. 2002;195:789–794. doi: 10.1084/jem.20011756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Unutmaz D, Pileri P, Abrignani S. Antigen-independent activation of naive and memory resting T cells by a cytokine combination. J. Exp. Med. 1994;180:1159–1164. doi: 10.1084/jem.180.3.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Geginat J, Sallusto F, Lanzavecchia A. Cytokine-driven proliferation and differentiation of human naive, central memory, and effector memory CD4(+) T cells. J. Exp. Med. 2001;194:1711–1719. doi: 10.1084/jem.194.12.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neidhart M, Pataki F, Schonbachler J, Bruhlmann P. Flow cytometric characterisation of the “false naive” (CD45RA+, CD45RO–, CD29 bright+) peripheral blood T-lymphocytes in health and in rheumatoid arthritis. Rheumatol. Int. 1996;16:77–87. doi: 10.1007/BF01816439. [DOI] [PubMed] [Google Scholar]
- 25.Murali-Krishna K, Ahmed R. Cutting edge: naive T cells masquerading as memory cells. J. Immunol. 2000;165:1733–1737. doi: 10.4049/jimmunol.165.4.1733. [DOI] [PubMed] [Google Scholar]
- 26.Goldrath AW, Bogatzki LY, Bevan MJ. Naive T cells transiently acquire a memory-like phenotype during homeostasis-driven proliferation. J. Exp. Med. 2000;192:557–564. doi: 10.1084/jem.192.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roth MD. Interleukin 2 induces the expression of CD45RO and the memory phenotype by CD45RA+ peripheral blood lymphocytes. J. Exp. Med. 1994;179:857–864. doi: 10.1084/jem.179.3.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen W, et al. Conversion of peripheral CD4+CD25– naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J. Exp. Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sereti I, et al. CD4 T cell expansions are associated with increased apoptosis rates of T lymphocytes during IL-2 cycles in HIV infected patients. AIDS. 2001;15:1765–1775. doi: 10.1097/00002030-200109280-00004. [DOI] [PubMed] [Google Scholar]
- 30.Cho BK, Rao VP, Ge Q, Eisen HN, Chen J. Homeostasis-stimulated proliferation drives naive T cells to differentiate directly into memory T cells. J. Exp. Med. 2000;192:549–556. doi: 10.1084/jem.192.4.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tanchot C, Le Campion A, Leaument S, Dautigny N, Lucas B. Naive CD4(+) lymphocytes convert to anergic or memory-like cells in T cell-deprived recipients. Eur. J. Immunol. 2001;31:2256–2265. doi: 10.1002/1521-4141(200108)31:8<2256::aid-immu2256>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 32.Unutmaz D, Baldoni F, Abrignani S. Human naive T cells activated by cytokines differentiate into a split phenotype with functional features intermediate between naive and memory T cells. Int. Immunol. 1995;7:1417–1424. doi: 10.1093/intimm/7.9.1417. [DOI] [PubMed] [Google Scholar]
- 33.Emery S, et al. The evaluation of subcutaneous proleukin (interleukin-2) in a randomized international trial: rationale, design, and methods of ESPRIT. Control Clin. Trials. 2002;23:198–220. doi: 10.1016/s0197-2456(01)00179-9. [DOI] [PubMed] [Google Scholar]
- 34.Grossman Z, Paul WE. The impact of HIV on naive T-cell homeostasis. Nat. Med. 2000;6:976–977. doi: 10.1038/79667. [DOI] [PubMed] [Google Scholar]
- 35.Godfrey WR, et al. Cord blood CD4+CD25+-derived T regulatory cell lines express FoxP3 protein and manifest potent suppressor function. Blood. 2005;105:750–758. doi: 10.1182/blood-2004-06-2467. [DOI] [PubMed] [Google Scholar]
- 36.Bayer AL, Yu A, Adeegbe D, Malek TR. Essential role for interleukin-2 for CD4+CD25+ T regulatory cell development during the neonatal period. J. Exp. Med. 2005;201:769–777. doi: 10.1084/jem.20041179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Setoguchi R, Hori S, Takahashi T, Sakaguchi S. Homeostatic maintenance of natural Foxp3+ CD25+ CD4+ regulatory T cells by interleukin (IL)-2 and induction of autoimmune disease by IL-2 neutralization. J. Exp. Med. 2005;201:723–735. doi: 10.1084/jem.20041982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Farel CE, et al. Induction and maintenance therapy with intermittent interleukin-2 in HIV-1 infection. Blood. 2004;103:3282–3286. doi: 10.1182/blood-2003-09-3283. [DOI] [PubMed] [Google Scholar]
- 39.Hazenberg MD, et al. T-cell division in human immunodeficiency virus (HIV)-1 infection is mainly due to immune activation: a longitudinal analysis in patients before and during highly active antiretroviral therapy (HAART) Blood. 2000;95:249–255. [PubMed] [Google Scholar]
- 40.Prussin C, Metcalfe DD. Detection of intracytoplasmic cytokine using flow cytometry and directly conjugated anti-cytokine antibodies. J. Immunol. Methods. 1995;188:117–128. doi: 10.1016/0022-1759(95)00209-x. [DOI] [PubMed] [Google Scholar]
- 41.Baecher-Allan C, Viglietta V, Hafler DA. Inhibition of human CD4(+)CD25(+high) regulatory T cell function. J. Immunol. 2002;169:6210–6217. doi: 10.4049/jimmunol.169.11.6210. [DOI] [PubMed] [Google Scholar]
- 42.Natarajan V, et al. Increased peripheral expansion of naive CD4+ T cells in vivo after IL-2 treatment of patients with HIV infection. Proc. Natl. Acad. Sci. U. S. A. 2002;99:10712–10717. doi: 10.1073/pnas.162352399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Elbeik, T., Dewar, R.L., and Natarajan, V. 1997. Isolation and detection of human immunodeficiency virus. In Manual of clinical laboratory immunology. 5th edition. N.R. Rose et al., editors. ASM Press. Washington, DC, USA. 781–787.
- 44.Connors M, et al. HIV infection induces changes in CD4+ T-cell phenotype and depletions within the CD4+ T-cell repertoire that are not immediately restored by antiviral or immune-based therapies. Nat. Med. 1997;3:533–540. doi: 10.1038/nm0597-533. [DOI] [PubMed] [Google Scholar]
- 45.Sereti I, Gea-Banacloche J, Kan MY, Hallahan CW, Lane HC. Interleukin 2 leads to dose-dependent expression of the alpha chain of the IL-2 receptor on CD25-negative T lymphocytes in the absence of exogenous antigenic stimulation. Clin. Immunol. 2000;97:266–276. doi: 10.1006/clim.2000.4929. [DOI] [PubMed] [Google Scholar]
- 46.Walker MR, et al. Induction of FoxP3 and acquisition of T regulatory activity by stimulated human CD4+CD25– T cells. J. Clin. Invest. 2003;112:1437–1443. doi:10.1172/JCI200319441. doi: 10.1172/JCI19441. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.