Abstract
Background
Acute kidney injury (AKI) frequently occurs as a complication of sepsis. PANoptosis refers to a type of inflammatory programmed cell death that exhibits key characteristics of apoptosis, necroptosis, and pyroptosis. Here, we evaluated the role of absent in melanoma 2 (AIM2) and eukaryotic translation initiation factor 2 alpha kinase 2 (EIF2AK2) in septic AKI.
Methods
A septic AKI model was created through cecal ligation and puncture (CLP), while an in vitro model was developed using lipopolysaccharide (LPS)-stimulated HK2 cells. Hematoxylin and eosin (HE), Periodic acid-Schiff (PAS), and TUNEL staining were conducted to assess kidney injury in mice. Levels of serum creatinine (Scr) and blood urea nitrogen (BUN) were detected by kits. Gene expression was detected utilizing RT-qPCR, and Western blot was used to test protein levels. Immunofluorescence was employed to measure EIF2AK2 and AIM2 expression in mouse kidney tissue. Lactate dehydrogenase (LDH) activity assay was conducted to evaluate cytotoxicity. Co-immunoprecipitation (Co-IP) was performed to verify the binding relationship between EIF2AK2 and AIM2.
Results
AIM2 expression was increased in the renal tissue of mice subjected to CLP. Activation of the inflammasome and PANoptosis were observed in the renal tissue of CLP mice. AIM2 depletion attenuated PANoptosis in LPS-treated HK-2 cells. Additionally, EIF2AK2 could directly target AIM2, leading to a positive regulation of AIM2 expression. Notably, EIF2AK2 induced PANoptosis through upregulating AIM2 in HK-2 cells stimulated by LPS.
Conclusions
Our results revealed the important role of EIF2AK2-induced AIM2 upregulation in the activation of PANoptosis during septic AKI.
Keywords: Acute kidney injury, AIM2, EIF2AK2, PANoptosis, sepsis
Highlights
Renal tissue from CLP mice exhibited an increase in AIM2 expression.
Renal tissue from CLP mice demonstrated inflammasome activation and PANoptosis.
AIM2 silencing reduced PANoptosis in LPS-treated HK-2 cells.
EIF2AK2 directly targeted AIM2 and positively regulated its expression.
EIF2AK2 promoted PANoptosis via AIM2 in LPS-triggered HK-2 cells.
1. Introduction
Sepsis is a severe syndrome of organ dysfunction that arises from an imbalance in the host’s response to infection, triggered by excessive inflammatory factors, with high morbidity and mortality [1]. Acute kidney injury (AKI) stands as a critical and significant complication frequently observed in cases of sepsis. Due to the complexity of the pathophysiological mechanism of AKI in sepsis, there is currently no effective specific therapy [2]. Therefore, exploring the potential mechanism of sepsis-induced AKI is of great significance. Emerging evidence indicates that apoptosis, necroptosis, and pyroptosis play significant roles in sepsis-triggered AKI [3–6]. PANoptosis, a recently identified form of programmed cell death, encompasses three primary modes of programmed cell death: apoptosis, necroptosis, and pyroptosis. This process is closely associated with infectious diseases induced by bacteria, fungi, viruses, and parasites [7]. The signal transduction process of PANoptosis typically initiates with the recognition of specific danger signals, either endogenous or exogenous, by receptor proteins. The downstream adaptor proteins receive signals identified by receptor proteins and transmit them to effector proteins such as caspase (CASP), RIP kinase (RIPK), and mixed lineage kinase domain-like (MLKL) through protein–protein interactions. CASP1 can cleave gasdermin D (GSDMD) to localize and aggregate into pores on the cell membrane. CASP8 triggers the activation of CASP3 and CASP7. RIPK1 engages with RIPK3 to activate the latter, leading to the phosphorylation of MLKL and ultimately inducing programmed death, such as pyroptosis, apoptosis, and necroptosis, and promoting the release of inflammatory factors [8, 9]. Nevertheless, the contribution of PANoptosis to septic renal injury has not been investigated yet.
The absent in melanoma 2 (AIM2) inflammasome functions as a sensor for dsDNA and plays a crucial role in various conditions, including infectious diseases, inflammatory diseases, and cancer [10]. A recent study demonstrated that AIM2 has the ability to modulate inflammatory signaling and PANoptosis by regulating the innate immune sensors pyrin and ZBP1 [11]. In sepsis, AIM2 holds potential diagnostic value for sepsis diagnosis [12]. Moreover, AIM2 is involved in the pathogenesis of kidney injury. For example, proinflammatory phenotype, which contributes to chronic kidney injury, is driven by activating AIM2 inflammasome through DNA derived from necrotic cells [13]. The calpain inhibitor calpeptin-mediated AIM2 inflammasome may provide a potential novel preventative and therapeutic target for AKI [14]. Nevertheless, the precise function of AIM2 in the context of sepsis-induced AKI remains unclear. Whether AIM2 regulates PANoptosis to promote AKI caused by sepsis remains to be explored.
The eukaryotic translation initiation factor 2 alpha kinase 2 (EIF2AK2, also known as PKR) was originally identified as a pathogen recognition molecule that binds to double-stranded RNA at the N-terminus, initiating activation of the C-terminal catalytic region [15]. Activation of EIF2AK2 plays a pivotal role in severe sepsis [16]. Targeting EIF2AK2 inhibition presents a promising therapeutic approach to mitigate septic AKI [17]. Studies have indicated a relationship between the activation of AIM2 and EIF2AK2, where pharmacological and genetic inhibition of EIF2AK2 reduces the activation of AIM2 inflammasome [18]. Through GeneMANIA database analysis, we discovered a physical interaction between AIM2 and EIF2AK2 proteins. Therefore, we speculate that the EIF2AK2 protein may bind to AIM2 to induce its activation and jointly mediate PANoptosis.
In this study, we developed both in vivo and in vitro models of sepsis-induced AKI to examine the impact of AIM2. We hypothesize that in sepsis-triggered AKI, EIF2AK2 can target activation of AIM2 to form a complex and mediate renal cell PANoptosis by activating the inflammasome. This study has the potential to offer a theoretical foundation for the treatment of sepsis-associated AKI.
2. Materials and methods
2.1. Septic AKI animal model
Male C57BL/6 mice (7–8 weeks old) weighing 18–22 g were acquired from Shanghai SLAC Laboratory Animal Co., Ltd. (Shanghai, China). Throughout the feeding procedure, each cage housed four animals, with a daily provision of ample water and feed. Additionally, a 12-h light cycle was maintained consistently. To establish a sepsis model in mice, we employed the method of cecal ligation and puncture (CLP) as previously recorded [19]. For the CLP group, a midline laparotomy was performed to expose the cecum. Subsequently, the distal end of cecal valve was ligated. Using a 20-gauge needle, the cecum was punctured multiple times. Gentle pressure was applied to expel a small quantity of stool from the punctured cecum into the abdominal cavity. The cecum was repositioned within the peritoneal cavity. Subsequently, the incision was closed using two sutures. Conversely, the Sham group underwent an externalization of the cecum without any ligation or puncture. For A151 (AIM2 inhibitor) administration, the mice underwent CLP were then randomly assigned to treatment groups with A151 (Tsingke Biotechnology Co., Ltd., Beijing, China) or PBS. A151 was administered by IP injection at 300 μg in 0.2-mL PBS per mouse immediately after CLP, and control mice were injected with the same volumes of PBS. Animals were sacrificed by cervical dislocation 12 h following the CLP surgery. Subsequently, both blood and kidney tissues were collected for further experiments. Approval for the research program was obtained from Laboratory Animal Welfare and Ethical Committee of the Hospital.
2.2. Hematoxylin and eosin (HE) staining
Kidney tissues from mice were sectioned into slices with a thickness of 5 μm. These sections were then preserved in 10% (w/v) neutral-buffered formalin at 4 °C overnight. Following this, the tissue sections underwent dehydration through a series of graded ethanol solutions, followed by cleaning, and embedding in paraffin. The sections were stained with hematoxylin for a duration of 10 min, followed by eosin staining for 5 min. Slides were examined and visualized using a microscopy (Olympus, Tokyo, Japan). Histopathological alterations were assessed based on the proportion of affected renal tubules, characterized by tubular disintegration, expansion, breakdown, and cast formation. The degree of tissue injury was graded on a scale from 0 to 4, with 0, 1, 2, 3, and 4 representing 0%, less than 25%, 26% to 50%, 51% to 75%, and 76% or more of affected renal tubules, respectively.
2.3. Periodic acid-Schiff (PAS) staining
Sections of mouse kidney tissues (5 μm-thick) were prepared and subjected to PAS staining, following the provided instructions from manufacturer. In brief, the sections were incubated with periodic acid solution (Beyotime, Shanghai, China) for 15 min, followed by rinsing twice. Subsequently, the sections were darkly incubated with Schiff solution (Beyotime, Shanghai, China) for 30 min and then rinsed with running water. The stained sections were observed utilizing a microscope (Olympus, Tokyo, Japan).
2.4. TUNEL
TUNEL Apoptosis Assay kit (Beyotime, Shanghai, China) was adopted to determine apoptotic cells. Sections of mouse kidney tissues were deparaffinized and subjected to proteinase K treatment at a temperature of 37 °C for 30 min. Following two washes with PBS, the slides were incubated with a TUNEL detection solution for 1 h. A fluorescent microscope (Olympus, Tokyo, Japan) was used to observe TUNEL-positive cells. For comparison, five randomly selected fields were examined, and the number of positive cells per unit area was calculated.
2.5. Renal function assessment
Mouse tail vein blood samples were subjected to centrifugation at 4 °C and 3000 rpm for 20 min. Subsequently, levels of serum creatinine (Scr) and blood urea nitrogen (BUN) were measured utilizing a colorimetric method and a creatinine assay kit (Nanjing Jiancheng, Nanjing, China) following the manufacturer’s instructions.
2.6. Quantitative real-time PCR
Total RNA from HK-2 cells was isolated with TRIzol reagent (Invitrogen, Carlsbad, CA), and reverse-transcribed in cDNA using HiScript® III RT SuperMix (Invitrogen, Carlsbad, CA). The qPCR was conducted with SYBR® qPCR Master Mix (TaKaRa, Dalian, China) on DNA Engine Opticon 2 System. The primer sequences were as follows: AIM2 F: 5′-CTGCAGTGATGAAGACCATTCGTA-3′, R: 5′-GGTGCAGCACGTTGCTTTG-3′. β-actin was used as the internal control. Relative expression was quantified using the comparative CT method (2−(Gene Ct − Normalizer Ct)).
2.7. Western blot
Tissue or cell proteins were extracted using RIPA buffer (Beyotime, Shanghai, China). The protein content was determined utilizing a BCA protein assay kit (Beyotime, Shanghai, China). Subsequently, the proteins were separated using SDS-PAGE and transferred onto PVDF membranes (Millipore, Bedford, MA). Following blocking with 5% nonfat dry milk, the membranes were incubated overnight at 4 °C with primary antibodies: AIM2 (14-6008-93, 1:1000, Invitrogen, Carlsbad, CA), EIF2AK2 (ab184257, 1:1000, Abcam, Cambridge, MA), CASP1 (PA5-87536, 1:1000, Invitrogen, Carlsbad, CA), CASP3 (700182, 1:1000, Invitrogen, Carlsbad, CA), CASP8 (PA5-87373, 1:1000, Invitrogen, Carlsbad, CA), GSDMD (PA5-116815, 1:1000, Invitrogen, Carlsbad, CA), p-RIPK1 (PA5-105363, 1:1000, Invitrogen, Carlsbad, CA), RIPK1 (PA5-20811, 1:1000, Invitrogen, Carlsbad, CA), p-MLKL (ab196436, 1:1000, Abcam, Cambridge, MA), MLKL (ab243142, 1:1000, Abcam, Cambridge, MA), and ZBP1 (703166, 1:1000, Invitrogen, Carlsbad, CA). Then, HRP-conjugated secondary antibodies (#7074, 1:1,000, Cell Signaling Technology, Danvers, MA) were incubated for 1 h. Band detection was performed using an ECL system and an ECL kit (Amersham, GE Healthcare, Buckinghamshire, UK). The intensity of the bands was analyzed utilizing ImageJ software (NIH, Bethesda, MD), with β-actin and vinculin as internal controls.
2.8. Immunofluorescence (IF)
The mouse kidney tissue sections were initially subjected to a 2-h baking process at 55 °C in an oven. Subsequently, deparaffinization and rehydration steps were performed, followed by antigen retrieval utilizing sodium citrate. Immunostaining was conducted on the samples using anti-AIM2 (14-6008-93, 1:500, Invitrogen, Carlsbad, CA) and anti-EIF2AK2 (ab184257, 1:500, Abcam, Cambridge, MA) antibodies at 4 °C overnight. Subsequently, the sections were incubated with a fluorescently labeled secondary antibody for 1 h. Finally, the sections were counterstained with DAPI and examined using a fluorescence microscope (Olympus, Tokyo, Japan).
2.9. Lactate dehydrogenase (LDH) detection
The kidney tissue of mice and HK-2 cells was homogenized and centrifuged at 12,000 × g for 15 min. The supernatant was collected. LDH levels were assessed utilizing an LDH cytotoxicity detection kit (Nanjing Jincheng Biology Engineering Institute, Nanjing, China), following the provided instructions. The absorbance of the sample was determined at 450 nm with a microplate reader (Perkin Elmer, Waltham, MA).
2.10. Cell culture and treatment
Human proximal tubular epithelial cells (HK-2) were obtained from the American Type Cell Culture (ATCC) and cultured in DMEM (Roche, Basel, Switzerland) supplemented with 10% FBS (Roche, Basel, Switzerland) and 1% penicillin–streptomycin at 37 °C with 5% CO2. HK-2 cells were treated with lipopolysaccharide (LPS, 1 μg/mL; Sigma, St. Louis, MO) for 24 h.
2.11. Cell transfection
shRNAs targeting AIM2 (sh-AIM2#1, #2, #3) and EIF2AK2 (sh-EIF2AK2), along with the negative control sh-NC, were obtained from Sangon Biotech (Shanghai, China). The overexpression vector for AIM2 (OE-AIM2) and an empty vector were provided by GeneChem (Shanghai, China). Plasmids were transfected into HK-2 cells utilizing Lipofectamine 3000 (Invitrogen, Carlsbad, CA) for 48 h.
2.12. Co-immunoprecipitation (Co-IP)
A Co-IP assay was conducted to detect the binding relationship between EIF2AK2 and AIM2 utilizing the Dynabeads™ Co-Immunoprecipitation Kit (ThermoFisher Scientific, Waltham, MA). The extracted protein was incubated with antibodies specific to EIF2AK2 (ab184257, Abcam, Cambridge, MA), AIM2 (14-6008-93, Invitrogen, Carlsbad, CA), or IgG (ab2410, Abcam, Cambridge, MA) overnight at 4 °C. Following that, the mixture was subjected to centrifugation at 4 °C for a duration of 3 h after adding protein A + G agarose beads (Beyotime, Shanghai, China). Subsequently, the beads were eluted using SDS loading buffer. Finally, the protein levels of EIF2AK2 and AIM2 were analyzed by Western blot.
2.13. Calcein–propidium iodide (PI) staining
For the detection of cell death, a fluorescent signal, PI, was employed, while calcein fluorescently labeled living cells. Briefly, transfected cells (2 × 105 cells/well) were seeded in six-well plates and allowed to incubate overnight. Following this, the cells were exposed to LPS treatment for 24 h. After being washed twice with PBS, the cells were then incubated at 37 °C for 30 min with the PI and calcein fluorescent dye (Beyotime, Shanghai, China). Following another round of PBS washing, images were captured using a fluorescence microscope (Olympus, Tokyo, Japan).
2.14. Statistical analysis
All values are shown as mean ± standard deviation (SD) of three independent experiments. SPSS software 17.0 (SPSS, Chicago, IL) was conducted for data analysis. Student’s t-test (two groups) and one-way ANOVA (multiple groups) were performed on comparisons. p < .05 was considered statistically significant.
3. Results
3.1. AIM2 was highly expressed in septic AKI mice
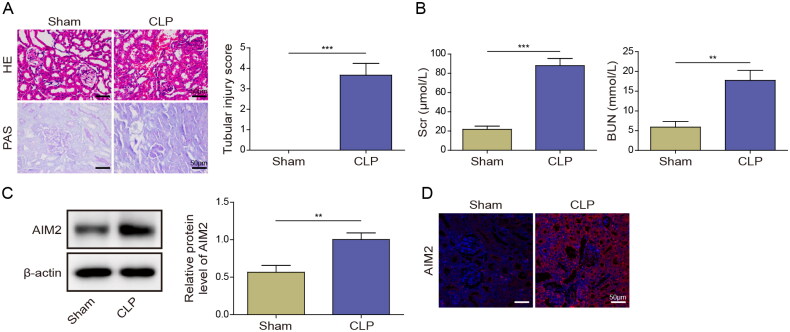
Initially, we induced septic AKI in mice through CLP. HE staining revealed that compared with the Sham group, renal tubular dilatation, swelling, cast formation, and loss of brush border were observed in the CLP group. PAS staining exhibited notable glomerular and tubulointerstitial damage in the CLP group (Figure 1(A)). Moreover, the serum levels of Scr and BUN in CLP mice were significantly elevated, which reflected kidney injury (Figure 1(B)). Western blot and IF analyses further demonstrated that the expression of AIM2 protein was elevated in the renal tissue of CLP mice, comparing with that in Sham mice (Figure 1(C,D)). Thus, the upregulation of AIM2 in sepsis-triggered AKI suggests its potential involvement in septic AKI.
Figure 1.
AIM2 was highly expressed in septic AKI mice. Sepsis mouse model was established utilizing CLP surgery. (A) HE staining and PAS staining were used to detect the changes of renal tissue in mouse model. (B) Serum levels of Scr and BUN in mice were tested by kits. (C, D) AIM2 expression in renal tissue of mice was assessed with Western blot and IF. **p < .01 and ***p < .001. n = 5 mice/group.
3.2. PANoptosis regulated sepsis-induced renal injury in mice
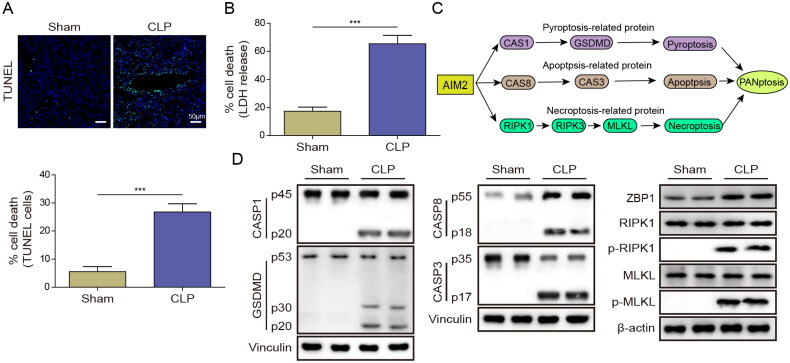
Next, TUNEL staining was utilized to evaluate apoptosis in mouse kidney tissue. Compared to Sham mice, the number of TUNEL-positive cells in the kidney tissue of CLP mice was obviously increased (Figure 2(A)). In addition, CLP treatment enhanced LDH release, indicating cytotoxicity in kidney tissue (Figure 2(B)). As shown in Figure 2(C), we speculate that AIM2 may regulate PANoptosis by affecting the expression of these proteins. Further, we investigated whether PANoptosis is involved in the regulation of septic AKI. Western blot analysis illustrated that inflammasome activation and cell death, including pyroptosis, apoptosis, and necroptosis, were observed in the kidneys of CLP mice, indicating the occurrence of PANoptosis (Figure 2(D)). Taken together, these findings reveal that PANoptosis may be the key driver of septic AKI development.
Figure 2.
PANoptosis regulated sepsis-induced renal injury in mice. (A) Images of TUNEL staining and TUNEL-positive cells in mouse kidney tissue. (B) LDH kit was adopted to test cytotoxicity. (C) AIM2 induces PANoptosis. (D) Western blot was utilized to determine the levels of pyroptosis, apoptosis, and necroptosis-related proteins in renal tissue of mice. ***p < .001. n = 5 mice/group.
3.3. AIM2 mediated sepsis-induced renal injury by regulating PANoptosis
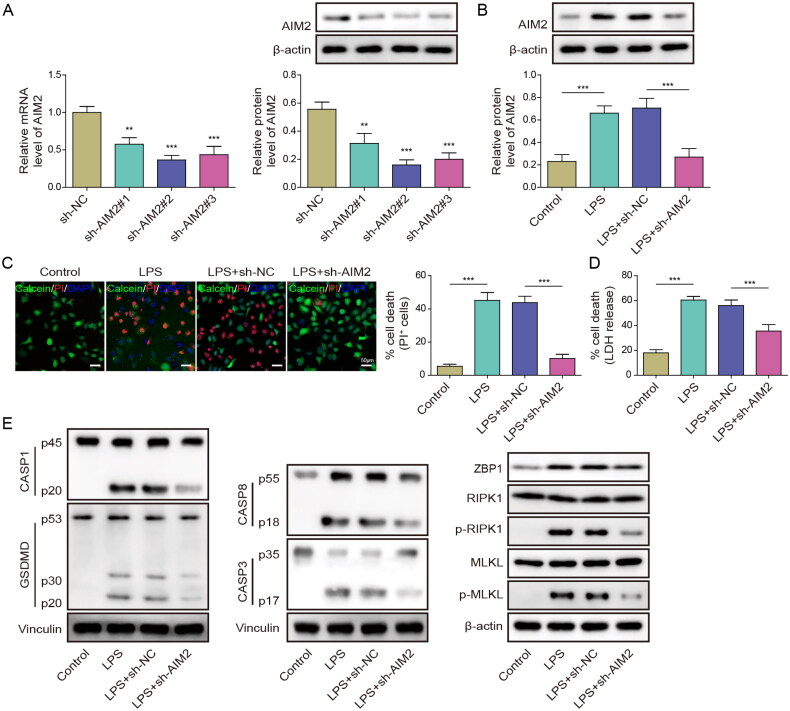
Subsequently, we investigated whether AIM2 regulates septic AKI by promoting PANoptosis. HK-2 cells were transfected with sh-AIM2#1, sh-AIM2#2 or sh-AIM2#3, and sh-AIM2#2 with the best knockdown efficiency was selected for further experiments (Figure 3(A)). Next, we established an AKI model in LPS-treated HK-2 cells. The HK-2 cells were transfected with sh-AIM2 or sh-NC, and then treated with LPS. AIM2 expression was elevated by LPS, but it was recovered by AIM2 depletion in LPS-treated cells (Figure 3(B)). PI staining indicated an increase in cell death following LPS stimulation, while cell death decreased after AIM2 knockdown (Figure 3(C)). In addition, LPS treatment induced LDH release, which was reversed by AIM2 silencing (Figure 3(D)). Furthermore, Western blot analysis demonstrated that LPS-induced HK-2 cells exhibited PANoptosis, while knockdown of AIM2 decreased PANoptosis (Figure 3(E)). Altogether, the above data indicate that AIM2 induces AKI in vitro by promoting PANoptosis.
Figure 3.
AIM2 mediated sepsis-induced renal injury by regulating PANoptosis. (A) Transfection efficiency of sh-AIM2 was assessed in HK-2 cells by RT-qPCR. Next, HK-2 cells were transfected with sh-AIM2 or sh-NC, and treated with LPS. (B) Western blot detection of AIM2 expression. (C) PI staining was used to detect cell death rate. (D) LDH kit was used to test cytotoxicity. (E) Western blot was performed to determine the levels of pyroptosis, apoptosis, necroptosis and PANoptosis-related proteins. Data are presented as mean ± SD of three independent experiments. **p < .01 and ***p < .001.
3.4. EIF2AK2 protein targeted and activated AIM2
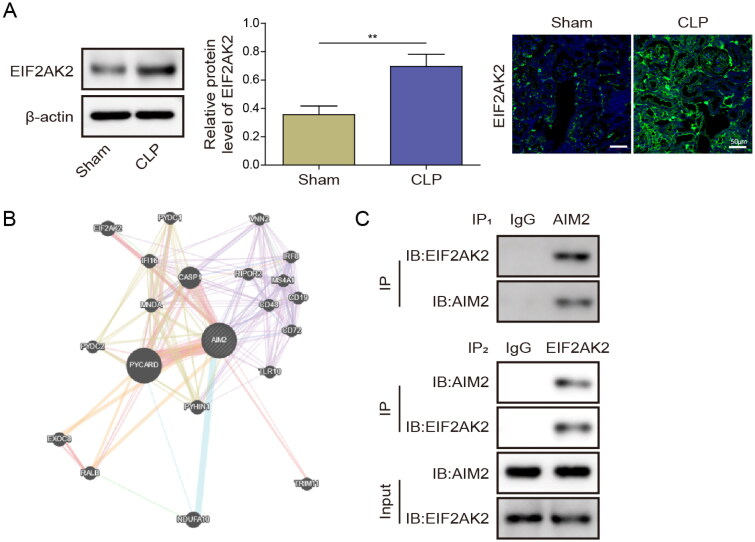
To delve deeper into the specific mechanisms through which AIM2 regulates septic AKI, Western blot and IF analyses were conducted to detect EIF2AK2 expression in the renal tissue of Sham and CLP mice. Results showed that the level of EIF2AK2 in the renal tissue of CLP mice was increased significantly (Figure 4(A)). Through GeneMANIA prediction, we found that EIF2AK2 interacted with AIM2 (Figure 4(B)). Furthermore, Co-IP assay verified that EIF2AK2 directly bound to AIM2 (Figure 4(C)). These results imply that EIF2AK2 has a direct relationship with AIM2.
Figure 4.
EIF2AK2 protein targeted and activated AIM2. (A) Western blot and IF were used to detect EIF2AK2 expression in renal tissue of Sham and CLP mice. (B) GeneMANIA database was utilized to predict the binding relationship between EIF2AK2 and AIM2. (C) Co-IP assay was used to verify the binding relationship between EIF2AK2 and AIM2. Data are the means ± SD for three independent experiments. **p < .01.
3.5. EIF2AK2 targeted activation of AIM2 mediated PANoptosis in sepsis-induced renal injury
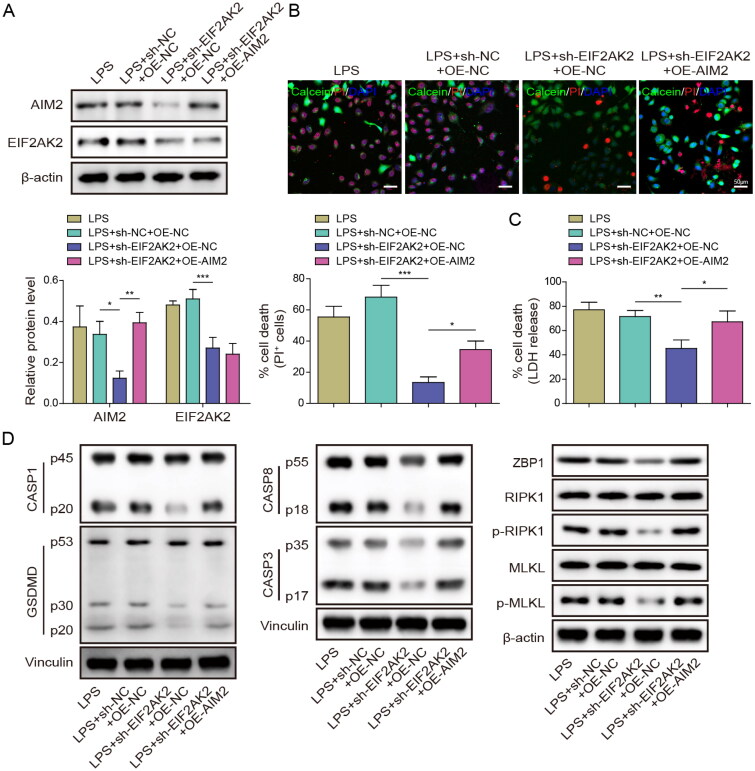
We further investigated the function of the EIF2AK2/AIM2 axis in PANoptosis during septic AKI in vitro. HK-2 cells were transfected with sh-EIF2AK2 or OE-AIM2, and then treated with LPS. AIM2 and EIF2AK2 expression was decreased after knockdown of EIF2AK2, while AIM2 expression was restored after overexpression of AIM2, but EIF2AK2 expression was not affected by AIM2 upregulation (Figure 5(A)). PI staining revealed that cell death was suppressed by sh-EIF2AK2, and it was recovered by AIM2 overexpression (Figure 5(B)). Additionally, LDH release was decreased when cells were transfected with sh-EIF2AK2, whereas co-transfection of OE-AIM2 reversed the reduction of LDH release (Figure 5(C)). Moreover, Western blot results showed that EIF2AK2 depletion decreased PANoptosis, while overexpressed AIM2 further increased PANoptosis (Figure 5(D)). Therefore, these results demonstrate that the EIF2AK2/AIM2 axis can induce PANoptosis in septic AKI.
Figure 5.
EIF2AK2 targeted activation of AIM2 mediated PANoptosis in sepsis-induced renal injury. HK-2 cells were transfected with sh-EIF2AK2 or OE-AIM2, and treated with LPS. (A) Western blot detection of EIF2AK2 and AIM2 expression. (B) PI staining was used to detect cell death rate. (C) LDH kit was used to test cytotoxicity. (D) Western blot was conducted to determine the levels of pyroptosis, apoptosis, necroptosis, and PANoptosis-related proteins. Values were expressed as mean ± SD of three separate determinations. *p < .05, **p < .01, and ***p < .001.
3.6. Inhibition of AIM2-mediated PANoptosis alleviated sepsis-induced renal injury in mice
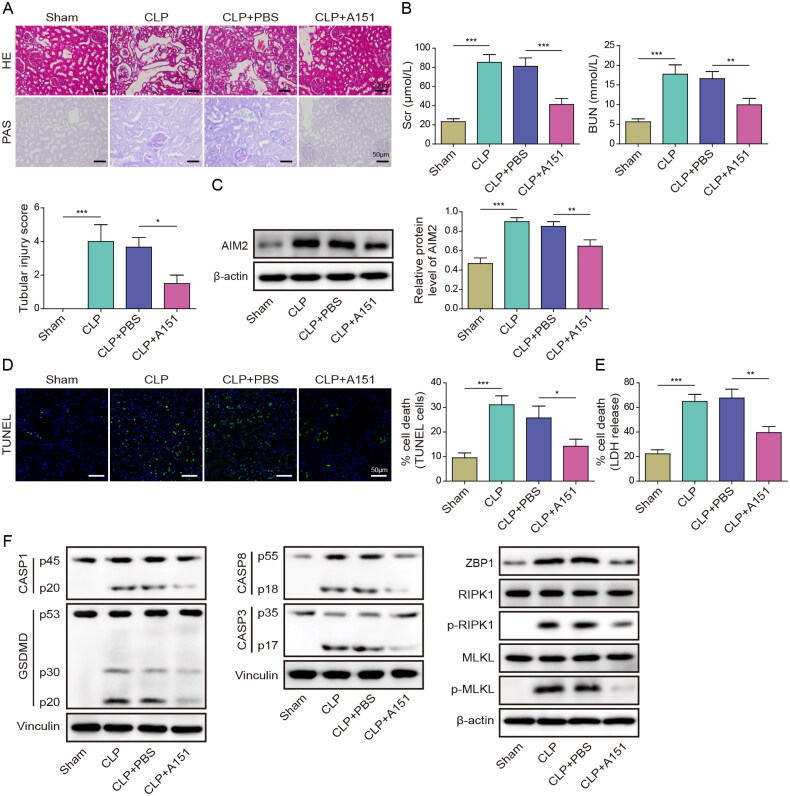
Subsequently, we investigated whether AIM2 could mediate sepsis-induced AKI by regulating PANoptosis in vivo. The mice were subjected to different groups: Sham group, CLP group, CLP + PBS group, and CLP + A151 (AIM2 inhibitor) group. Histological examination using HE staining indicated that the CLP group displayed renal tubular dilatation, swelling, cast formation, and loss of brush border, contrasting with the findings in the Sham group. PAS staining highlighted significant glomerular and tubulointerstitial injuries in the CLP group. However, the damage was alleviated after adding AIM2 inhibitor (Figure 6(A)). The Scr and BUN in the kidney tissue of mice in the CLP model group increased, while the Scr and BUN in the kidney tissue of mice decreased after the addition of A151 (Figure 6(B)). The AIM2 protein level increased in the renal tissue of CLP model mice, and the AIM2 expression was decreased after the addition of inhibitor (Figure 6(C)). The renal cell death of mice in the CLP group increased, and the apoptosis decreased after adding AIM2 inhibitor (Figure 6(D)). The LDH release in the CLP group increased, suggesting the cytotoxicity increased, and the toxicity decreased with AIM2 inhibitor (Figure 6(E)). Inflammasome activation and cell death, including pyroptosis, apoptosis, and programmed necrosis, occurred in the renal tissue of mice in the CLP group, indicating that PANoptosis occurred. However, the PANoptosis index decreased after adding AIM2 inhibitor (Figure 6(F)). Taken together, these results suggest that AIM2 inhibition suppresses PANoptosis and reduces sepsis-induced renal injury in mice.
Figure 6.
Inhibition of AIM2-mediated PANoptosis alleviated sepsis-induced renal injury in mice. The mice were subjected to different groups: Sham group, CLP group, CLP + PBS group, and CLP + A151 (AIM2 inhibitor) group. (A) HE staining and PAS staining were used to detect the changes of renal tissue in mouse model. (B) Serum levels of Scr and BUN in mice were tested by kits. (C) AIM2 expression in renal tissue of mice was assessed with Western blot. (D) Images of TUNEL staining and TUNEL-positive cells in mouse kidney tissue. (E) LDH kit was adopted to test cytotoxicity. (F) Western blot was utilized to determine the levels of pyroptosis, apoptosis and necroptosis-related proteins in renal tissue of mice. *p < .05, **p < .01, and ***p < .001. n = 5 mice/group.
4. Discussion
PANoptosis, a newly discovered form of programmed cell death, not only involves intricate regulatory mechanisms but also exhibits significant associations with various diseases [20]. Current evidence suggests that PANoptosis is an important mechanism for body to fight infection [21]. Sepsis, resulting from an infection, triggers a systemic inflammatory response and frequently leads to widespread cellular demise. It has been shown that PANoptosis is activated simultaneously in rats with sepsis-associated encephalopathy [22]. In addition, PANoptosis plays an important role in renal injury. For example, 3,4-methylenedioxy-β-nitrostyrene (MNS) significantly protects the kidney against renal ischemia–reperfusion injury by reducing PANoptosis [23]. Aspera water-soluble extract (AAW) downregulates genes and signaling pathways associated with oxidative stress, inflammation, and PANoptosis, thereby reducing cisplatin-induced AKI [24]. In the current study, a successful establishment of the sepsis-induced AKI model in mice was achieved. Additionally, our findings indicated the presence of PANoptosis in the kidneys of mice with septic AKI, suggesting the concurrent activation of multiple cell death pathways in response to sepsis.
AIM2 is known to play an essential role in host defense against pathogens, inflammatory diseases, and cancer [25]. A previous study indicated that the potential of AIM2 in sepsis diagnosis holds significant promise [12]. Especially in AKI, roxadustat-conveyed protection against AKI may be mediated by the suppression of the AIM2 inflammasome [26]. Calpain can improve renal functional deterioration, attenuate pathological structure damage, and decrease tubular cell apoptosis in AKI mice by suppressing the AIM2 inflammasome [14]. Results from our group indicated that AIM2 expression was increased in septic AKI mice and AIM2 depletion alleviated sepsis-induced renal injury. Notably, by regulating the innate immune sensors pyrin and ZBP1, AIM2 demonstrates the capacity to modulate both inflammatory signaling and PANoptosis [11]. Through in vitro experiments, we discovered that AIM2 promoted AKI through enhancing PANoptosis, suggesting an inductive role for AIM2 in septic AKI.
EIF2AK2 has been shown to act as an activator of the inflammasome [27]. The activation of AIM2 inflammasome is associated with EIF2AK2, and inhibition of EIF2AK2 leads to a decrease in AIM2 inflammasome activation [18]. Here, we additionally validated that EIF2AK2 physically interacted with AIM2. In addition, EIF2AK2 activation plays a critical role in driving severe sepsis [16]. Moreover, inhibiting EIF2AK2 shows promise as a therapeutic approach to alleviate septic AKI [17]. We further found that EIF2AK2 knockdown inhibited PANoptosis by decreasing AIM2 expression in LPS-treated HK-2 cells. Thus, our findings revealed EIF2AK2 and AIM2 as master regulators of PANoptosis in sepsis-induced AKI.
In summary, we demonstrated that PANoptosis occurred simultaneously in septic AKI. AIM2 activated PANoptosis and AIM2 knockdown provided a renal protective effect in septic AKI. Further, EIF2AK2 targeted AIM2 and the EIF2AK2/AIM2 axis promoted PANoptosis. Hence, EIF2AK2 and AIM2 may function as potential targets for the treatment of septic AKI. However, our research also has some limitations. It is still unclear whether the effects of various inflammatory corpuscles on PANoptosis of septic AKI are synergistic or superimposed. A previous report indicated that NLRP3 inflammasome, AIM2 inflammasome, NLRC4, and pyrin are combined to form a large multiprotein complex, which drives PANoptosis together with ASC, CASP-1, CASP-8, and RIPK3 [28]. A recent review showed that several PANoptosis complexes (including ZBP1-, AIM2-, RIPK1-, and NLRP12-PANoptosomes) have been identified [29]. Interestingly, it was proposed in a recent report that NLRP3 inflammasome might mediate PANoptosis in AKI [24]. It can be seen that NLRP3 inflammatory may also regulate the PANoptosis of septic AKI, and the possible regulatory relationship between NLRP3 inflammatory and AIM2 inflammatory will also be the direction of our follow-up research.
Supplementary Material
Acknowledgements
We would like to give our sincere gratitude to the reviewers for their constructive comments.
Funding Statement
This study was supported by Hunan Provincial Natural Science Foundation of China (No. 2023JJ40348).
Author contributions
Conceptualization: Siwei Wei, Zhen Du. Methodology: Lei Wu, Dongjie Pei. Software: Zhen Xiang, Xiaoxiao Yang. Validation: Siwei Wei, Zhen Xiang, Xiaoxiao Yang, Liubing Jiang. Formal analysis: Lei Wu, Dongjie Pei. Investigation: Siwei Wei, Zhen Xiang. Resources: Zhen Xiang, Liubing Jiang. Data curation: Lei Wu, Dongjie Pei. Writing – original draft: Siwei Wei. Writing – review and editing: Siwei Wei. Visualization: Xiaoxiao Yang, Liubing Jiang. Supervision: Zhen Du, Siwei Wei. Project administration: Zhen Du. All authors read and approved the final manuscript.
Ethics statement
The Animal Experiment was approved by the Laboratory Animal Welfare and Ethical Committee of the Hospital.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All data generated or analyzed during this study are included in this article.
References
- 1.Srzić I, Nesek Adam V, Tunjić Pejak D.. Sepsis definition: what’s new in the treatment guidelines. Acta Clin Croat. 2022;61(Suppl. 1):67–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peerapornratana S, Manrique-Caballero CL, Gómez H, et al. Acute kidney injury from sepsis: current concepts, epidemiology, pathophysiology, prevention and treatment. Kidney Int. 2019;96(5):1083–1099. doi: 10.1016/j.kint.2019.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu Z, Deng J, Zhou H, et al. Programmed cell death in sepsis associated acute kidney injury. Front Med. 2022;9:883028. doi: 10.3389/fmed.2022.883028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang G, Bao J, Shao X, et al. Inhibiting pannexin-1 alleviates sepsis-induced acute kidney injury via decreasing NLRP3 inflammasome activation and cell apoptosis. Life Sci. 2020;254:117791. doi: 10.1016/j.lfs.2020.117791. [DOI] [PubMed] [Google Scholar]
- 5.An S, Yao Y, Wu J, et al. Gut-derived 4-hydroxyphenylacetic acid attenuates sepsis-induced acute kidney injury by upregulating ARC to inhibit necroptosis. Biochim Biophys Acta Mol Basis Dis. 2023;1870(1):166876. doi: 10.1016/j.bbadis.2023.166876. [DOI] [PubMed] [Google Scholar]
- 6.Sun J, Ge X, Wang Y, et al. USF2 knockdown downregulates THBS1 to inhibit the TGF-β signaling pathway and reduce pyroptosis in sepsis-induced acute kidney injury. Pharmacol Res. 2022;176:105962. doi: 10.1016/j.phrs.2021.105962. [DOI] [PubMed] [Google Scholar]
- 7.Zheng M, Kanneganti TD.. The regulation of the ZBP1–NLRP3 inflammasome and its implications in pyroptosis, apoptosis, and necroptosis (PANoptosis). Immunol Rev. 2020;297(1):26–38. doi: 10.1111/imr.12909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Christgen S, Zheng M, Kesavardhana S, et al. Identification of the PANoptosome: a molecular platform triggering pyroptosis, apoptosis, and necroptosis (PANoptosis). Front Cell Infect Microbiol. 2020;10:237. doi: 10.3389/fcimb.2020.00237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malireddi RKS, Kesavardhana S, Karki R, et al. RIPK1 distinctly regulates yersinia-induced inflammatory cell death, PANoptosis. Immunohorizons. 2020;4(12):789–796. doi: 10.4049/immunohorizons.2000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharma BR, Karki R, Kanneganti TD.. Role of AIM2 inflammasome in inflammatory diseases, cancer and infection. Eur J Immunol. 2019;49(11):1998–2011. doi: 10.1002/eji.201848070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee S, Karki R, Wang Y, et al. AIM2 forms a complex with pyrin and ZBP1 to drive PANoptosis and host defence. Nature. 2021;597(7876):415–419. doi: 10.1038/s41586-021-03875-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liang S, Xing M, Chen X, et al. Predicting the prognosis in patients with sepsis by a pyroptosis-related gene signature. Front Immunol. 2022;13:1110602. doi: 10.3389/fimmu.2022.1110602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Komada T, Chung H, Lau A, et al. Macrophage uptake of necrotic cell DNA activates the AIM2 inflammasome to regulate a proinflammatory phenotype in CKD. J Am Soc Nephrol. 2018;29(4):1165–1181. doi: 10.1681/ASN.2017080863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu Y, Yang H, Cheng M, et al. Calpain inhibitor calpeptin alleviates ischemia/reperfusion-induced acute kidney injury via suppressing AIM2 inflammasome and upregulating Klotho protein. Front Med. 2022;9:811980. doi: 10.3389/fmed.2022.811980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu B, Nakamura T, Inouye K, et al. Novel role of PKR in inflammasome activation and HMGB1 release. Nature. 2012;488(7413):670–674. doi: 10.1038/nature11290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hato T, Maier B, Syed F, et al. Bacterial sepsis triggers an antiviral response that causes translation shutdown. J Clin Invest. 2019;129(1):296–309. doi: 10.1172/JCI123284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huen SC. Targeting protein translation to prevent septic kidney injury. J Clin Invest. 2019;129(1):60–62. doi: 10.1172/JCI125432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xie M, Yu Y, Kang R, et al. PKM2-dependent glycolysis promotes NLRP3 and AIM2 inflammasome activation. Nat Commun. 2016;7(1):13280. doi: 10.1038/ncomms13280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Q-L, Xing W, Yu C, et al. ROCK1 regulates sepsis-induced acute kidney injury via TLR2-mediated endoplasmic reticulum stress/pyroptosis axis. Mol Immunol. 2021;138:99–109. doi: 10.1016/j.molimm.2021.07.022. [DOI] [PubMed] [Google Scholar]
- 20.Zhu P, Ke Z-R, Chen J-X, et al. Advances in mechanism and regulation of PANoptosis: prospects in disease treatment. Front Immunol. 2023;14:1120034. doi: 10.3389/fimmu.2023.1120034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Place DE, Lee S, Kanneganti TD.. PANoptosis in microbial infection. Curr Opin Microbiol. 2021;59:42–49. doi: 10.1016/j.mib.2020.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou R, Ying J, Qiu X, et al. A new cell death program regulated by toll-like receptor 9 through p38 mitogen-activated protein kinase signaling pathway in a neonatal rat model with sepsis associated encephalopathy. Chin Med J. 2022;135(12):1474–1485. doi: 10.1097/CM9.0000000000002010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uysal E, Dokur M, Kucukdurmaz F, et al. Targeting the PANoptosome with 3,4-methylenedioxy-β-nitrostyrene, reduces PANoptosis and protects the kidney against renal ischemia–reperfusion injury. J Invest Surg. 2022;35(11–12):1824–1835. doi: 10.1080/08941939.2022.2128117. [DOI] [PubMed] [Google Scholar]
- 24.Lin S-Y, Chang C-L, Liou K-T, et al. The protective role of Achyranthes aspera extract against cisplatin-induced nephrotoxicity by alleviating oxidative stress, inflammation, and PANoptosis. J Ethnopharmacol. 2023;319(Pt 1):117097. doi: 10.1016/j.jep.2023.117097. [DOI] [PubMed] [Google Scholar]
- 25.Kumari P, Russo AJ, Shivcharan S, et al. AIM2 in health and disease: inflammasome and beyond. Immunol Rev. 2020;297(1):83–95. doi: 10.1111/imr.12903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang H, Wu Y, Cheng M, et al. Roxadustat (FG-4592) protects against ischaemia-induced acute kidney injury via improving CD73 and decreasing AIM2 inflammasome activation. Nephrol Dial Transplant. 2023;38(4):858–875. doi: 10.1093/ndt/gfac308. [DOI] [PubMed] [Google Scholar]
- 27.Varghese GP, Uporova L, Halfvarson J, et al. Polymorphism in the NLRP3 inflammasome-associated EIF2AK2 gene and inflammatory bowel disease. Mol Med Rep. 2015;11(6):4579–4584. doi: 10.3892/mmr.2015.3236. [DOI] [PubMed] [Google Scholar]
- 28.Oh S, Lee J, Oh J, et al. Integrated NLRP3, AIM2, NLRC4, pyrin inflammasome activation and assembly drive PANoptosis. Cell Mol Immunol. 2023;20(12):1513–1526. doi: 10.1038/s41423-023-01107-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pandeya A, Kanneganti TD.. Therapeutic potential of PANoptosis: innate sensors, inflammasomes, and RIPKs in PANoptosomes. Trends Mol Med. 2024;30(1):74–88. doi: 10.1016/j.molmed.2023.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this article.